Abstract
AIM: To study the metabolic profile of human umbilical mesenchymal stem cells (HUMSC) and adipogenic differentiation by nuclear magnetic resonance (NMR) spectroscopy.
METHODS: HUMSC isolated from human umbilical cord stroma were induced to adipocytes over 2 wk by adding dexamethasone, 3-isobutyl-1-methylxanthine, indomethacin, and insulin to the culture medium. Adipogenic differentiation was confirmed by Red O staining and transcription-polymerase chain reaction. Perchloric acid extracts of the HUMSCs and adipocytes (about 7 × 106) were characterized for metabolites by using in vitro high resolution 9.4T NMR spectroscopy.
RESULTS: Several major metabolites, such as: choline, creatine, glutamate and myo-inositol, acetate, and some fatty acids/triglycerides, were observed in the MR spectroscopic pattern of HUMSCs and their adipogenic differentiation. HUMSCs are characterized by an unusually low number of NMR-detectable metabolites, high choline, acetate, glutamate and creatine content. However, the metabolic profiles of adipogenic differentiation demonstrated considerably higher methionine and fatty acids, and non-detectable creatine.
CONCLUSION: The biomarkers of HUMSCS and adipocytes were obtained and assigned. NMR spectroscopy will be a promising tool for monitoring stem cell differentiation.
Keywords: Human umbilical mesenchymal stem cell, Adipogenic differentiation, MR spectroscopy, Biomarker
INTRODUCTION
Cellular-based therapies using mesenchymal stem cells (MSCs) are being evaluated as promising treatment options for many diseases and injuries. MSCs are of great interest in regenerative medicine, because of their properties of pluripotency[1,2], and low immunologic rejection. They are capable of reconstructing a tissue and a coordinating function. Human umbilical MSCs (HUMSC) isolated from human umbilical cord Wharton’s Jelly called fetal appendage, were considered to be a new stem cell line for cellular-based therapy[3]. Increasingly, nowadays, researches are focusing on the HUMSC. Extensive gene expression and immunocytochemistry studies were recently performed on HUMSC, but despite the demanding effort and expense involved, they yielded surprisingly incoherent results[4]. However, so far, the information about metabolites of HUMSC is limited, and there are unexplored areas in this field. HUMSC should be studied further to obtain more information before considering clinical applications.
Nuclear magnetic resonance (NMR) spectroscopy can act as a powerful window into the metabolic machinery of cells[5-7]. Because of its noninvasive nature and high chemical specificity, it is well suited for studying and quantifying cellular metabolism in tissue and organ, reflecting levels of endogenous metabolites involved in key cellular pathways. Recently, NMR spectroscopy in vitro has been shown to readily identify many cell types under culture conditions on the basis of their metabolic properties; such as neural stem cells and embryonic stem cells[7], neural progenitor cells[8], bone MSCs (BMSCs)[9], and cell apoptosis[10]. The changes in the intracellular metabolites associated with the pathological change, can be detected by NMR or MR spectroscopy, in vitro and in vivo, respectively. MRS studies in vitro provide a more comprehensive metabolic profile of the low molecular weight components. In the present study, we attempt to identify the metabolic profile of HUMSC, and characterize the changes to intracellular metabolites of HUMSC undergoing adipogenic differentiation, using 9.4T high-resolution 1H NMR spectroscopy. The results show that the MR spectroscopic pattern of HUMSC was identified, and the change in metabolites was obvious after adipogenic differentiation. Furthermore, it will provide a theoretical basis for future HUMSC monitoring after transplantation by using in vivo MR spectroscopy.
MATERIALS AND METHODS
HUMSCs and adipogenic differentiation
The HUMSCs were obtained from human umbilical cord stroma, received as a gift from Stem Cell Laboratory (Multidisciplinary Research Center of Shantou University, China). HUMSCs were cultivated as described[2]. 80%-85% subconfluent HUMSCs from passage 4 in culture underwent a differentiation process described earlier by Karahuseyinoglu et al[11]. Briefly, the HUMSCs were induced for adipogenic differentiation by an administration of 1 μmol/L dexamethasone, 500 μmol/L 3-isobutyl-1-methylxanthine, 60 μmol/L indomethacin, and 5 μg/mL insulin in DMEM-low glucose (low glucose, 1 g/L) supplemented with 10% fetal bovine serum. Induced cells were maintained at 37 °C in 5% CO2/95% air incubator under sterile conditions, and the culture medium was changed every 3-4 d.
Adipogenic differentiation identification with Red-O staining and reverse reverse transcriptase-polymerase chain reaction
When the cells were induced for adipogenic differentiation for 2 wk, the culture medium was changed 12 h before Oil Red-O staining. After removing the medium, the cells were washed with PBS 3 times, after that ten percent buffered formalin-fixed cells (15 min at room temperature) were stained by 1% (wt/vol) oil red O in 60% isopropanol for 15 min. After washing cells with distilled H2O 3 times, the cells were observed under a microscope. In order to confirm that the lipid body formed is mainly due to adipogenic induction, the mRNA expression test was required. Total cellular RNA was isolated using the RNeasy mini kit (Qiagen, GmbH, and Hilden, Germany) according to the manufacturer’s instructions. Several main primers such as: peroxisome proliferator-activated receptor (PPAR)-γ, acyl-CoA synthetase (ACS), and the enzyme lipoprotein lipase (LPL), etc., were used for reverse reverse transcriptase-polymerase chain reaction (RT-PCR); the expression level of GAPDH was used as the house keeping gene control. We described the examination, in detail, in a previous publication[12].
Cells extracts and 1H-NMR spectroscopy data acquisition
When the HUMSCs grew to sufficient numbers for the experiment (about 7 × 106) and cell differentiation induced for 2 wk, the cells were harvested quickly with a vulcanite scraper and washed three times with PBS to remove potential medium residue. The cells were collected for future study by centrifugation (1200 r/min at 4 °C) for 8 min. Metabolite extraction was performed on ice in 2 mL perchloric acid PCA (0.5 mmol/L) for these cell pellets. Briefly, the samples were sonicated (BILON99-11DL Instruments, China) for 6 min and then the sample pH values were neutralized with ice-cold KOH (1 mmol/L) and PCA (0.5 mmol/L). Then the mixture was centrifuged (BECKMAN USA) for 25 min (15 000 r/min at 4 °C). The resulting supernatant was lyophilized into powder, and then stored at -80 °C for 1H NMR analysis. Weighed 10 mg lyophilized extracts were transferred to 5 mm NMR tubes and redissolved in 500 μL D2O containing 0.5 mmol/L 2, 2-3, 3-tetradeutero-trimethyl-sylilpropionate (TMSP), which was used as an external standard [0.00 part per million (ppm)] for sample quantification and assignment of the metabolites. The samples were analyzed on an in vitro 9.4 T high resolution magnetic resonance spectrometer (Bruker Avance 400 MHz) at 27 °C. NMR spectra were obtained using presaturation of the residual water protons in the solvent with the Bruker ZGPR pulse program. The spectral parameters were: sweep width = 5 kHz, data size = 4096 points, TR = 20 s and the number of scans = 128. The chemical shift was assigned according to the internal standard (TMSP), and was analyzed by MestRe-c 4.3.
Quantification of the interesting metabolites
We compared the integration of TMSP signal with intracellular metabolites after baseline and phase correction for quantification. The concentration of the metabolite was calculated according to this equation[10]. Metabolite = [Square (metabolite)]/[square (TMSP)] × [Number of protons of metabolite/9] × TMSP. Square (metabolite) and square (TMSP) stand for the area of metabolite peak of interest and TMSP signals; (TMSP) and (metabolite) represent the concentration of TMSP and metabolites, respectively; 9 is the number of protons giving rise to the TMSP resonance. Statistical comparisons of the metabolite level in HUMSC and differentiated cells were made using the SPSS13.0 software. Absolute concentrations were given as mean ± SD and statistically analyzed using the Student’s t-test for the comparison of two groups, with P < 0.05 considered to be significant.
RESULTS
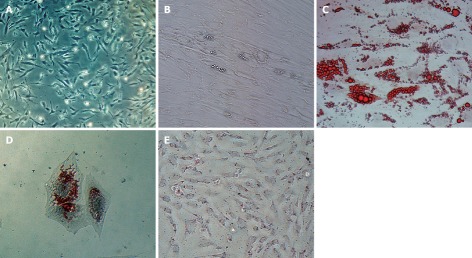
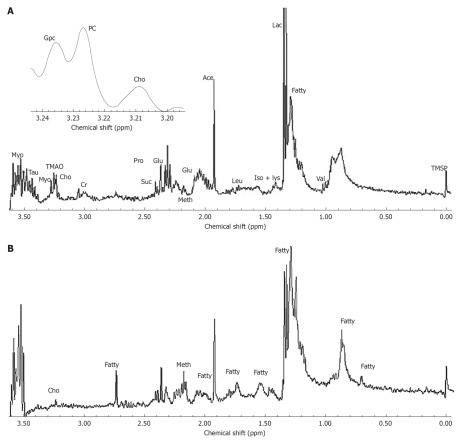
The HUMSCs isolated from human umbilical cord stroma were serial sub-cultivation and adipogenic differentiation induced. Figure 1A shows HUMSCs as a monolayer of large, fat cells (about 30 μm in diameter) in culture medium. As the HUMSCs approached confluence, they assumed a more spindle-shaped, fibroblastic morphology. The onset of phenotypic changes was noticed as early as the first days of induction. Figure 1B shows that after 14 d adipogenic treatment the cell shape had transformed into an ovoid/round morphology, together with increased number and size of mobile lipid droplets which coalesced, and showed a tendency to accumulate in the cell periphery. Figure 1C and D are the results for Oil Red-O stain of the HUMSCs after and before undergoing adipogenic induction. After 14 d under adipogenic conditions, the lipid droplets in the cytoplasm were clearly visualized, with Oil Red-O stain. However, no lipid body was observed in the cytoplasm of the HUMSCs before differentiation. Figure 2 demonstrates that the PPAR-γ, ACS, FABP4, LPL gene expression increased with adipogenic differentiation induced for 0, 7 and 14 d. At 14 d, all the above genes were expressed to different degrees. Figure 3A and B represent a typical 1H NMR spectrum of HUMSCs before and after adipogenic treatment, respectively. There are two obviously distinct spectra in 0.00-3.50 ppm region of the spectrum. Several resonance signals seem particularly outstanding within 0.00-3.50 ppm chemical shifts; for instance, choline compound (3.21-3.24 ppm) consists of choline, phosphocholine and glyocerphosphocholine, creatine (3.05 ppm), acetate (1.91 ppm), methionine (2.19 ppm), succinate (2.41 ppm), lactate (1.31 ppm) and fatty acid (1.28 ppm), and so on. These visible metabolites in the two spectra and their corresponding chemical shifts, also including the respective groups contributing to these signals, are all shown in Table 1. It is noticeable that in the spectra of HUMSCs with and without differentiation, the amplitudes of metabolic resonances are distinct. Quantification of metabolite concentrations was performed; the levels of intracellular metabolites, such as choline (Cho), creatine (Cr), glutamate (Glu) and acetate (Ace) all decreased, with an increased level of methionine (Meth), succinate (Suc) and fatty acids after HUMSCs differentiation for 2 wk. Intracellular choline, acetate, glutamate and creatine reduced from 6.3 ± 0.68, 0.97 ± 0.23, 0.3 ± 0.05 and 0.1 ± 0.02 mmol/L to 1.1 ± 0.06 (P < 0.01), 0.45 ± 0.1 (P < 0.01), 0.16 ± 0.08 mmol/L (P < 0.05) and non-detected, respectively. Inversely, the methionine, succinate increased from 0.03 ± 0.01, 0.11 ± 0.02 mmol/L to 0.12 ± 0.05 (P < 0.01) and 0.15 ± 0.05 mmol/L (P > 0.05), in addition the fatty acids also increased remarkably (Table 2).
Figure 1.
Human umbilical mesenchymal stem cells, adipogenic differentiation and red-o staining. A is the human mesenchymal stem cells at passage 4, the cells assumed a more spindle-shaped, fibroblastic morphology; B-D demonstrate the human umbilical mesenchymal stem cells (HUMSCs) after 2 wk adipogenic differentiation without and with the Red-O staining, respectively. The differentiated cells’ shape had transformed into an ovoid/round morphology and there were abundant lipid droplets presented in the cell periphery; E is the HUMSCs with Red-O staining without differentiation, no lipid body is visible.
Figure 2.
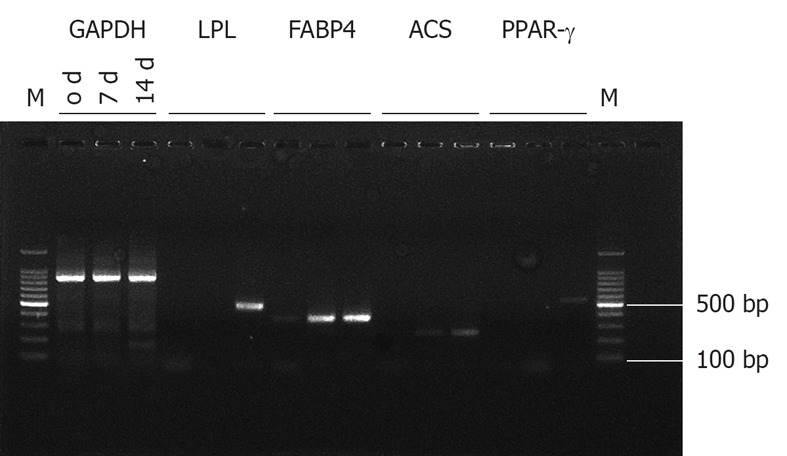
Reverse reverse transcriptase-polymerase chain reaction of the human umbilical mesenchymal stem cells and adipogenic differentiation. After adipogenic differentiation induced for 1 and 2 wk, the mRNA expression of adipocyte-specific genes, such as peroxisome proliferator-activated receptor (PPAR)-γ, FABP4, acyl-CoA synthetase (ACS), and lipoprotein lipase (LPL) are visible more or less. This expression is absent in human umbilical mesenchymal stem cells, without differentiation.
Figure 3.
Metabolic profile of human umbilical mesenchymal stem cells before and after adipogenic differentiation. A and B are the nuclear magnetic resonance Spectrums of human umbilical mesenchymal stem cells (HUMSCs) and adipogenic differentiation, respectively. The two spectrums demonstrated that the levels of intracellular metabolites, such as choline, creatine, glutamate and acetate all decreased with the increased level of methionine, succinate and fatty acids after the HUMSCs differentiation 2 wk.
Table 1.
Intracellular metabolites and their chemical shifts, before and after differentiation of human umbilical mesenchymal stem cells (Sitter et al[20], 2002 and Govindaraju et al[21], 2000)
| Metabolites | 1H or spin | Chemical shift (ppm) |
| Valine (Val) | γCH3 | 0.98 1.02 |
| Fatty acids | -CH2-(CH2)n-CH2- | 1.28 |
| Lactate (Lac) | CH3 | 1.32 |
| Fatty acids | (-CH2-CH3) | 1.35 |
| Fatty acids | (-CO-CH2-CH2-) | 1.54 |
| Acetate (Ace) | CH3 | 1.92 |
| Glutamate (Glu) | βCH2 γCH2 | 2.05 2.34 |
| Methionine (Meth) | βCH2 | 2.19 |
| Succinate (Suc) | (α,βCH2) | 2.41 |
| Fatty acids | -CH=CH-Ch2-CH=CH- | 2.75 |
| Creatine (Cr) | CH3 | 3.05 |
| Choline (Cho) | N-(CH3)3 | 3.21 |
| Phosphocholine (PC) | N-(CH3)3 | 3.22 |
| Glyocerphosphocholine (GPC) | N-(CH3)3 | 3.23 |
| Taurine (Tau) | N-CH2 S-CH2 | 3.27 3.43 |
| Myo-Inosito (Myo) | C1H C3H | 3.54 |
Table 2.
Concentrations of major metabolites in mesenchymal stem cells and mesenchymal stem cell-differentiated cells
| Metabolites | Cho (mmol/L) | Ace (mmol/L) | Cr (mmol/L) | Glu (mmol/L) | Meth (mmol/L) | Suc (mmol/L) |
| MSCs (n = 6) | 6.30 ± 0.68 | 0.97 ± 0.23 | 0.10 ± 0.02 | 0.30 ± 0.05 | 0.03 ± 0.01 | 0.11 ± 0.02 |
| Differentiated cells (n = 4) | 1.10 ± 0.06 | 0.45 ± 0.10 | ND | 0.16 ± 0.08 | 0.15 ± 0.05 | 0.12 ± 0.05 |
| P value | < 0.01 | < 0.01 | < 0.01 | < 0.01 | > 0.05 |
MSCs: Mesenchymal stem cells; Cho: Choline; Ace: Acetate; Cr: Creatine; Glu: Glutamate; Meth: Methionine; Suc: Succinate.
DISCUSSION
HUMSCs, pluripotent progenitors derived from human umbilical cord Wharton’s Jelly, were capable of differentiating into the mesoderm lineage, such as adipocytes, chondrocytes and osteoblasts[1,2]. The adipogenic induction in HUMSCs was described by Karahuseyinoglu et al[11], and was simply verified by Oil Red-O, a histological stain used to detect neutral lipids. In this study, a significant quantity of lipid particles occurred after 3 d in HUMSCs undergoing adipogenic differentiation. After 14 d adipogenic treatment, mobile lipid droplets of increased number and size, stained positive with Oil Red O stain, were observed gathering in the cytoplasm. However, previous studies also indicated that lipid bodies are a reference for apoptosis evaluation[13,14]. Therefore, in the present study, the adipogenesis-specific genes, such as PPAR-γ, LPL, ACS and FABP4 were confirmed. The PPAR-γ is important in initiating the transcription of adipogenic genes and this protein can be activated by fatty acids. In lipoprotein metabolism, LPL breaks the large molecules of triglycerides into chylomicron and very light density lipoprotein into small molecules of fatty acids to facilitate absorption into tissues through the endothelium of the capillary. Synthesis of LPL is found to be dominant in adipose tissue. In the present study, RT-PCR was performed at HUMSCs adipogenic induction at 7th and 14th day. This demonstrated that the expression of PPAR-γ, ACS and LPL were all increased gradually, but at different rates. In the present study we found: (1) HUMSCs are promising seeding cells in tissue engineering, as they can be differentiated into mature fat cells; and (2) the method used for inducing HUMSCs to became adipocytes was successful.
Biological construction of the metabolome in an organism is now fully understood. The metabolites located “downstream” of the genome will fluctuate naturally along with changes in genome, transcription, proteome[5], and enzyme activation. Thus, metabolites contain abundant biological information and indicate cells' physiological function and activity. Several metabolites are usually predominant in the spectrum of biological samples; these metabolites include Cho, Glu, Cr and Lac, which demonstrate different physiological function[15]. Whereas, some metabolites are the typical biomarker for a particular cell line, this can contribute to identify, trace and purify that specific cell line. One of the notable findings in present series was that the HUMSC spectrum pattern was established; Cho, Glu, Cr, MI and Lac peaks were predominant. The biggest difference between the HUMSC spectrum and the adipocyte spectrum is that some peaks located at 1.35 ppm, 1.54 ppm and 2.75 ppm arose in the latter. A previous study showed that these resonances were attributable to fatty acid or neutral lipid[16]. However, in the present study, we assumed that they most likely arose from the neutral lipid, especially triglycerides, accumulating in lipid bodies in the cells. Abundant cytoplasmic lipid bodies were observed in HUMSCs undergoing adipogenic differentiation. The relationship between 1H NMR-visible mobile lipid domains with cytoplasmic lipid bodies had been revealed by many previous studies[17,18]. Here, there would be one question; whether the PCA extraction is unable to get comprehensive lipid signals in the cells. However, to a certain extent, some information of water-soluble fatty acids or neutral lipid can be accessed. We found, typically, that these lipid signals arose in the spectra of adipogenic differentiation. The level of these metabolites would vary with the specific cell line and process. The level of metabolites would vary with the process of cell development, differentiation and damage due to functional gene expression, transcription level and enzyme activation changes. Interestingly, we found that, in HUMSCs undergoing adipogenic differentiation, the reduction of Cr, Cho, Ace and Glu was remarkable. It indicates that the intracellular metabolites will meet the requirements of the cell’s function and activity variation. We presumed the metabolite variation can be explained by: (1) Initially, there is growth arrest of proliferating pre-adipocytes, which was demonstrated by Hansen et al[19]. The PPAR-γ activation is the main reason for promoting growth arrest and the establishment of the differentiated adipocyte phenotype[19]. In the present study, PPAR-γ expression increased after 2 wk adipogenic differentiation. Lower levels of metabolites might be associated with cells in growth arrest; and (2) it is expected that adipocytes have much lower intracellular metabolite levels compared to HUMSCs. The results appeared to be similar to that of Shi et al[9], so that it seemed that this had little novelty value but, in fact, this is not the case. Several important distinctions were obvious. Firstly, human UMSCs were used in this study, instead of Balb/c mouse BMSCs, namely, the metabolic profile of HUMSCs was established. It is surprising that the metabolic features of an MSC line in human and mouse was almost the same. Therefore, combining both studies, showed that using mouse MSCs to replace human MSCs for pre-clinical MRS research is feasible. Secondly, using RT-PCR, as performed in this research, to verify adipogenic differentiation was scientifically exact. Moreover, a routine PCA extraction, not HRMAS 1H-NMR spectroscopy, was used in present series, although the spectrum of MSC with and without adipogenic differentiation was similar. The two experimental results confirm each other. Although many results of the two studies were similar, the concentration of the metabolites is lower in the present series. We hypothesized that these distinctions may be attributed to differences in the detection methods used, as well as in the differentiation time. Using high-resolution 1H NMR spectroscopy for cell extracts, metabolite loss would be greater. Even more surprisingly, methionine was increased after differentiation, which had not been reported before. As an essential amino acid, the methionine consumption decreased with the cell differentiation stage, but uptake was not decreased correspondingly. This may be an anomaly in the present study, further research is required.
However, there are some limitations to the current study. Firstly, a large number of cultured cells are required to achieve excellent NMR resonance intensity. These are in vitro studies, so the results cannot really reflect the in vivo metabolism of HUMSCs. Furthermore, as a routine method, PCA extraction cannot provide overall metabolic information. Nonetheless, we have reported the first established metabolic profile of HUMSC. In addition, these experiments confirmed the metabolite features of adipogenic differentiation in HUMSCs by a traditional and effective method. This will, in future, provide a theoretical basis for identifying HUMSCs and monitoring their differentiation using MR spectroscopy.
ACKNOWLEDGMENTS
The majority of work in this study was carried out in the Multidisciplinary Research Center (MRC) of Shantou University. Many techniques and materials were supplied and supported by researchers in the Stem Cell Laboratory of MRC.
COMMENTS
Background
Cellular-based therapies using mesenchymal stem cells (MSCs) are being evaluated as promising treatment options for many diseases and injuries. Before clinical application, an effective method for monitoring the cell’s physiological function and activity should be established. NMR spectroscopy can act as a powerful window into the metabolic machinery of cells, so that it can reflect the function and activity of the cells. However, so far, the information about metabolites of human umbilical MSCs (HUMSC) has been limited, and there are unexplored areas in this field. This project was aimed to explore the characteristic biomarkers of HUMSCs, and identify their variation after adipogenic differentiation using in vitro nuclear magnetic resonance (NMR) spectroscopy. This has not been reported previously.
Research frontiers
The research hotspot is to establish the metabolic profile of HUMSCs. Additionally, the results demonstrated that NMR spectroscopy would be a promising technology for identifying stem cell type and the physiological function and activity change.
Innovations and breakthroughs
In the present study, the authors report a novel strategy for investigating intracellular metabolites of HUMSCs and adipocytes using NMR spectroscopy, which allows identification of the stem cell and changes in physiological function and activity. The current study established the metabolic profile of HUMSCs, successfully. It will provide a theoretical basis for future use of NMR spectroscopy to identify HUMSCs and monitor their differentiation.
Applications
The present study not only identified the endogenous metabolites of HUMSCs, but also provided a novel strategy for stem cell identification and evaluation of physiological function and activity.
Terminology
HUMSC isolated from human umbilical cord Wharton’s Jelly called fetal appendage, were considered to be a new stem cell line for cellular-based therapy. NMR spectroscopy can act as a powerful window into the metabolic machinery of cells, and it can reflect the function and activity of cells non-invasively.
Peer review
In this study the authors attempt to identify the metabolic profile of HUMSC during adipogenic differentiation by NMRs. The method which used for evaluating of differentiation is interesting.
Footnotes
Supported by Grants from the National Natural Science Foundation of China (Key program 30930027) and Natural Science Foundation of Guangdong Province (No. 8151503102000032)
Peer reviewer: Arash Zaminy, PhD, Anatomy and Cell Biology Department, Medicine School, Shahid Beheshti University of Medical Sciences, Evine 1985717443, Tehran, Iran
S- Editor Wang JL L- Editor Hughes D E- Editor Zheng XM
References
- 1.Ding DC, Shyu WC, Lin SZ. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- 2.Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH. Human umbilical cord Wharton’s Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl) 2005;118:1987–1993. [PubMed] [Google Scholar]
- 3.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 4.Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. doi: 10.1186/1478-811X-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu ZF, Xiao YY, Wu RH. NMR in metabolic profiles of neural stem/progenitor cells: Current status and relevant problems. Curr Med Imaging Rev. 2009;5:144–149. [Google Scholar]
- 6.Haddadin IS, McIntosh A, Meisamy S, Corum C, Styczynski Snyder AL, Powell NJ, Nelson MT, Yee D, Garwood M, Bolan PJ. Metabolite quantification and high-field MRS in breast cancer. NMR Biomed. 2009;22:65–76. doi: 10.1002/nbm.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen JF, Shamblott MJ, van Zijl PC, Lehtimäki KK, Bulte JW, Gearhart JD, Hakumäki JM. Stem cell profiling by nuclear magnetic resonance spectroscopy. Magn Reson Med. 2006;56:666–670. doi: 10.1002/mrm.20968. [DOI] [PubMed] [Google Scholar]
- 8.Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, et al. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi C, Wang X, Wu S, Zhu Y, Chung LW, Mao H. HRMAS 1H-NMR measured changes of the metabolite profile as mesenchymal stem cells differentiate to targeted fat cells in vitro: implications for non-invasive monitoring of stem cell differentiation in vivo. J Tissue Eng Regen Med. 2008;2:482–490. doi: 10.1002/term.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Z, Wu LP, Li YX, Guo YB, Chen YW, Wu RH. Change of choline compounds in sodium selenite-induced apoptosis of rats used as quantitative analysis by in vitro 9.4T MR spectroscopy. World J Gastroenterol. 2008;14:3891–3896. doi: 10.3748/wjg.14.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karahuseyinoglu S, Kocaefe C, Balci D, Erdemli E, Can A. Functional structure of adipocytes differentiated from human umbilical cord stroma-derived stem cells. Stem Cells. 2008;26:682–691. doi: 10.1634/stemcells.2007-0738. [DOI] [PubMed] [Google Scholar]
- 12.Xu ZF, Shen CY, Zhu HQ, Chen YW, Wu LP, Huang P, Xiao YY, Shen ZW, Pang L, Guo XQ, et al. Is 1.28 parts per million biomarker specific for neural progenitor cells. Neural Regen Res. 2010;5:1125–1129. [Google Scholar]
- 13.Schmitz JE, Kettunen MI, Hu DE, Brindle KM. 1H MRS-visible lipids accumulate during apoptosis of lymphoma cells in vitro and in vivo. Magn Reson Med. 2005;54:43–50. doi: 10.1002/mrm.20529. [DOI] [PubMed] [Google Scholar]
- 14.Iorio E, Di Vito M, Spadaro F, Ramoni C, Lococo E, Carnevale R, Lenti L, Strom R, Podo F. Triacsin C inhibits the formation of 1H NMR-visible mobile lipids and lipid bodies in HuT 78 apoptotic cells. Biochim Biophys Acta. 2003;1634:1–14. doi: 10.1016/j.bbalip.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Martínez-Bisbal MC, Martí-Bonmatí L, Piquer J, Revert A, Ferrer P, Llácer JL, Piotto M, Assemat O, Celda B. 1H and 13C HR-MAS spectroscopy of intact biopsy samples ex vivo and in vivo 1H MRS study of human high grade gliomas. NMR Biomed. 2004;17:191–205. doi: 10.1002/nbm.888. [DOI] [PubMed] [Google Scholar]
- 17.Di Vito M, Lenti L, Knijn A, Iorio E, D’Agostino F, Molinari A, Calcabrini A, Stringaro A, Meschini S, Arancia G, et al. 1H NMR-visible mobile lipid domains correlate with cytoplasmic lipid bodies in apoptotic T-lymphoblastoid cells. Biochim Biophys Acta. 2001;1530:47–66. doi: 10.1016/s1388-1981(00)00165-7. [DOI] [PubMed] [Google Scholar]
- 18.Gasparovic C, Rosenberg GA, Wallace JA, Estrada EY, Roberts K, Pastuszyn A, Ahmed W, Graham GD. Magnetic resonance lipid signals in rat brain after experimental stroke correlate with neutral lipid accumulation. Neurosci Lett. 2001;301:87–90. doi: 10.1016/s0304-3940(01)01616-0. [DOI] [PubMed] [Google Scholar]
- 19.Hansen JB, Petersen RK, Larsen BM, Bartkova J, Alsner J, Kristiansen K. Activation of peroxisome proliferator-activated receptor gamma bypasses the function of the retinoblastoma protein in adipocyte differentiation. J Biol Chem. 1999;274:2386–2393. doi: 10.1074/jbc.274.4.2386. [DOI] [PubMed] [Google Scholar]
- 20.Sitter B, Sonnewald U, Spraul M, Fjösne HE, Gribbestad IS. High-resolution magic angle spinning MRS of breast cancer tissue. NMR Biomed. 2002;15:327–337. doi: 10.1002/nbm.775. [DOI] [PubMed] [Google Scholar]
- 21.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]