Abstract
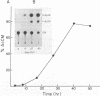
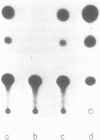
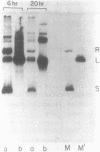
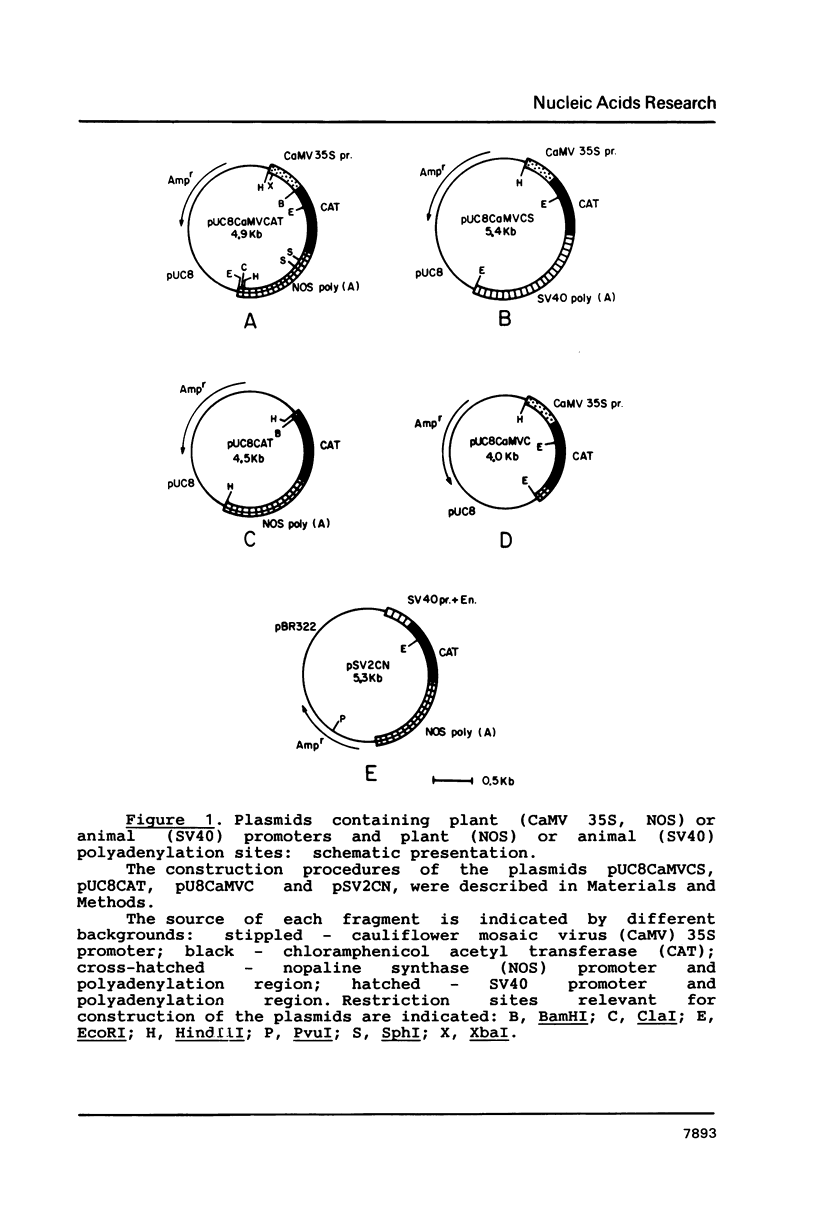
Mature Xenopus oocytes were challenged with DNA constructs including plant regulatory elements, namely, the Cauliflower mosaic virus (CaMV) 35S promoter as well as the nopaline synthase (NOS) promoter and polyadenylation signal. The bacterial chloramphenicol acetyl transferase (CAT) was used as a reporter gene. When microinjected into these cells, the plant-derived DNA constructs effectively promoted CAT synthesis in a manner dependent on the presence of the plant promoters and probably also on the polyadenylation signals. Structural studies revealed that the supercoiled structures of the above DNA plasmids were much more active in supporting CAT synthesis in microinjected oocytes than their linear forms, with clear correlation between efficient gene expression and DNA topology. In contrast, the linear forms of these plasmids were considerably more active than the supercoiled ones in transfected plant protoplasts. These findings demonstrate, for the first time, the activity of regulatory elements from plant genes in Xenopus oocytes and shed new light on the specific rules applicable for gene expression in plant and animal cells.
Full text
PDF


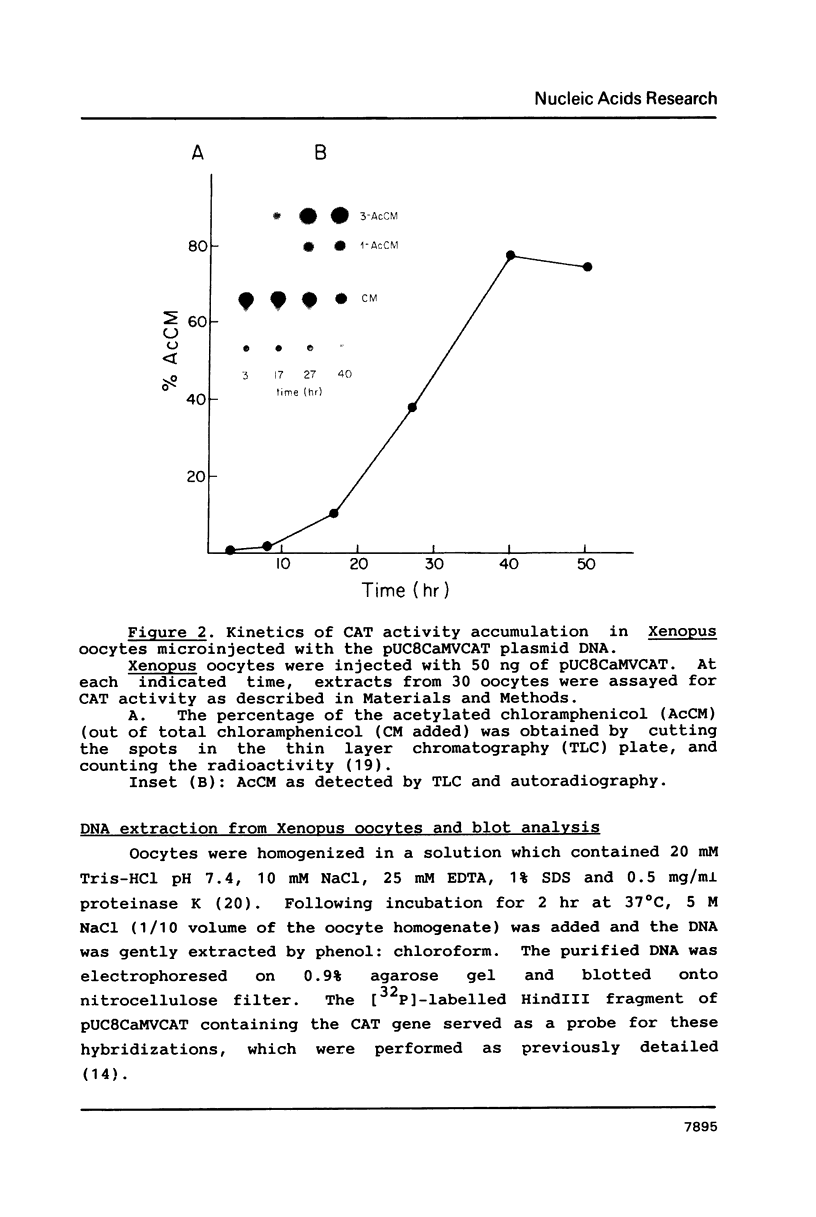
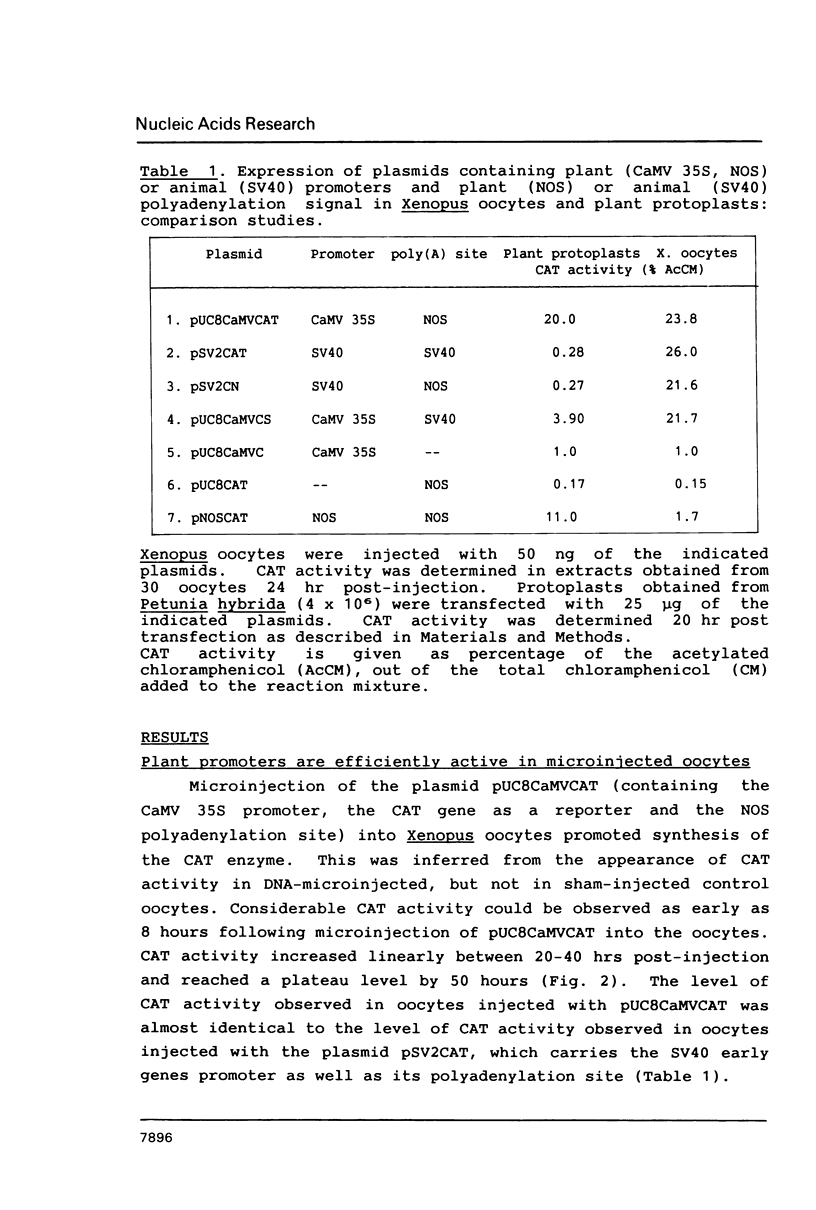



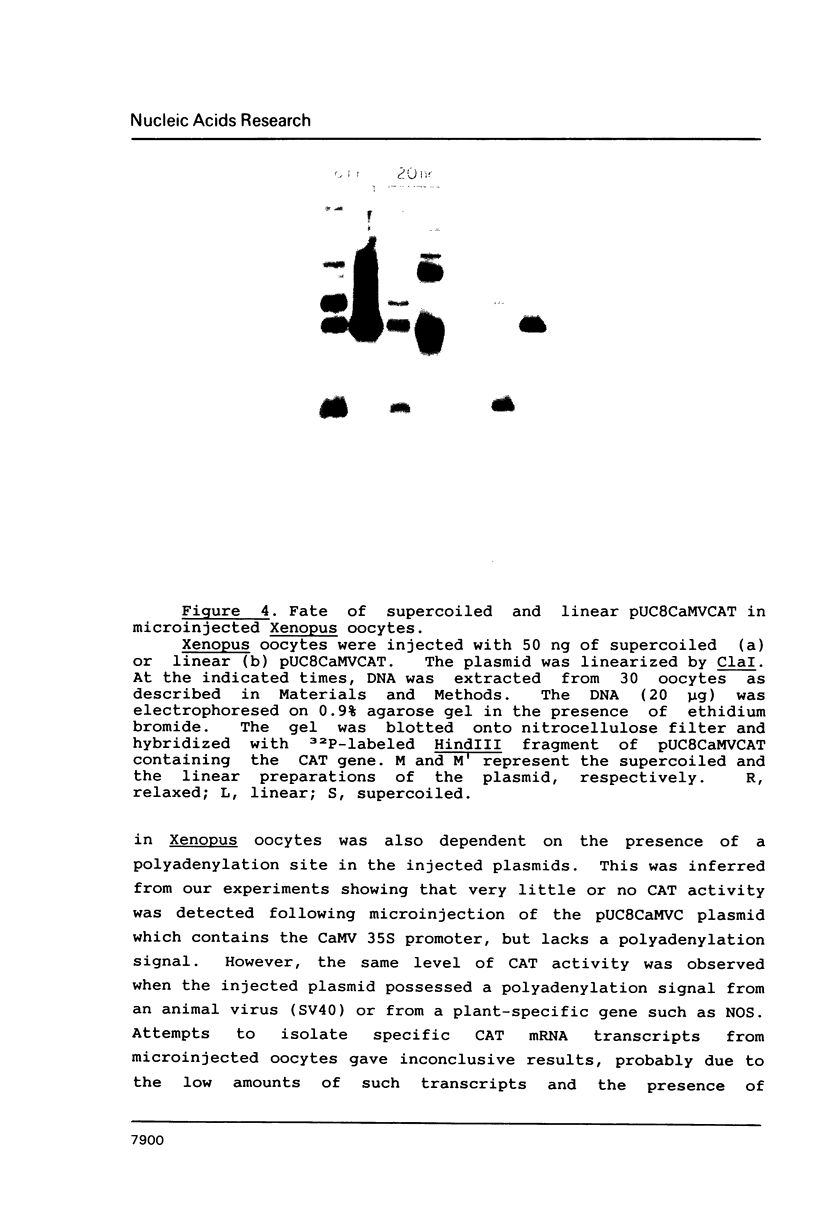



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballas N., Zakai N., Loyter A. Transient expression of the plasmid pCaMVCAT in plant protoplasts following transformation with polyethyleneglycol. Exp Cell Res. 1987 May;170(1):228–234. doi: 10.1016/0014-4827(87)90132-7. [DOI] [PubMed] [Google Scholar]
- Barton K. A., Binns A. N., Matzke A. J., Chilton M. D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell. 1983 Apr;32(4):1033–1043. doi: 10.1016/0092-8674(83)90288-x. [DOI] [PubMed] [Google Scholar]
- Caplan A., Herrera-Estrella L., Inzé D., Van Haute E., Van Montagu M., Schell J., Zambryski P. Introduction of genetic material into plant cells. Science. 1983 Nov 18;222(4625):815–821. doi: 10.1126/science.222.4625.815. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Fromm M., Taylor L. P., Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5824–5828. doi: 10.1073/pnas.82.17.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Weintraub H., McKnight S. L. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983 Mar 3;302(5903):38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- Koncz C., Kreuzaler F., Kalman Z., Schell J. A simple method to transfer, integrate and study expression of foreign genes, such as chicken ovalbumin and alpha-actin in plant tumors. EMBO J. 1984 May;3(5):1029–1037. doi: 10.1002/j.1460-2075.1984.tb01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel M. G., Adams T. L., Hazlet M. A., Damm D., Miller J., Dahlbeck D., Jayne S., Staskawicz B. J. A cauliflower mosaic virus promoter directs expression of kanamycin resistance in morphogenic transformed plant cells. J Mol Appl Genet. 1984;2(6):549–562. [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Miller T. J., Mertz J. E. Template structural requirements for transcription in vivo by RNA polymerase II. Mol Cell Biol. 1982 Dec;2(12):1595–1607. doi: 10.1128/mcb.2.12.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scangos G., Ruddle F. H. Mechanisms and applications of DNA-mediated gene transfer in mammalian cells - a review. Gene. 1981 Jun-Jul;14(1-2):1–10. doi: 10.1016/0378-1119(81)90143-8. [DOI] [PubMed] [Google Scholar]
- Shaw C. H., Leemans J., Shaw C. H., van Montagu M., Schell J. A general method for the transfer of cloned genes to plant cells. Gene. 1983 Sep;23(3):315–330. doi: 10.1016/0378-1119(83)90021-5. [DOI] [PubMed] [Google Scholar]
- Soreq H. The biosynthesis of biologically active proteins in mRNA-microinjected Xenopus oocytes. CRC Crit Rev Biochem. 1985;18(3):199–238. doi: 10.3109/10409238509085134. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Cheng P. F., Conrad K. Expression of transfected DNA depends on DNA topology. Cell. 1986 Jul 4;46(1):115–122. doi: 10.1016/0092-8674(86)90865-2. [DOI] [PubMed] [Google Scholar]