Abstract
Objective:
To describe 16 patients with a coincidence of 2 rare diseases: aquaporin-4 antibody (AQP4-Ab)–mediated neuromyelitis optica spectrum disorder (AQP4-NMOSD) and acetylcholine receptor antibody (AChR-Ab)–mediated myasthenia gravis (AChR-MG).
Methods:
The clinical details and antibody results of 16 patients with AChR-MG and AQP4-NMOSD were analyzed retrospectively.
Results:
All had early-onset AChR-MG, the majority with mild generalized disease, and a high proportion achieved remission. Fifteen were female; 11 were Caucasian. In 14/16, the MG preceded NMOSD (median interval: 16 years) and 11 of these had had a thymectomy although 1 only after NMOSD onset. In 4/5 patients tested, AQP4-Abs were detectable between 4 and 16 years prior to disease onset, including 2 patients with detectable AQP4-Abs prior to thymectomy. AChR-Abs decreased and the AQP4-Ab levels increased over time in concordance with the relevant disease. AChR-Abs were detectable at NMOSD onset in the one sample available from 1 of the 2 patients with NMOSD before MG.
Conclusions:
Although both conditions are rare, the association of MG and NMOSD occurs much more frequently than by chance and the MG appears to follow a benign course. AChR-Abs or AQP4-Abs may be present years before onset of the relevant disease and the antibody titers against AQP4 and AChR tend to change in opposite directions. Although most cases had MG prior to NMOSD onset, and had undergone thymectomy, NMOSD can occur first and in patients who have not had their thymus removed.
Neuromyelitis optica (NMO) is a recurrent inflammatory and demyelinating CNS disorder that affects predominantly the optic nerve and spinal cord.1 In the majority of patients, the disease is associated with immunoglobulin G1–complement activating antibodies (Ab) against aquaporin-4 (AQP4).2–4 The detection of these antibodies helps distinguish between NMO and other inflammatory and autoimmune disorders of the CNS2,4 and characterizes a form of NMO spectrum disorder (NMOSD) that includes spatially limited forms of disease such as longitudinally extensive transverse myelitis (LETM) or optic neuritis (ON).5,6 The pathogenicity of the antibodies has been shown by passive transfer in different animal models,7–9 as reviewed elsewhere.10
Myasthenia gravis (MG) is a well-recognized antibody-mediated disease affecting the neuromuscular junction, caused in around 85% of patients by immunoglobulin G (IgG)1- and IgG3-complement activating antibodies against the nicotinic acetylcholine receptor (AChR, AChR-Ab); for review, see Hoedemaekers et al.11
Both AQP4-Ab-positive NMOSD and AChR-Ab-positive MG are associated with other autoimmune diseases and autoantibodies, both organ-specific and systemic.5,12–15 Despite the rarity of MG and of NMO, several cases or small series of patients with both disorders have been reported over the years, most published prior to the identification of AQP4-Abs. In the English language literature, only 7 cases of AChR-Ab positive MG and AQP4-Ab-positive NMO/NMOSD have been reported.16–20
Here we describe 16 patients with MG and NMOSD from 9 neurology centers around the world, and describe the clinical features, serologic, and temporal associations of the diseases.
METHODS
Clinical data.
Sixteen patients with MG with AChR-Abs and NMOSD with AQP4-Abs were identified from the databases of neurology centers in the United Kingdom, Brazil, Portugal, Japan, and Argentina. One patient with AChR-MG and NMO-IgG seronegative NMOSD was not included because serum was not available to be tested for AQP4-Abs. Data on the clinical features, associated diseases, investigation results, treatments, and outcomes were collected from the patients' notes. All additional serum samples available from those patients were tested for AChR and AQP4 antibodies.
Standard protocol approvals, registrations, and patient consents.
This retrospective study was approved by the Medical Ethics Committee of each center and conducted in accordance with the ethical standards recognized internationally. Written consent to collect and use anonymized clinical data, as well as serum and thymus tissue, was obtained from each patient prior to the study.
RESULTS
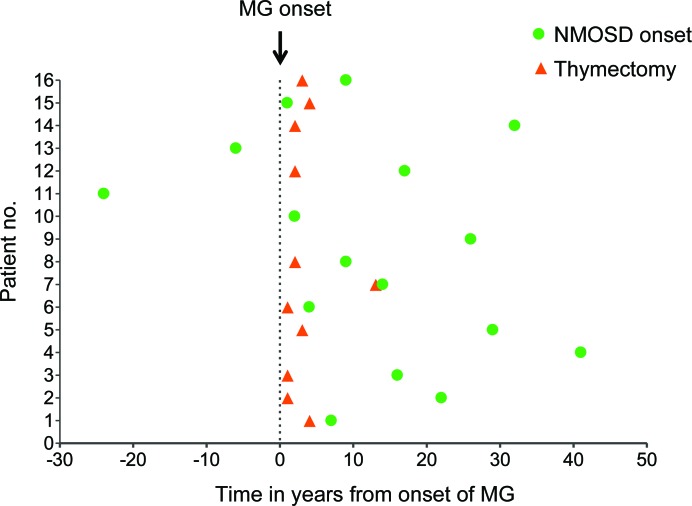
Eight of the 16 patients were identified from the UK NMO service. Fifteen of the 16 were female, and 11 were Caucasian. The clinical and laboratory data of each patient are summarized in table e-1 and table e-2 on the Neurology® Web site at www.neurology.org. Figure 1 illustrates the timing of the thymectomy and the NMOSD onset in relation to the onset of MG for each patient.
Figure 1. Time, in years, to thymectomy and to neuromyelitis optica spectrum disorder (NMOSD) onset in relation to the myasthenia gravis (MG) onset for each patient.
Patients are numbered as in the tables. MG onset: time point 0; red triangle: thymectomy; green dot: NMOSD onset.
Myasthenia gravis features.
MG onset occurred between the ages of 12 and 47 years (median: 26.5) and all patients were positive for AChR antibodies. The time to the diagnosis of MG ranged from 0 to 3 years (median 0.5). The initial presentation was typical with ocular muscle weakness in 14 patients, and all but 3 became generalized over time. In general the MG was mild to moderate (at maximal severity none of the patients had respiratory symptoms, and none required intensive care/respiratory support, except perioperatively related to thymectomy) and, in 6 patients, the response to pyridostigmine was sufficient to control their myasthenic symptoms. Nine patients required immunosuppressive medications such as oral steroids with or without azathioprine. IV immunoglobulin and plasma exchange was only required early on in the disease course. Thymectomy was performed in 11 of the 13 patients with generalized MG. Time to thymectomy varied from 1 to 13 years (median 2) after MG onset. Eight patients had thymic hyperplasia; the remaining 3 had normal histology. All responded well to treatments, as seen by their MGFA score at maximal severity and MGFA postintervention status (table e-1).
Myasthenia gravis in relation to the presentation and course of NMOSD.
In 14/16 patients, the MG presented prior to NMOSD, and the time between the onset of both conditions ranged from 1 to 41 years (median 16). Among those 14 patients, only 11 were thymectomized, 10 before NMOSD onset (3 to 30 years of interval; median 11 years) and 1 after. Seven of those 14 patients needed immunosuppression for their MG, but 4 had stopped that treatment between 2 and 16 years prior to the NMOSD onset; only 3 were still taking immunosuppression at a low dose when NMOSD presented. There was no correlation between timing of discontinuation of immunosuppressive treatment for MG and time to onset of NMOSD. Eleven had minimal myasthenic symptoms or had been in remission for months to 25 years at the time of NMOSD presentation. At the onset of NMOSD, MG was quiescent and remained so in all but 2 patients who had a mild exacerbation of myasthenic symptoms. There was no clinical evidence of deterioration or flare up of any of the other associated autoimmune conditions at, or following onset of, NMOSD, nor was there any clinical evidence of new autoimmune diseases.
The other 2 patients developed MG 6 and 24 years after the first manifestations of NMOSD. These patients were not thymectomized. Both patients were taking azathioprine at low dose when the MG started. There was no apparent exacerbation of NMOSD at the MG onset.
NMOSD features.
The clinical presentation of NMOSD occurred between the ages of 23 and 67 years (median 39.5). All patients were positive for AQP4-Abs using the cell-based assay previously described.2,21 In the first 10 patients, the median time from onset of NMOSD to serologic diagnosis was 4 years, but in the 6 recently diagnosed patients the delay did not exceed 1 year. The initial NMOSD symptoms were ON in 8 patients (1 bilateral ON and 7 unilateral ON) and TM (associated with LETM) in the other 8. The disease was monophasic in 3 patients (2 had severe ON and 1 had LETM); 1 of them was already on steroid treatment for her MG, another started immunosuppression immediately after the NMOSD onset attack, and the third started immunosuppression 4 years after the onset symptoms when the diagnosis was made. Their follow-up times ranged from 6 months to 6 years from NMOSD onset.
In the 13 patients with relapsing disease, 4/13 had relapses affecting the same system (rON in 2 and rLETM in 2), whereas 9 experienced attacks involving both optic nerve and spinal cord fulfilling the diagnostic criteria for NMO.
Brain MRI was performed during the first attack in 13 patients, and was normal in 10 except for increased T2 signal in the optic nerve or the optic chiasm in 4. The other 3 patients had small nonspecific/vascular white matter lesions. Spinal cord MRI showed LETM in all the patients who had transverse myelitis. CSF examination was performed in 10 patients, of whom 1 had positive oligoclonal bands (OCB) and 4 had a mild pleocytosis. We did not find any obvious relationships between the NMOSD features and the MG characteristics or treatments, such as thymectomy.
Treatments and evolution of NMOSD.
In general, all patients responded well to immunosuppressive (IS) therapy (reduction in relapse rate), that was started (or increased) at variable times in the disease course. At the last visit, all 15 patients with available treatment information were on IS, the majority on a combined regimen of prednisolone and azathioprine. Despite this, a considerable proportion of patients had profound deficits: overall, 10 out of the 16 patients were blind in at least 1 eye or unable to walk. One patient died of complications related to severe NMO at age 54 (12 years post NMO onset and 28 years post MG onset). The disability at last follow-up did not correlate with the age at onset of any of the diseases, the severity of MG, or whether or not they had undergone thymectomy. However, patients with longer disease duration and delayed diagnosis were more disabled.
Evidence of antibodies preceding the onset of the diseases: AQP4 and AChR antibodies.
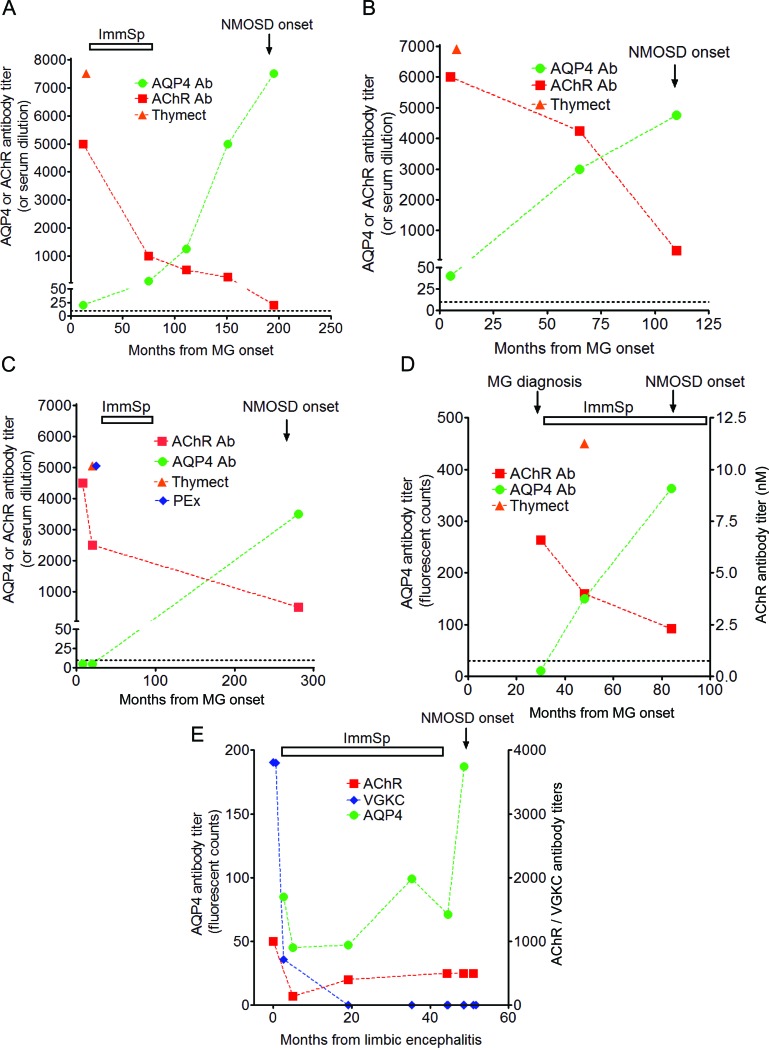
Four of the 5 patients on whom stored serum samples were available had AQP4-Abs for 4 to 16 years before onset of NMOSD (patients 3, 8, 1, and 4) (figure 2, A–E). In 2 patients, AQP4-Abs were detectable at the time of presentation of MG and prior to thymectomy (8 and 16 years before first manifestation of the NMOSD). In all 5 cases, the AQP4-Ab levels increased until NMOSD onset, whereas the AChR-Abs declined following treatments for their MG, although remaining positive as is usually observed. The MG at NMOSD onset was in remission in 2 of these and mild in 3 and only 1 was still on low-dose immunosuppression. Similarly, the only patient with serum available, whose NMOSD presented before MG (patient 13), had detectable AChR-Abs at the time of NMOSD presentation.
Figure 2. Sequential titers of serum autoantibodies against acetylcholine receptor (AChR) and aquaporin-4 (AQP4) (A–D) and also against voltage-gated potassium channel (VGKC) (E), and their correlation with clinical manifestations and treatments of the respective diseases (myasthenia gravis [MG], neuromyelitis optica spectrum disorder [NMOSD], and limbic encephalitis [LE]).
Clinical features of each of the diseases in all patients illustrated were temporally correlated with high titers of the respective antibodies. In all patients, acetylcholine receptor antibody (AChR-Ab) titers were highest in the first sample available (in the majority of patients at the MG onset or MG diagnosis), and decreased following MG treatments (either thymectomy or immunosuppression or both). AQP4-Ab titers were low in 3 patients (A, patient 3; B, patient 8; E, patient 4) or undetectable in 2 patients (C, patient 2; D, patient 1) in the first sample tested. Regardless of the treatment for LE or MG, AQP4-Ab titers were increased at NMOSD onset (C, patient 2) or increased throughout up to 16 years (A, patient 3), peaking at the first manifestations of NMOSD.
Other associated autoimmunity.
Thirteen patients had laboratory manifestations of other autoimmunity, 7 of whom had clinical manifestations. Of these, 5 patients had autoimmune thyroid disease with thyroid peroxidase antibodies and hypothyroidism, treated with thyroxine; 1 patient had mild/moderately severe systemic lupus erythematosus (SLE); and 1 had had an episode of limbic encephalitis and neuromyotonia associated with voltage-gated potassium channel complex (VGKC) antibodies. In the latter case the VGKC antibodies fell rapidly after treatment, as is usual for these antibodies when treated adequately.22 The remaining 6 patients had 1 or more autoantibodies, predominantly antinuclear, anti-DNA, antithyroid peroxidase, and anticardiolipin antibodies. In the majority of cases, the associated autoimmune conditions and laboratory markers were apparent at the time of presentation with the primary disease.
A family history of other autoimmune diseases was recorded in 4 patients, 1 of whom had 3 affected family members; the diseases were autoimmune thyroid disease (3), type I diabetes (2), and rheumatoid arthritis (1).
Comparison between patients with both early-onset AChR-MG and AQP4-NMOSD and populations with each of those diseases alone.
The clinical features and severity of the diseases in patients with both conditions were compared to those in patients with either disease alone (51 patients with early-onset AChR-MG23; 106 patients with AQP4-NMOSD24); see table e-3. There were no significant differences among these 3 cohorts apart from an increase in other autoimmune manifestations in patients with both diseases compared to the AChR-MG cohort (p < 0.0001) and a trend when compared to AQP4-NMOSD alone (p = 0.0146 [p < 0.0026 is significant after Bonferroni correction for multiple comparisons]). The patients with both diseases tended to have milder MG than patients with AChR-Ab MG only (p = 0.0058). There was a greater female predominance in the group of patients with both diseases than in those with either of the diseases, although this difference was not statistically significant after Bonferroni correction.
DISCUSSION
We describe a cohort of 16 patients with 2 rare organ-specific autoimmune diseases mediated by 2 distinct antibodies, to AChR and to AQP4, which are both predominantly IgG1 complement activating antibodies.2,25,26 The features of the 2 diseases were typical, although the MG tended to be relatively mild and treatment responsive.
Among the few English-language publications of patients with MG and demyelinating CNS diseases, only 7 patients were known to have antibody-positive MG and NMO.16–20 The prevalence of early-onset AChR-MG (rather than late-onset MG, which is now much more common) is about 6.2:100,000 (Carr and McConville, in preparation) and AQP4-NMOSD prevalence is about 2.7:100,000.27 Thus by chance one would expect only 1:600 million. Since the population of the United Kingdom is 66 million, the existence of 8 UK cases is approximately 70 times higher than expected by chance (based on the prevalence of the individual diseases), suggesting a predisposition to autoimmunity in these patients.
AQP4-NMOSD is recognized to be associated with other autoimmune manifestations in about 25%–50% of cases, both organ-specific and systemic. These include SLE, Sjögren syndrome, rheumatoid arthritis, autoimmune thyroid disease, and their respective antibodies.12,13,28 The same associations are reported in MG, although less frequently (5%–30%) with thyroid autoimmunity, particularly Graves disease, predominating.14,15 In our series, 44% of the patients had clinical and laboratory features of thyroiditis with hypothyroidism associated with thyroid peroxidase Abs, and 81% had clinical or laboratory evidence of other autoimmune conditions such as Sjögren syndrome, SLE, or antiphospholipid syndrome. This is significantly higher than their frequency in isolated NMOSD or MG, further reflecting an increased risk of multiple autoimmune manifestations in this subgroup of patients.12–15,28
In 90% of our patients, MG preceded NMOSD, and nearly 70% had had a preceding thymectomy (as is standard treatment in early-onset generalized AChR-MG), as was found in all 6 previous AChR-Ab and AQP4-Ab positive cases reported.16–20 The thymic pathology showed the expected hyperplasia in the majority of our patients but was normal in some. Although 81% of our patients had generalized MG, none were severely affected and a high proportion achieved remission. It was rare for the MG to relapse once NMOSD developed, and the AChR-Ab levels correlated with the MG clinical course. However, more severely affected AChR-MG patients may be on more aggressive treatment that could prevent the clinical expression of NMOSD. A screening of patients with MG for AQP4-Abs is needed to clarify this finding.
A link between the thymus gland and MG has been demonstrated previously. Human thymus tissue has been shown to express AChR,29,30 which is widely thought to be a triggering mechanism in early-onset AChR-MG.31–33 Recent evidence suggests that AQP4 is also expressed in human thymus34 (Marx and Strobel, unpublished data; Leite and Vincent, unpublished data), suggesting a similar, and early, involvement of the thymus in NMOSD; this is supported by our findings of AQP4-Abs being detectable years prior to the onset of NMOSD, including at MG onset.
The clinical and laboratory features of the individual diseases and their time courses remain unchanged in patients with both conditions suggesting coincident diseases. However, the relative increase in prevalence of patients with both AQP4-NMOSD and AChR-MG suggests these patients have a predisposition to autoimmunity, but the dynamics of the individual diseases remain unchanged. An interesting finding in this study was the frequent detection of antibodies associated with a second autoimmune disease many years before any clinical signs. This study also implies that the thymus may have a role in the immunopathogenic mechanisms triggering those 2 diseases and possibly other associated autoimmune manifestations, but could not find clear evidence for the role of thymectomy on the development of AQP4-NMOSD.
Given the risk of concurrent autoimmune diseases in patients with either MG or NMO, we suggest routine evaluation of thyroid antibodies, and where possible, AQP4-Abs in all patients with early-onset AChR-MG. When AQP4-Abs are detected in patients with MG without symptoms of NMO, careful observation is strongly encouraged and patients should be warned of a possible increased risk of developing other antibody-mediated conditions. NMOSD should be first considered in patients with MG who develop inflammatory demyelinating conditions of the CNS, and their sera should be tested for AQP4-Abs. In cases where sera are AQP4-Ab negative, other diagnoses such as multiple sclerosis, seronegative NMO, acute disseminated encephalomyelitis, SLE, or Sjögren syndrome may also be considered. Conversely, the development of MG in 2 patients with a primary diagnosis of NMO, and the coexistence of other antibody-mediated diseases in NMO, means that testing for antibodies to AChR and other autoantigens would be appropriate in patients with NMO in whom the clinical features were not fully consistent with the radiologic findings.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. L. Jacobson and Dr. M. Woodhall (UK) for their technical assistance in the antibody assays and Dr. C. Capurro (Argentina) for providing clinical information and reviewing the manuscript.
GLOSSARY
- Ab
antibodies
- AChR-Ab
acetylcholine receptor antibody
- AChR-MG
acetylcholine receptor antibody–mediated myasthenia gravis
- AQP4-Ab
aquaporin-4 antibody
- AQP4-NMOSD
aquaporin-4 antibody–mediated neuromyelitis optica spectrum disorder
- IgG
immunoglobulin G
- IS
immunosuppressive
- LETM
longitudinally extensive transverse myelitis
- MG
myasthenia gravis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- OCB
oligoclonal bands
- ON
optic neuritis
- SLE
systemic lupus erythematosus
- VGKC
voltage-gated potassium channel
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Leite: study concept, acquisition of clinical and laboratory data, analysis of data, drafting and revising the manuscript. Dr. Coutinho: acquisition of clinical data, analysis of data, drafting the manuscript. Prof. Lana-Peixoto: acquisition of clinical data, revising the manuscript. Dr. Apostolos: acquisition of clinical data, analysis of data, revising the manuscript. Dr. Waters: supervision and acquisition of laboratory data, revising the manuscript. Dr. Sato: acquisition of clinical data, analysis of data, drafting and revising the manuscript. Dr. Melamud: acquisition of clinical data, revising the manuscript. Dr. Marta: acquisition of clinical data, revising the manuscript. Dr. Graham: acquisition of clinical data, revising the manuscript. Dr. Spillane: acquisition of clinical data. Dr. Villa: acquisition of clinical data, revising the manuscript. Prof. Callegaro: acquisition of clinical data, revising the manuscript. Dr. Santos: acquisition of clinical data. Dr. Martins da Silva: acquisition of clinical data. Dr. Jarius: study concept, revising the manuscript. Dr. Howard: acquisition of clinical data, revising the manuscript. Dr. Nakashima: study concept, acquisition of clinical data, revising the manuscript. Prof. Giovannoni: acquisition of clinical data, revising the manuscript. Dr. Buckley: acquisition of clinical data, revising the manuscript. Dr. Hilton-Jones: acquisition of clinical data, revising the manuscript. Prof. Vincent: study concept, study supervision, analysis and interpretation of laboratory data, revising manuscript. Dr. Palace: study supervision, analysis and interpretation of clinical data, revising manuscript.
DISCLOSURE
Dr. Leite, Dr. Coutinho, Prof. Lana-Peixoto, Dr. Apostolos, and Dr. Waters report no disclosures. Dr. Sato receives scholarship from the Ministry of Education, Culture, Sports, Science & Technology of Japan (MEXT) and research support from Ichiro Kanehara Foundation. Dr. Melamud reports no disclosures. Dr. Marta receives fellowship from Merck-Serono UK for an Investigator-Led Study. Dr. Graham reports no disclosures. Dr. Spillane receives a grant from Myasthenia Gravis Association, UK. Dr. Villa reports no disclosures. Prof. Callegaro has received travel grant support and scholarships for congresses and teaching activities from Bayer, Biogen, Merck, Novartis, and Teva. Dr. Santos, Dr. Martins da Silva, Dr. Jarius, and Dr. Howard report no disclosures. Dr. Nakashima has received funding for travel and received speaker honoraria from Bayer Schering Pharma and Biogen Idec; and has received research funding from Mitsubishi Chemical Medience Corporation and the Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology of Japan. Prof. Giovannoni has received research grant support from Bayer-Schering Healthcare, Biogen-Idec, GW Pharma, Merck Serono, Merz, Novartis, Teva, and Sanofi-Aventis. He has received personal compensation for participating on advisory boards in relation to clinical trial design, trial steering committees, and data and safety monitoring committees from Bayer-Schering Healthcare, Biogen-Idec, Eisai, Elan, Fiveprime, Genzyme, Genentech, GSK, Ironwood, Merck-Serono, Novartis, Pfizer, Roche, Sanofi-Aventis, Synthon BV, Teva, UCB Pharma, and Vertex Pharmaceuticals. Dr. Buckley is funded by the Medical Research Council of United Kingdom. Dr. Hilton-Jones reports no disclosures. Prof. Vincent and the Department of Clinical Neurology in Oxford hold patents and receive royalties and payments for performing antibody assays. Dr. Palace has received support for MS-related scientific meetings and honorariums for advisory committees from Merck Serono, Biogen Idec, Novartis, Teva, and Bayer Schering and support for investigator-led research from Merck Serono and Bayer Schering. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Wingerchuk DM, Hogancamp WF, O'Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic's syndrome). Neurology 1999; 53: 1107– 1114 [DOI] [PubMed] [Google Scholar]
- 2. Waters P, Jarius S, Littleton E, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol 2008; 65: 913– 919 [DOI] [PubMed] [Google Scholar]
- 3. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004; 364: 2106– 2112 [DOI] [PubMed] [Google Scholar]
- 4. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005; 202: 473– 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006; 66: 1485– 1489 [DOI] [PubMed] [Google Scholar]
- 6. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805– 815 [DOI] [PubMed] [Google Scholar]
- 7. Kinoshita M, Nakatsuji Y, Kimura T, et al. Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun 2009; 386: 623– 627 [DOI] [PubMed] [Google Scholar]
- 8. Bradl M, Misu T, Takahashi T, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol 2009; 66: 630– 643 [DOI] [PubMed] [Google Scholar]
- 9. Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain 2010; 133: 349– 361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jarius S, Wildemann B. AQP4 antibodies in neuromyelitis optica: diagnostic and pathogenetic relevance. Nat Rev Neurol 2010; 6: 383– 392 [DOI] [PubMed] [Google Scholar]
- 11. Hoedemaekers AC, van Breda Vriesman PJ, De Baets MH. Myasthenia gravis as a prototype autoimmune receptor disease. Immunol Res 1997; 16: 341– 354 [DOI] [PubMed] [Google Scholar]
- 12. McKeon A, Lennon VA, Jacob A, et al. Coexistence of myasthenia gravis and serological markers of neurological autoimmunity in neuromyelitis optica. Muscle Nerve 2009; 39: 87– 90 [DOI] [PubMed] [Google Scholar]
- 13. Pittock SJ, Lennon VA, de Seze J, et al. Neuromyelitis optica and non organ-specific autoimmunity. Arch Neurol 2008; 65: 78– 83 [DOI] [PubMed] [Google Scholar]
- 14. Nakamura H, Usa T, Motomura M, et al. Prevalence of interrelated autoantibodies in thyroid diseases and autoimmune disorders. J Endocrinol Invest 2008; 31: 861– 865 [DOI] [PubMed] [Google Scholar]
- 15. Thorlacius S, Aarli JA, Riise T, Matre R, Johnsen HJ. Associated disorders in myasthenia gravis: autoimmune diseases and their relation to thymectomy. Acta Neurol Scand 1989; 80: 290– 295 [DOI] [PubMed] [Google Scholar]
- 16. Nakamura M, Nakashima I, Sato S, Miyazawa I, Fujihara K, Itoyama Y. Clinical and laboratory features of neuromyelitis optica with oligoclonal IgG bands. Mult Scler 2007; 13: 332– 335 [DOI] [PubMed] [Google Scholar]
- 17. Kay CS, Scola RH, Lorenzoni PJ, Jarius S, Arruda WO, Werneck LC. NMO-IgG positive neuromyelitis optica in a patient with myasthenia gravis but no thymectomy. J Neurol Sci 2008; 275: 148– 150 [DOI] [PubMed] [Google Scholar]
- 18. Uzawa A, Mori M, Iwai Y, et al. Association of anti-aquaporin-4 antibody-positive neuromyelitis optica with myasthenia gravis. J Neurol Sci 2009; 287: 105– 107 [DOI] [PubMed] [Google Scholar]
- 19. Kister I, Gulati S, Boz C, et al. Neuromyelitis optica in patients with myasthenia gravis who underwent thymectomy. Arch Neurol 2006; 63: 851– 856 [DOI] [PubMed] [Google Scholar]
- 20. Gotkine M, Fellig Y, Abramsky O. Occurrence of CNS demyelinating disease in patients with myasthenia gravis. Neurology 2006; 67: 881– 883 [DOI] [PubMed] [Google Scholar]
- 21. Waters P, McKeon A, Leite M, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 2012; 78: 665– 671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain 2004; 127: 701– 712 [DOI] [PubMed] [Google Scholar]
- 23. Beekman R, Kuks JB, Oosterhuis HJ. Myasthenia gravis: diagnosis and follow-up of 100 consecutive patients. J Neurol 1997; 244: 112– 118 [DOI] [PubMed] [Google Scholar]
- 24. Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in 106 aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder patients from the United Kingdom and Japan Brain (in press 2012). [DOI] [PubMed] [Google Scholar]
- 25. Vincent A, Newsom-Davis J. Acetylcholine receptor antibody characteristics in myasthenia gravis: I: patients with generalized myasthenia or disease restricted to ocular muscles. Clin Exp Immunol 1982; 49: 257– 265 [PMC free article] [PubMed] [Google Scholar]
- 26. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007; 69: 2221– 2231 [DOI] [PubMed] [Google Scholar]
- 27. Asgari N, Lillevang ST, Skejoe HP, Falah M, Stenager E, Kyvik KO. A population-based study of neuromyelitis optica in Caucasians. Neurology 2011; 76: 1589– 1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Collongues N, Marignier R, Zephir H, et al. Neuromyelitis optica in France: a multicenter study of 125 patients. Neurology 2010; 74: 736– 742 [DOI] [PubMed] [Google Scholar]
- 29. Schluep M, Willcox N, Vincent A, Dhoot GK, Newsom-Davis J. Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol 1987; 22: 212– 222 [DOI] [PubMed] [Google Scholar]
- 30. Wekerle H, Ketelsen UP. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet 1977; 1: 678– 680 [DOI] [PubMed] [Google Scholar]
- 31. Leite MI, Jones M, Strobel P, et al. Myasthenia gravis thymus: complement vulnerability of epithelial and myoid cells, complement attack on them, and correlations with autoantibody status. Am J Pathol 2007; 171: 893– 905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roxanis I, Micklem K, McConville J, Newsom-Davis J, Willcox N. Thymic myoid cells and germinal center formation in myasthenia gravis; possible roles in pathogenesis. J Neuroimmunol 2002; 125: 185– 197 [DOI] [PubMed] [Google Scholar]
- 33. Giraud M, Taubert R, Vandiedonck C, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature 2007; 448: 934– 937 [DOI] [PubMed] [Google Scholar]
- 34. Chan KH, Kwan JS, Ho PW, et al. Aquaporin-4 water channel expression by thymoma of patients with and without myasthenia gravis. J Neuroimmunol 2010; 227: 178– 184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.