Abstract
Studies on bone development, formation and turnover have grown exponentially over the last decade in part because of the utility of genetic models. One area that has received considerable attention has been the PI3K signaling pathway, which has emerged as a major survival network for osteoblasts. Genetic engineering has enabled investigators to study downstream effectors of PI3K by directly over-expressing activated forms of AKT in cells of the skeletal lineage or deleting Pten which leads to a constitutively active AKT. The results from these studies have provided novel insights into bone development and remodeling, critical processes in the lifelong maintenance of skeletal health. This paper reviews those data in relation to recent advances in osteoblast biology and their potential relevance to chronic disorders of the skeleton and their treatment.
I. Introduction
Skeletal tissue in vertebrates develops in two ways, through either intramembranous or endochondral ossification. The former is required for cranial development, while the latter is the mechanism for bone formation in the limb and axial skeleton. Osteoblasts which are the bone forming cells of the skeleton, are essential in both these pathways (Kronenberg 2003). Osteoblasts originate from mesenchymal stem cells (MSC) which are multipotent and can differentiate into a number of lineages including bone, fat, muscle and cartilage. This allocation is dependent on extracellular signaling and the activation or repression of transcription factors that affect common intracellular signaling networks. The Wnt/β-catenin and the insulin/growth factor/PI3K pathways, have been the focus of recent work and both have emerged as critical for bone development, skeletal remodeling and energy metabolism. The Wnt/β-catenin pathway has been the subject of several very recent reviews (Baron and Rawadi 2007, Secreto et al 2009, Willams and Insoga 2009) whereas the latter (i.e. insulin/growth factor/PI3K pathway) is often considered solely within the framework of ligand activation rather than as a final common pathway that targets critical transcription factors to promote MSC growth and differentiation. This review outlines some of the recent advances in our understanding of the PI3K signaling system and its role in bone formation. We will first discuss the osteoblast transcriptional factors that control osteogenesis and then delineate how these are related to PI3K signaling and its downstream targets. Finally we will discuss the potential therapeutic applications of these studies.
II. PI3K and transcriptional control of osteoblastogenesis
Phosphatidyl inositol 3 kinase (PI3K) is an important lipid kinase that controls a number of cellular functions including proliferation, survival and motility in response to ligand activation. In contrast, constitutively active PI3K signaling can lead to neoplastic proliferation because of unrestricted cell growth. Therefore cells have developed a mechanism to regulate this process utilizing Phosphatase and tensin homologue deleted on chromosome ten (PTEN) (Franke et al 1997, Manning and Cantley 2007). Pten was first identified as a tumor suppressor gene and later on was characterized as a lipid phosphatase (Li et al 1997; Maehama and Dixon 1998). PTEN works mainly by acting as a direct antagonist to the actions of PI3K, where it can dephosphorylate phosphatidyl inositiol 3,4,5 tris phosphate (PIP3) to phosphatidyl inositiol 4,5 bis phosphate (PIP2) thereby negatively regulating PI3K signaling as shown in Fig 1 (Myers et al 1998). In the absence of Pten there is accumulation of PIP3 at the membrane to recruit PDK1 which can phosphorylate Akt at its threonine 308 residue (Alessi et al 1997). This, in turn, leads to mammalian target of rapamycin complex 2 (mTORC2) phosphorylating Akt at serine 473 (Sarbassov et al 2005), resulting in a completely active Akt pathway.
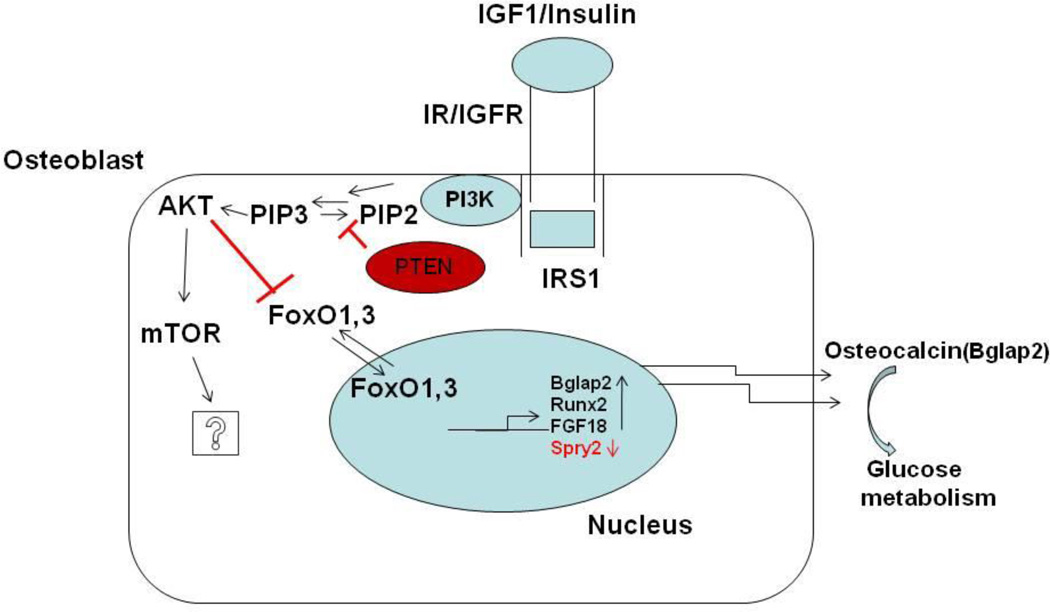
Figure 1.
The model depicted above shows an osteoblast cell where Phosphatidyl inositol 3 kinase (PI3K) signaling is activated by a growth factor in this case Insulin or Insulin like growth factor 1 (IGF1) by binding to their respective tyrosine kinase receptors which leads to binding of substrate proteins like Insulin receptor substrate 1(IRS1) that can bind to PI3K and activate it. PI3K can phosphorylate and convert Phosphatidyl inositol 4,5 bis phosphate (P1P2) to Phosphatidyl inositol 3,4,5 bis phosphate (P1P3) which leads to activation of AKT. AKT can phosphorylate and activate mammalian target of Rapamycin (mTOR), the downstream effects of this action have not been elucidated in detail during osteogenesis. AKT can also phosphorylate and inactivate Forkhead group of transcriptional factors 1 and 3 (FOXO1,3) which leads to regulation of a number of genes that are important during osteoblastogenesis,the figure shows Fibroblast growth factor 18 (FGF18), Runx2 and Sprouty2 (Spry2). Osteocalcin (Bglap2) which is also among the genes regulated then acts in an endocrine fashion and effects glucose homeostasis.
Recent genetic studies of PI3K signaling reveal that Akt and its downstream targets are critical regulators of endochondral ossification. For example, double Akt1/Akt2 knockout mice have delayed bone ossification (Peng et al 2003), whereas Akt1 knockout mice have shorter bones and delayed formation of secondary ossification centers (Ulici et al 2009). Akt has also been shown to affect bone formation and osteoblast survival by regulating FOXO3 (Kawamura et al 2007). In addition, Akt in concert with bone morphogenetic protein 2 (BMP2) mediates osteoblast differentiation from mesenchymal stromal cells (Ghosh-choudhary et al 2002; Mukherjee and Rotwein 2010).
a. FOXOs
In addition to its relatively direct role in bone formation via mTOR, AKT is an AGC family kinase, which can also phosphorylate other families of substrates that are critical for bone formation. Among those is the Forkhead group of transcriptional factors (FOXOs). These transcriptional factors are not osteoblast specific as opposed to other nuclear factors such as Runx2, Osterix and ATF4. The Forkhead group of transcriptional family members FOXO1, FOXO3 and FOXO4 are highly expressed in chondrocytes and osteoblasts (Ambrogini et al 2010). Activated Akt can phosphorylate FOXOs and control their transcriptional activity by maintaining their retention in the cytoplasm rather than promoting their nuclear transport (Brunet et al 1999). FOXO1 and FOXO3 have a major role in bone development by negatively regulating the transcriptional expression of key osteoblastic genes such as osteocalcin (Bglap2), Esp and ATF4 (Rached et al 2010). FOXO1 by modulating the expression of osteocalcin also plays a role in glucose homeostasis (Rached et al 2010; Kousteni 2010) and both these studies showed that deletion of FOXO’S strongly affects osteoblast function (Table 1). Furthermore a study when all three FOXO factors were deleted showed an increase in osteoblast and osteocyte apoptosis due to enhanced oxidative stress resulting in loss of bone mass. Most importantly, Amborgini et al demonstrated that FOXO3 was the major factor expressed in bone and overexpression in bone led to an increase in bone mass (Amborgini et al 2010). The role of FOXO4 is not clear in osteoblasts and chondocytes even though its expression has been shown in these cells.
Table 1.
Summarizes all the different knockouts and promoter cre lines utilized to delete Pten and its downstream targets in different skeletal lineages along with some of the key observations.
| Conditional knockouts of Pten generated by using the following mice |
References | Skeletal phenotypes observed |
|---|---|---|
| 1) a)Col2a1cre/ Pten flox/flox (exon4 and 5) | Ford-Hutchinson, et al 2007 | Disrupted normal growth plate organization, increased skeletal formation with increased AKT and mTOR activity |
| b)Col2a1cre/ Ptenflox/flox (exon4 and exon5) | Yang et al 2008 | Caused dyschondroplasia, decreased chondrocyte proliferation and differentiation with increased endoplasmic stress. |
| c)Col2a1cre/Pten flox/flox (exon5) | Hsieh, et al 2009 | Kyphosis, larger disorganized growth plates and vertebrae, Increased marrow adipocytes. |
| 2)Osteocalcin cre / Pten flox/flox (exon4 and 5) | Liu, et al 2007 | Decreased apoptosis of osteoblasts leading to increased bone formation throught the animals life span |
| 3)Dermo1cre/ Pten flox/flox (exon4 and 5) | Guntur, et al 2011 | Deletion in osteoprogenitors led to increased osteoblast differentiation due to increased FGF signaling |
| 4)Akt1/Akt2 knockouts | Peng, et al 2003 | Delayed bone formation |
| 5) Akt1 | Kawamura, et al 2007 | Decreased bone formation and osteoclast mediated resorption |
| 6) myrAKT (under the control of Col2a1 promoter) | Rokutanda, et al 2009 | AKT regulates endochondral bone formation (acting on GSK3,mTOR,FOXO) |
| 7)Col1a1cre/ FOXO1 | Rached, et al 2010 | Osteoblast specific knockout led to decreased bone mass |
| 8) a)FOXO1,3,4 and b) FOXO3 over expression | Amborgini, et al 2010 | a) Decreased bone formation b) Increased bone formation |
b. Runx2
MSCs which enter the osteogenic lineage can be identified by expression of an important transcriptional factor called Runx2. Runx2 is a member of the RUNX family of transcriptional factors. In humans, haploinsufficiency of Runx2 causes cleidocranial dysplasia which leads to delays in ossification of the skull and bone formation. Mouse studies of Runx2 deletion reveal embryonic lethality due to the absence of bone formation. Runx2 controls osteoblast differentiation by upregulating the expression of several critical osteoblastic genes including Osteopontin (Spp1), Bone sailoprotein (Bsp) and osteocalcin (Bglap2) (Otto et al 1997; Ducy et al 1997; Mundlos et al 1997>). Runx2 also interacts with PI3K signaling networks at various levels to control expression of p85 and p110β subunits of the PI3K complex (Fujita et al 2004). Runx2 binds to the promoter regions of these genes and interacts with co-activators thereby inducing gene expression. Recent studies have shown that FOXO1 which can be negatively regulated by active AKT signaling, can bind to RUNX2 and negatively regulate its transcriptional activity specifically relative to osteocalcin gene expression (Yang et al 2011).
c. Osterix
Another important osteoblastic gene which is a target of Runx2 during osteoblast differentiation is Osterix (Sp7). It is a zinc finger containing protein homologous to the Sp group of transcriptional factors. Gene deletion studies have shown that in the absence of Osterix there is no bone formation even though there is expression of Runx2 suggesting that Osterix is downstream of Runx2 (Nakashima et al 2002). There is also some evidence suggesting that Osterix can be transcriptionally upregulated by Runx2 (Nishio et al 2006). The expression of this transcriptional factor starts around 12.5dpc and has a major role during embryonic bone development. But deleting Osterix at different time points post natally showed that it is also necessary for adult bone growth and resorption, by affecting osteoblast and osteocyte development and function (Zhou et al 2010). Osterix has been shown to transcriptionally upregulate Col1a1 expression through its interaction with NFATc1 (Koga et al 2005). However there is no direct evidence showing that osterix gene expression can be regulated through PI3K signaling although it has recently been suggested that Osterix gene expression can be upregulated by PI3K signaling in concert with BMP2 signaling (Mandal et al 2010). Additional evidence from targeted FOXO1 knockout mice show there is a decrease in Osterix expression implicating PI3K in the regulation of Osterix (Rached et al 2010).
d. ATF4
Another major transcriptional factor that is osteoblast specific is ATF4 (CREB2, cyclic AMP response element binding protein2) which belongs to the CREB group of transcriptional factors. Deletion of ATF4 in mice proved that it was essential for terminal osteoblast differentiation (Yang et al 2004). ATF4 is phosphorylated by RSK2 during bone development and accumulates in osteoblasts where it enhances the synthesis of matrix proteins required for bone formation by stimulating amino acid transport into osteoblasts. ATF4 also controls the expression of 1) osteocalcin which in its uncarboxylated form modulates not only bone formation but also glucose metabolism, and 2) the expression of Esp which codes for a tyrosine phosphatase (OST-PTP) in osteoblasts and acts as a key negative regulator of insulin receptor activity (Yoshizawa et al 2009). Under conditions of increased oxidative stress, FOXO1 has been shown to physically interact with ATF4 in osteoblasts to upregulate protein synthesis (Rached et al 2010 a,b). ATF4 is also important for modulating bone resorption by regulating the expression of RANKL, the protein required for osteoclast differentiation (Yoshizawa et al 2009).
A number of other important transcriptional factors have emerged in the last half decade that regulate osteoblast differentiation such as CREB (Oury et al 2010), β-catenin (Day et al 2005; Holmen et al 2005), Twist1, and Twist 2 (Bialek et al 2004) among others. Published data reveals that PI3K signaling and its downstream effectors like AKT interact mainly with these transcriptional factors at the level of gene transcription (Fujita etal 2004, Rached et al 2010 a,b). Insulin signaling and bone development also are intricately linked, since osteoblasts secrete and deposit osteocalcin, which can act as hormone to enhance insulin secretion and promote insulin sensitivity. Interestingly, the AKT-FOXO axis in osteoblasts also has been shown to regulate glucose homeostasis. Osteoblasts also express tyrosine kinase receptors for Insulin and Insulin like growth factors which through ligand binding can activate downstream P13K related events. This might be one of the major mechanisms through which PI3K signaling activates bone formation (Figure 1). Not surprisingly, emerging evidence suggests there is also significant synergy between the AKT/PI3K pathway and Wnt activation of β-catenin. Although the interactions between P13K and other signaling pathways and transcription factors in osteoblasts are critical, it is clear that this system must be tightly regulated and most importantly, context-specific.
III. Pten signaling and Endochondral ossification
Through genetic engineering studies a key role in bone development has emerged for Pten, the lipid phosphatase that inactivates PI3K as well as for the PI3K/AKT pathway. Three chondrocyte and one osteoblast specific Pten conditional knockout studies have been published delineating the role of Pten during endochondral ossification. These studies are extremely valuable since they shed light on the context specific nature of the PI3K signaling pathway in bone and the potential interaction with other signaling pathways.
a. Chondrocyte specific deletion of Pten
Ford-Hutchinson et al 2007 used a Col2a1Cre to selectively delete Pten in mice. The cre recombinase is under the control of the collagen2a1 promoter and starts expression at 9.5dpc and its expression in chondrocytes is specific (Ovchinnikov et al 2000). The authors utilized the Pten flox/flox mice described by Suzuki et al 2001 with flox sites flanking exon 4 and exon 5 of Pten. Deletion of Pten led to increased chondrocyte differentiation and trabecular bone. Histological survey showed that the growth plates were abnormal with disruption in the arrangement of chondrocytes. The increase in trabecular bone was also followed by an increase in marrow adipocytes in 5 week old animals. Another group (Yang et al 2008) utilizing the same mice to look at chondrocyte specific deletion of Pten, identified that loss of PTEN in the growth plate led to an increase in endoplasmic stress levels which induced HIF1α activation. They concluded there was impairment in chondrocyte differentiation with Pten ablation in chondrocytes leading to dyschondroplasia thereby suggesting a mechanism through which the growth plate phenotype occurs.
A third study (Hsieh S-C et al 2009) that was also a chondrocyte specific model, used the Col2cre crossed to Pten flox/flox mice. These mice have only exon5 of Pten floxed, but on utilization of the Cre showed a similar loss in PTEN protein levels in a manner identical to other Pten floxed mice (Lesche et al 2002). The chondrocyte specific knockout of Pten in this case also showed increased chondrocyte differentiation and growth plate defects similar to the above-mentioned studies. Additionally the long bones of these mice had an increase in marrow adipogenesis with lipoma formation. Through the use of a Rosa 26 reporter expression to study the cre expression the authors raised the possibility that Pten also plays a role in regulating adipocyte formation.
The increased adipocyte phenotype can be explained by the activity of the cre targeting adipocyte precursors among MSCs leading to the increase seen in adipogenesis as AKT has been shown to be necessary for adipogenesis. The phenotypes of the different chondrocyte specific knockouts show a reasonably similar phenotype when growth plate development is compared. But, the difference among these knockouts cannot be attributed solely to deletion of Pten as there was an efficient knockout of Pten and activation of pAKT. On the other hand, the differences could be due to variations in the genetic background of the mice utilized. The phenotypes of the different conditional knockouts that have been reported have been summarized in Table I.
b. Osteoblast specific knockout of Pten
Deletion of Pten in late stage osteoblasts using a Cre that is under the control of an osteocalcin promoter which starts expression at around 17dpc (Zhang et al 2002) showed there is accumulation (increase) of bone mineral density in bones that develop through both intramembranous and endochondral ossification. This would suggest a cell autonomous effect of loss of PTEN on bone development. This potential mechanism was studied with calvarial osteoblasts and the authors observed a decrease in osteoblast apoptosis and an increase in mTOR signaling pathway molecules in cells from PTEN-null mice (Liu et al 2007).
Another study using a conditional knockout approach was performed using Dermo1Cre to delete Pten in osteoprogenitors (Guntur et al 2011). Dermo1 or twist2 cre starts expression at around 9.5dpc and targets mesenchymal cells so it works on precursors that are destined to become skeletal cells (Li et al 1995; Yu et al 2003). Loss of Pten in osteoprogenitors resulted in an increase in osteoblast progenitor proliferation specifically in the perichondrium and was accompanied by 1) enhanced FGF signaling; 2) by increased Fgf18 expression and 3) by a decrease in Sprouty2, an inhibitor of FGF signaling, which when activated increases perichondral osteoprogenitor proliferation and differentiation. Not surprisingly, the expression of Sprouty2, at the transcriptional level, is regulated by the FoxO group of transcriptional factors. For example, we found that over expression of FOXO3 in C3H10T1/2 cells leads to suppression of Fgf18 expression. FGF signaling in turn activates GLI2 activity via downstream effectors, leading to osteoblast differentiation. (summarized in Table I).
Marrow adipogenesis is an alternative pathway for mesenchymal stromal cell differentiation and is dependent on the activation of several critical transcription factors, including Pparγ. As noted, chondrocyte specific deletion of Pten resulted in the appearance of marrow adipocytes (Hsieh S-C et al 2009). These findings have significant clinical implications. First, in age-related osteoporosis, osteoblast progenitor numbers are reduced while marrow adipogenesis is enhanced suggesting that lineage allocation is a critical determinant of subsequent bone turnover even in the elderly (Nutall et al 1998). Second, increased Pparγ activation with agents such as the TZDs, force mesenchymal progenitors into the adipocyte pathway and this results in significant bone loss and a greater risk of fracture in Type II diabetes mellitus. From a therapeutic perspective, agents that selectively target lineage allocation in progenitor cells might offer promise as anabolic agents, or to prevent age-related marrow adipogenesis or to set the stage for more robust skeletal formation under the appropriate circumstances.
The latter is provocative and may be critical in a number of scenarios. As shown, the Pten and the PI3K signaling pathway clearly play an important role in regulating tissue specific progenitor cell fate (Yilmaz et al 2007; Hill and Wu 2009). This is further supported by findings that an increase in the activity of the Forkhead group of transcriptional factors is associated with a loss in bone mass and an increase in marrow adipogenesis (Moerman et al 2004; Almeida et al 2007). Additional evidence from AKT1/AKT2 knockout mice shows that in the absence of AKT there is an inhibition of adipocyte differentiation and that AKT is important for adipogenesis by inducing Pparγ expression. On the other hand, it could be argued that the increase in proliferation in Pten deleted cells maintains precursor cells as an immature population allowing for expansion of marrow adipocytes under the appropriate conditions. Experimental studies in our laboratory suggest that marrow adipogenesis is an early feature of injury models such as irradiation post bone marrow transplant or during the first days post fracture repair. Maintenance of precursor cells in their “stem-like” state by marrow adipocytes could be a critically important function by acting as ‘place-holders’ to allow progenitor cells time to be primed prior for differentiation. Ultimately, in the bone marrow irradiation/transplant model, adipocytes in the bone marrow disappear either by apoptosis or conversion to a new cell type, and this is followed by rapid hematopoietic repopulation. Thus fully understanding lineage allocation and the role of PI3K in this process is an important clinical and translational goal.
c. PI3K, Pten signaling and Intramembranous ossification
All the above studies examined the role of PI3K signaling during endochondral ossification. However, changes in PI3K signaling during intramembranous ossification have not been studied in a systematic manner. For example, examination of the Akt1/Akt2 knockout animals shows a clear defect in intramembranous ossification which could be attributed to PI3K signaling regulating RUNX2. One study utilized the Pten flox/flox/col2a1 cre as a model to study the effect of variation of phenotypes on the shape of the skull. As the base of the skull develops through endochondral ossification the authors observed a shortening of the skull base (Hallgrimsson et al 2007). Furthermore, the osteocalcin cre Pten conditional knockout exhibit increased calvarial bone density suggesting that the Pten knockout has a cell autonomous effect on cranial bone development. In another study where AKT signaling was modulated in the skeleton by expressing a dnAKT (dominant negative) form under the control of a col2a1 promoter, skull base formation (i.e. shortening of the skull base) was affected, a finding which is nearly identical to the effect of Pten deletion on the skull base (Rokutanda et al 2009). Studying the effect of PI3K signaling or its downstream effectors during intramembranous ossification using mouse models that have lesions in the PI3K signaling effectors might provide novel information about skull development and provide new therapeutic avenues to treat pathological conditions, particularly in respect to craniofacial disorders.
IV. Diverse effects of the PI3K pathway
One of the most exciting developments in skeletal biology has been the establishment of the skeleton as a modulator of glucose and metabolic homeostasis (Lee et al 2007; Ferron et al 2010, Clemens and Karsenty 2011). For example a recent study in which FOXO1 was deleted only in osteoblasts revealed that not only was bone mass affected but so was glucose metabolism (Rached et al 2010). Though Pten specific knockouts should inactivate FOXO1 in chondrocytes and osteoblasts, and thereby lead to the metabolic effects seen with the osteoblast specific deletion of FOXO1, none of the studies to date have addressed this question. Furthermore, osteoprogenitor specific deletion of Pten led to FGF signaling mediated activation of MAPK signaling showing that this arm of the signaling mechanism downstream of tyrosine kinase receptors like FGFR is also activated. Thus studying the energy sensor kinase mammalian target of rapamycin (mTOR,) the other major target of AKT (Fingar and Blenis 2004), and its role in osteoblast differentiation would be provocative. For example, the Pten conditional knockout using an osteocalcin cre by Liu et al clearly showed that deletion in calvarial osteoblasts was associated with an increase in mTOR activity. Additionally the study by Ford-Hutchinson et al revealed an increase in pP70S6K (a downstream direct target of mTOR) in the growth plate when the chondrocytes were PTEN-null. The only studies that have directly addressed these issues however, have utilized preosteoblastic cells MC3T3E1 rats and mouse ex vivo organ cultures to inhibit mTOR using rapamycin (Alvarez-Garcia et al 2007; Sanchez and He 2009; Rokutanda et al 2009; Phornphutkul et al 2008, Singha et al 2008). Therefore it would be essential to study the role of mTOR signaling both in the context of loss of Pten or downstream of activation PI3K signaling during osteogenesis. In that respect, a recent study showed that loss of insulin like growth factor binding factor2 (IGFBP2) in male mice led to an increase in PTEN protein expression in bone marrow stromal cells and calvarial osteoblasts (Demambro et al 2008, Kawai et al 2011). This was accompanied by an increase in marrow adiposity suggesting that enhanced Pten activity played a role in regulating bone development by controlling mesenchymal stem cell fate. Not surprisingly, Igfbp2−/− mice develop mild insulin resistance and glucose intolerance with age. In that same model, treatment of the Igfbp2−/− mice with a short polypeptide for the heparin binding domain of the IGFBP2 molecule rescued the low bone mass phenotype and markedly suppressed PTEN expression. (Kawai et al 2011). Though there have been no lesions in humans that have been identified that affect P13K signaling directly, syndromes like growth hormone deficiency that cause Laron’s syndrome and skeletal deficiencies, affect IGF1 levels in the body and thereby potentially modulate PI3K signaling. Moreover, PTH 1–34 and 1–84, are two peptides approved for the treatment of postmenopausal osteoporosis by enhancing bone formation. Although the primary mechanisms that underlie the skeletal anabolic activity of PTH are not totally defined, activation of the AKT/PI3K pathway via induction of IGF-1 in osteoblasts is one pathway proven to be operative when PTH is administered.
V. Conclusions
In sum, we have shown that Pten and PI3K are part of a critical nodal pathway in skeletal development and bone turnover. Neither the growth factor/ PI3K nor Wnt/β-catenin pathways exist in a vacuum; rather these two networks are integrated with other signaling network. The context specific nature of these pathways and their overlap lead to a significant level of complexity that will require further studies. Notwithstanding, experimental attempts to modify PI3K signaling to favorably impact progenitor recruitment and skeletal acquisition, or reduce the severity of craniofacial disorders, represent a major therapeutic challenge that could be extremely rewarding.
Acknowledgments
Supported by NIH grants: AR45433 and AR 54604
References
- Alessi DR, James SR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B[alpha] Current Biology. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, et al. Oxidative Stress Antagonizes Wnt Signaling in Osteoblast Precursors by Diverting β-Catenin from T Cell Factor- to Forkhead Box O-mediated Transcription. Journal of Biological Chemistry. 2007;282(37):27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- Alvarez-Garcia O, Carbajo-Pérez E, et al. Rapamycin retards growth and causes marked alterations in the growth plate of young rats. Pediatric Nephrology. 2007;22(7):954–961. doi: 10.1007/s00467-007-0456-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini E, Maria A, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell metabolism. 2010;11(2):136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Rawadi G. Targeting the Wnt/{beta}-Catenin Pathway to Regulate Bone Formation in the Adult Skeleton. Endocrinology. 2007;148(6):2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- Bialek P, Kern B, et al. A Twist Code Determines the Onset of Osteoblast Differentiation. Developmental cell. 2004;6(3):423–435. doi: 10.1016/s1534-5807(04)00058-9. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, et al. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The Phosphoinositide 3-Kinase Pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Clemens TL, Karsenty G. The osteoblast: An insulin target cell controlling glucose homeostasis. Journal of Bone and Mineral Research. 2011;26(4):677–680. doi: 10.1002/jbmr.321. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, et al. Wnt/2-Catenin Signaling in Mesenchymal Progenitors Controls Osteoblast and Chondrocyte Differentiation during Vertebrate Skeletogenesis. Developmental cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- DeMambro VE, Clemmons DR, et al. Gender-Specific Changes in Bone Turnover and Skeletal Architecture in Igfbp-2-Null Mice. Endocrinology. 2008;149(5):2051–2061. doi: 10.1210/en.2007-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Zhang R, et al. Osf2/Cbfa1: A Transcriptional Activator of Osteoblast Differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Ferron M, Wei J, et al. Insulin Signaling in Osteoblasts Integrates Bone Remodeling and Energy Metabolism. Cell. 2010;142(2):296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 23(18):3151–3171. doi: 10.1038/sj.onc.1207542. (0000). [DOI] [PubMed] [Google Scholar]
- Ford-Hutchinson AF, Ali Z, et al. Inactivation of Pten in Osteo-Chondroprogenitor Cells Leads to Epiphyseal Growth Plate Abnormalities and Skeletal Overgrowth. Journal of Bone and Mineral Research. 2007;22(8):1245–1259. doi: 10.1359/jbmr.070420. [DOI] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, et al. PI3K: Downstream AKTion Blocks Apoptosis. Cell. 1997;88(4):435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- Fujita T, Azuma Y, et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. The Journal of Cell Biology. 2004;166(1):85–95. doi: 10.1083/jcb.200401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh-Choudhury N, Abboud SL, et al. Requirement of BMP-2-induced Phosphatidylinositol 3-Kinase and Akt Serine/Threonine Kinase in Osteoblast Differentiation and Smad-dependent BMP-2 Gene Transcription. Journal of Biological Chemistry. 2002;277(36):33361–33368. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- Guntur AR, Reinhold MI, et al. Conditional ablation of Pten in osteoprogenitors stimulates FGF signaling. Development. 2011;138(7):1433–1444. doi: 10.1242/dev.058016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgrímsson B, Lieberman DE, et al. Epigenetic interactions and the structure of phenotypic variation in the cranium. Evolution & Development. 2007;9(1):76–91. doi: 10.1111/j.1525-142X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- Hill R, Wu H. PTEN, Stem Cells, and Cancer Stem Cells. Journal of Biological Chemistry. 2009;284(18):11755–11759. doi: 10.1074/jbc.R800071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Zylstra CR, et al. Essential Role of β-Catenin in Postnatal Bone Acquisition. Journal of Biological Chemistry. 2005;280(22):21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- Hsieh S-C, Chen N-T, et al. Conditional loss of PTEN leads to skeletal abnormalities and lipoma formation. Molecular Carcinogenesis. 2009;48(6):545–552. doi: 10.1002/mc.20491. [DOI] [PubMed] [Google Scholar]
- Kawai M, Breggia AC, et al. The heparin-binding domain of IGFBP-2 has IGF binding-independent biologic activity in the growing skeleton. Journal of Biological Chemistry. doi: 10.1074/jbc.M110.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura N, Kugimiya F, et al. Akt1 in Osteoblasts and Osteoclasts Controls Bone Remodeling. PLoS ONE. 2007;2(10):e1058. doi: 10.1371/journal.pone.0001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Matsui Y, et al. NFAT and Osterix cooperatively regulate bone formation. Nat Med. 2005;11(8):880–885. doi: 10.1038/nm1270. [DOI] [PubMed] [Google Scholar]
- Kousteni S. FoxO1: A molecule for all seasons. Journal of Bone and Mineral Research. 2010 doi: 10.1002/jbmr.306. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- Lee NK, Sowa H, et al. Endocrine Regulation of Energy Metabolism by the Skeleton. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesche R, Groszer M, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32(2):148–149. doi: 10.1002/gene.10036. [DOI] [PubMed] [Google Scholar]
- Li J, Yen C, et al. PTEN, a Putative Protein Tyrosine Phosphatase Gene Mutated in Human Brain, Breast, and Prostate Cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- Li L, Cserjesi P, et al. Dermo-1: A Novel Twist-Related bHLH Protein Expressed in the Developing Dermis. Developmental Biology. 1995;172(1):280–292. doi: 10.1006/dbio.1995.0023. [DOI] [PubMed] [Google Scholar]
- Liu X, Bruxvoort KJ, et al. Lifelong accumulation of bone in mice lacking Pten in osteoblasts. Proceedings of the National Academy of Sciences. 2007;104(7):2259–2264. doi: 10.1073/pnas.0604153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. Journal of Biological Chemistry. 1998;273(22):13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- Mandal C, Drissi H, et al. Integration of Phosphatidylinositol 3-Kinase, Akt Kinase, and Smad Signaling Pathway in BMP-2-Induced Osterix Expression. Calcified tissue international. 2010:1–8. doi: 10.1007/s00223-010-9419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB Signaling: Navigating Downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Wilson EM, et al. Selective Signaling by Akt2 Promotes Bone Morphogenetic Protein 2-Mediated Osteoblast Differentiation. Mol. Cell. Biol. 2010;30(4):1018–1027. doi: 10.1128/MCB.01401-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MP, Pass I, et al. The lipid phosphatase activity of PTEN is critical for its tumor supressor function. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(23):13513–13518. doi: 10.1073/pnas.95.23.13513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, et al. The Novel Zinc Finger-Containing Transcription Factor Osterix Is Required for Osteoblast Differentiation and Bone Formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Nishio Y, Dong Y, et al. Runx2-mediated regulation of the zinc finger Osterix/Sp7 gene. Gene. 2006;372:62–70. doi: 10.1016/j.gene.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Nuttall ME, Patton AJ, et al. Human Trabecular Bone Cells Are Able to Express Both Osteoblastic and Adipocytic Phenotype: Implications for Osteopenic Disorders. Journal of Bone and Mineral Research. 1998;13(3):371–382. doi: 10.1359/jbmr.1998.13.3.371. [DOI] [PubMed] [Google Scholar]
- Otto F, Thornell AP, et al. Cbfa1, a Candidate Gene for Cleidocranial Dysplasia Syndrome, Is Essential for Osteoblast Differentiation and Bone Development. Cell. 1997;89(5):765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- Oury F. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes & development. 2010;24(20):2330–2342. doi: 10.1101/gad.1977210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov DA, Deng JM, et al. Col2a1-directed expression of Cre recombinase in differentiating chondrocytes in transgenic mice. Genesis. 2000;26(2):145–146. [PubMed] [Google Scholar]
- Peng X-D. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes & development. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phornphutkul C, Wu K-Y, et al. mTOR signaling contributes to chondrocyte differentiation. Developmental Dynamics. 2008;237(3):702–712. doi: 10.1002/dvdy.21464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rached M-T. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. The Journal of clinical investigation. 2010;120(1):357–368. doi: 10.1172/JCI39901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rached M-T, Kode A, et al. FoxO1 Is a Positive Regulator of Bone Formation by Favoring Protein Synthesis and Resistance to Oxidative Stress in Osteoblasts. Cell metabolism. 2010;11(2):147–160. doi: 10.1016/j.cmet.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rokutanda S, Fujita T, et al. Akt regulates skeletal development through GSK3, mTOR, and FoxOs. Developmental Biology. 2009;328(1):78–93. doi: 10.1016/j.ydbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Sanchez C, He Y-Z. Bone growth during rapamycin therapy in young rats. BMC Pediatrics. 2009;9(1):3. doi: 10.1186/1471-2431-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, et al. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Secreto FJ. Wnt signaling during fracture repair. Current osteoporosis reports. 2009;7(2):64–69. doi: 10.1007/s11914-009-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. Journal of Cellular Biochemistry. 2008;103:434–446. doi: 10.1002/jcb.21411. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, et al. T Cell-Specific Loss of Pten Leads to Defects in Central and Peripheral Tolerance. Immunity. 2001;14(5):523–534. doi: 10.1016/s1074-7613(01)00134-0. [DOI] [PubMed] [Google Scholar]
- Ulici V, Hoenselaar KD, et al. The role of Akt1 in terminal stages of endochondral bone formation: Angiogenesis and ossification. Bone. 2009;45(6):1133–1145. doi: 10.1016/j.bone.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Williams BO, Insogna KL. Where Wnts Went: The Exploding Field of Lrp5 and Lrp6 Signaling in Bone. Journal of Bone and Mineral Research. 2009;24(2):171–178. doi: 10.1359/jbmr.081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Sun Q, et al. PTEN deficiency causes dyschondroplasia in mice by enhanced hypoxia-inducible factor 1α signaling and endoplasmic reticulum stress. Development. 2008;135(21):3587–3597. doi: 10.1242/dev.028118. [DOI] [PubMed] [Google Scholar]
- Yang S, Xu H, et al. FOXO1 mediates IGF1/insulin regulation of osteocalcin expression by antagonizing RUNX2 in osteoblasts. Journal of Biological Chemistry. 2011 doi: 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Matsuda K, et al. ATF4 Is a Substrate of RSK2 and an Essential Regulator of Osteoblast Biology: Implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Hinoi E, et al. The transcription factor ATF4 regulates glucose metabolism in mice through its expression in osteoblasts. The Journal of clinical investigation. 2009;119(9):2807–2817. doi: 10.1172/JCI39366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development. 2003;130(13):3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xuan S, et al. Osteoblast-specific Knockout of the Insulin-like Growth Factor (IGF) Receptor Gene Reveals an Essential Role of IGF Signaling in Bone Matrix Mineralization. Journal of Biological Chemistry. 2002;277(46):44005–44012. doi: 10.1074/jbc.M208265200. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang Z, et al. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proceedings of the National Academy of Sciences. 2010;107(29):12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]