Abstract
Objective:
To delineate the temporal patterns of outcome and to determine the probability of seizure freedom with successive antiepileptic drug regimens in newly diagnosed epilepsy.
Methods:
Patients in whom epilepsy was diagnosed and the first antiepileptic drug prescribed between July 1, 1982, and April 1, 2006, were followed up until March 31, 2008. Outcomes were categorized into 4 patterns: A) early and sustained seizure freedom; B) delayed but sustained seizure freedom; C) fluctuation between periods of seizure freedom and relapse; and D) seizure freedom never attained. Probability of seizure freedom with successive drug regimens was compared. Seizure freedom was defined as no seizures for ≥1 year.
Results:
A total of 1,098 patients were included (median age 32 years, range 9–93). At the last clinic visit, 749 (68%) patients were seizure-free, 678 (62%) on monotherapy. Outcome pattern A was observed in 408 (37%), pattern B in 246 (22%), pattern C in 172 (16%), and pattern D in 272 (25%) patients. There was a higher probability of seizure freedom in patients receiving 1 compared to 2 drug regimens, and 2 compared to 3 regimens (p < 0.001). The difference was greater among patients with symptomatic or cryptogenic than with idiopathic epilepsy. Less than 2% of patients became seizure-free on subsequent regimens but a few did so on their sixth or seventh regimen.
Conclusions:
Most patients with newly diagnosed epilepsy had a constant course which could usually be predicted early. The chance of seizure freedom declined with successive drug regimens, most markedly from the first to the third and among patients with localization-related epilepsies.
Seventy million people have epilepsy, with 34–76 per 100,000 developing the condition every year.1 To formulate rational treatment plans, it is important to understand the different clinical courses and patterns of response to antiepileptic drugs (AEDs), ideally by following outcomes from the point of treatment initiation. Most studies conducted at specialist centers were limited by selection bias toward patients with chronic refractory epilepsy.2–5 The few reported cohorts of newly diagnosed patients tended to include only children6,7 or focus on response over time irrespective of treatment status.8
We have previously reported outcomes in adolescent and adult patients with newly diagnosed epilepsy.9,10 Over the ensuing years many new AEDs have become available, but their impact on the overall prognosis of epilepsy remains unclear. The main objectives of the present analysis were to delineate the temporal patterns of seizure outcome and to determine the probability of seizure freedom with successive regimens in an expanded cohort of 1,098 newly diagnosed patients, who were recruited between 1982 and 2006 and followed for up to 26 years.
METHODS
Patients.
Patients in whom epilepsy was diagnosed and the first AED prescribed at the Epilepsy Unit in the Western Infirmary in Glasgow, Scotland, between July 1, 1982, and April 1, 2006, were included in the analysis. All were followed up prospectively until March 31, 2008, or death. Patients with persistent poor adherence to treatment (unrelated to efficacy or tolerability), seizures secondary to drug or alcohol abuse, or psychogenic nonepileptic seizures were excluded. The source of referrals, approach to evaluation and treatment, and follow-up arrangements have been described previously.9 Most were referred by their primary care physicians with less than 10% by the hospital's accident and emergency department. The study population included the patients analyzed in our previous reports (n = 470 and 890, respectively),9,10 thus expanding the cohort and extending the follow-up period.
Treatment.
Upon diagnosis, an appropriate AED was chosen taking into account seizure type and side-effect and interaction profiles.11 In principle, treatment was commenced after 2 or more seizures,10 although it was infrequently (<5%) also offered to patients who experienced 1 seizure if there was evidence of enduring alteration in the brain that increased the likelihood of future seizures such as a structural abnormality.12 We adopted a staged approach to epilepsy management. In general, if the first AED is poorly tolerated at low dosage or fails to improve seizure control, an alternative should be substituted. If the first well-tolerated AED greatly improved, but does not completely abolish the seizures, combination therapy could be tried.13 Reasons for poor adherence to treatment were thoroughly explored, and drug levels were checked as clinically indicated.14 If a problem arose between scheduled appointments, the patient or the primary care physician could contact the Epilepsy Unit using a dedicated telephone line. Drug doses were adjusted as clinical circumstances dictated, with particular attention being paid to efficacy and tolerability. Patients were treated with a single drug whenever possible.15 Treatment was changed if seizures continued or if the patient developed an idiosyncratic reaction or reported intolerable adverse effects. A combination of drugs could be used if the epilepsy remained uncontrolled despite treatment with monotherapy trials.16
Definitions.
Seizures and epileptic syndromes were classified according to the guidelines of the International League Against Epilepsy (ILAE).17,18 The epilepsy was classified as idiopathic, symptomatic, or cryptogenic, according to the putative cause and depending on factors such as age, type of seizure, family history, electroencephalographic changes, and presence or absence of an anatomic brain lesion. Idiopathic epilepsies were presumed to have a genetic origin. Symptomatic epilepsies were considered to be the consequence of an identified structural abnormality. Cryptogenic epilepsy was presumed to be due to an unidentified focal abnormality on the basis of clinical information and results of investigations. Patients were considered seizure-free if they experienced no seizures for at least the previous year on unchanged treatment. Time to seizure freedom was expressed as the time to the start of a seizure-free period of a year or more. An AED regimen was defined as a trial of either a single drug (monotherapy) or a combination of 2 or more drugs. Thus the first regimen was always monotherapy, whereas the second regimen could be an alternative monotherapy, or a combination of the first monotherapy together with a second AED, and so on.
Patterns of outcome over time.
Seizure outcomes were classified into 4 mutually exclusive temporal patterns. In pattern A, patients became seizure-free early (either immediately after or within 6 months of commencing treatment) and remained so throughout follow-up. In pattern B, seizure freedom was delayed for more than 6 months after starting treatment but patients remained seizure-free throughout follow-up. Patients with pattern C outcome had a fluctuating course with periods of seizure freedom (some immediate) lasting more than a year interspersed with relapses. These patients were either seizure-free or not at the time of analysis. Patients exhibiting pattern D never became seizure-free for any complete year.
Statistical analysis.
Continuous demographic variables were summarized using the median, interquartile range (IQR), and range. Categorical variables were summarized using counts and percentages. Kruskal-Wallis and Pearson χ2 tests were employed to compare continuous and categorical variables among the outcome patterns. All tests were 2-sided. A competing risks analysis of time to seizure freedom at the end of the study was carried out accounting for the competing risk of death. Cumulative incidence curves for time to final seizure-free period were constructed.19 Patients not seizure-free (or dead) were censored at March 31, 2008. Previous seizure-free periods were discounted if there had been a relapse before the end of the study, although all seizure-free periods contributed to the outcome pattern analysis. The cumulative incidence curves for time to final seizure-free period were compared for different numbers of drug regimens using Gray's test.20 Because they have similar prognoses,9 patients with symptomatic and cryptogenic epilepsies were combined in some of the subgroup analyses under the general heading of localization-related epilepsies.
RESULTS
A total of 1,098 untreated patients (575, 52% male), who were prescribed their first AED on being diagnosed with epilepsy, were included (outcome of 890 patients up to May 2003 had been reported previously10). Their median age at treatment initiation was 32 years (range 9–93; IQR 20–51). Epilepsy was classified as idiopathic in 251 (23%), symptomatic in 447 (41%), and cryptogenic in 400 (36%) patients. Among the 208 patients who were commenced on treatment between 9 and 18 years of age (inclusive), epilepsy was classified as idiopathic in 110 (52.9%), symptomatic in 33 (15.9%), and cryptogenic in 65 (31.2%). Seventy-six (7%) patients (46 seizure-free, 30 uncontrolled) died during the study period (12 within 2 years of starting treatment). Seven died of sudden unexplained death in epilepsy (2 seizure-free) and the others died from non-epilepsy-related causes. Excluding the 12 patients who died within 2 years of starting treatment, the median duration of follow-up was 7.5 years (IQR 4.7–12.0) since starting treatment.
Outcome at last follow-up.
At the time of the last clinic visit, 913 (83%) patients were receiving treatment with 1 AED. The remaining 185 (17%) patients took a combination of 2 or more drugs. A total of 543 (49%) patients remained seizure-free on their first AED; 398 were treated with a second regimen (as monotherapy in 254, in combination with the first drug in 144), 146 (37%) of whom became seizure-free. The corresponding seizure-free rates for those receiving between 3 and 9 regimens are listed in table 1. Just 70 (6%) patients remained seizure-free on more than 1 drug, with the majority (67 patients) taking 2 drugs. Only 2 patients were controlled on 3 drugs and 1 on 4.
Table 1.
Seizure-free rates with successive antiepileptic drug regimens

A total of 749 patients (68% of 1,098) were seizure-free at the end of the study. Based on the cohorts followed up for at least the respective number of years at the end of the study, 69% (747/1,086), 61% (461/759), and 52% (188/360) of patients remained seizure-free continuously for at least 2, 5, and 10 years, respectively (longest 25 years).
Outcome over time.
Figure 1 displays the progress of the patient population in terms of seizure outcome over time. Outcome pattern A was observed in 408 (37%) patients, who either became seizure-free immediately (n = 262; 24%) or within 6 months of commencing treatment with their first AED (n = 146; 13%). Among these 146 patients, 105 received 1 regimen, 37 were given 2, and 4 required 3. These patients remained seizure-free for the remainder of the follow-up period.
Figure 1. Patient flow throughout the study in terms of seizure outcome.
Seizure-free represents a patient with no seizure for at least the previous 12 months. Pattern A (early and sustained seizure freedom): patients became seizure-free within 6 months of starting treatment and remained seizure-free. Pattern B (delayed and sustained seizure freedom): patients became seizure-free after 6 months of starting treatment and remained seizure-free. Pattern C (fluctuating course): patients fluctuating between periods of seizure freedom and relapse. Pattern D: patients never seizure-free for any complete year.
Outcome pattern B was observed in 246 (22%) patients who continued to report seizures after commencing treatment for more than 6 months, but later became and remained seizure-free until the end of follow-up. The median time to seizure freedom in this group was 15.4 months (range 6–216; IQR 10–27), while the median duration of the seizure-free period was 64.5 months (range 12–287; IQR 38–109).
A total of 172 patients (16%) demonstrated a more fluctuating course (outcome pattern C). After attaining a seizure-free period of a year or more (either early [immediately, n = 88; within 6 months after initial recurrence, n = 28] or with a delay [after 6 months, n = 56]), they had up to 5 periods of relapse (149 had 1 period of relapse, 17 had 2, 4 had 3, 1 had 4, and 1 had 5) intercalated with periods of seizure freedom. The median duration of seizure freedom before relapse was 30 months (range 12–154; IQR 19–53). Among patients with outcome pattern C, 77 (45%) had not attained seizure freedom at the last follow-up visit. Patients who became seizure-free early (immediately or within 6 months of commencing treatment after initial recurrence) were just as likely to relapse (116/524, 22%) as those who did so after a delay of more than 6 months (56/302, 19%; p = 0.26).
A total of 272 (25%) patients never became seizure-free for any complete year throughout follow-up (pattern D), among whom 154 had tried 2 or more AEDs rising to a maximum of 9 regimens in 2 patients.
Patients with outcome patterns A and D had a slightly older age at epilepsy onset (median age 34 and 36 years, respectively) compared to those with patterns B and C (median age 31 and 28 years, respectively). There were slightly more men among patients with pattern A and more women among those with pattern D. Epilepsy syndrome classification was not associated with any particular outcome pattern (table 2).
Table 2.
Patterns of outcome over time in 1,098 newly treated epilepsy patients
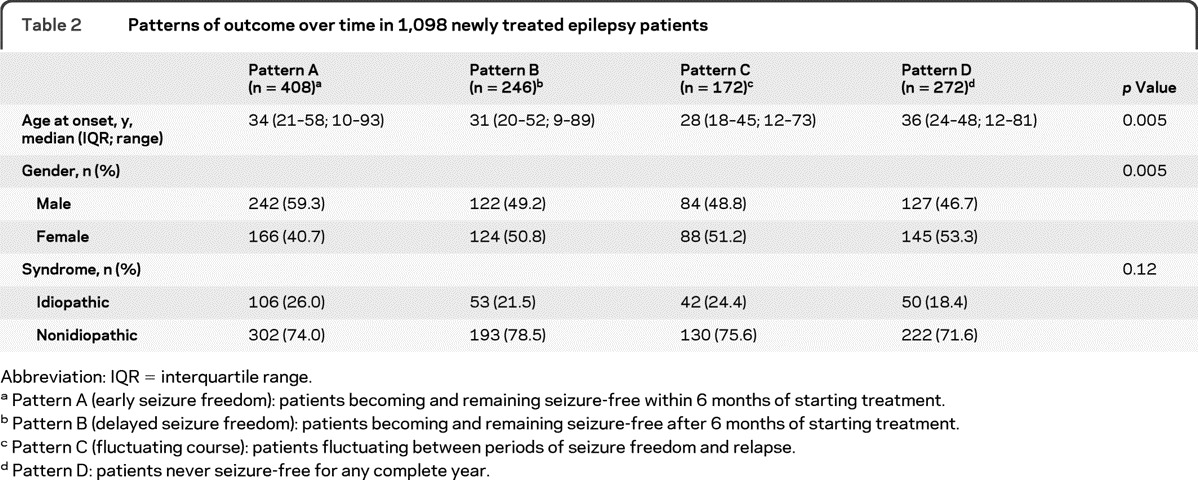
Abbreviation: IQR = interquartile range.
Pattern A (early seizure freedom): patients becoming and remaining seizure-free within 6 months of starting treatment.
Pattern B (delayed seizure freedom): patients becoming and remaining seizure-free after 6 months of starting treatment.
Pattern C (fluctuating course): patients fluctuating between periods of seizure freedom and relapse.
Pattern D: patients never seizure-free for any complete year.
Response to successive drug regimens.
Survival analysis over time confirmed that the chance of seizure freedom declined with the number of AED regimens. There was a significant difference in overall probability of seizure freedom between patients treated with 1 and with 2 regimens (p < 0.001) (figure 2A). The difference was equally marked when comparing those receiving treatment with 2 and 3 regimens (p < 0.001). This was not statistically significant when comparing patients treated with 3, 4, and 5 regimens, although a trend could still be observed. When stratifying the analysis according to epilepsy syndrome classification, patients were grouped into 1, 2, or ≥3 regimens owing to small group sizes for 3, 4, or 5 regimens. Similar differences in probability of seizure freedom among patients taking 1, 2, or ≥3 regimens was noted, although a greater difference between 1 and ≥3 regimens was observed for localization-related (symptomatic or cryptogenic) epilepsy compared with the idiopathic group (figure 2, B and C).
Figure 2. Cumulative probability of being seizure-free by time from start of treatment and number of antiepileptic drug regimens tried.
(A) All patients (n = 1,098; 700 tried 1, 230 tried 2, 100 tried 3, 36 tried 4, and 32 tried 5 or more regimens); (B) patients with idiopathic epilepsies (n = 251; 220 tried 1–2, 31 tried 3 or more regimens); and (C) patients with symptomatic or cryptogenic (nonidiopathic) epilepsies (n = 847; 710 tried 1–2, 137 tried 3 or more regimens).
DISCUSSION
By utilizing the high volume of referrals from primary care physicians, this study included a large number of patients at the point of treatment initiation with a long duration of follow-up. Most modern studies exploring response to sequential treatment regimens in adults have been conducted at single epilepsy centers.5,21,22 As a result, they typically followed patients who had already failed at least 1 or 2 drug regimens and were in need of specialist intervention. Hence, sample sizes tended to be small. The British National General Practice Study of Epilepsy (NGPSE), which recruited over 500 newly diagnosed patients (mainly adults) from the community, last reported seizure freedom rates in the 1990s before many of the newer AEDs became available, and did not analyze relapse rates or sequential responses.8 Both approaches of including newly diagnosed and chronic cohorts have strengths and weaknesses. The former is more representative of the general patient population, but recruitment of a sufficiently large sample often requires multicenter involvement (275 primary care practices in the case of NGPSE) with the associated challenges in quality control or a long duration of recruitment. The latter cohort can be assembled relatively quickly from specialist centers. However, the findings are largely relevant to patients whose epilepsy has already demonstrated a poor prognosis and in whom a history of drug response prior to enrollment cannot be reliably guaranteed. These approaches should, therefore, be regarded as different but complementary. Their findings are valuable in synthesizing a better understanding of how different epilepsies respond to different AED schedules.
The present study with increased number of patients substantiated previous results9,10 and extended the analysis of treatment outcomes and patterns. We observed 4 distinct outcome patterns in patients with newly diagnosed epilepsy. A constant course was apparent in 62% of patients, who either became and remained seizure-free shortly after commencing treatment (outcome pattern A, 37%) or had persistent seizures despite repeated trials of different medications used singly or in combination (outcome pattern D, 25%). Prognosis in the other 38% of patients was less predictable, although 22% appeared to enter remission after a delay varying between 6 months and 18 years (outcome pattern B). The remaining 16% had a “remitting-relapsing” course (outcome pattern C), fluctuating between periods of seizure freedom and recurrence. Recognizing the latter 2 outcome patterns is important because they suggest that drug response can be a dynamic process.
Our observation of a “remitting-relapsing” course in approximately 1 in 6 of patients corresponds well with data from studies in chronic cohorts, in which seizure freedom could be achieved even after failure of 2 or more medications at a rate of approximately 3%–5% per year, although the epilepsy subsequently relapsed within 5 years in up to 80% of these patients.5,19,22 Relapse was often not temporally related to medication change or environmental triggers, suggesting that other unknown factors came into play after a significant period of seizure freedom. This is perhaps not surprising since AEDs treat the symptoms (seizures) but not the cause of the epilepsy.23 In the present cohort, patterns of outcome were not associated with epilepsy syndrome classification, and patient groups differed little in age at onset and gender distribution. Multivariate analyses in other studies have consistently shown that seizure freedom and relapse were not associated with a range of clinical factors including age, gender, epilepsy syndrome, and preremission seizure frequency, but only predicted by the number of drug regimens failed.5,21
The progressive decline in the likelihood of producing seizure freedom with successive AED regimens was clearly demonstrated in the present study, most markedly from the first to the third treatment schedule. These differences did not reach statistical significance thereafter, probably owing to the smaller number of patients trying more than 3 regimens. This pattern of response was particularly impressive in patients with localization-related epilepsies, who should, therefore, be evaluated early for resective surgery.24 Nonetheless, we did find that a few patients (2%) could demonstrate a lasting response to their fourth, fifth, sixth, or even seventh regimen (but none beyond), whether as monotherapy or in a combination. This observation concurs with the view that, at least in the short term, prognosis for patients who do not respond to their first few drug regimens should not always be viewed nihilistically.21 Even if other treatment options, such as surgery, are not appropriate, further pharmacologic modifications can still occasionally produce a worthwhile and sustained response.25
Despite being the largest studied cohort of newly diagnosed patients with a follow-up duration of up to 26 years, the present analysis has highlighted some of the major challenges in studying seizure outcomes in epilepsy. Cohorts of this size still have limited statistical power to identify predictive factors for the various outcome patterns. Another challenge is the classification of different outcomes, which was dichotomized in the present study into absolute seizure freedom or not, although it can be argued that seizure frequency should be regarded as a continuous trait. Moreover, different cutoff points can be chosen according to the purpose of the analysis (e.g., selecting patients for epilepsy surgery, designing an epidemiologic study). We chose our definition of seizure freedom (≥12 months) prior to the publication of the ILAE's consensus which requires no seizures for 12 months or 3 times the longest pretreatment interseizure interval, whichever is longer.26 Using the latter definition would be more rigorous in patients with infrequent seizures, but applying the former has facilitated comparison with previous reports. In addition, 1 year as the minimum duration of seizure-free has been consistently associated with meaningful improvement in quality of life.26
There is limited evidence endorsing improved outcomes in the common adult epilepsies over the past 20 years despite the global introduction of more than 12 new AEDs.27 Compared with our initial analysis in the first 470 patients of this expanding cohort,28 the seizure-free rate 10 years later has increased from 64% to 68%. One interpretation of this observation could be that these patients have been followed up for longer, thus allowing them more time to become seizure-free. This difference could also be explained by an increase in successful combination therapy (usually just 2 drugs), rising from 3% to 6% over the past decade, implying a positive, albeit limited, effect of the introduction of a range of newer agents as add-on treatment.
This analysis illustrates that there are identifiable outcome patterns in newly diagnosed epilepsy, and that treatment resistance can be defined successively after the failure of 2 drugs. Nevertheless, all hope for a good prognosis is not lost even after further drug trials. Larger cooperative studies are needed to better understand the risk factors for outcome and the neurobiologies underpinning pharmacoresistance so that this modest but encouraging picture can be improved.
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- ILAE
International League Against Epilepsy
- IQR
interquartile range
- NGPSE
National General Practice Study of Epilepsy.
Footnotes
Editorial, page 1542
AUTHOR CONTRIBUTIONS
M.J.B. is Director of the Epilepsy Unit where the data were collected and conceived the project. S.J.E.B. and G.A.B. analyzed and managed the data under the supervision of J.D.N. and M.J.B. M.J.B. and P.K. coordinated the data analysis plan and interpretation and drafted the paper. All authors contributed to the revision for important intellectual content and approved the final version.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Ngugi AK, Kariuki SM, Bottomley C, Kleinschmidt I, Sander JW, Newton CR. Incidence of epilepsy: a systematic review and meta-analysis. Neurology 2011; 77: 1005– 1012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Callaghan BC, Anand K, Hesdorffer D, Hauser WA, French JA. Likelihood of seizure remission in an adult population with refractory epilepsy. Ann Neurol 2007; 62: 382– 389 . [DOI] [PubMed] [Google Scholar]
- 3. Luciano AL, Shorvon SD. Results of treatment changes in patients with apparently drug-resistant chronic epilepsy. Ann Neurol 2007; 62: 375– 381 . [DOI] [PubMed] [Google Scholar]
- 4. Choi H, Heiman G, Pandis D, et al. Seizure remission and relapse in adults with intractable epilepsy: a cohort study. Epilepsia 2008; 49: 1440– 1445 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiller Y. Seizure relapse and development of drug resistance following long-term remission. Arch Neurol 2009; 66: 1233– 1239 . [DOI] [PubMed] [Google Scholar]
- 6. Sillanpäa M, Schmidt D. Natural history of treated childhood-onset epilepsy: prospective long-term population-based study. Brain 2006; 129: 617– 624 . [DOI] [PubMed] [Google Scholar]
- 7. Berg AT, Levy SR, Testa FM, D'Souza R. Remission of epilepsy after failures in children: a prospective study. Ann Neurol 2009; 65: 510– 519 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995; 346: 140– 144 . [DOI] [PubMed] [Google Scholar]
- 9. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med 2000; 342: 314– 319 . [DOI] [PubMed] [Google Scholar]
- 10. Mohanraj R, Brodie MJ. Diagnosing refractory epilepsy: response to sequential treatment schedules. Eur J Neurol 2006; 13: 277– 282 . [DOI] [PubMed] [Google Scholar]
- 11. Stephen LJ, Brodie MJ. Selection of antiepileptic drugs in adults. Neurol Clin 2009; 27: 967– 992 . [DOI] [PubMed] [Google Scholar]
- 12. Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr Epilepsy seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005; 46: 470– 472 . [DOI] [PubMed] [Google Scholar]
- 13. Brodie MJ, Kwan P. Staged approach to epilepsy management. Neurology 2002; 58 (suppl 5): S2– S8 . [DOI] [PubMed] [Google Scholar]
- 14. Patsalos PN, Berry DJ, Bourgeois BFD, et al. Antiepileptic drugs: best practice guidelines for therapeutic drug monitoring: a position paper by the Subcommission on Therapeutic Drug Monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia 2008; 49: 1239– 1276 . [DOI] [PubMed] [Google Scholar]
- 15. French JA, Pedley TA. Initial management of epilepsy. N Engl J Med 2008; 359: 166– 176 . [DOI] [PubMed] [Google Scholar]
- 16. Kwan P, Brodie MJ. Combination therapy in epilepsy: when and what to use. Drugs 2006; 66: 1817– 1829 . [DOI] [PubMed] [Google Scholar]
- 17. Commission on classification and terminology of the ILAE: proposal for revised clinical classification of epileptic seizures. Epilepsia 1981; 22: 489– 501 . [DOI] [PubMed] [Google Scholar]
- 18. Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsy and epileptic syndromes. Epilepsia 1989; 30: 389– 399 . [DOI] [PubMed] [Google Scholar]
- 19. Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data, 2nd ed. New York: Springer-Verlag; 2003 [Google Scholar]
- 20. Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1141– 1154 . [Google Scholar]
- 21. Callaghan B, Schlesinger M, Rodemer W, et al. Remission and relapse in a drug-resistant epilepsy population followed prospectively. Epilepsia 2011; 52: 619– 626 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Choi H, Heiman GA, Clary HM, Etienne M, Resor SR, Hauser WA. Seizure remission in adults with long-standing intractable epilepsy: an extended follow-up. Epilepsy Res 2011; 93: 115– 119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dichter MA. Emerging concepts in the pathogenesis of epilepsy and epileptogenesis. Arch Neurol 2009; 66: 443– 447 . [DOI] [PubMed] [Google Scholar]
- 24. Engel J, Jr, Wiebe S, French J, et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 2003; 44: 741– 751 . [DOI] [PubMed] [Google Scholar]
- 25. Schiller Y, Najjar Y. Quantifying response to antiepileptic drugs: effect of past treatment history. Neurology 2008; 70: 54– 65 . [DOI] [PubMed] [Google Scholar]
- 26. Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010; 50: 1069– 1077 . [DOI] [PubMed] [Google Scholar]
- 27. Löscher W, Schmidt D. Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 2011; 52: 657– 678 . [DOI] [PubMed] [Google Scholar]
- 28. Kwan P, Brodie MJ. Definition of refractory epilepsy: defining the indefinable? Lancet Neurol 2010; 9: 27– 29 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




