Abstract
The use of umbilical cord blood (UCB) grafts for hematopoietic stem cell transplantation (HSCT) is a promising technique that permits a degree of human leukocyte antigen mismatch between the graft and the host without the concomitant higher rate of graft-versus-host disease that would be observed between an adult marrow graft and a mismatched host. A disadvantage to the use of UCB for HSCT is that immune reconstitution may be significantly delayed because of the low stem cell dose available in the graft. Ex vivo expansion of UCB CD34 cells would provide a greater number of stem cells; however, there are persistent concerns that ex vivo-expanded CD34 cells may lose pluripotency and the ability to contribute meaningfully to long-term engraftment. To address this issue, we transduced CD34-selected UCB cells with a lentiviral construct expressing luciferase, and determined homing and engraftment patterns in vivo by noninvasive bioluminescent imaging in sublethally irradiated NOD/SCID/IL-2Rγ−/− (NSG) mice. Graft contribution to multilineage commitment was also confirmed by analysis of primary and secondary transplants by flow cytometry and immunohistochemistry. Our results demonstrate that, other than a mild delay at the onset of engraftment, there were no significant differences in lineage repopulation or in long-term or secondary engraftment between culture-expanded and unexpanded UCB CD34-selected cells. The results suggest that multipotent stem cells can be expanded ex vivo and can contribute meaningfully to long-term hematopoietic engraftment.
Keywords: Bioluminescent imaging, Umbilical cord blood, Hematopoiesis, Transplantation, Engraftment, Luciferase, Calvarium
Introduction
Umbilical cord blood (UCB) has become an important source of stem cells for use in hematopoietic stem cell (HSC) transplantation. The advantages of cord over mobilized peripheral blood or bone marrow are numerous and include its ease of collection, storage, and distribution; its lower incidence and severity of post-transplant graft-versus-host disease; its tolerance of significant human leukocyte antigen mismatching between donor and recipient; and the ability of cord blood banks to obtain units that proportionately represent underserved minority populations. One downside, however, is the limited cell dose available for transplant in the UCB graft, especially for recipients >70 kg. This is a significant issue and often results in delayed platelet and neutrophil engraftment and a greater risk for graft failure than with other sources of HSCs [1–11]. The hematopoietic progenitor cell content of the UCB unit has been shown to be a reliable predictor of platelet and neutrophil engraftment [12]; hence, a number of strategies have been developed with the goal of improving the dose of UCB hematopoietic progenitor cells in the graft. These strategies have included the tandem transplantation of two different UCB units [13–15] as well as the use of ex vivo expansion techniques, some of which have resulted in significant proliferation of pluripotent progenitors [16–25] and have also identified the importance of insulin-like and angiopoietin-like growth factors in the biology of expansion [26–29].
Many groups have attempted to identify ex vivo culture conditions that best preserve and expand the “stem cell reserve” of a graft, thereby generating a product that might improve neutrophil and platelet engraftment for cord blood recipients; however, ex vivo culture conditions drive hematopoietic differentiation and increase the numbers of mature hematopoietic progenitors at the expense of the most primitive, pluripotent hematopoietic progenitors. The mature hematopoietic progenitors generated by an ex vivo expansion product may therefore allow for a rapid, transient short-term engraftment, but may lack the long-term repopulating activity required for transfusion-independent hematopoiesis [16, 30–38]. And, although we have previously hypothesized that the culture conditions employed by our group do allow for the expansion of primitive, pluripotent progenitors [20], in vivo experimental verification of this hypothesis is lacking.
Hematopoietic engraftment following transplantation is a poorly understood biological process. In an attempt to better understand the real-time dynamic nature of hematopoietic engraftment, we used a noninvasive bioluminescent-based imaging technique [39, 40] to follow the in vivo engraftment kinetics of expanded and unexpanded UCB CD34+ cells following transplantation. Our results demonstrate long-term persistence of human hematopoiesis in mice receiving both unexpanded and ex vivo-expanded UCB CD34+ cells. Further, we were also able to visualize human hematopoiesis in secondary recipients of bone marrow from mice receiving ex vivo-expanded UCB CD34+ cells, demonstrating the presence of primitive, pluripotent stem cells with long-term marrow repopulating activity in the primary graft. The data confirm the hypothesis that our UCB CD34+ expansion protocol allows for the maintenance of primitive hematopoietic progenitors capable of long-term multilineage engraftment.
Materials and Methods
Mice
Six- to 12-week-old NOD.Cg-Prkdcscid IL2rgtmWjl/Sz (NSG) mice (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) were used for these studies. Unlike regular nonobese diabetic severe combined immunodeficient (NOD-SCID) mice, which possess some T and natural killer (NK) cells, NSG mice are completely devoid of T-cell and NK-cell activity, allowing the robust detection of peripheral blood engraftment using as few as 102 total progenitor cells. Use of 105 progenitors can result in peripheral blood engraftment ≥70% [41–43].
Lentivirus Production
The plasmid pHIV-GFPFFLuc was constructed by insertion of the enhanced green fluorescent protein (eGFP) firefly Luciferase (FFLuc) fusion cassette in place of the internal ribosome entry site (IRES) enhanced yellow fluorescent protein (eYFP) sequence in the plasmid vector pHIV-IRES-eYFP (kindly provided by Dr. Richard Sutton, Baylor College of Medicine, Houston, Texas). To produce the lentiviral supernatant, 293T cells were cotransfected with lentiviral vectors, pHIV-GFPFFLuc, and pDRF containing the sequence for the RD114 envelope [44], using the Fugene6 transfection reagent (Roche, Indianapolis, IN, http://www.roche.-com) according to the manufacturer’s instructions. Lentiviral supernatant was collected 48 and 72 hours later and immediately filtered and frozen.
Human UCB CD34 Selection and Lentiviral Transduction
UCB units were obtained from the MD Anderson Cord Blood Bank as stipulated by institutional review board protocol LAB03-0796. Mononuclear cells (MNCs) were separated from red cells using a Ficoll gradient. CD34+ cells were isolated from recovered UCB MNCs by magnetic selection (Miltenyi Biotec, Auburn, CA, http://www.miltenyibiotec.com). Percent purity was determined by flow cytometry. For transduction, 24-well nontissue culture plates precoated with retronectin (Takara Shuzo, Otsu, Japan, http://www.takara.co.jp) were incubated twice with 0.5 ml lentiviral supernatant for 30 minutes. CD34-enriched cells were then plated in 0.5 ml complete medium—α-minimal essential medium (MEM) (Gibco-BRL, Gaithersburg, MD, http://www.gibcobrl.-com) supplemented with 10% fetal calf serum and 2 mM L-glutamine—at a concentration of 0.3–0.8 × 106 cells per well mixed with an additional 1.5 ml lentiviral supernatant. After addition of the virus, the plates were centrifuged and incubated at 37°C overnight. Subsequently, 50%–70% of the medium was removed and replaced with fresh HSC expansion medium. Transduction efficiency was determined 72 hours post-transduction by flow cytometric detection of CD34+GFP+ cells within the stem cell gate. Cells were then infused into mice or expanded further in culture.
UCB HSC Expansion
Transduced UCB cells were expanded for 10 days in HSC expansion medium consisting of either α-MEM (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) or SCGM CellGro (CellGenix, Antioch, IL, http://www.cellgenix.com) supplemented with 50 µg/ml gentamycin (Abbott Laboratories, Chicago, IL, http://www.abbott.com), 100 ng/ml stem cell factor (CellGenix), 100 ng/ml Flt3-L (CellGenix), 100 ng/ml thrombopoietin (CellGenix), and 100 ng/ml G-CSF (Amgen, Thousand Oaks, CA, http://www.amgen.com).
Engraftment of Mice With CD34+ UCB Cells
Adult NSG mice were irradiated with 270 cGy from a 137-Cs source. Mice were injected i.v. through the tail vein with 0.8 × 105 to 3.5 × 105 transduced, unexpanded cells or the equivalent number (with regard to CD34+ content) of 10-day expanded cells.
Analysis of Engraftment by Flow Cytometry
Mice were bled retroorbitally, and red cells were removed by treatment with PharmLyse (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) MNCs were labeled with anti-mouse CD45 and anti-human CD45 monoclonal antibodies as well as a variety of lineage-specific antibodies including anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD19, anti-CD33, anti-CD34, and anti-CD56 (all from BD Biosciences). Labeled cells were fixed in 4% paraformaldehyde and analyzed on a FACScan flow cytometer using CellQuest software (BD Biosciences).
Noninvasive Bioluminescent Imaging
Cultured, transduced, expanded or unexpanded human UCB cells were injected into conditioned NSG mice, and human cell engraftment in the bone marrow of the recipients was determined by bioluminescent imaging every other day during the first 2 weeks as well as 3, 6, and 12 months post-transplant. For in vivo imaging of engrafted GFP-FFLuc-expressing cells, mice were injected i.p. with D-luciferin (150 mg/kg) and analyzed using the Xenogen-IVIS Imaging System (Caliper Life Sciences, Hopkinton, MA, http://www.caliperls.com). A constant region of interest was drawn and the intensity of the signal was measured as total photons/second per cm2/sr.
Immunohistochemistry
Mouse spines were dissected and fixed in 3% formaldehyde in phosphate-buffered saline (PBS) for 2 hours at 4°C, washed in PBS, and decalcified by immersion in a 10% solution of EDTA changed daily for 10 days. Before embedding, spines were incubated in a Ca2+-containing solution for 1 hour. Spines were cut into two sections for embedding in OCT and in paraffin. For immunostaining, 8-µm cryosections were prepared on a Microm HM525 cryostat (Microm, Walldorf, Germany, http://www.microm-online.com). Sections were dried overnight, rehydrated in PBS, washed in PBS-0.05% Tween-10 (PBS-T), and incubated in blocking buffer (5% bovine serum albumin, 5% skim milk powder, 0.05% Triton-X100, 2% normal donkey serum, and 2% rat anti-mouse CD16/32 in 4× SSC) for 30 minutes. Sections were then incubated with mouse anti-human CD45 monoclonal antibody and isotype control (BD Biosciences) overnight at 4°C in a humidified chamber, washed in PBS-T, and incubated with biotinylated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, http://www.jacksonimmuno.com) for 1 hour at room temperature in a humidified chamber, and washed again in PBS-T. Endogenous peroxidase activity was blocked by incubation of the slides in 6% H2O2 in methanol for 30 minutes. Slides were washed in PBS-T, incubated for 1 hour at room temperature with streptavidin-biotinylated horseradish peroxidase complex, then washed in PBS. Peroxidase activity was detected by incubation with a solution of 0.25 mg/ml diaminobenzidine (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) in PBS supplemented with 3% H2O2 for 6 minutes. Slides were dehydrated in successive baths of ethanol and xylene and mounted in DPX mounting medium (Sigma-Aldrich). Images were visualized using a BX51 Olympus microscope and acquired by a DP71 Olympus camera (Olympus, Tokyo, http://www.olympus-global.com).
Results
CD34+ Human UCB Transduction
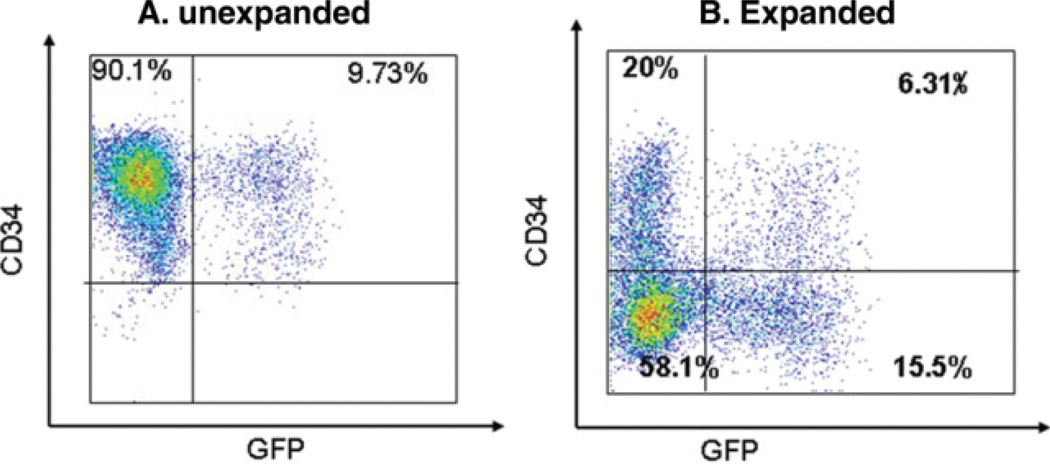
CD34+ UCB cells were isolated by magnetic selection to an average purity of 25%–50%; however, >95% of cells within the stem cell gate were CD34+. CD34-selected cells were then transduced with lentivector expressing the gene marker eGFP or the fusion protein eGFPFFLuc. Transduction efficiency, as measured by flow cytometry, indicated a percentage of 10%–15% CD34+GFP+ cells on day 3 post-transduction (Fig. 1A). The transduced cells were divided into two equal fractions, and one fraction was injected directly into irradiated NSG mice while the other half was expanded ex vivo for an additional 7 days. The percentage of CD34+GFP+ cells was determined postexpansion (Fig. 1B), and expanded cells were injected into irradiated NSG mice. Typically, the number of expanded cells injected was three- to fourfold greater than the number of unexpanded cells, so that the number of CD34+ cells in the expanded cell fraction was equivalent to the number of unexpanded CD34+ cells injected in any given experiment. We observed up to a tenfold expansion of CD34+ progenitors in some experiments; however, expansion data in aggregate were quite similar to what we have reported previously [20].
Figure 1.
Lentiviral transduction efficiency of CD34-selected UCB cells pre- and postexpansion. CD34-selected UCB cells were transduced with a lentiviral construct expressing both GFP and luciferase and were then cultured for 72 hours to allow reporter gene expression. (A): After 72 hours in culture, approximately 10%–15% of cells were GFP+ as determined by flow cytometry. All cells at this stage were typically still CD34+. (B): After 7–10 days of expansion in culture, approximately one third of CD34-selected UCB cells remained CD34+ and 3%–10% of the total cell population remained CD34+GFP+. x-axis, GFP content; y-axis, CD34 content. Abbreviations: GFP, green fluorescent protein; UCB, umbilical cord blood.
Engraftment of Cultured Human UCB Cells in NSG Mice
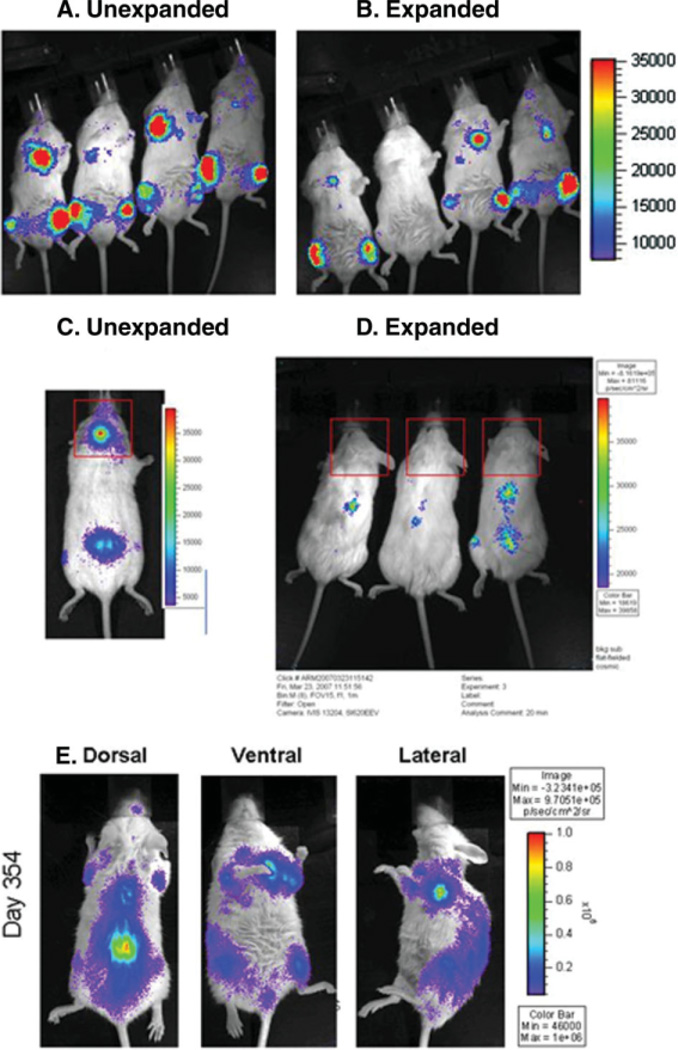
To assess the engraftment capabilities of the transduced HSCs, we examined the mice by noninvasive bioluminescent imaging. The first clear signals of engraftment were observed between days 7 and 9. Mice that received the expanded fraction exhibited engraftment signals 2–5 days later than those that received the unexpanded fraction; however, few quantitative differences were discernable beyond day 20–25 (Fig. 2A, 2B). In contrast, peripheral blood engraftment of cord blood in the mice could not be detected by flow cytometry until day 21–28 at the earliest, >2 weeks after engraftment was apparent by bioluminescent imaging.
Figure 2.
Bioluminescent imaging demonstrates repopulation of hematopoietic niche compartments by transduced human CD34-selected umbilical cord blood (UCB) cells. By post-transplant day 20, differences in the kinetics of engraftment between unexpanded (A) and expanded (B) CD34-selected UCB cells are negligible in the long bones, spine, sternum, and pelvis. Calvarial engraftment (C), however, remained strikingly absent among recipients of expanded CD34-selected UCB cells (D) until 3–6 months post-transplant. At 1 year post-transplant (E), the signal intensity of repopulated niche areas remained very strong among mice transplanted with either expanded CD34 UCB cells (shown) or unexpanded CD34 UCB cells. Dorsal, ventral, and lateral views are shown.
Expanded HSC Engraftment
There was little variation between expanded and unexpanded UCB cells in terms of the sites at which early engraftment was observed. All mice demonstrated early engraftment in the long bones, sternum, spine, and pelvis with equal frequency; however, 45% of mice receiving unexpanded UCB cells exhibited an engraftment signal in the area of the calvarium by day 14. Such a signal was never detected early among mice receiving expanded UCB cells (representative mice shown in Fig. 2C, 2D; p < .001 by χ2). Calvarial engraftment signals were eventually seen at about post-transplant day 90 among mice who received expanded UCB grafts. These differences were significant and observed exclusively in the calvarium. By harvesting tissues from mice and incubating them with D-luciferin and ATP, we demonstrated that the source of the signal originated genuinely within the calvarium (Fig. 3) and not within the brain (data not shown). This was the only temporal or spatial variation observed between expanded and unexpanded UCB grafts.
Figure 3.
Ex vivo bioluminescent imaging identifies a localized niche of calvarial hematopoiesis in the mouse. As demonstrated by incubation of intact cranial bones in D-luciferin substrate and ATP, calvarial hematopoiesis in the mouse appears to be restricted to a specific niche located at the junction of the parietal and interparietal fissures. The calvarial hematopoietic niche is significant in that it is the only site at which expanded and unexpanded CD34-selected umbilical cord blood cells displayed differential engraftment kinetics.
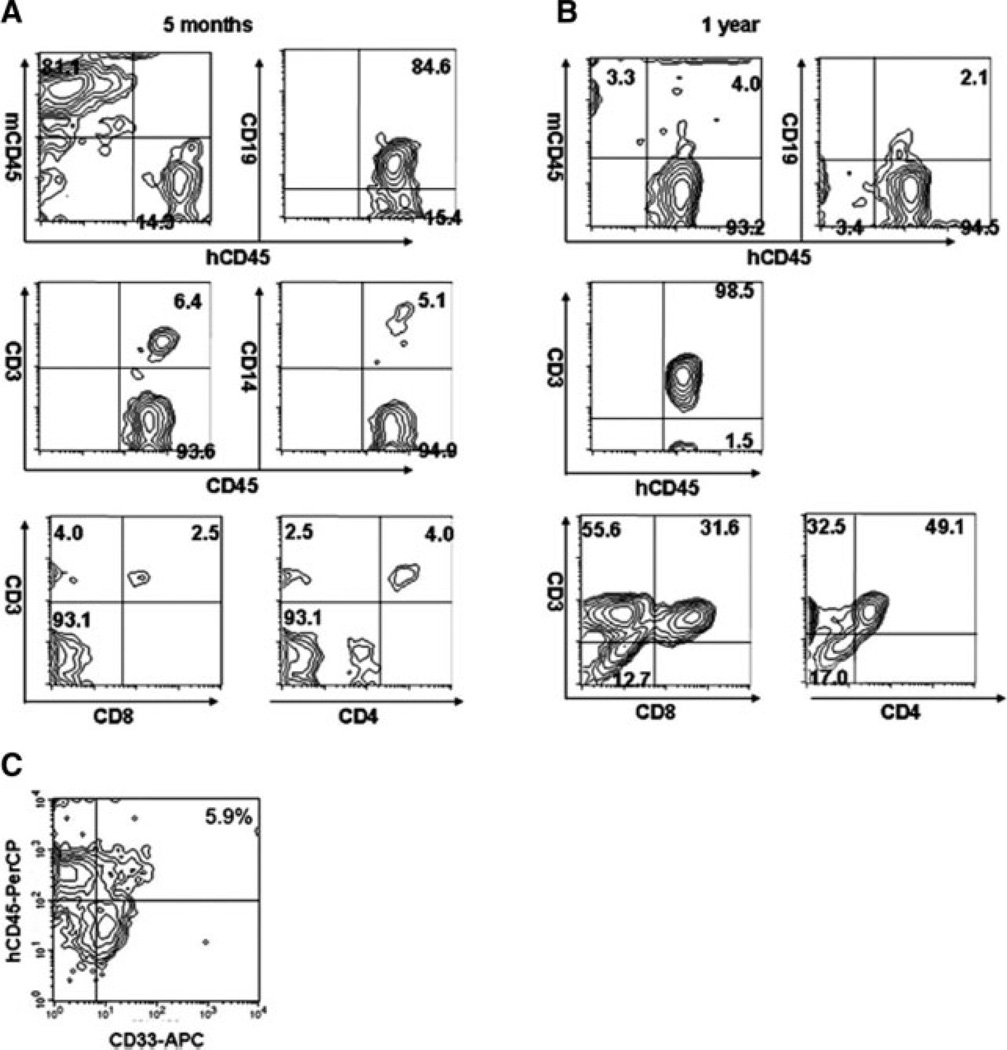
To determine lineage of engrafted cells, we analyzed the subset of human CD45+ cells present in the periphery of mice that received unexpanded or expanded UCB grafts. CD19 was the dominant lineage observed within 3 months post-transplant. When peripheral human CD45 was measured 3–6 months post-transplant, broad multilineage engraftment was observed, including human CD3, CD4, CD8, CD19, and CD14 (Fig. 4A). Over time, the T-cell subset gradually increased, so that by 10–12 months post-transplant the T-cell subpopulation comprised the majority of peripheral human CD45+ cells in the engrafted mice (Fig. 4B). Thirteen mice analyzed for peripheral myeloid engraftment demonstrated an average of 4.6% ± 2.7% CD33+ cells (range, 0.4%–9.4%) (Fig. 4C). In some mice (but not all), low levels of CD56+ cells were observed; however, this population never comprised >2% of the total human cell population (data not shown). No significant differences in peripheral blood engraftment characteristics were observed between mice receiving expanded and those receiving unexpanded cord blood cells.
Figure 4.
Multilineage engraftment of mice repopulated with expanded CD34-selected umbilical cord blood cells is dominated early by a CD19+ lymphoid subset and late by a CD3+ lymphoid subset. (A): At 5 months post-transplant, >80% of human cells in the peripheral blood are CD19+. CD14+ and CD3+ (including both CD4+ and CD8+) subsets can also be identified. (B): By 1 year post-transplant, >90% of human cells in the peripheral blood are CD3+. Though the ratio of CD4+ to CD8+ human cells varied by recipient, both subsets were adequately represented. (C): Typical myeloid engraftment characteristics are shown: 5.9% of total peripheral blood mononuclear cells are positive for human CD33. x-axis, human CD33-allophycocyanin (APC); y-axis, human CD45-peridinin-chlorophyll-protein complex (PerCP).
Secondary Transplantation
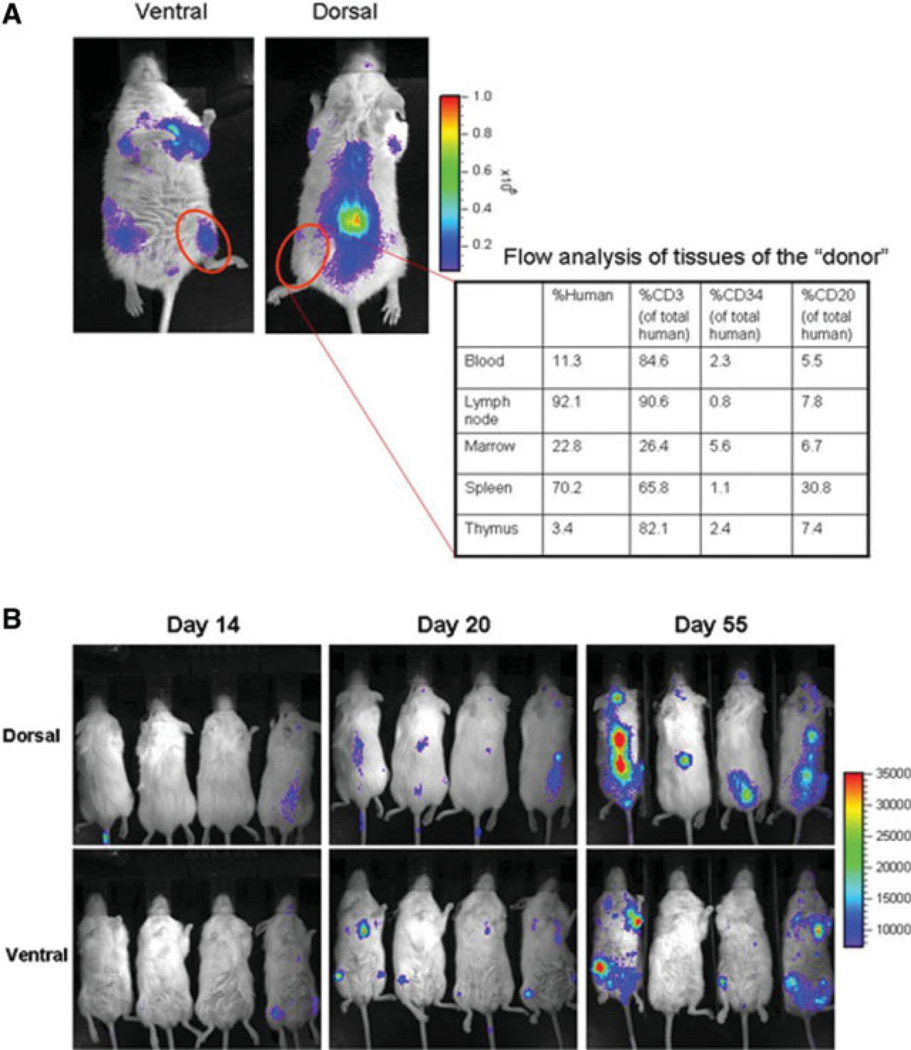
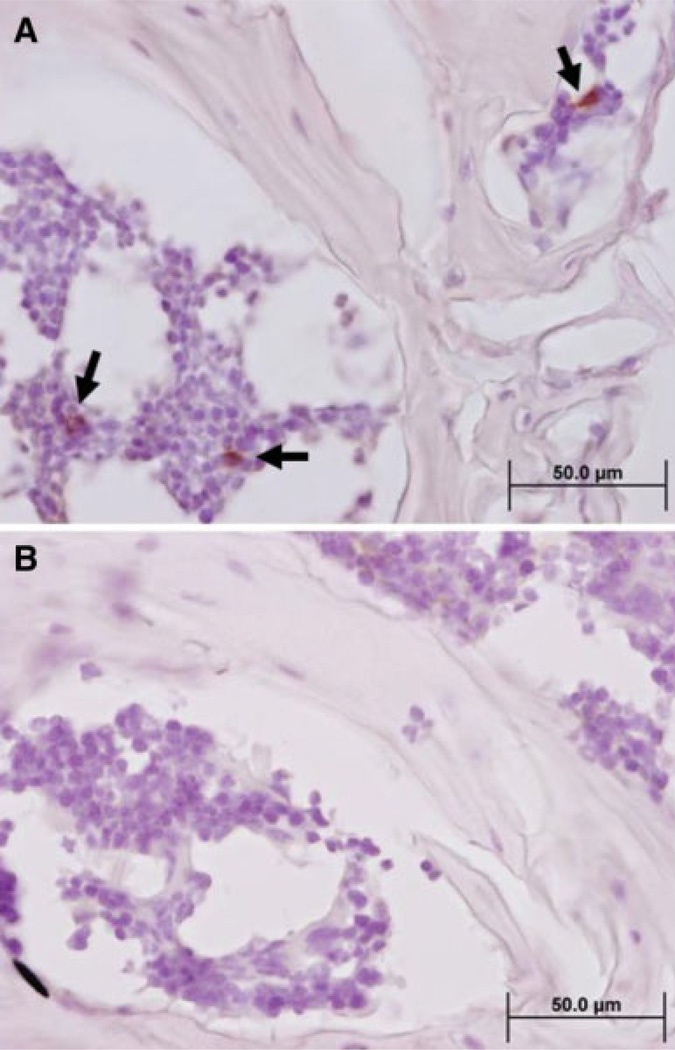
At 1 year post-transplant, mice receiving both expanded and unexpanded grafts were still displaying powerful engraftment signals (representative mouse shown in Fig. 2E). At this point, organs were harvested and the organ cell phenotype was determined by flow cytometry, and secondary mice were transplanted by reinfusion of 2–3 × 106 unmanipulated chimeric bone marrow cells derived from expanded CD34 UCB primary transplants. The phenotypic characteristics of a representative graft as determined by flow cytometry are displayed in Figure 5A. Myeloid engraftment characteristics of donors were typical of the group as a whole (data not shown). Engraftment signals in secondarily transplanted mice were detectable within 2–3 weeks post-transplant. Two months after secondary transplant, clear engraftment signals were detectable by imaging (Fig. 5B), but only low levels of peripheral blood engraftment ([ltequ]5%) could be detected by flow cytometry. Nevertheless, we were able to verify the presence of CD45+ human cells in the marrow spaces of secondary transplants by immunohistochemistry (Fig. 6).
Figure 5.
Secondary transplantation. (A):. At 1 year post-transplant of expanded CD34-selected umbilical cord blood cells, tissues were harvested from engrafted mice and characterized by flow cytometry (table shows composition of typical mouse). Total bone marrow was harvested and 2–3 × 106 marrow cells/mouse were used for secondary transplantation of irradiated recipients. (B): Kinetics of secondary transplantation on days 14, 20, and 55. Dorsal and ventral views are shown.
Figure 6.
Demonstration of human CD45+ cells in the marrow spaces of secondary transplants by immunohistochemistry. (A): Spines with intact marrow spaces were decalcified by EDTA immersion, paraffin embedded, and sliced into 8-µm sections for analysis by immunohistochemistry. Staining with an anti-human CD45 monoclonal antibody demonstrated the engraftment of human cells in hematopoietic spaces. Black arrows point to examples of positively stained cells. (B): Isotype (negative) control demonstrates the absence of background and specificity of the anti-CD45 antibody.
Discussion
Here, we have used in vivo bioluminescent imaging to compare the engraftment potential of human cord blood CD34+ cells expanded in culture for 10 days with the equivalent unexpanded fraction. Our results validate the hypothesis that expanded UCB progenitors can produce durable, multilineage engraftment, nearly comparable with that of unexpanded UCB progenitors.
A recent publication by Giassi et al. [45] demonstrated that cultured human UCB cells could generate myeloid and erythroid lineages, but not lymphoid lineage cells in NSG mice unless the mice were pretreated with tumor necrosis factor α. In our model, the majority of detectable human cells were CD19+ following the onset of engraftment; however, as early as 8 weeks post-transplant, we were able to detect CD3+ human cells as well. Furthermore, by 10 months post-transplant, a majority of cells in the periphery were CD3+. Although the only physical difference between our expansion protocol and that of Giassi et al. [45] is the use of G-CSF, this cytokine is known to promote the enhancement of myeloid, not lymphoid, lineages. Given this, it is unlikely that this modification to our expansion protocol contributed significantly to our differential results. More likely, Giassi et al. [45] did not report results that extended beyond 8 weeks post-transplant. Because of the time that it might take for T cells to mature in the thymus and for the thymus itself to become populated with de novo-generated human dendritic cells, it is unlikely that T cells would be observed prior to this time point. Indeed, we ourselves did not observe the development of T cells until the later stages of engraftment. The shift from a predominantly CD19+ lymphoid subset to a predominantly CD3+ subset approximately 10 months post-transplant was curious. Preliminarily, we have hypothesized that this shift may have been a result of physiologic events related to aging and not necessarily a result of events that were hematopoietic in nature. Histologic analysis of the long bones revealed an abnormal cellular distribution. Although we found human cells in the epiphysis, the diaphysis was calcified and completely a cellular, suggesting that the ability of older NSG mice to produce B cells could be significantly impaired. However, we did not examine untransplanted controls, and we cannot rule out the hypothesis that the grafts themselves were the cause of the diaphysis calcification.
Our lentiviral transduction efficiency was consistent with that reported in a recent publication by Liu et al. [46], which outlines the major parameters for efficient lentiviral transduction and engraftment of human CD34+ UCB. Liu [46] demonstrated that optimal transduction and engraftment efficiency is achieved by transducing unstimulated stem cells for 5 hours followed by 3 days of culture. We also observed the importance of incorporating a short culture period subsequent to an overnight transduction. In order to allow the viral vector to survive an overnight incubation period, it would have been necessary to replace the RD114 viral envelope coat protein with vesicular stomatitis virus G (VSV-G). Though VSV-G is able to more efficiently transduce CD34+ stem cells, it is also much more toxic, and cell viability following a VSV-G transduction is unacceptable. Because RD114 is not able to form viable virus particles with any third-generation, self-inactivated lentiviral vectors, we did all the described studies with first-generation vectors. Such a protocol is fine for in vitro studies, but would be unacceptable in a clinical setting. If we are to use our vectors clinically, we will likely have to adopt a transduction period similar to that of Liu [46], though they did not demonstrate the potential of their transduced cells to mediate long-term engraftment.
The sensitivity of luciferase-based bioluminescent imaging is much greater than that of engraftment screening assays that detect the presence of human cells in peripheral blood. Although we were able to detect clear signals of engraftment in the marrow spaces no later than 10 days post-transplant and predict successful engraftment as early as day 6, we were unable to detect human cells in the periphery by flow cytometry until post-transplant day 28 at the earliest. Although bioluminescent imaging is not applicable clinically, we show, in principle, that engraftment failure can be detected very early. We determined that transduction of as few as 3% of cells could still result in a detectable image (data not shown), and we speculate that the labeling of patient products prior to transplantation could conceivably have predictive merit in a number of clinical protocols if used in conjunction with appropriate technologies that allow imaging of cells within human recipients.
In our imaging study, we were able to detect hematopoiesis in a defined area within the calvarium. This area is not generally appreciated to be hematopoietically active; however, a recent report by Lo Celso et al [47]. demonstrates the importance of the calvarium as a focus of hematopoietic activity. By imaging, we demonstrated that UCB cells migrate to a specific calvarial focus and expand significantly (per available volume niche). This area, which does not exist at the time of birth, develops during the postnatal period and may sustain unique hematopoietic and niche migration characteristics. At 2 weeks post-transplant, there were significant differences in the calvarial engraftment signal between unexpanded and expanded HSC grafts. With time, this difference diminished, and no significant differences were observed long term. The significance of this phenomenon is unclear and requires further study. It is our hypothesis that stem cells lose the ability to migrate to the calvarium during expansion, and that expanded grafts can only populate the calvarium after de novo hematopoiesis occurs at other sites, that is, in the long bones or the spine. If this is indeed the case, a better understanding of the mechanism of calvarial engraftment could conceivably improve the rate of engraftment in expanded UCB clinical protocols.
In summary, with the exception of a small delay in early engraftment, human UCB cells expanded 10 days in culture could engraft and differentiate into multiple hematopoietic lineages in NSG mice in virtually the same manner as unexpanded human UCB cells. The ability to image transduced stem cells might allow the prediction of engraftment failure a few days after transplant, allowing the clinical decision-making process to be significantly enhanced.
Acknowledgments
This work was supported in part by NIH grant no. 5 R01 CA061508-13 (to E.J.S.).
Footnotes
Author contributions: D.S.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; J.G.: data analysis and interpretation, final approval of manuscript; B.S.: collection and assembly of data; S.R.: manuscript writing, provision of study materials; W.D.: provision of study materials, collection and assembly of data, data analysis and interpretation, manuscript writing; N.B.: collection and assembly of data, data analysis and interpretation; A.N.: conception and design, provision of study materials; D.X.: collection and assembly of data; H.Y.: provision of study materials, data analysis and interpretation; S.L.: collection and assembly of data; F.M.: provision of study materials, data analysis and interpretation, manuscript writing; P.Z.: conception and design, provision of study materials, data analysis and interpretation; C.B.: data analysis and interpretation; E.S.: conception and design, financial support, provision of study materials, data analysis and interpretation, final approval of manuscript; G.D.: conception and design, collection and assembly of data, data analysis and interpretation; P.S.: conception and design, data analysis and interpretation, final approval of manuscript; D.S. and J.G. contributed equally to this work.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Barker JN, Davies SM, DeFor T, et al. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: Results of a matched-pair analysis. Blood. 2001;97:2957–2961. doi: 10.1182/blood.v97.10.2957. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Weisdorf DJ, DeFor TE, et al. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 3.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: Impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Chevret S. Results of unrelated umbilical cord blood hematopoietic stem cell transplantation. Rev Clin Exp Hematol. 2001;5:87–99. doi: 10.1046/j.1468-0734.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman E, Rocha V, Arcese W, et al. Factors associated with outcomes of unrelated cord blood transplant: Guidelines for donor choice. Exp Hematol. 2004;32:397–407. doi: 10.1016/j.exphem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 8.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 9.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 11.Wagner JE, Barker JN, DeFor TE, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: Influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100:1611–1618. doi: 10.1182/blood-2002-01-0294. [DOI] [PubMed] [Google Scholar]
- 12.Migliaccio AR, Adamson JW, Stevens CE, et al. Cell dose and speed of engraftment in placental/umbilical cord blood transplantation: Graft progenitor cell content is a better predictor than nucleated cell quantity. Blood. 2000;96:2717–2722. [PubMed] [Google Scholar]
- 13.Barker JN, Weisdorf DJ, Wagner JE. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N Engl J Med. 2001;344:1870–1871. doi: 10.1056/NEJM200106143442417. [DOI] [PubMed] [Google Scholar]
- 14.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 15.De Lima M, St Johns LS, Wieder ED, et al. Double-chimaerism after transplantation of two human leucocyte antigen mismatched, unrelated cord blood units. Br J Haematol. 2002;119:773–776. doi: 10.1046/j.1365-2141.2002.03893.x. [DOI] [PubMed] [Google Scholar]
- 16.McNiece IK, Almeida-Porada G, Shpall EJ, et al. Ex vivo expanded cord blood cells provide rapid engraftment in fetal sheep but lack long-term engrafting potential. Exp Hematol. 2002;30:612–616. doi: 10.1016/s0301-472x(02)00805-6. [DOI] [PubMed] [Google Scholar]
- 17.McNiece I, Harrington J, Turney J, et al. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6:311–317. doi: 10.1080/14653240410004871. [DOI] [PubMed] [Google Scholar]
- 18.McNiece I, Kubegov D, Kerzic P, et al. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol. 2000;28:1181–1186. doi: 10.1016/s0301-472x(00)00520-8. [DOI] [PubMed] [Google Scholar]
- 19.Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: A phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao M, Broxmeyer HE, Horie M, et al. Extensive proliferative capacity of single isolated CD34 human cord blood cells in suspension culture. Blood Cells. 1994;20:455–466. discussion 466–467. [PubMed] [Google Scholar]
- 23.Traycoff CM, Abboud MR, Laver J, et al. Ex vivo expansion of CD34+ cells from purified adult human bone marrow and umbilical cord blood hematopoietic progenitor cells. Prog Clin Biol Res. 1994;389:385–391. [PubMed] [Google Scholar]
- 24.Moore MA, Hoskins I. Ex vivo expansion of cord blood-derived stem cells and progenitors. Blood Cells. 1994;20:468–479. discussion 479–481. [PubMed] [Google Scholar]
- 25.Broxmeyer HE, Hangoc G, Cooper S, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A. 1992;89:4109–4113. doi: 10.1073/pnas.89.9.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang CC, Kaba M, Iizuka S, et al. Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood. 2008;111:3415–3423. doi: 10.1182/blood-2007-11-122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med. 2006;12:240–245. doi: 10.1038/nm1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang CC, Lodish HF. Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood. 2005;105:4314–4320. doi: 10.1182/blood-2004-11-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang CC, Lodish HF. Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood. 2004;103:2513–2521. doi: 10.1182/blood-2003-08-2955. [DOI] [PubMed] [Google Scholar]
- 30.Abkowitz JL, Taboada MR, Sabo KM, et al. The ex vivo expansion of feline marrow cells leads to increased numbers of BFU-E and CFU-GM but a loss of reconstituting ability. Stem Cells. 1998;16:288–293. doi: 10.1002/stem.160288. [DOI] [PubMed] [Google Scholar]
- 31.Guenechea G, Segovia JC, Albella B, et al. Delayed engraftment of nonobese diabetic/severe combined immunodeficient mice transplanted with ex vivo-expanded human CD34(+) cord blood cells. Blood. 1999;93:1097–1105. [PubMed] [Google Scholar]
- 32.Holyoake TL, Alcorn MJ, Richmond L, et al. CD34 positive PBPC expanded ex vivo may not provide durable engraftment following myeloablative chemoradiotherapy regimens. Bone Marrow Transplant. 1997;19:1095–1101. doi: 10.1038/sj.bmt.1700799. [DOI] [PubMed] [Google Scholar]
- 33.Peters SO, Kittler EL, Ramshaw HS, et al. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23:461–469. [PubMed] [Google Scholar]
- 34.Peters SO, Kittler EL, Ramshaw HS, et al. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 35.Tisdale JF, Hanazono Y, Sellers SE, et al. Ex vivo expansion of genetically marked rhesus peripheral blood progenitor cells results in diminished long-term repopulating ability. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 36.Traycoff CM, Cornetta K, Yoder MC, et al. Ex vivo expansion of murine hematopoietic progenitor cells generates classes of expanded cells possessing different levels of bone marrow repopulating potential. Exp Hematol. 1996;24:299–306. [PubMed] [Google Scholar]
- 37.Von Drygalski A, Alespeiti G, Ren L, et al. Murine bone marrow cells cultured ex vivo in the presence of multiple cytokine combinations lose radioprotective and long-term engraftment potential. Stem Cells Dev. 2004;13:101–111. doi: 10.1089/154732804773099308. [DOI] [PubMed] [Google Scholar]
- 38.Williams DA. Ex vivo expansion of hematopoietic stem and progenitor cells–robbing Peter to pay Paul? Blood. 1993;81:3169–3172. [PubMed] [Google Scholar]
- 39.De A, Lewis XZ, Gambhir SS. Noninvasive imaging of lentiviral-mediated reporter gene expression in living mice. Mol Ther. 2003;7(suppl 5):681–691. doi: 10.1016/s1525-0016(03)00070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y, Molter J, Lee Z, et al. Bioluminescence imaging of hematopoietic stem cell repopulation in murine models. Methods Mol Biol. 2008;430:295–306. doi: 10.1007/978-1-59745-182-6_20. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor γchain (null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008;126:303–314. doi: 10.1016/j.clim.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Shultz LD, Pearson T, King M, et al. Humanized NOD/LtSz-scid IL2 receptor common gamma chain knockout mice in diabetes research. Ann N Y Acad Sci. 2007;1103:77–89. doi: 10.1196/annals.1394.002. [DOI] [PubMed] [Google Scholar]
- 44.Kelly PF, Vandergriff J, Nathwani A, et al. Highly efficient gene transfer into cord blood nonobese diabetic/severe combined immunodeficiency repopulating cells by oncoretroviral vector particles pseudo-typed with the feline endogenous retrovirus (RD114) envelope protein. Blood. 2000;96:1206–1214. [PubMed] [Google Scholar]
- 45.Giassi LJ, Pearson T, Schultz LD, et al. Expanded CD34+ human umbilical cord blood cells generate multiple lymphohematopoietic lineages in NOD-scid IL2rγ (null) mice. Exp Biol Med. 2008;233:997–1012. doi: 10.3181/0802-RM-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y, Hangoc G, Campbell TB, et al. Identification of parameters required for efficient lentiviral vector transduction and engraftment of human cord blood CD34(+) NOD/SCID-repopulating cells. Exp Hematol. 2008;36:947–956. doi: 10.1016/j.exphem.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lo Celso C, Fleming HE, Wu JW, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]