Abstract
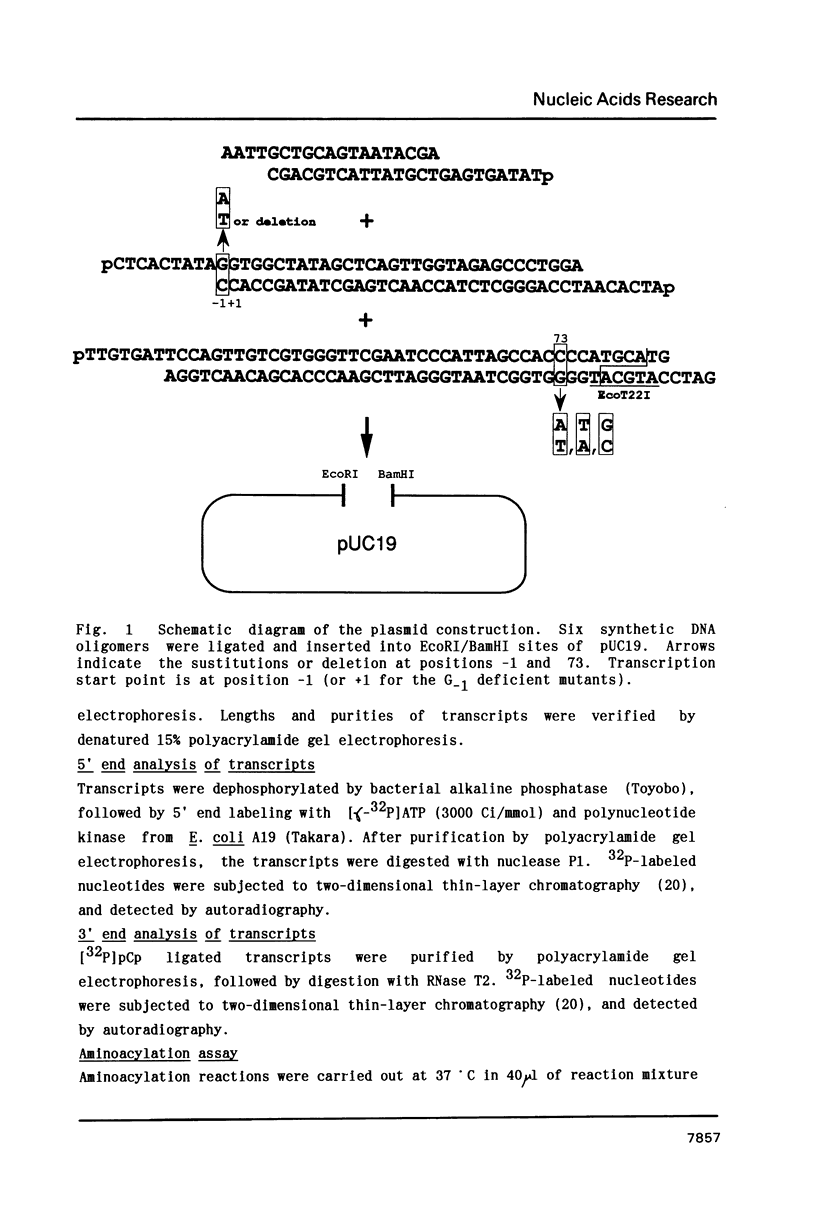
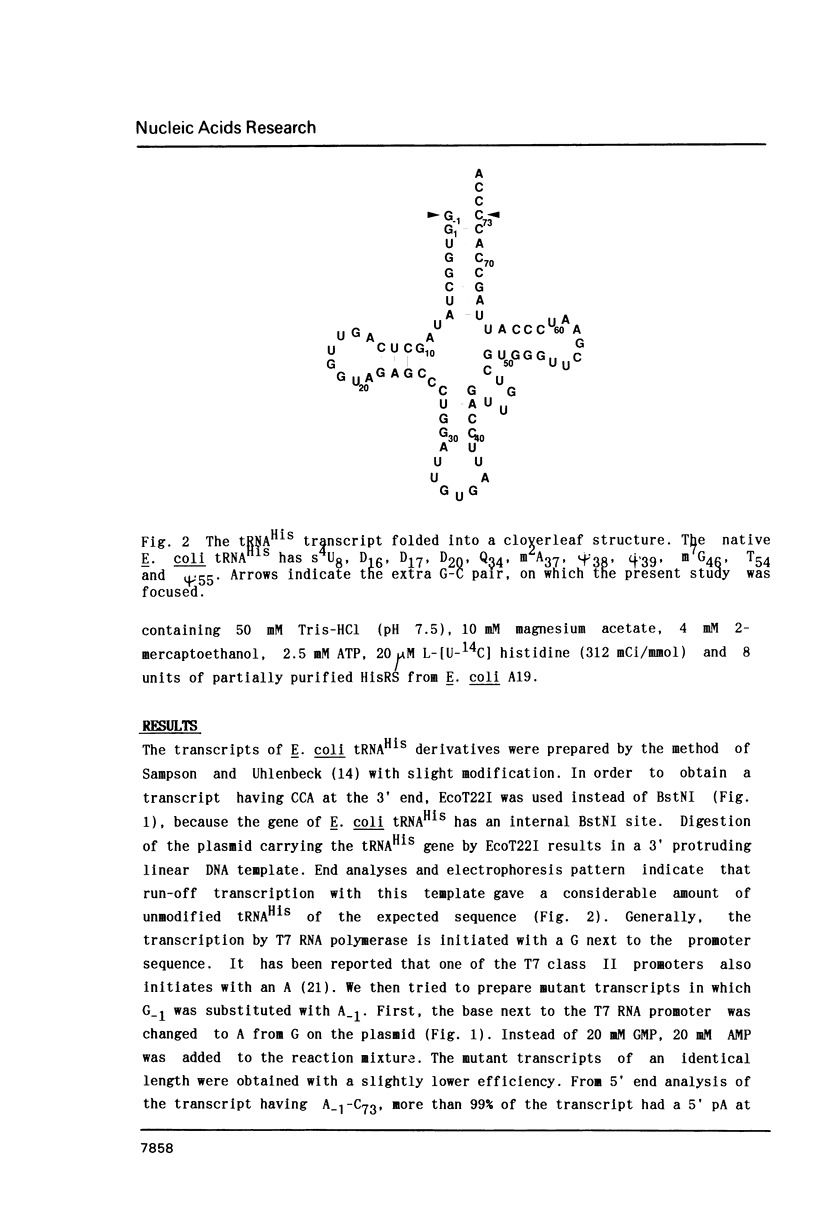
All sequenced histidine tRNAs have one additional nucleotide at the 5' end compared with other tRNA species. To investigate the role of this unique structure in aminoacylation, we constructed in vitro transcripts corresponding to the E. coli histidine tRNA sequence and its variants at the G-1-C73 base pair, by using T7 RNA polymerase transcription system. A transcript having a wild-type sequence with no modified bases was a good substrate for histidyl-tRNA synthetase (HisRS), and aminoacylation activity was affected by introduction of a triphosphate at the 5' terminus. Base replacements at position 73 caused a marked decrease of Vmax, and deletion and substitution of the G-1 had a remarkable effect on the aminoacylation. A mutant having an A-1-U73 pair was also not a good substrate for HisRS. Comparison among G-1-deficient mutants showed that A was preferable rather than C as the base at position 73. These data demonstrate that the set of the G-1-C73 pair at the end of the acceptor stem of histidine tRNA is crucial for the catalytic process of aminoacylation.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner M., Ames B. N. Histidine regulation in Salmonella typhimurium. IX. Histidine transfer ribonucleic acid of the regulatory mutants. J Biol Chem. 1972 Feb 25;247(4):1080–1088. [PubMed] [Google Scholar]
- Burkard U., Söll D. The 5'-terminal guanylate of chloroplast histidine tRNA is encoded in its gene. J Biol Chem. 1988 Jul 15;263(20):9578–9581. [PubMed] [Google Scholar]
- Burkard U., Willis I., Söll D. Processing of histidine transfer RNA precursors. Abnormal cleavage site for RNase P. J Biol Chem. 1988 Feb 15;263(5):2447–2451. [PubMed] [Google Scholar]
- Celis J. E., Piper P. W. Compilation of mutant suppressor tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r83–r91. doi: 10.1093/nar/10.2.762-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L., Appel B., Söll D. Post-transcriptional nucleotide addition is responsible for the formation of the 5' terminus of histidine tRNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6475–6479. doi: 10.1073/pnas.79.21.6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Ferber S., Ciechanover A. Transfer RNA is required for conjugation of ubiquitin to selective substrates of the ubiquitin- and ATP-dependent proteolytic system. J Biol Chem. 1986 Mar 5;261(7):3128–3134. [PubMed] [Google Scholar]
- Freedman R., Gibson B., Donovan D., Biemann K., Eisenbeis S., Parker J., Schimmel P. Primary structure of histidine-tRNA synthetase and characterization of hisS transcripts. J Biol Chem. 1985 Aug 25;260(18):10063–10068. [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Structural requirements for processing of synthetic tRNAHis precursors by the catalytic RNA component of RNase P. J Biol Chem. 1988 Jan 15;263(2):652–657. [PubMed] [Google Scholar]
- Grodberg J., Dunn J. J. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J Bacteriol. 1988 Mar;170(3):1245–1253. doi: 10.1128/jb.170.3.1245-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Sato S., Nishimura S. Unusual CCA-stem structure of E. coli B tRNAH(His)(1). FEBS Lett. 1972 Jan 1;19(4):352–354. doi: 10.1016/0014-5793(72)80078-4. [DOI] [PubMed] [Google Scholar]
- Kalousek F., Konigsberg W. H. Purification and characterization of histidyl transfer ribonucleic acid synthetase of Escherichia coli. Biochemistry. 1974 Feb 26;13(5):999–1006. doi: 10.1021/bi00702a026. [DOI] [PubMed] [Google Scholar]
- Labouze E., Bedouelle H. Structural and kinetic bases for the recognition of tRNATyr by tyrosyl-tRNA synthetase. J Mol Biol. 1989 Feb 20;205(4):729–735. doi: 10.1016/0022-2836(89)90317-3. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Natsoulis G., Hilger F., Fink G. R. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986 Jul 18;46(2):235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Harada F., Narushima U., Seno T. Purification of methionine-, valine-, phenylalanine- and tyrosine-specific tRNA from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):133–148. doi: 10.1016/0005-2787(67)90522-9. [DOI] [PubMed] [Google Scholar]
- Normanly J., Ogden R. C., Horvath S. J., Abelson J. Changing the identity of a transfer RNA. Nature. 1986 May 15;321(6067):213–219. doi: 10.1038/321213a0. [DOI] [PubMed] [Google Scholar]
- Orellana O., Cooley L., Söll D. The additional guanylate at the 5' terminus of Escherichia coli tRNAHis is the result of unusual processing by RNase P. Mol Cell Biol. 1986 Feb;6(2):525–529. doi: 10.1128/mcb.6.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona J. J., Swanson R., Steitz T. A., Söll D. Overproduction and purification of Escherichia coli tRNA(2Gln) and its use in crystallization of the glutaminyl-tRNA synthetase-tRNA(Gln) complex. J Mol Biol. 1988 Jul 5;202(1):121–126. doi: 10.1016/0022-2836(88)90524-4. [DOI] [PubMed] [Google Scholar]
- Podjarny A., Rees B., Thierry J. C., Cavarelli J., Jésior J. C., Roth M., Lewitt-Bentley A., Kahn R., Lorber B., Ebel J. P. Yeast tRNA(Asp)-aspartyl-tRNA synthetase complex: low resolution crystal structure. J Biomol Struct Dyn. 1987 Oct;5(2):187–198. doi: 10.1080/07391102.1987.10506389. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., DiRenzo A. B., Behlen L. S., Uhlenbeck O. C. Nucleotides in yeast tRNAPhe required for the specific recognition by its cognate synthetase. Science. 1989 Mar 10;243(4896):1363–1366. doi: 10.1126/science.2646717. [DOI] [PubMed] [Google Scholar]
- Sampson J. R., Uhlenbeck O. C. Biochemical and physical characterization of an unmodified yeast phenylalanine transfer RNA transcribed in vitro. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1033–1037. doi: 10.1073/pnas.85.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman L. H., Abelson J. Recent excitement in understanding transfer RNA identity. Science. 1988 Jun 17;240(4859):1591–1592. doi: 10.1126/science.2454505. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Anticodon switching changes the identity of methionine and valine transfer RNAs. Science. 1988 Nov 4;242(4879):765–768. doi: 10.1126/science.3055296. [DOI] [PubMed] [Google Scholar]
- Schulman L. H., Pelka H. Structural requirements for aminoacylation of Escherichia coli formylmethionine transfer RNA. Biochemistry. 1977 Sep 20;16(19):4256–4265. doi: 10.1021/bi00638a020. [DOI] [PubMed] [Google Scholar]
- Scornik O. A. Faster protein degradation in response to decreases steady state levels of amino acylation of tRNAHis in Chinese hamster ovary cells. J Biol Chem. 1983 Jan 25;258(2):882–886. [PubMed] [Google Scholar]
- Singhal R. P., Vakharia V. N. The role of queuine in the aminoacylation of mammalian aspartate transfer RNAs. Nucleic Acids Res. 1983 Jun 25;11(12):4257–4272. doi: 10.1093/nar/11.12.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Hartmann T., Weber J., Blank J., Zeidler R. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 1989;17 (Suppl):r1–172. doi: 10.1093/nar/17.suppl.r1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H., Imai M., Ohtsuka E., Ikehara M., Söll D. E. coli initiator tRNA analogs with different nucleotides in the discriminator base position. Nucleic Acids Res. 1982 Oct 25;10(20):6531–6539. doi: 10.1093/nar/10.20.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. tRNA identity: a hair of the dogma that bit us. Cell. 1988 Dec 2;55(5):739–741. doi: 10.1016/0092-8674(88)90127-4. [DOI] [PubMed] [Google Scholar]



