Following treatment with the growth hormone–releasing hormone analogue tesamorelin, patients who experience reduced visceral adiposity have improved lipid levels and relatively unchanged glucose homeostasis, whereas patients who do not experience decreased visceral fat have worsened glucose homeostasis and unchanged lipid levels.
Abstract
Background. Tesamorelin, a growth hormone–releasing hormone analogue, decreases visceral adipose tissue (VAT) by 15%–20% over 6–12 months in individuals with human immunodeficiency virus (HIV)–associated abdominal adiposity, but it is unknown whether VAT reduction is directly associated with endocrine and metabolic changes.
Methods. In 2 phase III, randomized, double-blind studies, men and women with HIV-associated abdominal fat accumulation were randomly assigned (ratio, 2:1) to receive tesamorelin or placebo for 26 weeks. At week 26, patients initially receiving tesamorelin were randomly assigned to continue receiving tesamorelin or to receive placebo for an additional 26 weeks. In per-protocol analysis of 402 subjects initially randomly assigned to receive tesamorelin, those with ≥8% reduction in VAT were defined a priori as responders per the statistical analysis plan. Post hoc analyses were performed to assess differences between responders and nonresponders.
Results. Compared with tesamorelin nonresponders, responders experienced greater mean (±SD) reduction in triglyceride levels (26 weeks: −0.6 ± 1.7 mmol/L vs −0.1 ± 1.2 mmol/L [P = .005]; 52 weeks: −0.8 ± 1.8 mmol/L vs 0.0 ± 1.1 mmol/L [P = .003]) and attenuated changes in fasting glucose levels (26 weeks: 1 ± 16 mg/dL vs 5 ± 14 mg/dL [P = .01]; 52 weeks: −1 ± 14 mg/dL vs 8 ± 17 mg/dL [P < .001]), hemoglobin A1c levels (26 weeks: 0.1 ± 0.3% vs 0.3 ± 0.4% [P < .001]; 52 weeks: 0.0 ± 0.3% vs 0.2 ± 0.5% [P = .003]), and other parameters of glucose homeostasis. Similar patterns were seen for adiponectin levels, with significant improvement in responders vs nonresponders. Changes in lipid levels and glucose homeostasis were significantly associated with percentage change in VAT.
Conclusions. In contrast to nonresponders, HIV-infected patients receiving tesamorelin with ≥8% reduction in VAT have significantly improved triglyceride levels, adiponectin levels, and preservation of glucose homeostasis over 52 weeks of treatment.
Clinicaltrials.gov Registration. NCT00123253, NCT00435136, NCT00608023.
Human immunodeficiency virus (HIV)–infected patients, particularly those treated with antiretroviral therapy, often experience significant accumulation of visceral fat [1, 2]. In addition to causing appearance-related distress, increased visceral adiposity is associated with dyslipidemia [3, 4], impaired glucose homeostasis [4, 5], worsened measures of cardiovascular risk [6–9], and increased mortality [10] among HIV-infected patients. Tesamorelin, a synthetic 44–amino acid growth hormone–releasing hormone analogue, decreases visceral adipose tissue (VAT) area by approximately 15% over 26 weeks of treatment and by 18% over 52 weeks [11]. In addition, tesamorelin significantly reduces levels of triglycerides and non–high-density lipoprotein cholesterol [11], increases levels of adiponectin [12], and reduces levels of tissue plasminogen activator antigen [12, 13]. In combined data from 2 phase III studies of tesamorelin, fasting glucose, fasting insulin, and 2-hour glucose levels did not significantly change after treatment periods of 26 weeks or 52 weeks [11]. There was a small but significant increase in hemoglobin A1c (HbA1c) level (treatment effect, 0.12%) at 26 weeks, but the HbA1c level was not significantly different from the baseline level after 52 weeks of treatment [11]. In November 2010, the US Food and Drug Administration approved tesamorelin to reduce visceral adiposity in individuals with HIV-associated abdominal fat accumulation.
In the current analysis, we sought to determine whether changes in VAT were associated with the metabolic effects of tesamorelin.
METHODS
The current analysis combines data from 2 similar multicenter, randomized, placebo-controlled, double-blind phase III studies of tesamorelin (Theratechnologies, Montreal, Canada) [12, 14, 15]. Both studies included a primary 26-week treatment phase, in which subjects were randomly assigned in a 2:1 ratio to receive tesamorelin (2 mg subcutaneously in the morning) or placebo, and a subsequent 26-week extension phase, in which individuals who received placebo were switched to tesamorelin and individuals who received tesamorelin were randomly assigned to continue receiving tesamorelin or to switch to placebo.
Eligible subjects were HIV-infected men and women between the ages of 18–65 years who had abdominal fat accumulation (defined as an elevated waist circumference [ie, ≥95 cm for men and ≥94 cm for women] and an elevated waist-to-hip ratio [≥0.94 for men and ≥0.88 for women]), had been receiving stable antiretroviral therapy for 8 weeks, had a CD4 cell count of >100 cells/mm3, had an HIV RNA load of <10 000 copies/mL, and had a fasting glucose level of <150 mg/dL [16]. Exclusion criteria included known history of diabetes mellitus requiring medication, history of malignancy, or active neoplasm. Both studies were approved by the institutional review boards at each study site, and all participants provided written informed consent.
In consultation with the Food and Drug Administration, a decrease of ≥8% in VAT area was determined to be clinically significant as per a consensus roundtable [17] and was used to define “responders,” as specified a priori in the data analysis plan. By use of this threshold to define “responders,” we compared metabolic and endocrine responses by responder status in a post hoc analysis of the combined per protocol data from the 2 phase III studies.
Study Procedures
Fasting blood samples were collected to measure glucose, insulin, and insulin-like growth factor-1 (IGF-I) levels. A 75-g 2-hour oral glucose tolerance test, a single-slice computed tomography (CT) scan for VAT and subcutaneous adipose tissue (SAT) [12], and a dual-energy x-ray absorptiometry (DXA) scan were performed. Subjects also completed a self-assessment of distress caused by belly appearance (the belly appearance distress [BAD] score), in which a score of 0 indicated extremely upsetting and distressing and a score of 100 indicated extremely encouraging (Phase V Technologies, Wellesley, MA) [12]. Tests were repeated at weeks 26 and 52. IGF-I levels were measured at Esoterix; fasting glucose levels, insulin levels, and lipid panels were assessed using standard laboratory methods. C-reactive protein (CRP) and adiponectin levels were measured only in the first phase III study [12]. CRP levels were measured by nephelometry (Siemens Diagnostics). Serum adiponectin levels were determined by enzyme-linked immunosorbant assay (B-Bridge International).
Statistical Analysis
In this analysis, responder status was determined independently at both 26 weeks and 52 weeks. To analyze the clinical effects of tesamorelin in individuals who were relatively adherent to treatment, a per-protocol analysis was used. The per-protocol population included individuals who demonstrated >80% compliance with study drug injections, had no major protocol violations, and underwent ≥1 postdose abdominal CT for measurement of VAT. Responders are individuals whose CT scan showed ≥8% reduction in VAT from baseline. The current analysis focuses primarily on the changes within the tesamorelin group, for the purpose of understanding the clinical and metabolic changes by responder status in this group. A sensitivity analysis for glucose parameters was performed in the intent to treat (ITT) population with available paired data at baseline and 26 weeks and baseline and 52 weeks (ITT population observed case analysis).
Baseline comparisons between groups (responder vs nonresponder) were made using the Cochran-Mantel-Haenszel statistic, for categorical variables, and analysis of variance, for continuous variables. Spearman correlations were performed to evaluate the relationships between baseline values and changes from baseline. To compare changes from baseline to 26 and 52 weeks between responder and nonresponder groups, analysis of covariance was used, with control for baseline value and study. Within-group comparisons were done using a mixed repeated measure model, with control for study. Mean values ± SD are reported.
RESULTS
In total, 402 (73%) of the tesamorelin subjects and 197 (74%) of the placebo subjects met criteria for per-protocol analysis at 26 weeks. Of this group, 337 subjects receiving tesamorelin for 26 weeks who had paired VAT data at baseline and 26 weeks (n = 337) were included in the 26-week analysis. A total of 176 subjects randomly assigned to receive tesamorelin for a full 52 weeks met criteria for per-protocol analysis, and, of these, 152 subjects who had paired VAT data for baseline and 52 weeks were included in the 52-week analysis. The responder rate (VAT reduction, ≥8%) was higher for tesamorelin-treated patients than for placebo recipients at 26 weeks (69% vs 33%; P < .001) and rose slightly among those who continued to receive tesamorelin for 52 weeks (72%). Sixteen of 39 nonresponders at week 26 became responders by week 52.
Baseline Characteristics of Responders and Nonresponders
Baseline clinical characteristics according to responder status are shown in Table 1. Responders were not significantly different from nonresponders with respect to demographic, clinical, or immunological variables (Tables 1–3).
Table 1.
Baseline Characteristics of Study Subjects, by Response or Nonresponse to Tesamorelin
| Subjects Included in 26-Week Responder Analysis | Subjects Included in 52-Week Responder Analysis | |||||
|---|---|---|---|---|---|---|
| Responders | Nonresponders | Pa | Responders | Nonresponders | Pa | |
| Male sex, (%) | 87.5 | 84.8 | .509 | 86.4 | 83.3 | .644 |
| Age (years) | 47.6 ± 7.0 | 47.4 ± 7.7 | .770 | 48.3 ± 7.4 | 47.1 ± 7.6 | .348 |
| Race (%) | .466 | .418 | ||||
| White | 81.5 | 76.2 | 78.2 | 73.8 | ||
| Black | 9.9 | 13.3 | 14.5 | 11.9 | ||
| Other | 8.6 | 10.5 | 7.3 | 14.3 | ||
| Use of testosterone (%) | 21.1 | 20.0 | .712 | 23.6 | 23.8 | .893 |
| Use of lipid-lowering agents (%) | 46.6 | 42.9 | .596 | 45.5 | 38.1 | .395 |
| Current use of ART (%) | .190 | .101 | ||||
| NRTI + NNRTI and no PI | 34.1 | 43.8 | 30.9 | 50.0 | ||
| NRTI + NNRTI + PI | 9.1 | 9.5 | 10.9 | 7.1 | ||
| NRTI + PI and no NNRTI | 47.8 | 34.3 | 50.9 | 31.0 | ||
| NRTI alone | 3.9 | 5.7 | 4.6 | 4.8 | ||
| Other | 5.2 | 6.7 | 2.7 | 7.1 | ||
| CD4+ cell count (cells/mm3) | 609 ± 289 | 609 ± 279 | .895 | 607 ± 294 | 615 ± 261 | .963 |
| Undetectable viral load (%) | 77.6 | 72.1 | .177 | 75.5 | 73.8 | .91 |
| BMI | 28.4 ± 3.7 | 29.4 ± 4.7 | .033 | 28.5 ± 3.4 | 28.3 ± 4.5 | .698 |
| Presence of lipoatrophyb (%) | 73.7 | 68.6 | .380 | 70.9 | 78.6 | .371 |
| Waist circumference (cm) | 103.7 ± 8.6 | 106.1 ± 10.4 | .03 | 103.6 ± 8.2 | 102.8 ± 9.6 | .564 |
| VAT (cm2) | 187 ± 82 | 193 ± 84 | .67 | 190 ± 79 | 163 ± 70 | .08 |
| VAT:SAT | 1.28 ± 1.41 | 1.30 ± 1.35 | .886 | 1.34 ± 1.46 | 1.44 ± 1.58 | .724 |
| IGF-I level (ng/mL) | 152 ± 61 | 162 ± 66 | .11 | 163 ± 60 | 161 ± 79 | .849 |
Values are mean ± standard deviation, unless otherwise indicated.
Abbreviations: ART, antiretroviral therapy; BMI, body mass index (defined as the weight in kilograms divided by the height in meters squared); IGF-I, insulin-like growth factor-1; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse-transcriptase inhibitor; PI, protease inhibitor; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
For comparison of values for responders with those for nonresponders at each time point. Analysis of variance modeling (controlling for study [first vs second phase III study]) was used for continuous variables, and the Cochran-Mantel-Haenszel statistic (controlling for study) was used for categorical variables, including viral load (detectable vs undetectable).
Defined as atrophy of the face and/or limbs, determined by the presence of ≥1 sign involving the face, lower limbs, or upper limbs.
Table 2.
Change in Abdominal Adiposity, Insulin-Like Growth Factor-1 Levels, and Metabolic Parameters Between Baseline and Week 26 Among 232 Responders and 105 Nonresponders to Tesamorelin
| Baseline Value, Mean ± SD | Change at 26 Weeks, Mean ± SD | ||||
| Responders | Nonresponders | Responders | Nonresponders | Pa | |
| VAT (cm2) | 187 ± 82 | 193 ± 84 | −50 ± 34 | 16 ± 28 | NDb |
| SAT (cm2) | 220 ± 117 | 231 ± 125 | −8 ± 36c | 12 ± 33c | <.001 |
| Waist circumference (cm) | 103.7 ± 8.6 | 106.1 ± 10.4 | −4.2 ± 5.7c | −0.4 ± 4.4 | <.001 |
| Trunk fat (kg) | 14.4 ± 4.7 | 15.8 ± 6.0 | −1.8 ± 2.0c | 0.6 ± 1.8c | <.001 |
| Fat in limbs (kg) | 6.7 ± 3.8 | 7.4 ± 4.6 | −0.3 ± 1.0c | 0.5 ± 0.8c | <.001 |
| Lean mass (kg) | 62.2 ± 9.6 | 62.8 ± 11.4 | 1.6 ± 2.4c | 1.2 ± 2.5c | .226 |
| IGF-I level (ng/mL) | 152 ± 61 | 162 ± 66 | 136 ± 106c | 85 ± 104c | <.001 |
| CRP leveld (mg/L) | 4.3 ± 7.8 | 3.9 ± 5.2 | 0.1 ± 14.1 | 0.1 ± 5.4 | .926 |
| Adiponectin leveld (μg/mL) | 5.7 ± 4.5 | 5.0 ± 3.3 | 1.0 ± 3.0c | −0.30 ± 1.8 | .011 |
| Total cholesterol level (mmol/L [mg/dL]) | 5.1 ± 1.2 [195 ± 45] | 4.9 ± 1.0 [190 ± 40] | −0.2 ± 0.9c [−8 ± 34] | 0.1 ± 1.0 [4 ± 37] | .014 |
| HDL-C level (mmol/L [mg/dL]) | 1.18 ± 0.38 [46 ± 15] | 1.15 ± 0.34 [45 ± 13] | 0.03 ± 0.22 [1.2 ± 8.6] | −0.01 ± 0.17 [−0.4 ± 6.7] | .118 |
| Triglycerides level (mmol/L [mg/dL]) | 2.7 ± 2.2 [240 ± 197] | 2.6 ± 1.7 [226 ± 153] | −0.6 ± 1.7c [−50 ± 147] | −0.1 ± 1.2 [−12 ± 105] | .005 |
| Fasting glucose level (mg/dL) | 98 ± 14 | 99 ± 13 | 1 ± 16 | 5 ± 14c | .010 |
| Fasting insulin level (μIU/mL) | 21.6 ± 25.1 | 18.8 ± 13.0 | −1.1 ± 25.2 | 5.7 ± 13.5c | .011 |
| HOMA-IR score | 5.4 ± 7.7 | 4.7 ± 3.6 | −0.4 ± 7.9 | 1.8 ± 4.3c | .006 |
| HbA1c level (%) | 5.3 ± 0.5 | 5.3 ± 0.5 | 0.1 ± 0.3c | 0.3 ± 0.4c | <.001 |
| 2-h glucose level (mg/dL) | 113 ± 37 | 114 ± 36 | −1 ± 34 | 10 ± 44c | .009 |
| CD4+ cell count (cells/mm3) | 609 ± 289 | 609 ± 279 | −9 ± 146 | 0 ± 175 | .590 |
| BAD scoree | 21.4 ± 21.7 | 23.0 ± 26.0 | 15.5 ± 30.9c | 3.4 ± 28.1 | <.001 |
Abbreviations: BAD, belly appearance distress; CRP, C-reactive protein; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment—insulin resistance; IGF-I, insulin-like growth factor-1; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
For comparison of the change at 26 weeks between responders and nonresponders, using analysis of covariance to control for baseline value, study (first vs second phase III study), and lipid-lowering treatment (for lipid parameters only).
Statistical comparison was not done (ND) because the change in VAT was the basis of stratification into responder and nonresponder groups.
Indicates P < .05 for within group comparison of baseline vs 26 weeks, using a mixed repeated measure model controlling for study.
Measured only in the first phase III study [12].
Higher scores indicate less distress.
Table 3.
Change in Abdominal Adiposity, Insulin-Like Growth Factor-1 Levels, and Metabolic Parameters Between Baseline and Week 52 Among 110 Responders and 42 Nonresponders to Tesamorelin
| Baseline Value, Mean ± SD | Change at 52 Weeks, Mean ± SD | ||||
| Responders | Nonresponders | Responders | Nonresponders | Pa | |
| VAT (cm2) | 190 ± 79 | 163 ± 70 | −61 ± 48 | 11 ± 23 | NDb |
| SAT (cm2) | 226 ± 126 | 185 ± 109 | −11 ± 48c | 14 ± 30c | .011 |
| Waist circumference (cm) | 103.6 ± 8.2 | 102.8 ± 9.6 | −4.7 ± 6.4c | −1.0 ± 4.9 | .001 |
| Trunk fat (kg) | 14.4 ± 4.4 | 13.5 ± 6.1 | −2.2 ± 2.3c | 0.4 ± 1.6 | <.001 |
| Fat in limbs (kg) | 6.6 ± 3.8 | 6.7 ± 4.8 | −0.3 ± 1.1c | 0.4 ± 1.0c | .002 |
| Lean mass (kg) | 62.1 ± 9.1 | 61.0 ± 11.4 | 1.2 ± 2.8c | 2.0 ± 2.6c | .162 |
| IGF-I level (ng/mL) | 163 ± 60 | 161 ± 79 | 91 ± 100c | 76 ± 138c | .515 |
| CRP leveld (mg/L) | 3.9 ± 4.3 | 4.5 ± 6.3 | −1.0 ± 5.1 | −0.3 ± 8.0 | .381 |
| Adiponectin leveld (μg/mL) | 6.1 ± 5.6 | 4.5 ± 2.5 | 2.3 ± 3.2c | 0.3 ± 1.6 | .008 |
| Total cholesterol level (mmol/L [mg/dL]) | 5.0 ± 1.2 [194 ± 46] | 4.8 ± 1.2 [187 ± 48] | −0.2 ± 0.9c [−9 ± 33] | 0.0 ± 0.9 [1 ± 35] | .205 |
| HDL-C level (mmol/L [mg/dL]) | 1.18 ± 0.39 [46 ± 15] | 1.15 ± 0.37 [45 ± 14] | −0.02 ± 0.26 [−0.8 ± 9.9] | −0.02 ± 0.24 [−0.8 ± 9.3] | .773 |
| Triglycerides level (mmol/L [mg/dL]) | 2.8 ± 2.3 [247 ± 202] | 2.4 ± 1.8 [213 ± 158] | −0.8 ± 1.8c [−68 ± 159] | 0.0 ± 1.1 [3 ± 102] | .003 |
| Fasting glucose level (mg/dL) | 96 ± 12 | 98 ± 14 | −1 ± 14 | 8 ± 17c | <.001 |
| Fasting insulin level (μIU/mL) | 19.3 ± 20.3 | 19.1 ± 15.0 | −2.5 ± 19.1 | 4.9 ± 17.9 | .002 |
| HOMA-IR score | 4.6 ± 5.6 | 4.7 ± 4.4 | −0.7 ± 5.2 | 1.6 ± 5.5 | <.001 |
| HbA1c level (%) | 5.2 ± 0.5 | 5.3 ± 0.5 | 0.0 ± 0.3 | 0.2 ± 0.5c | .003 |
| 2-h glucose level (mg/dL) | 110 ± 33 | 115 ± 32 | −5 ± 37 | 10 ± 31c | .006 |
| CD4+ cell count (cells/mm3) | 607 ± 294 | 615 ± 261 | 35 ± 183 | 22 ± 191 | 0.610 |
| BAD scoree | 19.9 ± 21.3 | 30.5 ± 30.1 | 20.7 ± 33.0b | 2.44 ± 36.1 | .166 |
Abbreviations: BAD, belly appearance distress; CRP, C-reactive protein; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment—insulin resistance; IGF-I, insulin-like growth factor-1; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
For comparison of the change at 52 weeks between responders and nonresponders, using analysis of covariance to control for baseline value, study (first vs second phase III study), and lipid-lowering treatment (for lipid parameters only).
Statistical comparison was not done (ND) because the change in VAT was the basis of stratification into responder and nonresponder groups.
Indicates P < .05 for within group comparison of baseline vs 52 weeks, using a mixed repeated measure model controlling for study.
Measured only in the first phase III study [12].
Higher scores indicate less distress.
In univariate analysis, the change in VAT after 26 weeks of tesamorelin treatment was significantly associated with the baseline VAT (ρ = −0.265; P < .001) and the VAT:SAT ratio (ρ = −0.201; P < .001), such that subjects with a higher initial VAT and/or VAT:SAT ratio had greater absolute decreases in VAT. The change in VAT after 26 weeks was not significantly associated with any other baseline variables.
Effects of Tesamorelin in Responders and Nonresponders
Changes in body composition and metabolic parameters according to responder status are shown in Table 2 (26 weeks) and Table 3 (52 weeks). Responders experienced significantly greater decreases in waist circumference and trunk fat than nonresponders in both the 26-week and 52-week analyses. The mean changes in waist circumference among responders were −4.2 cm after 26 weeks and −4.7 cm after 52 weeks (Tables 2 and 3). Changes in SAT were significantly different in responders, compared with nonresponders, with responders experiencing small decreases in SAT and nonresponders demonstrating small increases in SAT in both 26-week and 52-week analyses (Tables 2 and 3). The magnitude of reduction in SAT seen in responders was modest, approximately one-fifth to one-sixth of the magnitude of reduction in VAT. Similarly, changes in limb fat were significantly different in responders versus nonresponders: in within-group analyses, responders experienced a small but statistically significant reduction in limb fat (−0.3 ± 1.0 kg at 26 weeks and −0.3 ± 1.1 kg at 52 weeks), whereas nonresponders experienced a modest but significant increase in limb fat. These differences were significant in between-group analyses for both 26 weeks (P < .001) and 52 weeks (P = .002).
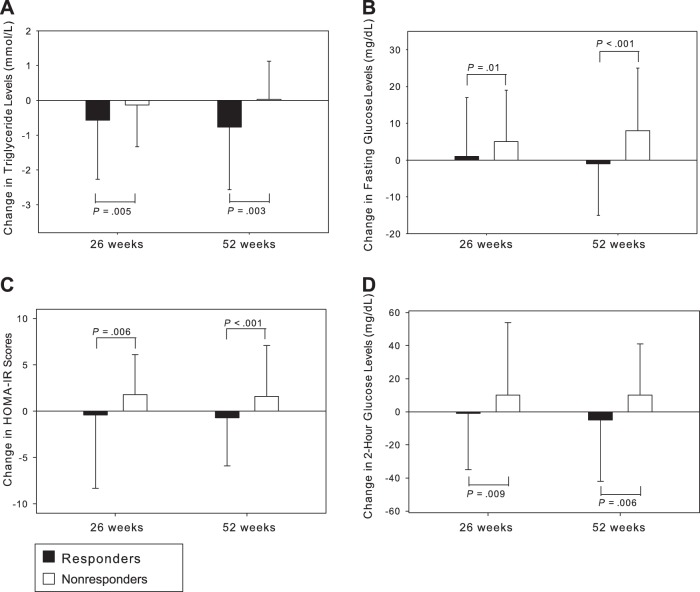
IGF-I levels increased significantly more in responders, compared with nonresponders, in the 26-week analysis (136 ± 106 ng/mL vs 85 ± 104 ng/mL; P < .001), but there was no significant difference in change in IGF-I levels between groups in the 52-week analysis. As previously reported, CRP levels did not change significantly with treatment [12], and this was not affected by responder status. Adiponectin levels increased in responders in both 26-week and 52-week within-group analyses, whereas there were no significant changes in adiponectin levels in nonresponders. Differences in adiponectin levels between responders and nonresponders were significant for both 26-week (P = .011) and 52-week (P = .008) analyses. Triglyceride levels decreased significantly more in the responders, compared with the nonresponders, in both 26-week (−0.6 ± 1.7 mmol/L vs −0.1 ± 1.2 mmol/L; P = .005) and 52-week (−0.8 ± 1.8 mmol/L vs 0.0 ± 1.1 mmol/L; P = .003) analyses (Figure 1A). In within-group analyses, triglyceride levels significantly decreased in responders but did not significantly change in nonresponders (Tables 2 and 3). Changes in total cholesterol levels were significantly different in between-group analyses at 26 weeks (−0.20 ± 0.9 mmol/L vs 0.10 ± 1.0 mmol/L; P = .014) but not 52 weeks (P = .205); moreover, total cholesterol levels decreased significantly in within-group analyses of responders at both 26 and 52 weeks but did not significantly change in nonresponders (Tables 2 and 3). There were no significant changes in high-density lipoprotein cholesterol levels in either responders or nonresponders (Tables 2 and 3).
Figure 1.
Changes in triglyceride level (A), fasting glucose level (B), homeostasis model assessment—insulin resistance [HOMA-IR] value (C), and 2-h glucose following 75-g oral glucose load (D) in responders and nonresponders to tesamorelin at 26 and at 52 weeks. P values are based on comparisons of responders with nonresponders, using analysis of covariance to control for baseline values and study (first vs second phase III study).
With respect to measures of glucose homeostasis, changes were significantly different in responders versus nonresponders for all parameters (Tables 2 and 3). In both 26-week and 52-week analyses, fasting glucose levels did not change in responders but increased significantly in nonresponders, resulting in significant between-group differences (Figure 1B). Likewise, fasting insulin levels, homeostasis model assessment—insulin resistance (HOMA-IR) score (Figure 1C), and 2-hour glucose levels (Figure 1D) did not change in responders by within-group analysis, but all increased significantly in nonresponders at 26 weeks, with a similar trend observed at 52 weeks. HbA1c levels increased modestly but significantly in both groups at 26 weeks, but this change was significantly attenuated in the responder group, compared with nonresponders (0.1 ± 0.3% vs 0.3 ± 0.4%; P < .001). In the 52-week analysis, HbA1c levels did not significantly change from baseline in responders but were significantly increased in nonresponders, again with a significant between-group difference (0.0 ± 0.3% vs 0.2 ± 0.5%; P = .003). Nearly identical results were obtained in a sensitivity analysis that used the ITT observed case population (n = 543 at week 26 and n = 246 at week 52) (Supplementary Tables 1A and 1B). Subjects’ BAD score, a self-assessment in which higher scores indicate less distress, improved significantly more in responders versus nonresponders at 26 weeks (Tables 2 and 3).
Safety was not different between responders and nonresponders, with rates of serious adverse events of 2.2% and 1.9%, respectively, over 26 weeks and comparable rates between weeks 26 and 52. Compliance was similar between responders and nonresponders in the analysis (98% vs 97%).
Relationship Between Percentage Change in VAT and Changes in Other Variables
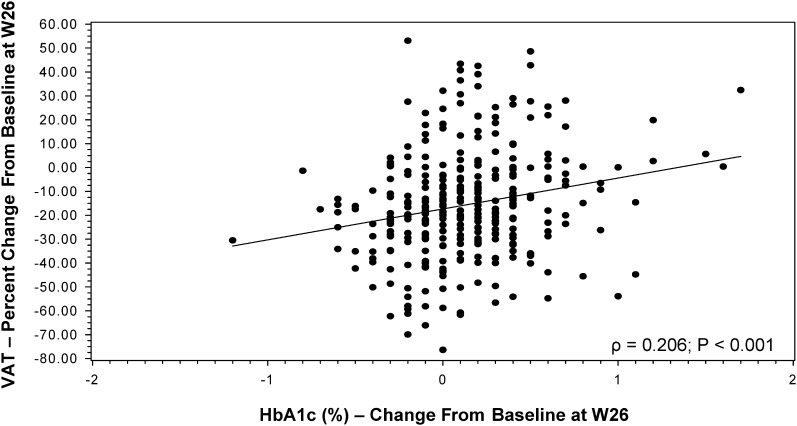
Percentage changes in VAT were negatively associated with changes in IGF-I levels (ρ = −0.239; P < .001), such that subjects with larger increases in IGF-I levels experienced larger percentage decreases in VAT (Table 4). Percentage changes in VAT were strongly positively associated with changes in waist circumference (ρ = 0.442; P < .001) and trunk fat (ρ = 0.626; P < .001), as well as with changes in waist-to-hip ratio (ρ = 0.275; P < .001). Improvements in VAT were also associated with reductions in levels of cholesterol (ρ = 0.164; P = .003) and triglycerides (ρ = 0.174; P = .001). Percentage changes in VAT were also significantly associated with changes in HbA1c levels (ρ = 0.206 [P < .001]; Figure 2) and changes in other measures of glucose homeostasis (Table 4). Of note, changes in limb fat were not associated with changes in levels of triglycerides (P = .25) or glucose (P = .31) in sensitivity analyses that controlled for changes in VAT.
Table 4.
Univariate Associations Between Percentage Changes in Visceral Adipose Tissue and Change in Metabolic Variables at Week 26 Among Responders and Nonresponders
| Variable | ρa | P |
| IGF-I level | −0.239 | <.001 |
| Fasting glucose level | 0.109 | .050 |
| HbA1c level | 0.206 | <.001 |
| HOMA-IR score | 0.115 | .043 |
| Fasting insulin level | 0.119 | .033 |
| 2-h glucose level | 0.172 | .003 |
| Total cholesterol level | 0.164 | .003 |
| HDL-C level | −0.105 | .057 |
| Triglycerides level | 0.174 | .001 |
Abbreviations: HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment—insulin resistance; IGF-I, insulin-like growth factor-1.
Spearman rank correlation coefficient.
Figure 2.
Association between change in hemoglobin A1c (HbA1c) level and percentage change in visceral adipose tissue (VAT) at 26 weeks. The regression line for Spearman correlation (ρ = 0.206; P < .001) is shown.
Metabolic Effects of VAT Loss Among Placebo-Treated Patients
Among the smaller number of placebo-treated patients who did have a reduction in VAT of ≥8% over 26 weeks, changes in levels of triglycerides (−0.4 ± 1.1 mmol/L vs 0.2 ± 1.4 mmol/L; P < .001) and glucose (−0.1 ± 0.7 mmol/L vs 0.1 ± 1.0 mmol/L; P = .04) were significant compared with those for placebo-treated patients who did not experience an 8% loss in VAT.
DISCUSSION
In the current analysis, we show that, in contrast to nonresponders, individuals who responded to tesamorelin, defined as an ≥8% reduction in VAT, experienced significantly greater improvements in levels of triglycerides and adiponectin and preservation of long-term glucose homeostasis over 52 weeks, suggesting metabolic benefits associated with reducing VAT in this population.
Baseline IGF-I levels were not related to tesamorelin response, but the 26-week data suggest that the magnitude of the change in IGF-I levels during tesamorelin therapy was significantly associated with the percentage decreases in VAT, with responders having greater increases in IGF-I levels than nonresponders at 26 weeks. Changes in IGF-I levels were not significantly different between responders and nonresponders at 52 weeks.
Tesamorelin significantly reduced triglyceride levels by approximately 0.5 mmol/L (40 mg/dL) in pooled analysis of the ITT population [11]. This reduction in triglyceride levels is likely mediated at least in part by the direct effect of tesamorelin in augmenting growth hormone levels, which, in turn, increases lipolysis, increases lipid β-oxidation, and reduces de novo lipogenesis [18–22]. The current data, demonstrating that reductions in triglyceride levels were strongly associated with reductions in VAT, also suggest a possible role of VAT reduction itself in decreasing triglyceride levels. Mechanistically, VAT is highly lipolytic and contributes free fatty acids to the portal circulation, [23], potentially leading to increased hepatic production of very-low-density lipoprotein cholesterol. The improvement in triglyceride levels seen among the small number of placebo-treated patients with VAT loss further suggests the importance of VAT loss in this group to the improvement of metabolic parameters.
The novel associations between percentage reductions in VAT and changes in glucose parameters in the current data, whereby the more that VAT is reduced, the more that glucose control improves, lend strong support to a potential relationship between VAT and glucose homeostasis. Exogenous administration of recombinant human growth hormone is well-known to exacerbate insulin resistance in both the HIV-infected population [24–26] and in non-HIV infected cohorts [27, 28]. However, longer-term studies of recombinant human growth hormone in obese adults have shown that initial exacerbations in insulin resistance may be reversed with longer-term treatment in association with improvements in VAT [29, 30]. The current results suggest, similarly, that any adverse effects that augmentation of growth hormone levels might have on glucose metabolism may be counterbalanced by the beneficial effects that reductions in VAT have on glucose homeostasis. VAT may affect glucose homeostasis through multiple mechanisms, including its contribution to increased systemic inflammation [31] and increased hepatic delivery of free fatty acids, resulting in increased hepatic gluconeogenesis [32]. Indeed, individuals who did not experience VAT reduction with tesamorelin had significant increases in fasting glucose levels, fasting insulin levels, and HOMA-IR score, as well as increased HbA1c levels, whereas changes in glucose homeostasis were significantly attenuated in responders, who experienced only a slight increase in HbA1c levels in 26-week analysis and no significant changes in the 52-week analysis in association with a highly significant reduction in VAT. One potential mechanism for the preservation of glucose homeostasis in responders is the increase in adiponectin levels seen among responders compared with nonresponders.
The current analysis has some limitations. The analysis was designed to explore the differences between responders and nonresponders who were taking the drug as prescribed (ie, individuals in the per-protocol population). However, nearly identical data were seen in sensitivity analyses among the larger population finishing the study, demonstrating that responders had significantly less deterioration of glucose levels among all patients finishing the study, regardless of compliance. A direct measure of insulin sensitivity was not used, but all the measures of glucose homeostasis that were evaluated, including glucose level, insulin level, HOMA-IR score, 2-hour glucose level, and HbA1c level, were consistent, suggesting a true effect related to responder status.
The current study suggests that achieving an 8% or greater reduction in visceral adiposity as a result of tesamorelin is associated with metabolic benefits. In the clinical setting, however, it is often not possible to determine the precise degree of VAT loss. Clinicians can measure waist circumference response [33] in subjects receiving tesamorelin, as the change in waist circumference is highly correlated with the reduction in VAT. Moreover, the majority of fat loss represented by the decrease in waist circumference is loss of VAT, as the VAT decrease is approximately five to six times the SAT decrease in responders. In this regard, even a 1 cm reduction in waist circumference corresponded to a significant reduction in VAT greater than 8% in response to tesamorelin and might be useful as an initial minimal acceptable reduction for continuation of tesamorelin. Additional considerations as to whether to continue patients should also include assessment of overall clinical status, with consideration to safety, tolerability and mood. Of note, the change in trunk fat by DXA was even more highly associated with VAT reduction than waist circumference, but performance of DXA is not routinely available to clinicians or standardized, as in this study, for the assessment of changes in regional body composition. In contrast, there was no change in BMI between tesamorelin vs placebo, and thus change in BMI is not useful as a predictor for change in VAT.
Although IGF-I levels were somewhat higher in responders vs nonresponders over the initial 26 weeks of treatment, no differences in safety (ie, frequency of serious adverse events) were seen in responders versus nonresponders over 26 and 52 weeks, including malignancy rates. Nonetheless, clinicians should consider monitoring IGF-I level, not as a means to predict response but rather to ensure that it remains within an acceptable range if a patient is a responder and going on to longer-term use of tesamorelin. As the safety of longer-term tesamorelin use beyond 52 weeks is not known, it is prudent to keep IGF-I levels within the assay’s normal range or within 2–3 SD to minimize any potential long-term effects associated with tesamorelin use.
In addition to metabolic benefits, greater responses to tesamorelin in terms of VAT reduction are also associated with less distress regarding abdominal adiposity, and this may be an important benefit of therapy in patients among whom baseline body dysmorphia and related distress have been shown [34].
Overall, the current report demonstrates that reductions in VAT during tesamorelin therapy are associated with improvements in triglyceride levels, adiponectin levels, and long-term preservation of glucose homeostasis. In contrast, these benefits are not seen in individuals who do not respond to tesamorelin with a reduction in VAT.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We are grateful to all of the research volunteers for their participation in the studies.
Study investigators.
Clinique Médicale du Quartier Latin, Montreal, Canada—P. Côté; Montreal General Hospital, Montreal, Canada—J. Falutz; St-Paul’s Hospital, Vancouver, Canada—J. Montaner; Southern Alberta HIV Clinic, Calgary, Canada—J. M. Gill; HIV Care Program, Windsor Regional Hospital, Windsor, Canada—C. Quan; Sunnybrook and Women’s College Health Sciences Centre, Toronto, Canada—A. Rachlis; New York University Medical Center, NY—J. Aberg; St-Luke’s-Roosevelt Hospital, New York, NY—J. Albu; Infectious Disease Physicians, Annandale, VA—S. Ambardar; University of Texas Medical School of Houston—R. Arduino; Northstar Healthcare, Chicago, IL—D. Berger; Dallas VA Medical Center, TX—R. Bedimo; Central Texas Clinical Research, Austin—C. Brinson; AIDS Research Alliance, West Hollywood, CA—S. Brown; Johns Hopkins University School of Medicine, Baltimore, MD—T. Brown; Center for Special Immunology, Fountain Valley, CA—P. Cimoch; Fanno Creek Clinic, Portland, OR—G. Coodley; Community Research Initiative of New England, Boston, MA—C. Cohen; UCLA School of Medicine, Los Angeles, CA—J. Currier; University of Maryland Institute of Human Virology, Baltimore—C. Davis; Orlando Immunology Center, FL—E. DeJesus; Indiana University Department of Medicine, Division of Infectious Diseases, Indianapolis—M. Dube; ACRIA, New York, NY—J. Ersnt; Kaiser Permanente, HIV Research Unit, San Francisco, CA—J. W. Fessel; University of Cincinnati Medical Center, OH—J. Feinberg; Bach & Godofsky, MD, PA, Bradenton, FL—E. Godofsky; Massachusetts General Hospital, Program in Nutritional Metabolism, Boston—S. Grinspoon; Hennepin County Medical Center, Minneapolis, MN—K. Henry; Rush University Medical Center, Chicago, IL—H. Kessler; Body Positive, Phoenix, AZ—R. Myers; UCSD Medical Center, Owen Clinic Antiviral Research Center, San Diego, CA—D. Lee; Treasure Coast Infections Disease Consultants, Vero Beach, FL—G. Pierone; Capital Medical Associates, Washington, DC—B. Rashbaum; Fort Lauderdale, FL—G. J. Richmond; Care Resource, Miami, FL—S. Santiago; Swedish Medical Center, Seattle, WA—P. Shalit; Community Research Initiative of New England, Springfield, MA—D. Skiest; Drexel University College of Medicine, Division of HIV/AIDS Medicine, Philadelphia, PA—P. Sklar; Infectious Disease, Palms Springs—M. Somero; AIDS Research Consortium of Atlanta, Atlanta, GA—M. Thompson; Saint-Vincent’s Hospital and Medical Center, New York, NY—A. Urbina; Infectious Diseases Associates, Sarasota, FL—W. Vega; Tufts New England Medical Center, Infectious Disease, Boston, MA—C. Wanke.
Manuscript preparation.
Theratechnologies provided assistance with study design and data collection and performed statistical analyses. T. L. S. and S. K. G. prepared the manuscript and were responsible for final edits.
Financial support.
This work was supported by Theratechnologies and by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grant K23 DK089910 to T. L. S. and grant K24 DK064545 to S. K. G.).
Potential conflicts of interest.
J. F. reports having received research support from Theratechnologies; having received payment for lectures from ViiV Canada, Abbott Canada, Gilead Canada, Bristol Myers Squibb Canada, and Merck Canada; having received payment for development of educational presentations from EMD Serono; and having received compensation for travel, accommodation, and meeting expenses from EMD Serono. C. M., J. M., G. S., J. C. M., and H. A. are employees of Theratechnologies. R. T. reports having served as a consultant to Theratechnologies and EMD Serono. S. K. G. reports having received research support from Theratechnologies and serving as a consultant to Theratechnologies and EMD Serono. T. L. S. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Shlay JC, Bartsch G, Peng G, et al. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. J Acquir Immune Defic Syndr. 2007;44:506–17. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen A, Calmy A, Schiffer V, et al. Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000–2006. HIV Med. 2008;9:142–50. doi: 10.1111/j.1468-1293.2007.00537.x. [DOI] [PubMed] [Google Scholar]
- 3.Wohl D, Scherzer R, Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 5.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–90. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–7. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Tong Q, Sankale JL, Hadigan CM, et al. Regulation of adiponectin in human immunodeficiency virus-infected patients: relationship to body composition and metabolic indices. J Clin Endocrinol Metab. 2003;88:1559–64. doi: 10.1210/jc.2002-021600. [DOI] [PubMed] [Google Scholar]
- 8.Masia M, Padilla S, Garcia N, et al. Endothelial function is impaired in HIV-infected patients with lipodystrophy. Antivir Ther. 2010;15:101–10. doi: 10.3851/IMP1491. [DOI] [PubMed] [Google Scholar]
- 9.Guaraldi G, Zona S, Orlando G, et al. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2011 doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 10.Scherzer R, Heymsfield SB, Lee D, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV Infection. AIDS. 2011 doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falutz J, Mamputu JC, Potvin D, et al. Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010;95:4291–304. doi: 10.1210/jc.2010-0490. [DOI] [PubMed] [Google Scholar]
- 12.Falutz J, Allas S, Blot K, et al. Metabolic effects of a growth hormone-releasing factor in patients with HIV. N Engl J Med. 2007;357:2359–70. doi: 10.1056/NEJMoa072375. [DOI] [PubMed] [Google Scholar]
- 13.Stanley TL, Falutz J, Mamputu JC, Soulban G, Potvin D, Grinspoon SK. Effects of tesamorelin on inflammatory markers in HIV patients with excess abdominal fat: relationship with visceral adipose reduction. AIDS. 2011;25:1281–8. doi: 10.1097/QAD.0b013e328347f3f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falutz J, Allas S, Mamputu JC, et al. Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS. 2008;22:1719–28. doi: 10.1097/QAD.0b013e32830a5058. [DOI] [PubMed] [Google Scholar]
- 15.Falutz J, Potvin D, Mamputu JC, et al. Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: a randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr. 2010;53:311–22. doi: 10.1097/QAI.0b013e3181cbdaff. [DOI] [PubMed] [Google Scholar]
- 16.Lemieux S, Prud'homme D, Bouchard C, Tremblay A, Despres JP. A single threshold value of waist girth identifies normal-weight and overweight subjects with excess visceral adipose tissue. Am J Clin Nutr. 1996;64:685–93. doi: 10.1093/ajcn/64.5.685. [DOI] [PubMed] [Google Scholar]
- 17.Snyder S. Forum for collaborative HIV research roundtable discussion. Washington, DC: George Washington University School of Public Health and Health Services; 2004. Regulatory considerations for the treatment of lipodystrophy; pp. 1–44. [Google Scholar]
- 18.Richelsen B, Pedersen SB, Borglum JD, Moller-Pedersen T, Jorgensen J, Jorgensen JO. Growth hormone treatment of obese women for 5 wk: effect on body composition and adipose tissue LPL activity. Am J Physiol. 1994;266:E211–16. doi: 10.1152/ajpendo.1994.266.2.E211. [DOI] [PubMed] [Google Scholar]
- 19.Richelsen B, Pedersen SB, Kristensen K, et al. Regulation of lipoprotein lipase and hormone-sensitive lipase activity and gene expression in adipose and muscle tissue by growth hormone treatment during weight loss in obese patients. Metabolism. 2000;49:906–11. doi: 10.1053/meta.2000.6738. [DOI] [PubMed] [Google Scholar]
- 20.Schwarz JM, Mulligan K, Lee J, et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J Clin Endocrinol Metab. 2002;87:942. doi: 10.1210/jcem.87.2.8391. [DOI] [PubMed] [Google Scholar]
- 21.Moller N, Jorgensen JO, Alberti KG, Flyvbjerg A, Schmitz O. Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. J Clin Endocrinol Metab. 1990;70:1179–86. doi: 10.1210/jcem-70-4-1179. [DOI] [PubMed] [Google Scholar]
- 22.Moller L, Norrelund H, Jessen N, et al. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. J Clin Endocrinol Metab. 2009;94:4524–32. doi: 10.1210/jc.2009-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kotler DP, Muurahainen N, Grunfeld C, et al. Effects of growth hormone on abnormal visceral adipose tissue accumulation and dyslipidemia in HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:239–52. doi: 10.1097/00126334-200403010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Grunfeld C, Thompson M, Brown SJ, et al. Recombinant human growth hormone to treat HIV-associated adipose redistribution syndrome: 12 week induction and 24-week maintenance therapy. J Acquir Immune Defic Syndr. 2007;45:286–97. doi: 10.1097/QAI.0b013e3180691145. [DOI] [PubMed] [Google Scholar]
- 26.Lo J, You SM, Canavan B, et al. Low-dose physiological growth hormone in patients with HIV and abdominal fat accumulation: a randomized controlled trial. JAMA. 2008;300:509–19. doi: 10.1001/jama.300.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krusenstjerna-Hafstrom T, Clasen BF, Moller N, et al. Growth hormone (GH)-induced insulin resistance is rapidly reversible: an experimental study in GH-deficient adults. J Clin Endocrinol Metab. 2011 doi: 10.1210/jc.2011-0273. [DOI] [PubMed] [Google Scholar]
- 28.Jessen N, Djurhuus CB, Jorgensen JO, et al. Evidence against a role for insulin-signaling proteins PI 3-kinase and Akt in insulin resistance in human skeletal muscle induced by short-term GH infusion. Am J Physiol Endocrinol Metab. 2005;288:E194–9. doi: 10.1152/ajpendo.00149.2004. [DOI] [PubMed] [Google Scholar]
- 29.Johannsson G, Marin P, Lonn L, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997;82:727–34. doi: 10.1210/jcem.82.3.3809. [DOI] [PubMed] [Google Scholar]
- 30.Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson B, Johansson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–74. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- 31.Harman-Boehm I, Bluher M, Redel H, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–7. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 32.Rebrin K, Steil GM, Getty L, Bergman RN. Free fatty acid as a link in the regulation of hepatic glucose output by peripheral insulin. Diabetes. 1995;44:1038–45. doi: 10.2337/diab.44.9.1038. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES) Anthropometry Procedures Manual. 2009. pp. 1–120. Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/BodyMeasures_09.pdf. [Google Scholar]
- 34.Turner RR, Testa MA, Su M, et al. The impact of HIV-associated adipose redistribution syndrome (HARS) on health-related quality of life. Antivir Ther. 2006;11:L25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.