Abstract
Mammalian retinas are innervated by histaminergic axons that originate from perikarya in the posterior hypothalamus. To identify the targets of these retinopetal axons, we localized histamine receptors (HR) in monkey and rat retinas by light and electron microscopy. In monkeys, puncta containing HR3 were found at the tips of ON-bipolar cell dendrites in cone pedicles and rod spherules, closer to the photoreceptors than the other neurotransmitter receptors. This is the first ultrastructural localization of any histamine receptor and the first direct evidence that HR3 is present on postsynaptic membranes in the central nervous system. In rat retinas, most HR1 were localized to dopaminergic amacrine cells. The differences in histamine receptor localization may reflect the differences in the activity patterns of the two species.
Indexing terms: retinopetal, centrifugal, bipolar, amacrine, primate, dopamine
Vertebrate retinas receive input from the brain via retinopetal axons, and this pathway has important modulatory effects on retinal neurons in birds and fish, the groups that have been studied most intensively. The functions of retinopetal axons in mammalian retinas are not well understood, however (Uchiyama, 1989). Histamine has been localized to retinopetal axons in the guinea pig (Airaksinen and Panula, 1988), monkey (Gastinger et al., 1999), and rat (Gastinger et al., 2001) retinas. These axons originate from perikarya in the tuberomammillary nucleus of the posterior hypothalamus (Manning et al., 1996; Panula et al., 1989). Mast cells, another possible source of histamine, are not present in the retina (Smelser and Silver, 1963). With the identification of the neurotransmitter of these retinopetal axons, it is now possible to predict how these inputs contribute to information processing in mammalian retinas.
These retinopetal axons are expected to be active at night in rats and during the day in monkeys. Histaminergic neurons play an important role in the ascending arousal system (Saper et al., 2001), firing at a steady rate during waking and becoming silent during sleep (Steininger et al., 1999; Vanni-Mercier et al., 2003). In rats, a nocturnal species, histaminergic neurons are most active at night, and the rate of histamine release in the posterior hypothalamus is higher at night than during the day (Prast et al., 1992). In macaques, a diurnal species, levels of histamine metabolites in the third ventricular cerebral spinal fluid are higher during the day than at night (Prell et al., 1989).
In the central nervous system, histamine acts on three types of G-protein-coupled receptors: histamine receptor 1 (HR1), histamine receptor 2 (HR2) and histamine receptor 3 (HR3). Among these, only HR1 has been characterized previously in mammalian retinas (Nowak, 1993; Sawai et al., 1988). However, there is indirect evidence that HR2 and HR3 are also present in mammalian retinas. Histamine inhibits a forskolin-induced increase in cAMP in the rabbit retina via HR2 (Nowak, 1991; Nowak et al., 1989). Histamine also increases a GABAA-mediated chloride current in amacrine cells from rat retinal slices via protein kinase A (PKA; Feigenspan and Bormann, 1994), the signaling pathway typically used by HR2 (Gantz et al., 1991). Electrically evoked dopamine release from the pig retina is inhibited by histamine via HR3 (Schlicker et al., 1990). To identify the targets of histaminergic retinopetal axons, we localized histamine receptors in monkey and rat retinas by using both light and electron microscopic immunolabeling techniques.
MATERIALS AND METHODS
Adult baboon (Papio anubis) eyes were obtained from the Biological Materials Distribution Program at the Southwest Foundation for Biomedical Research (San Antonio, TX). These animals were sedated with ketamine (15 mg/kg), exsanguinated via the femoral vein, and killed with an overdose of sodium pentobarbital (100 mg/kg), following protocols approved by the Institutional Animal Care and Use Committee and conforming to NIH guidelines. The eyes were quickly removed and hemisected; the vitreous was removed with fine forceps. Macaque (Macaca mulata) eyes fixed for immunohistochemistry were purchased from Covance Research Products (Alice, TX).
Adult Sprague-Dawley albino rat eyes were obtained from researchers at the University of Texas Medical School at Houston. These were controls or sham-operated rats that were either euthanized by CO2 inhalation, over-dosed with ketamine/xylazine, or else decapitated without anesthesia. In all cases, the protocols for euthanasia were approved by the Animal Welfare Committee for the University of Texas Health Science Center at Houston and conformed to NIH guidelines. All eyes were removed and hemisected under room illumination.
Light microscopy
Eyecups were immersion fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.4, for 2 hours at room temperature or overnight at 4°C. The retinas were isolated from the retinal pigment epithelium, and the vitreous was removed. Some pieces were embedded in 4% agarose in phosphate-buffered saline with 0.3% sodium azide (PBSa), and 100 μm vertical Vibratome (Oxford Laboratories, St. Louis, MO) sections were cut. Some other pieces were cryoprotected in 30% sucrose in PBSa, embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek Inc., Torrance, CA), frozen, and cut into 30-μm sections with a cryostat (International Equipment Corp., Needham Heights, MA). Some pieces were also processed as flat mounts. All the sections were incubated in 1:100 donkey serum in PBSa with 0.3% Triton X-100 at 4°C overnight or for 3 hours at room temperature. All sections were incubated in primary antibody with 0.3% Triton for 3–6 days; flat mounts were incubated for 8–10 days at 4°C. The rabbit polyclonal antibodies were either neat antisera or affinity purified and diluted in PBSa:HR1C (1:2,000–1:5,000, AB5652P; Chemicon, Temecula, CA), HR1CL (1:500–1:2,000, AB5654P; Chemicon), HR2 (1:2,000, AB5656P; Chemicon), HR3C (1:5,000, AB5660P; Chemicon), HR3C (1:1,000–1:5,000 H3R31; ADI, San Antonio, TX), HR3N (1:1,000–1:5,000 H3R32; ADI), and human metabotropic glutamate receptor 6 (mGluR6; 1:1,000; Vardi et al., 2000). Affinity-purified secondary antibodies, biotinylated donkey anti-rabbit, and Cy3-conjugated streptavidin (1:100; Jackson Immunoresearch, West Grove, PA) were applied in succession for 1–2 days at 4°C. For frozen sections, secondary antibodies were applied for 1–3 hours at room temperature.
Double labeling
Some sections and flat mounts were incubated in a second primary antibody: goat anti-choline acetyltransferase (ChAT 1:200, AB144P; Chemicon), mouse anti-GABAA receptor (GABAAR) α-chain (MAB339, clone bd24; Chemicon), mouse anti-human mGluR6 (1:100; Vardi et al., 2000), or mouse anti-tyrosine hydroxylase (TH, 1:10,000; T2928 Sigma, St. Louis, MO). Affinity-purified secondary antibodies conjugated to Alexa 488, donkey anti-goat or donkey anti-mouse (1:200; Molecular Probes, Eugene, OR), were applied for 1 day at 4°C. Tissue was mounted on slides and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA).
Confocal analysis
All images were acquired with a Zeiss confocal laser scanning microscope (LSM 410; Carl Zeiss, Thornwood, NY) with a krypton-argon laser. By using laser emission filters of 590–610 nm for Cy3 and 515–540 nm for Alexa 488, images were acquired with a ×63 oil-immersion objective as a series of optical sections (0.5-μm step size). Each marker was assigned a pseudocolor (red or green). The images were analyzed as single sections and stacks of optical sections projected along the x-axis. All images were processed in Adobe Photoshop (Adobe Systems, San Jose, CA) to enhance brightness and contrast; all retinal vertical sections were oriented with the photoreceptors upward. The alignment of the lasers was verified by imaging 1-μm beads that were excited by the same wavelengths as those used in our experiments.
The double-labeled vertical sections were analyzed in the LSM image browser (Carl Zeiss) and Photoshop. Rod spherules whose invaginating processes were oriented parallel to the plane of section were analyzed with a signal averaging program (Li et al., 2002). Images from single optical sections or small reconstructed stacks were imported into the signal-averaging program, and nine to 13 rod spherule-related puncta per image were selected with a 1.9-μm-square box (30 × 30 pixels). Each selection box was centered on the mGluR6 signal and encompassed the entire spherule. A 3 × 3 median filter was applied to the data for smoothing. The normalized mean intensity vs. pixel position for the selected areas was calculated and exported to an Excel worksheet (Microsoft Corp., Redmond, WA).
Surface plots of the data were generated in Excel and copied into Photoshop for analysis. In all cases, the graphs represent normalized pixel intensities from 100–255; pixel intensities less than 100 were considered background. To determine the distance between the centers of the two signals, the surface plots were overlaid, and the peak to peak separation was measured. All values are reported as mean ± SEM.
Electron microscopy
Eyecups were fixed in 4% paraformaldehyde, 0.5% glutaraldehyde, or 4% paraformaldehyde, 0.1% glutaraldehyde in 0.1 M PB (pH 7.4) for 2 hours at room temperature, followed by 4% paraformaldehyde 0.1 M PB (pH 10) at 4°C overnight. The tissue was treated with 1% sodium borohydride in PBS for 1 hour, followed by an ascending and descending series of graded ethanol solutions in PBS (Marshak et al., 1990). Retinas were cut into small pieces and incubated in 1:5,000 rabbit anti-HR3C (AB5660; Chemicon) for 8–10 days at 4°C. The pieces were incubated for 2 days in biotinylated goat anti-rabbit IgG (1: 100; Vector Laboratories), and the biotin was visualized with a Vectastain avidin-biotin-peroxidase kit (1:100, PK-4000; Vector Laboratories) overnight at 4°C, followed by diaminobenzidine (0.5 mg/ml) with hydrogen peroxide (0.005%, 30–45 minutes). The retina was then postfixed in 1% osmium tetroxide in 0.1 M PB and embedded in epon. Sections 80–100 nm in thickness were collected on formvar-coated grids. The sections were stained with 2% uranyl acetate in 50% methanol for 60 minutes, followed by 0.2% lead citrate for 1–2 minutes. Labeled processes were viewed at ×20,000–50,000 in a JEOL (Peabody, MA) 100CX electron microscope (EM). Images were acquired with a side-mounted AMT Advantage HR 1000 CCD camera system (Advanced Microscopy Techniques Corp., Danvers, MA) and processed in Adobe Photoshop.
Peptide control experiments
The immunogen for the HR1C antibody (AB5652P; Chemicon) and antiserum (H1R11-S; ADI) was CNENFKK-TFKKILHIRS, a peptide (H1R11P; ADI) corresponding to a sequence near the cytoplasmic C-terminus of the rat HR1 (Fujimoto et al., 1993). The immunogen for the HR1CL antibody (AB5654P; Chemicon) and antiserum (H1R12-S; ADI) was (C)PSFSEIKLRPENPKGDAKK, a peptide (H1R12P; ADI) corresponding to a sequence within the third cytoplasmic loop of the human HR1 (Moguilevsky et al., 1994); cysteine was added for coupling to the carrier protein and was not part of the original sequence. The immunogen for HR3N (H3R32; ADI) was (C)SGALAGDAAAAGGARGFS, a peptide (H3R32P; ADI) corresponding to a sequence within the N-terminus of the human HR3 (Lovenberg et al., 1999, 2000); cysteine was added for coupling to the carrier protein and was not part of the original sequence. The immunogen for the HR3C affinity-purified polyclonal antibody [AB5660P (Chemicon) and H3R32A (ADI)] was CPQKLKVQPHGSLEQCWK, a peptide (H3R31P; ADI) corresponding to a sequence within the extracellular N-terminus of the human HR3 (Lovenberg et al., 1999, 2000); cysteine was added for coupling to the carrier protein and was not part of the original sequence. A region of the HR3C peptide, LKVQPH, is homologous to a region in protocadherin 2A, an unrelated peptide that is expressed in the developing retina; this sequence was synthesized (98.4% HPLC purified; Chemicon).
The HR1 peptide control experiments were peformed on vertical sections of rat retina prepared as described above by incubating the peptides (20 μg/μl) in the antisera for 1.5 hours at 37°C, then made to volume with PBS. For the HR3 peptide control experiments, macaque retinas were processed with graded ethanol solutions as in the EM study, to enhance the permeation of the antibodies through the tissue. The HR3 antibodies were incubated in the peptides (20 μg/μl) for 1 hour at 4°C. In all instances, the preincubation with the immunogen blocked all labeling in the retinal sections. To test whether the HR3C antibody recognized the short homologous sequence of protocadherin 2A, the synthesized peptide (1 mg/ml) was incubated with the diluted antibody; the labeling was not blocked and appeared the same as with untreated antibody.
RESULTS
Light and electron microscopic localization of HR3 in monkey retinas
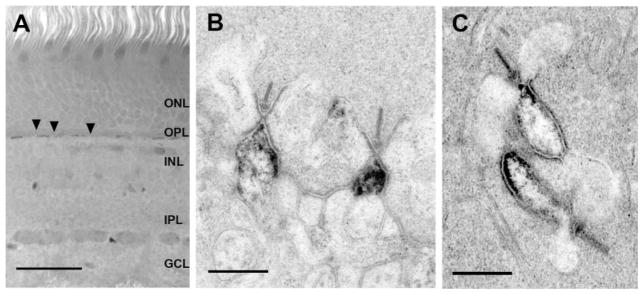
HR3-immunoreactive (-IR) puncta were restricted to the outer plexiform layer (OPL; Fig. 1A). Antibodies directed against two different regions of HR3 were used, and the results were the same with both (Fig. 2). Because the results were the same in baboon and macaque retinas, they are considered together here. Small HR3-IR puncta, approximately 0.3 μm in diameter, were arranged in a single row (Figs. 1, 2A). These puncta were aggregated in short bands, approximately 5–6 μm in length, beneath each cone pedicle; no labeling was found in perikarya in the INL. In the EM, reaction product was found at the apex of ON-bipolar cell dendrites, which were identified by their central position at ribbon synapses (Fig. 1). In most dendrites, the entire tip was filled with reaction product, and, in some cases, reaction product also extended vitreally or toward the ganglion cell layer (GCL). All cone pedicles analyzed had multiple labeled ON-bipolar cell dendrites. No reaction product was found in cone pedicles, in dendrites of horizontal cells, or in dendrites of OFF-bipolar cells.
Fig. 1.
HR3 localization in ON-bipolar cell dendrites of macaque and baboon retinas. A: A vertical section of macaque retina labeled with HR3C. Puncta associated with several cone pedicles (arrows) and rod spherules (arrowheads) were labeled. No labeling was detected in any other part of the retina. B: Electron microscopic localization of HR3 receptors in ON-cone bipolar cell dendrites in a baboon retina. Reaction product was found at the tips of the ON-bipolar cell dendrites and along the electron-dense portion of the plasma membrane. C: Electron microscopic localization of HR3C in two rod bipolar cell dendrites of a macaque retina. Reaction product was found along the membrane of the rod bipolar cell dendrites and extended to the tip (arrow). Scale bars = 50 μm in A; 500 nm for B,C.
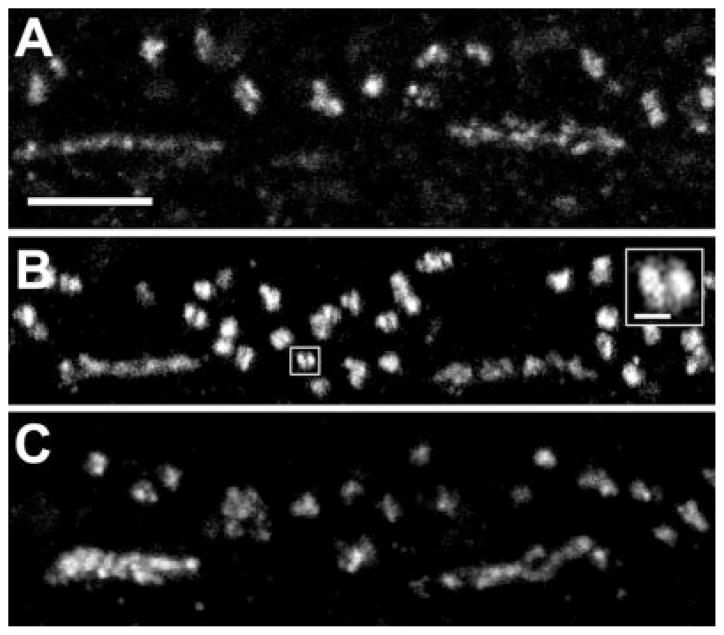
Fig. 2.
HR3 in the OPL of macaque (A,B) and baboon (C) retinas. The pattern of labeling in the OPL was identical with the two antibodies raised against HR3, HR3C (A) and HR3N (B). Clusters of small, HR3C-immunoreactive (IR) puncta formed a band at the bases of cone pedicles. Numerous larger puncta sclerad to the cone pedicles were associated with rod spherules. Some of the large puncta were composed of two smaller puncta in close apposition (inset). C: By using an antibody to HR3C, an identical pattern of labeling was found in baboon retina. Scale bars = 5 μm in A–C; 1 μm in inset.
Numerous larger HR3-IR puncta (0.7–1.2 μm) were found sclerad to, or toward the photoreceptor outer segments, and between the cone pedicles; they were associated with rod spherules (Fig. 2). Analysis of serial optical sections revealed that some of the large puncta were composed of two closely associated smaller puncta within the rod spherules (Fig. 2B, inset). Primate rod spherules have from two to seven central elements, consisting of rod bipolar cell dendrites, and two pairs of lateral elements, consisting of H1 horizontal cell axon terminals (Migdale et al., 2003). In the EM, reaction product was found on the apex of rod bipolar cell dendrites along the bifurcation ridge, the region of the synaptic cleft beneath the horizontal cell processes where the synaptic cleft bifurcates (Fig. 1C). Reaction product also extended at least 500 nm vitreally from the bifurcation ridge. No other elements associated with the rod spherules were labeled, but, in a few sections, HR3-IR puncta were observed in the neuropil, 2 μm vitread to the row of cone pedicles.
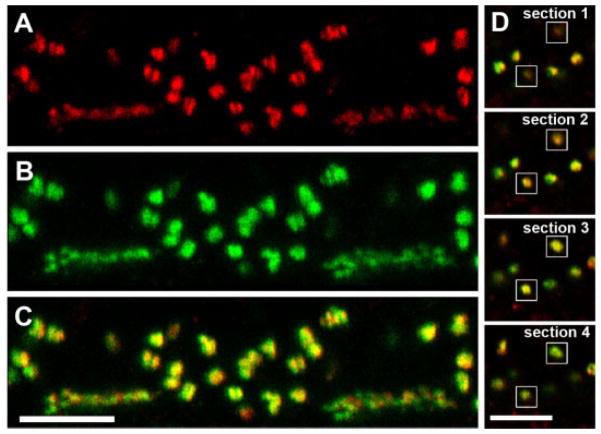
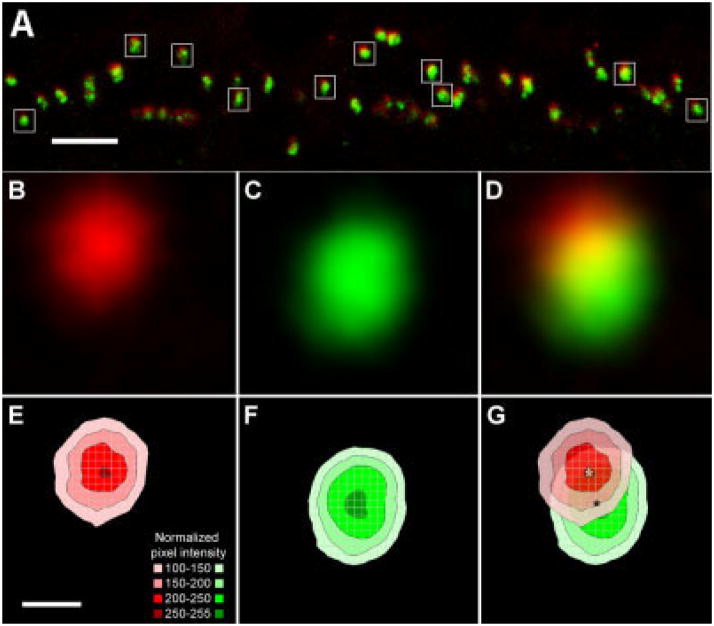
To compare the distribution of HR3 and the glutamate receptors of ON-bipolar cell dendrites, retinal sections were labeled with anti-mGluR6 and anti-HR3 (Figs. 3, 4). In primates, mGluR6 is found on all ON-bipolar cell dendrites, at both rod spherules and cone pedicles (Vardi et al., 2000). In the OPL, all HR3-IR puncta associated with the rod spherules were closely associated with mGluR6-IR puncta, but the two proteins did not have precisely the same distribution (Fig. 4). The HR3-IR puncta were sclerad relative to the mGluR6-IR puncta (Fig. 4A). This result is shown graphically in Figure 4E–G, as a plot of the normalized mean intensity vs. position for the HR3 and mGluR6 signals. An analysis of seven different double-labeled sections from two animals showed that the HR3 signal was shifted 0.286 ± 0.011 μm with respect to the peak of the mGluR6 signal. The HR3 immunoreactivity at the bases of cone pedicles was also sclerad relative to mGluR6 immunoreactivity (Fig. 3A–C), but individual puncta were not analyzed quantitatively.
Fig. 3.
HR3C-IR and mGluR6-IR puncta are closely associated (vertical section of a macaque retina). A: HR3C-IR (red) puncta at the bases of two cone pedicles and surrounding rod spherules in the OPL. B: In the same section, mGluR6-IR (green) puncta had a similar distribution. C: Composite of A and B showing that all HR3-IR puncta were associated with mGluR6-IR puncta. Note the sclerad shift in the HR3-IR puncta relative to the mGluR6-IR puncta. D: This series of four optical sections from C shows how the HR3-IR puncta on dendrites oriented perpendicular to the plane of section (white boxes) were more prominent in section 1 (top), whereas the mGluR6-IR puncta were more prominent in section 4. Puncta in sections 2 and 3 were yellow, indicating overlap between the two signals. Scale bars = 5 μm in C (applies to A–C); 5 μm in D (applies to all panels).
Fig. 4.
HR3-IR puncta are sclerad to MGluR6-IR puncta in rod bipolar cells. A: A single vertical section of macaque retina in which the rod bipolar cell dendrites were parallel to the plane of section with HR3C-IR (red) and mGluR6-IR (green) puncta. White boxes indicate examples of the rod-spherules selected for signal averaging analysis. B,C: Normalized mean intensity of the HR3C and mGluR6 signals from puncta in rod spherules. D: Composite figure showing both the red and the green signals; yellow indicates overlap. E,F: The normalized mean pixel intensities of the red HR3C and green mGluR6 signals are plotted as flat contour graphs. G: Superimposition of the two contour graphs showing that the HR3 signal was sclerad relative to the mGluR6 signal. Measurements were made from the peak of the red (white asterisk) to the peak of the green (black asterisk) signals. Scale bars = 5 μm in A; 0.5 μm in E (applies to B–G).
The HR3-IR puncta were further characterized by double labeling sections with a monoclonal antibody to GABAA receptor. In the OPL, GABAAR-IR puncta, arranged in bands approximately 5 μm in length and 2 μm thick, were labeled as described previously (Haverkamp et al., 2000; Vardi and Sterling, 1994), except that there was no labeling associated with rod spherules. HR3-IR puncta were sclerad to the GABAAR-IR band (Fig. 5A–C). The peak of the HR3 signal was shifted relative to the peak of the GABAAR signal, and the GABAAR signal was vitread to the mGluR6 signal (Fig. 5D–F).
Fig. 5.
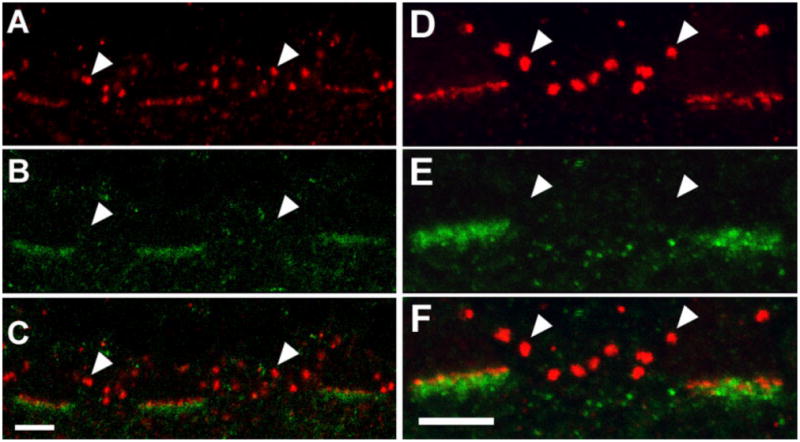
Localization of GABAA receptor α-chain (GABAAR), mGluR6, and HR3 in the OPL of a macaque. A: HR3C-IR (red) puncta are associated with three cone pedicles. Arrowheads indicate labeling in rod spherules. B: There are GABAAR-IR (green) puncta at the same three cone pedicles, but none was associated with rod spherules (arrowheads). C: Composite image of A and B showing that HR3C-IR puncta are sclerad relative to GABAAR-IR puncta. D: There are mGluR6-IR (red) puncta at the bases of two cone pedicles and several rod spherules (arrowheads). E: In the same section, GABAAR-IR puncta (green) are associated with the same cone pedicles. F: Composite image of E and F showing that mGluR6-IR puncta are sclerad to the GABAAR-IR puncta. Scale bars = 5 μm in C (applies to A–C), F (applies to D–F).
It was not possible to study the regional distribution of HR3 in monkey retinas, because whole-mount preparations could not be labeled. We were unable to detect HR3 in rat retinas, even though one of the antisera was raised against the cytoplasmic, C-terminus of the rat HR3.
Localization of HR1 in rat retinas
In rat retinas, the distribution of HR1 was analyzed by using neat antisera or affinity purified IgG from two antisera directed against different regions of the molecule, and the pattern of labeling was identical. HR1C and HR1CL both labeled large processes in stratum 1 (S1) of the IPL and large cell bodies in the inner nuclear layer (INL), approximately 15 μm in diameter. Smaller processes were also labeled in S4 of the IPL and in the OPL. HR1C and HR1CL both labeled all parts of dopaminergic amacrine cells, including somas, dendrites, and axons (Fig. 6). There were smaller HR1C-IR puncta in S1 of the IPL that were not associated with the TH-IR processes and also a few HR1C-IR puncta in S2 of the IPL. The HR1C-IR puncta in the IPL were not associated with ChAT-IR dendrites (not shown).
Fig. 6.
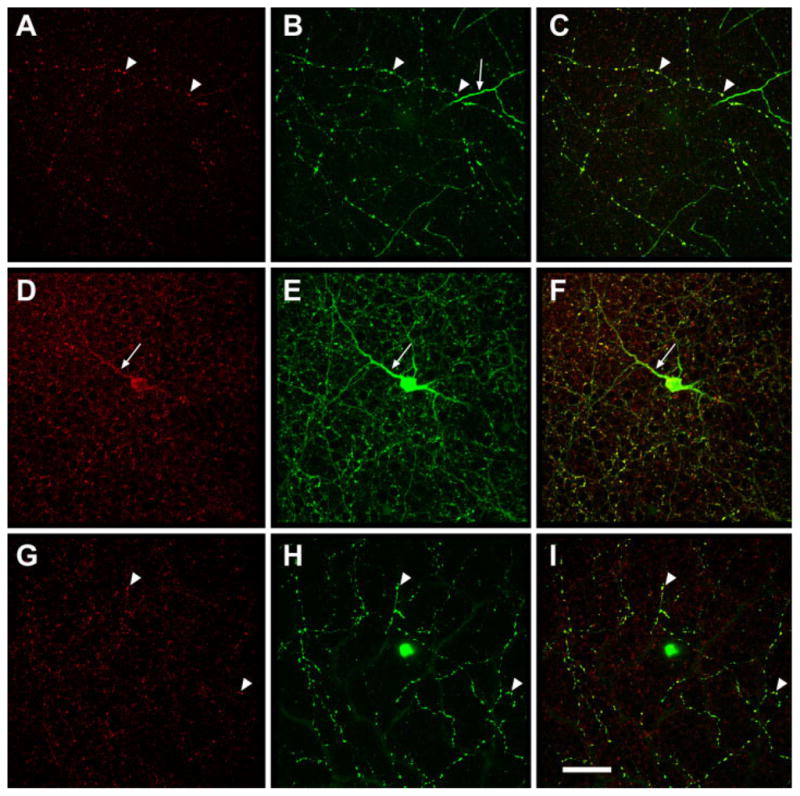
Dopaminergic amacrine cells in rat retina express HR1. Three stacks of optical sections showing red HR1C-IR puncta (A,D,G), green TH-IR amacrine cells (B,E,H), or both (C,F,I). A–C are from S4 of the IPL, D–F from S1, and G–I from the OPL. A dopaminergic cell body and nearly all of its processes (arrowheads), including the primary dendrite (arrow), were doubly labeled. Other HR1C-IR puncta in the IPL are not associated with dopaminergic cells. Scale bar = 50 μm in I (applies to A–I).
DISCUSSION
The first major finding was that HR3 receptors were localized to the tips of ON-bipolar cell dendrites in monkeys. These would be activated by histamine released tonically during the day. The receptors are strategically positioned to modulate transmission from photoreceptors and horizontal cells to ON-bipolar cells, because they are closer to the sites of glutamate release from the photoreceptors than either mGluR6 or GABAA receptors. This is the first direct evidence of HR3 at postsynaptic membranes, an unexpected result, in that HR3 are generally described as presynaptic receptors. Acting at HR3 autoreceptors, histamine inhibits its own release (Arrang et al., 1983). HR3 agonists inhibit the release of other neurotransmitters in the brain (Brown et al., 2001) and retina (Schlicker et al., 1990), and these effects have also been attributed to presynaptic receptors. However, the distribution of HR3 has been described in the brain using autoradiography (Pollard et al., 1993) and immunohistochemistry (Chazot et al., 2001), and both studies provide indirect evidence for HR3 on postsynaptic membranes.
The second major finding was that dopaminergic neurons in rat retinas express HR1. In rats, these receptors would be activated by tonic release of histamine at night. Histamine strongly inhibits the release of dopamine from guinea pig retinas (Weber and Schlicker, 2001), and the same is likely to be true in rats. Because dopamine plays a major role in light adaptation (Witkovsky, 2004), this reduction in dopamine release at night might facilitate dark adaptation. Dopaminergic neurons are also the targets of retinopetal axons in fish retinas (Zucker and Dowling, 1987). In mammalian retinas, dopaminergic neurons express receptors for other neuromodulators, somatostatin (Cristiani et al., 2000), and substance P (Casini et al., 2002; Catalani et al., 2004).
It is possible that there are regional differences in histamine receptor distribution that were undetected in this study. Histamine-IR axons are distributed differently in the nasal and temporal retina of macaques, but these differences are in the primary axons and their collaterals. These axons initially run toward the temporal retina in the optic fiber layer, encircle the fovea, and then return to the optic nerve. Axon terminals are distributed more uniformly; a few primary axons have branches that supply the rest of the retina, including the nasal retina and the parafovea (Gastinger et al., 1999). Rat retinas are typically supplied by only one histamine-IR axon. Although these branch extensively, there are regions of the retina that are apparently not innervated (Gastinger et al., 2001).
Histamine clearly exerts some of its effects in monkey and rat retinas via volume transmission, an ephaptic interaction in which the neurotransmitter diffuses through the extracellular space over a distance much greater than the width of the synaptic cleft before reaching its receptors. Histamine-IR retinopetal axons terminate exclusively in the IPL (Gastinger et al., 1999, 2001), but some histamine receptors were found in the OPL. In this respect, histamine acts as a typical retinal neuromodulator. There are receptors for neuropeptides (Bagnoli et al., 2003), dopamine (Witkovsky, 2004), serotonin (Pootanakit and Brunken, 2001), and acetylcholine (Yamada et al., 2003) in the OPL even though the processes of the neurons containing those modulators are very sparse or absent there.
It is possible that retinopetal axons also make synapses, because HR1 was localized in the IPL of rats. EM immunohistochemical studies will be required to determine whether these are sites of synaptic contacts. For mammalian brains, some groups have found a few synapses in histaminergic varicosities (Inagaki et al., 1987; Takagi et al., 1986) but others have found no synapses (Hayashi et al., 1984; Uhlrich et al., 1993; Wilson et al., 1999).
HR3 in monkey retinas
In cones, HR3 was localized at the tips of the ON-bipolar cell dendrites and typically extended only as far as the mouth of the invaginations. In macaque cone pedicles, mGluR6 is localized along the ON-bipolar cell membranes, at least 200 nm from the tips of the dendrites (Vardi and Sterling, 1994). In previous EM studies, GABAAR was localized to the bases of the cone pedicles, where it was found on both ON- and OFF-cone bipolar cell dendrites (Haverkamp et al., 2000; Vardi and Sterling, 1994). These results are summarized in Figure 7A. In rod spherules, HR3 and mGluR6 were both localized to rod bipolar cell dendrites, but, unlike mGluR6, HR3 was found at the tips of bipolar cell dendrites (Fig. 7B).
Fig. 7.
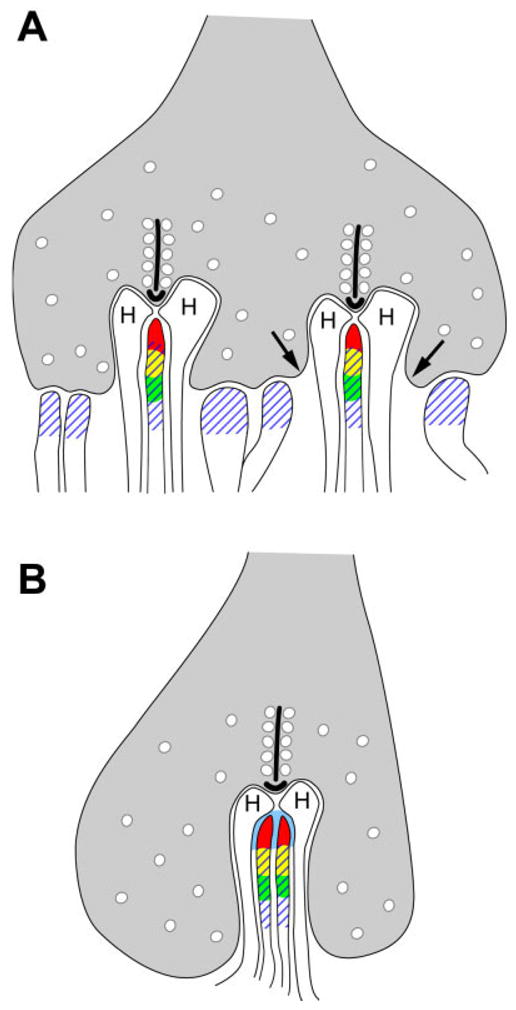
Summary diagram showing the distribution of HR3, mGluR6, and GABAAR in monkey OPL. A: At the cone pedicle, HR3 (red) are localized to the tips of ON-bipolar cell dendrites, and mGluR6 (green) are vitread to HR3. The overlap between mGluR6 and HR3 is represented by yellow. GABAAR (hatching) are localized to both ON- and OFF-bipolar cell dendrites. Arrows indicate the mouth of the invagination. B: In rod spherules, HR3 extends to the tips of rod bipolar cell dendrites along the bifurcation ridge (blue), and mGluR6 is located vitread to HR3. Based on previous electron microscopic studies, GABAAR are also shown on rod bipolar cell dendrites. H, horizontal cell.
The function of HR3 here is unknown; however, elsewhere in the central nervous system, HR3 activates either Go or Gi (Clark and Hill, 1996). The alpha subunit of Go (Goα) has been localized to ON-bipolar cell dendrites in macaques (Vardi, 1998) and shown to be coupled to mGluR6 (Weng et al., 1997). As with HR3, Goα is found at the tips of ON-bipolar cell dendrites (Vardi, 1998). The diameters of the labeled puncta cannot be measured accurately by light microscopy, but the results of the signal averaging analysis suggest that the distributions of mGluR6 and HR3 overlap to some extent. A comparison of our EM results with those published previously also suggests that they overlap (Vardi and Sterling, 1994). If HR3 activates Go associated with mGluR6, nonselective cation channels would close, hyperpolarizing ON-bipolar cells (Nawy, 2000).
Voltage-gated potassium (Kv) channels in bipolar cell dendrites might also be modulated by HR3. As with HR3, the Kv1.2 subunits have been localized by EM to the tips of ON-bipolar cell dendrites (Yazulla and Studholme, 1998). Other subunits, Kv1.1 and Kv1.3, have been localized to ON-bipolar cell dendrites by light microscopy as well (Klumpp et al., 1995). Some voltage-gated potassium channels in ON-bipolar cells are closed by cAMP-dependent PKA phosphorylation (Fan and Yazulla, 1999). Because HR3 is known to inhibit adenylate cyclase (Drutel et al., 2001), histamine acting via HR3 on macaque ON-bipolar cells would open more K+ channels, hyperpolarizing the ON-bipolar cells.
Other possible targets of HR3 are the excitatory amino acid transporters (EAAT) coupled to chloride channels, which are also found in ON-bipolar cells of fish retinas (Grant and Dowling, 1996). One of these, EAAT5, is expressed at high levels in human retinas (Arriza et al., 1997) and has been localized to rod spherules in macaque retinas by light microscopy (Pow et al., 2000). It is possible that EAAT5 or a related molecule is also present in the tips of rod bipolar cell dendrites. Because PKA stimulates EAATs (Lortet et al., 1999), HR3 activation would be expected to decrease the signaling through this pathway.
Histamine might also act by modulating the responses of ON-bipolar cells to GABA released from horizontal cells. HR3 might stimulate the cation-coupled chloride cotransporter, Na-K-Cl cotransporter (NKCC), which has also been localized to the apex of ON-bipolar cell dendrites (Vardi et al., 2002). In skeletal muscle, both mitogen-activated protein kinase and Gi-coupled mechanisms increase NKCC activity (Gosmanov et al., 2002; Wong et al., 2001), and HR3 can activate both of those signaling pathways (Drutel et al., 2001). Thus, histamine might increase the chloride influx, making the equilibrium potential for chloride (ECl) more positive. Histamine would then increase the efficacy of GABAA-mediated input from horizontal cells. However, there is only a small difference between ECl at the ON-bipolar cell dendrites and ECl at the axon terminals in rats (Billups and Attwell, 2002).
The close proximity of HR3 and GABAA receptors suggests that histamine may modulate the activity of ionotropic GABAA receptors of ON-bipolar cell dendrites. In slice preparations from ferret retinas, GABA applied to dendrites produces very similar responses mediated by GABAA receptors in all types of bipolar cells (Shields et al., 2000). However, the function of these receptors varies in different types of mouse bipolar cells (Dübel et al., 2004), and the effects on macaque bipolar cells would be expected to be variable as well. For example, the GABA antagonist picrotoxin has very little effect on the surround responses of parasol ganglion cells and, presumably, the bipolar cells that provide their input in macaque retinas (McMahon et al., 2004).
HR1 in rat retinas
The primary targets of histaminergic retinopetal axons in rats are HR1 on dopaminergic amacrine cells, and there is evidence suggesting that they are inhibitory. In rat retinas, dopamine release is lowest at night and highest during the day, even in constant darkness and in animals without photoreceptors, adult RCS/N-rdy rats homozygous for the rdy (retinal dystrophy) allele of photoreceptors (Doyle et al., 2002b). These rhythms might be maintained by melatonin release from the pineal gland (Doyle et al., 2002a) or by endogenous oscillations in the dopaminergic amacrine cells themselves (Gustincich et al., 2004; Witkovsky, 2004), but histaminergic retinopetal axons, which remained intact in these experiments, might also play an important role. Although HR1 receptors typically mediate excitatory effects, there are also known inhibitory effects (Brown et al., 2001). Histamine directly inhibits CA1 hippocampal neurons via HR1 (Selbach et al., 1997), possibly as a result of Ca2+ released from intracellular stores activating a calcium-dependent potassium conductance, as observed in cultured glial cells (Weiger et al., 1997). This current has been described previously in dopaminergic amacrine cells (Feigenspan et al., 1998).
CONCLUSIONS
One of the most striking results in this study was that the localization of histamine receptors was so different in rat and monkey retinas. This was unexpected, in that the morphology of the retinopetal axons themselves is quite similar in the two species. In monkeys, dendrites of ON-bipolar cells contained histamine receptors, and these were HR3. Acting at these receptors, histamine would be expected to influence the entire neural circuit that detects increments in light intensity. In rats, the major targets were dopaminergic amacrine cells, which express HR1. The effects of histamine would also be greatly amplified through this pathway because dopamine influences so many types of neurons in the retina. One possible explanation for the species differences is that they reflect differences in the photic environments that prevail when the animals are most active. This could be further tested by determining the distribution of histamine receptors in a variety of diurnal and nocturnal animals.
Acknowledgments
Grant sponsor: National Eye Institute; Grant number: EY06472; Grant number: EY11105; Grant number: EY12610; Grant number: Core Grant EY10608; Grant sponsor: Juvenile Diabetes Research Foundation; Grant sponsor: Pennsylvania Lion Sight Conservation and Eye Research Foundation; Grant sponsor: American Diabetes Association.
We are grateful to Mrs. Lillemor Krosby and Ms. Andrea Bordt for excellent technical assistance and to Drs. Stephen Mills, Brady Trexler, and Samuel Wu for valuable discussions. We also thank Drs. Karen Rice, Jerilyn Pecotte, and Gene Hubbard at the Southwest Foundation for Biomedical Research in San Antonio for providing monkey eyes and Dr. Pramod Dash at UT Medical School at Houston for providing rat eyes.
LITERATURE CITED
- Airaksinen MS, Panula P. The histaminergic system in the guinea pig central nervous system: an immunocytochemical mapping study using an antiserum against histamine. J Comp Neurol. 1988;273:163–186. doi: 10.1002/cne.902730204. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc Nat Acadl Sci U S A. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnoli P, Dal Monte M, Casini G. Expression of neuropeptides and their receptors in the developing retina of mammals. Histol Histopathol. 2003;18:1219–1242. doi: 10.14670/HH-18.1219. [DOI] [PubMed] [Google Scholar]
- Billups D, Attwell D. Control of intracellular chloride concentration and GABA response polarity in rat retinal ON bipolar cells. J Physiol. 2002;545:183–198. doi: 10.1113/jphysiol.2002.024877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Casini G, Sabatini A, Catalani E, Willems D, Bosco L, Brecha NC. Expression of the neurokinin 1 receptor in the rabbit retina. Neuroscience. 2002;115:1309–1321. doi: 10.1016/s0306-4522(02)00408-6. [DOI] [PubMed] [Google Scholar]
- Catalani E, Gangitano C, Bosco L, Casini G. Expression of the neurokinin 1 receptor in the mouse retina. Neuroscience. 2004;128:519–530. doi: 10.1016/j.neuroscience.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Chazot PL, Hann V, Wilson C, Lees G, Thompson CL. Immunological identification of the mammalian H3 histamine receptor in the mouse brain. Neuroreport. 2001;12:259–262. doi: 10.1097/00001756-200102120-00016. [DOI] [PubMed] [Google Scholar]
- Clark EA, Hill SJ. Sensitivity of histamine H3 receptor agonist-stimulated [35S]GTP gamma[S] binding to pertussis toxin. Eur J Pharmacol. 1996;296:223–225. doi: 10.1016/0014-2999(95)00800-4. [DOI] [PubMed] [Google Scholar]
- Cristiani R, Fontanesi G, Casini G, Petrucci C, Viollet C, Bagnoli P. Expression of somatostatin subtype 1 receptor in the rabbit retina. Invest Ophthal Vis Sci. 2000;41:3191–3199. [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002a;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002b;83:211–219. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- Drutel G, Peitsaro N, Karlstedt K, Wieland K, Smit MJ, Timmerman H, Panula P, Leurs R. Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Mol Pharmacol. 2001;59:1–8. [PubMed] [Google Scholar]
- Dübel J, Haverkamp S, Kuner T, Euler T. Chloride imaging in ON-type bipolar cells of a Clomeleon indicator mouse line [abstract] Invest Ophthal Vis Sci. 2004;45:1324. [Google Scholar]
- Fan SF, Yazulla S. Suppression of voltage-dependent K+ currents in retinal bipolar cells by ascorbate. Vis Neurosci. 1999;16:141–148. doi: 10.1017/s0952523899161091. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Facilitation of GABAergic signaling in the retina by receptors stimulating adenylate cyclase. Proc Nat Acadl Sci U S A. 1994;91:10893–10897. doi: 10.1073/pnas.91.23.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Gustincich S, Bean BP, Raviola E. Spontaneous activity of solitary dopaminergic cells of the retina. J Neurosci. 1998;18:6776–6789. doi: 10.1523/JNEUROSCI.18-17-06776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto K, Horio Y, Sugama K, Ito S, Liu YQ, Fukui H. Genomic cloning of the rat histamine H1 receptor. Biochem Biophys Res Commun. 1993;190:294–301. doi: 10.1006/bbrc.1993.1045. [DOI] [PubMed] [Google Scholar]
- Gantz I, Munzert G, Tashiro T, Schaffer M, Wang L, DelValle J, Yamada T. Molecular cloning of the human histamine H2 receptor. Biochem Biophys Res Commun. 1991;178:1386–1392. doi: 10.1016/0006-291x(91)91047-g. [DOI] [PubMed] [Google Scholar]
- Gastinger MJ, O’Brien JJ, Larsen NB, Marshak DW. Histamine immunoreactive axons in the macaque retina. Invest Ophthal Vis Sci. 1999;40:487–495. [PMC free article] [PubMed] [Google Scholar]
- Gastinger MJ, Barber AJ, Khin SA, McRill CS, Gardner TW, Marshak DW. Abnormal centrifugal axons in streptozotocin-diabetic rat retinas. Invest Ophthal Vis Sci. 2001;42:2679–2685. [PMC free article] [PubMed] [Google Scholar]
- Gosmanov AR, Wong JA, Thomason DB. Duality of G protein-coupled mechanisms for beta-adrenergic activation of NKCC activity in skeletal muscle. Am J Physiol Cell Physiol. 2002;283:C1025–1032. doi: 10.1152/ajpcell.00096.2002. [DOI] [PubMed] [Google Scholar]
- Grant GB, Dowling JE. On bipolar cell responses in the teleost retina are generated by two distinct mechanisms. J Neurophysiol. 1996;76:3842–3849. doi: 10.1152/jn.1996.76.6.3842. [DOI] [PubMed] [Google Scholar]
- Gustincich S, Contini M, Gariboldi M, Puopolo M, Kadota K, Bono H, LeMieux J, Walsh P, Carninci P, Hayashizaki Y, Okazaki Y, Raviola E. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc Natl Acad Sci U S A. 2004;101:5069–5074. doi: 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Takagi H, Takeda N, Kubota Y, Tohyama M, Watanabe T, Wada H. Fine structure of histaminergic neurons in the caudal magnocellular nucleus of the rat as demonstrated by immunocyto-chemistry using histidine decarboxylase as a marker. J Comp Neurol. 1984;229:233–241. doi: 10.1002/cne.902290208. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Yamatodani A, Shinoda K, Shiotani Y, Tohyama M, Watanabe T, Wada H. The histaminergic innervation of the mesencephalic nucleus of the trigeminal nerve in rat brain: a light and electron microscopical study. Brain Res. 1987;418:388–391. doi: 10.1016/0006-8993(87)90109-0. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Song EJ, Pinto LH. Identification and localization of K+ channels in the mouse retina. Vis Neurosci. 1995;12:1177–1190. doi: 10.1017/s0952523800006805. [DOI] [PubMed] [Google Scholar]
- Li W, Trexler EB, Massey SC. Glutamate receptors at rod bipolar ribbon synapses in the rabbit retina. J Comp Neurol. 2002;448:230–248. doi: 10.1002/cne.10189. [DOI] [PubMed] [Google Scholar]
- Lortet S, Samuel D, Had-Aissouni L, Masmejean F, Kerkerian-Le Goff L, Pisano P. Effects of PKA and PKC modulators on high affinity glutamate uptake in primary neuronal cell cultures from rat cerebral cortex. Neuropharmacology. 1999;38:395–402. doi: 10.1016/s0028-3908(98)00193-2. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- Lovenberg TW, Pyati J, Chang H, Wilson SJ, Erlander MG. Cloning of rat histamine H(3) receptor reveals distinct species pharmacological profiles. J Pharmacol Exp Ther. 2000;293:771–778. [PubMed] [Google Scholar]
- Manning KA, Wilson JR, Uhlrich DJ. Histamine-immunoreactive neurons and their innervation of visual regions in the cortex, tectum, and thalamus in the primate Macaca mulatta. J Comp Neurol. 1996;373:271–282. doi: 10.1002/(SICI)1096-9861(19960916)373:2<271::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marshak DW, Aldrich LB, Del Valle J, Yamada T. Localization of immunoreactive cholecystokinin precursor to amacrine cells and bipolar cells of the macaque monkey retina. J Neurosci. 1990;10:3045–3055. doi: 10.1523/JNEUROSCI.10-09-03045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated by a non-GABAergic pathway. J Neurosci. 2004;24:3736–3745. doi: 10.1523/JNEUROSCI.5252-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdale K, Herr S, Klug K, Ahmad K, Linberg K, Sterling P, Schein S. Two ribbon synaptic units in rod photoreceptors of macaque, human, and cat. J Comp Neurol. 2003;455:100–112. doi: 10.1002/cne.10501. [DOI] [PubMed] [Google Scholar]
- Moguilevsky N, Varsalona F, Noyer M, Gillard M, Guillaume JP, Garcia L, Szpirer C, Szpirer J, Bollen A. Stable expression of human H1-histamine-receptor cDNA in Chinese hamster ovary cells. Pharmacological characterisation of the protein, tissue distribution of messenger RNA and chromosomal localisation of the gene. Eur J Biochem. 1994;224:489–495. doi: 10.1111/j.1432-1033.1994.00489.x. [DOI] [PubMed] [Google Scholar]
- Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ J Neurosci. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ. Histamine in the retina. In: Watanabe T, Wada H, editors. Histaminergic neurons: morphology and function. Boca Raton, FL: CRC Press Inc; 1991. pp. 365–382. [Google Scholar]
- Nowak JZ. Histamine in the retina and some other components of the visual system. Prog Ret Res. 1993;12:41–74. [Google Scholar]
- Nowak JZ, Sek B, Szkiel B. Histamine-mediated regulation of cAMP levels and inositol phosphate metabolism in isolated rabbit retina. Agents Actions. 1989;27:131–134. doi: 10.1007/BF02222219. [DOI] [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Pollard H, Moreau J, Arrang JM, Schwartz JC. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience. 1993;52:169–189. doi: 10.1016/0306-4522(93)90191-h. [DOI] [PubMed] [Google Scholar]
- Pootanakit K, Brunken WJ. Identification of 5-HT(3A) and 5-HT(3B) receptor subunits in mammalian retinae: potential presynaptic modulators of photoreceptors. Brain Res. 2001;896:77–85. doi: 10.1016/s0006-8993(01)01998-9. [DOI] [PubMed] [Google Scholar]
- Pow DV, Barnett NL, Penfold P. Are neuronal transporters relevant in retinal glutamate homeostasis? Neurochem Int. 2000;37:191–198. doi: 10.1016/s0197-0186(00)00022-x. [DOI] [PubMed] [Google Scholar]
- Prast H, Dietl H, Philippu A. Pulsatile release of histamine in the hypothalamus of conscious rats. J Auton Nerv Syst. 1992;39:105–110. doi: 10.1016/0165-1838(92)90050-q. [DOI] [PubMed] [Google Scholar]
- Prell GD, Khandelwal JK, Burns RS, Green JP. Diurnal fluctuation in levels of histamine metabolites in cerebrospinal fluid of rhesus monkey. Agents Actions. 1989;26:279–286. doi: 10.1007/BF01967291. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Sawai S, Wang NP, Fukui H, Fukuda M, Manabe R, Wada H. Histamine H1-receptor in the retina: species differences. Biochem Biophys Res Commun. 1988;150:316–322. doi: 10.1016/0006-291x(88)90522-0. [DOI] [PubMed] [Google Scholar]
- Schlicker E, Schunack W, Gothert M. Histamine H3 receptor-mediated inhibition of noradrenaline release in pig retina discs. Naunyn-Schmiedebergs Arch Pharmacol. 1990;342:497–501. doi: 10.1007/BF00169035. [DOI] [PubMed] [Google Scholar]
- Selbach O, Brown RE, Haas HL. Long-term increase of hippocampal excitability by histamine and cyclic AMP. Neuropharmacology. 1997;36:1539–1548. doi: 10.1016/s0028-3908(97)00144-5. [DOI] [PubMed] [Google Scholar]
- Shields CR, Tran MN, Wong RO, Lukasiewicz PD. Distinct ionotropic GABA receptors mediate presynaptic and postsynaptic inhibition in retinal bipolar cells. J Neurosci. 2000;20:2673–2682. doi: 10.1523/JNEUROSCI.20-07-02673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelser GK, Silver S. The distribution of mast cells in the normal eye. Exp Eye Res. 1963;2:134–141. doi: 10.1016/s0014-4835(63)80005-6. [DOI] [PubMed] [Google Scholar]
- Steininger TL, Alam MN, Gong H, Szymusiak R, McGinty D. Sleep-waking discharge of neurons in the posterior lateral hypothalamus of the albino rat. Brain Res. 1999;840:138–147. doi: 10.1016/s0006-8993(99)01648-0. [DOI] [PubMed] [Google Scholar]
- Takagi H, Morishima Y, Matsuyama T, Hayashi H, Watanabe T, Wada H. Histaminergic axons in the neostriatum and cerebral cortex of the rat: a correlated light and electron microscopic immunocytochemical study using histidine decarboxylase as a marker. Brain Res. 1986;364:114–123. doi: 10.1016/0006-8993(86)90992-3. [DOI] [PubMed] [Google Scholar]
- Uchiyama H. Centrifugal pathways to the retina: influence of the optic tectum. Vis Neurosci. 1989;3:183–206. doi: 10.1017/s0952523800009950. [DOI] [PubMed] [Google Scholar]
- Uhlrich DJ, Manning KA, Pienkowski TP. The histaminergic innervation of the lateral geniculate complex in the cat. Vis Neurosci. 1993;10:225–235. doi: 10.1017/s0952523800003631. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Gigout S, Debilly G, Lin JS. Waking selective neurons in the posterior hypothalamus and their response to histamine H3-receptor ligands: an electrophysiological study in freely moving cats. Behav Brain Res. 2003;144:227–241. doi: 10.1016/s0166-4328(03)00091-3. [DOI] [PubMed] [Google Scholar]
- Vardi N. Alpha subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vis Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Vardi N, Duvoisin R, Wu G, Sterling P. Localization of mGluR6 to dendrites of ON bipolar cells in primate retina. J Comp Neurol. 2000;423:402–412. doi: 10.1002/1096-9861(20000731)423:3<402::aid-cne4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Vardi N, Dhingra A, Zhang L, Lyubarsky A, Wang TL, Morigiwa K. Neurochemical organization of the first visual synapse. Keio J Med. 2002;51:154–164. doi: 10.2302/kjm.51.154. [DOI] [PubMed] [Google Scholar]
- Weber B, Schlicker E. Modulation of dopamine release in the guinea-pig retina by G(i)- but not by G(s)- or G(q)-protein-coupled receptors. Fund Clin Pharmacol. 2001;15:393–400. doi: 10.1046/j.1472-8206.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- Weiger T, Stevens DR, Wunder L, Haas HL. Histamine H1 receptors in C6 glial cells are coupled to calcium-dependent potassium channels via release of calcium from internal stores. Naunyn-Schmiedebergs Arch Pharmacol. 1997;355:559–565. doi: 10.1007/pl00004983. [DOI] [PubMed] [Google Scholar]
- Weng K, Lu C, Daggett LP, Kuhn R, Flor PJ, Johnson EC, Robinson PR. Functional coupling of a human retinal metabotropic glutamate receptor (hmGluR6) to bovine rod transducin and rat Go in an in vitro reconstitution system. J Biol Chem. 1997;272:33100–33104. doi: 10.1074/jbc.272.52.33100. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Manning KA, Forestner DM, Counts SE, Uhlrich DJ. Comparison of cholinergic and histaminergic axons in the lateral geniculate complex of the macaque monkey. Anat Rec. 1999;255:295–305. doi: 10.1002/(SICI)1097-0185(19990701)255:3<295::AID-AR5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Wong JA, Gosmanov AR, Schneider EG, Thomason DB. Insulin-independent, MAPK-dependent stimulation of NKCC activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001;281:R561–R571. doi: 10.1152/ajpregu.2001.281.2.R561. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol. 2003;461:76–90. doi: 10.1002/cne.10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM. Differential distribution of Shaker-like and Shab-like K+-channel subunits in goldfish retina and retinal bipolar cells. J Comp Neurol. 1998;396:131–140. doi: 10.1002/(sici)1096-9861(19980622)396:1<131::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Zucker CL, Dowling JE. Centrifugal fibers synapse on dopaminergic interplexiform cells in the teleost retina. Nature. 1987;330:166–168. doi: 10.1038/330166a0. [DOI] [PubMed] [Google Scholar]