Abstract
Objective
To compare the course of HLHS patients diagnosed prenatally with any degree of atrial restriction with those without evidence of atrial restriction.
Design
Retrospective, cohort.
Methods
Prenatally diagnosed HLHS patients from 8/1999–1/2009 were categorized as non-restrictive versus restrictive, defined by left atrial hypertension on pulmonary venous Doppler and/or an interatrial septum.
Results
Of 73 total fetal patients identified, 49 were liveborn. Survival at 2 years was 29/35 (83% CI: 59.5%–88.9%) for the non-restrictive group and 6/14 (43% CI:17.7%–66.0%) for the restrictive group (p<0.0001). Of those who underwent stage 1 palliation (35 with nonrestrictive and 10 with restrictive atrial septa) both groups had a similar incidence of preoperative acidosis and need for ventilation and inotropic support. Postoperatively, there was no difference between groups in ventilator days, length of stay, or survival to discharge. There was decreased survival at 2 years in the restrictive group, 60% (CI: 26.2%–87.8%) versus 83% (CI: 66.4%–93.4%) in the non-restrictive group. Furthermore, a disproportionate number of interstage deaths was evident in the restrictive group.
Conclusion
Prenatal presence of any degree of atrial septal restriction in the setting of HLHS confers a significant survival disadvantage, with increases in both early and late mortality.
Introduction
HLHS is the most frequent congenital heart malformation diagnosis made prenatally1. Improvements in the outcome after surgical intervention have changed the paradigm in many centers from termination/comfort care or cardiac transplantation to aggressive surgical management with most patients surviving the first decade of life2,3. Though most fetuses and neonates with HLHS have a widely patent interatrial communication, in approximately 10–20% there is some degree of restriction to left atrial egress4. Though severe atrial restriction remains almost uniformly lethal in the neonatal period, there have been reports of success with early balloon septostomy, atrial stent placement, or surgical septectomy in stabilizing the neonate with atrial septal restricton5,6. Furthermore, not all neonates who appear to have restriction will require urgent postnatal intervention and may be medically managed in the neonatal period, including those with intact atrial septum with unobstructed decompressing venous pathways4.
Prenatal detection of atrial septal restriction and left atrial hypertension in the setting of HLHS is possible using a combination of 2D and Doppler echocardiographic techniques, though accurate prenatal prediction of need for urgent intervention at the time of birth may be difficult in the presence of a small but patent interatrial communication or decompressing levoatriocardinal vein. An abnormal pattern of pulmonary venous flow as recorded by the velocity time integral (VTI) Doppler signal7–11, namely a decrease in the ratio of forward to reverse flow in the pulmonary veins, has been described as a marker of moderate to severe elevation in left atrial pressure in the fetus. Previous reports have suggested that in patients with only moderate atrial restriction at birth (not requiring immediate decompression) survival after stage 1 (“Norwood”) palliation (S1P) is not adversely affected11, though less than severe degrees of restriction in fetal life or presence of decompressing venous communications with the systemic venous circulation may also cause adverse pulmonary vascular changes4,5 which may allow initial survival with shunt physiology but may impact survival with passive blood flow to the lungs (a superior cavopulmonary connection, bidirectional Glenn (BDG) or Stage 2 palliation). Vlahos et al.12have reported long-term outcomes in a cohort of HLHS patient that underwent immediate atrial decompression, however reports of patients with any degree of prenatal diagnosis of atrial level restriction are lacking. Therefore, we sought to determine the 2-year survival (beyond establishment of superior cavopulmonary anastamoses) of patients diagnosed in utero with atrial restriction of any degree
Our hypothesis was that chronic in utero left atrial hypertension from any degree of atrial restriction produces permanent adverse effects on pulmonary vasculature such that these patients would have increased postoperative morbidity and poorer tolerance of superior cavopulmonary (BDG) connections due to the pulmonary vascular changes. Our objective was to compare the early postoperative and 2-year postnatal course of HLHS patients diagnosed prenatally with atrial restriction with those without evidence of left atrial hypertension or restriction in fetal life, thus forming a better understanding of outcomes outside of the perinatal period in these patients with unique prenatal cardiovascular physiology.
Materials and Methods
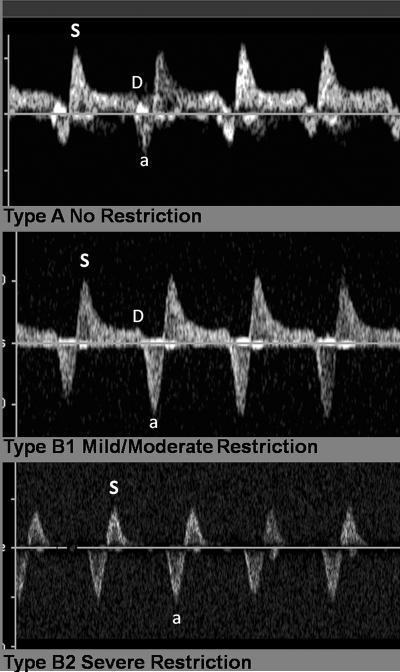
Review of medical records was approved by the Committee for Human Research (CHR) at the University of California, San Francisco (UCSF). We searched the UCSF Benioff Children's Hospital Fetal Cardiovascular Program echocardiography database for all fetal echocardiograms performed and interpreted as HLHS between 8/1999–1/2009, and reviewed these for signs of atrial level restriction or intactness. All echocardiograms were reviewed independently by two echocardiographers unaware of postnatal outcomes (AMG, AL) for confirmation of anatomy and degree of atrial level obstruction. HLHS was defined as hypoplasia of the left ventricle with aortic atresia or stenosis and mitral atresia or stenosis and included patients with severe aortic stenosis and patent mitral valve if left ventricular function was poor and there was reversal of flow in the transverse aortic arch and at the atrial septum in mid-gestation. Fetuses diagnosed prenatally with borderline left ventricle that underwent a two-ventricle repair after birth were excluded. Fetuses with other forms of a complex single ventricle or heterotaxy syndrome whose postnatal strategy may have included a Norwood or Damus–Kaye–Stansel palliation operation were excluded. Patients were divided into two groups: those with no evidence of atrial septal restriction (group A) and those evidence of left atrial hypertension on pulmonary venous Doppler (group B) as described previously10,11,13 and/or with an interatrial communication not detected on 2D or echocardiographic color Doppler flow with or without a decompressing levoatriocardinal vein. Group B patients were further characterized as having mild-moderate restriction versus severe restriction on the basis of prenatal echocardiographic appearance of the pulmonary venous Doppler signal as follows(Figure 1): qualitatively, restriction considered moderate if systolic peak (S wave) was followed by persistent but low velocity forward diastolic flow (D wave) and exaggerated flow reversal during atrial systole. Qualitatively restriction was considered severe if pulmonary venous Doppler patterns consisted of to-and-fro flow pattern with absent diastolic forward flow or if there was no detectable interatrial communication on color Doppler interrogation. Quantitative measurement of pulmonary venous Doppler forward and reverse velocity-time integral measurements were also performed and expressed as forward:reverse ratio (F/R ratio) and analyzed as a continuous variable.
Figure 1.
Pulmonary venous Doppler patterns in different degrees of obstruction.
General characteristics of the entire cohort (excluding termination of pregnancy) included gestational age at diagnosis, gestational age at birth, birth weight, lowest pre-surgical pH, highest pre-surgical lactate, need for intubation prior to surgery, need for inotropes prior to surgery and survival at two years of age. Postnatal echocardiogram data collected prior to surgery included: any degree of depression of right ventricular function, degree of tricuspid regurgitation (none/trace, mild, moderate, severe) and mean interatrial Doppler-derived gradient in mmHg. Additional clinical variables were collected for the subgroup of patients that underwent S1P, including time from admission to surgery, cardiopulmonary bypass time, cross clamp time, deep hypothermic circulatory arrest time, duration of post operative intubation, total length of stay, survival to discharge, interstage death, cardiac catheterization-derived pulmonary vascular resistance prior to superior cavopulmonary anastamoses, and survival at two years of age.
Statistical Analysis: Because of non-normal distribution of data, Wilcoxon rank-sum test was used for comparisons in the case of continuous variables and chi-squared or Fisher's Exact testing for nominal variables. A p value <0.05 was considered statistically significant. Receiver operator curves were generated for the fetal echocardiographic variables of forward/reverse ratio (F/R ratio). Differences in survival at two years between groups was assessed by log-rank test and Kaplan-Meier analysis.
Results
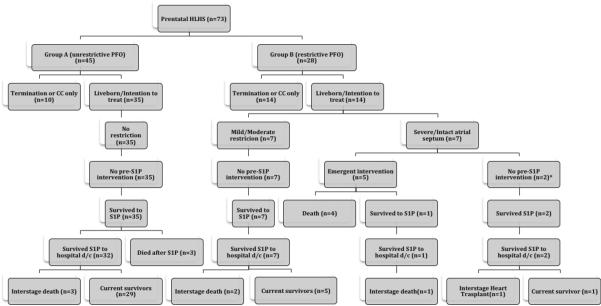
Between 8/1999–1/2009, 73 fetuses were diagnosed with HLHS at our institution, 45 without atrial level restriction (group A) and 28 with evidence of any level of atrial restriction (group B). Prenatal recognition of restriction did not increase during the study period: 12/34(35%) in the first half (1999–2004) and 16/39 (41%) in the second (2005–2009).Figure 2 depicts the subsequent clinical course of these patients. There were 10 pregnancy terminations in group A and 14 in group B (22% vs. 50%, p=0.021) for a total of 49 live births; 35 from group A and 14 from group B. None of the group B patients with mild-moderate restriction had atrial decompression prior to S1P, and all survived S1P, but there were 2 deaths prior to 2 years; one of the 5 children surviving to 2 years died at age 2 years 9 months of respiratory insufficiency, the remainder were still alive at last assessment. Seven of the 14 live born group B patients had severe restriction on prenatal echocardiogram, and 5 of these 7 patients underwent emergent atrial decompression (2 percutaneous atrial stent placement, 2 surgical septostomy and 1 hybrid procedure with direct atrial puncture) with 4 periprocedural deaths. The other 2 infants had intact interatrial septum and a decompressing levo-atrial cardinal vein and did not need an emergent atrial decompression procedure. Of the 3 survivors with severe restriction one had a hybrid procedure and died suddenly at home, another underwent orthotopic cardiac transplantation at 18 months of life and the last is alive at age 5 years.
Figure 2.
Flow diagram depicting outcomes through 2 years of followup for the entire cohort. All patients noted as alive were alive with superior cavopulmonary circulation unless noted otherwise in the figure.
S1P: Stage 1 palliation; CC: Comfort care (postnatal); PFO: Patent foramen ovale
*both with levo-atrial cardinal vein
Gestational age at birth was statistically significantly different between the two groups although both medians were at term and therefore likely not a clinically significant difference. There was no significant difference between the non-restrictive versus restrictive groups in gestational age at diagnosis, birth weight, pre-procedural pH, pre-procedural lactate, need for intubation, or right ventricular function (Table 1).
Table 1.
Characteristics of entire liveborn HLHS cohort (excludes pregnancy terminations): n=49
| Characteristic | No Restriction (n=35) | Restriction (n=14) | P* | ||
|---|---|---|---|---|---|
| Median | (Range) | Median | (Range) | Median | |
| Gestational age at Diagnosis (weeks) | 28 | (18, 39) | 29 | (21, 38) | 0.52 |
| Gestational age at birth (weeks) | 38 | (34, 40) | 37 | (36, 39) | <0.05 |
| Birth weight (grams) | 2995 | (1750, 3785) | 3000 | (2500, 5000) | 0.52 |
| Lowest pre-operative pH | 7.25 | (7.08, 7.49) | 7.23 | (7.01, 7.35) | 0.83 |
| Highest pre-operative lactate (mmol/L) | 2.9 | (0, 11.0) | 4.1 | (2.1, 11.0) | 0.09 |
| Pre-operative need for intubation, N(%) | 25 (74%) | 9 (75%) | 1.00† | ||
| Pre operative need for inotropes, N(%) | 6 (17%) | 9 (69%) | <0.05 | ||
| Depression of ventricular function, N(%) | 4 (12%) | 3 (27%) | 0.35 | ||
| Tricuspid regurgitation > mild on first post-natal echocardiogram N(%) | 3 (9.7%) | 5 (45%) | 0.02 | ||
| Atrial gradient, mmHg (range) | 0 (0, 8) | 6 (0, 10) | <0.05 | ||
| Alive at two years, N (%) | 29 (83%) | 6 (43%) | <0.0001 | ||
continuous data are expressed as medians with ranges. Statistical analysis consisting of Wilcoxon rank-sum test for continuous variables and chi-squared for nominal variables.
Fisher-Exact test
HLHS, Hypoplastic Left Heart Syndrome
The restrictive group were less hemodynamically stable in the immediate postnatal period as evidenced by significantly higher need for pre-procedural inotropic support, a higher incidence of mild tricuspid regurgitation on first postnatal echocardiogram (and higher left atrial pressure estimates than the non-restrictive group.
Forty five patients underwent S1P including all 35 live born group A patients without atrial septal restriction and 10 of the 14 live born group B patients. There was no difference between groups in time from admission to surgery, total cardiopulmonary bypass time, cross clamp time, deep hypothermic circulatory arrest time, duration of post operative ventilation, total length of stay, or survival to discharge (Table 2).
Table 2.
Characteristics of HLHS Patients Who Underwent Stage 1 Palliation (n=45)
| Characteristic | No Restriction (n=35) | Restriction(n=10) | P* | ||
|---|---|---|---|---|---|
| Median | (Range) | Median | (Range) | ||
| Time from admission to surgery (days) | 5 | (2, 14) | 5 | (3, 19) | 0.97 |
| Cardiopulmonary bypass time (min) | 119 | (60, 185) | 128 | (90, 174) | 0.83 |
| Cross clamp time (min) | 48.5 | (22, 69) | 46 | (42, 76) | 0.87 |
| Deep Hypothermic Circulatory Arrest (min) | 6 | (0.25, 21) | 19 | (9, 45) | 0.12 |
| Duration of post-operative intubation (days) | 7 | (0, 156) | 8 | (4,21) | 1.00 |
| Total length of stay (days) | 36 | (7, 223) | 58 | (27, 98) | 0.15 |
| Survival to discharge, N (%) | 31 (91%) | 10 (100%) | 1.00 | ||
| Interstage Death, N (%) | 3 (9%) | 4 (40%) | <0.05 | ||
| Alive at two years, N (%) | 29 (83%) | 6 (60%) | 0.19 | ||
Continuous data are expressed as medians with ranges. Statistical analysis consisting of Wilcoxon rank-sum test for continuous variables and chi-squared for nominal variables.
HLHS, Hypoplastic Left Heart Syndrome
There was no difference in cardiac catheterization-derived pulmonary vascular resistance between the two groups (Group A-2.4 Indexed Woods Units (0.7–6.6), Group B-2.9 Indexed Woods Units (1.4–4), p=0.67). However, within this surgical cohort there was proportionately, albeit not statistically significant, lower survival at 2 years associated with prenatal identification of atrial septal restriction Group B (83%(66.4–93.4) than in group A, 60% (26.2–87.8),) with a disproportionate number of interstage deaths in the restrictive patients (9.3% (2–25) in group A vs. 40% (12.2–73.8) in group B, p=0.04).
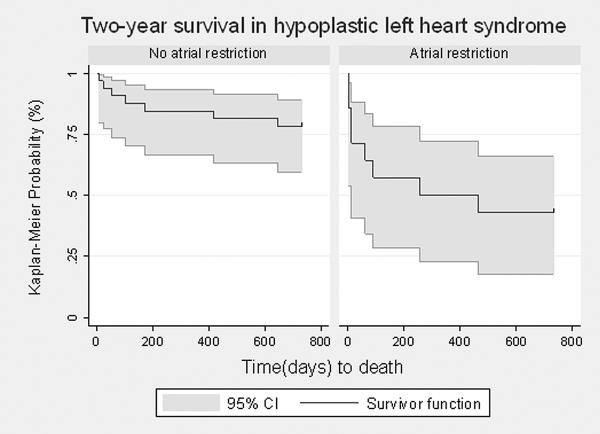
A comparison of the two-year survival between the two groups on an intent-to-treat basis (including those who died prior to S1P)using Kaplan-Meier techniques resulted in a statistically significant difference, as determined by the log-rank test (p<0.0001). Those in the non-restriction group had a two-year survival of 83% (95% CI 59.5% – 88.9%), while those with any degree of atrial restriction were much less likely to be alive at two years (42.9% (95% CI 17.7% – 66.0%)) (Figure 3).
Figure.3.
Two-year survival among all liveborn infants diagnosed prenatally with hypoplastic left heart syndrome, with and without atrial level restriction. The gray shaded areas represent the 95% confidence intervals. There was a statistically significant difference between the two groups (p<0.001) by log-rank testing.
Receiver operating characteristic (ROC) curves were developed for fetal pulmonary venous Doppler forward/reverse (F/R) Velocity Time Integral (VTI) ratio versus atrial septal procedure prior to S1P (AUC = 0.98) and 2 year survival (AUC =0.80) A ratio cutoff of <5.1 in fetal life produced 100% sensitivity and 91% specificity for prediction of need for atrial septal procedure prior to S1P but did not reliably predict two year survival.
Discussion
We have demonstrated that prenatal echocardiographic diagnosis of atrial septal restriction is associated with a significant survival disadvantage, with increased early and late mortality in these patients in comparison with prenatally diagnosed patients without evidence of left atrial hypertension. In terms of overall mortality, early mortality (prior to stage 1 palliation (S1P)) accounted for most of the observed difference; however for patients who were live born and survived to undergo S1P there was still proportionately lower survival at 2 years in those diagnosed with atrial restriction prenatally (60% vs. 83%), and though this difference did not achieve statistical significance this may have been due to the relatively small numbers of patients in the cohort. Additionally, there were a disproportionate number of interstage deaths in the patients identified in fetal life with any degree of restriction. The majority of the early and late deaths in our cohort were in the severely restrictive by prenatal echocardiographic criteria; fetuses with mild-moderate atrial septal restriction in utero had similar postoperative courses and 2 year survival to the group with no restriction, though there was proportionately more deaths prior to completion of superior cavopulmonary anastamoses in the restrictive group as a whole including 2/7 of the mild/moderately restrictive patients. On a descriptive basis, of those who lived to undergo S1P, 5/7(71%) mild/moderate restriction patients, and 1/3(33%) severe restriction patients survived to 2 years, versus 29/35(83%) survival for non-restrictive patients; our study did not have sufficient power to analyze the apparent difference in proportions from a statistical standpoint. However if larger studies can demonstrate a difference between mild/moderate restriction and no restriction in these patients it would suggest that there are factors other than relief of the atrial obstruction alone, that continue to confer disadvantage to these patients after they have undergone S1P.
Our early results with exclusively prenatally diagnosed patients with severe restriction are similar to those presented by Glatz et al.5 in 2007. This retrospective study of both prenatal and postnatal presentation showed that prenatal diagnosis versus postnatal diagnosis, did not improve initial hospital survival or cumulative survival for any degree of atrial restriction. In this single-center series, all seven patients diagnosed prenatally with severe restriction underwent immediate (surgical or catheter based) intervention with an overall survival to hospital discharge of 28%. Although the study did not evaluate longer term outcome, the estimated two year cumulative survival of all patients with severe restriction postnatally indicated only ~20% survival at two years. Interestingly, and similar to our experience, they also reported an unusually high late mortality in those with moderate restriction, with a three-year cumulative survival of 60%. However, they included preterm infants and infants with known or suspected genetic syndromes, factors that have previously been shown to independently affect outcome in HLHS14, thus this may influence their analysis of morbidity and mortality. In contrast, in a study by Vlahos et al., the postnatal courses of 33 babies born with HLHS and a restrictive or intact septum that needed immediate postnatal intervention were described12. They reported that those who survived the neonatal period had late survival, pulmonary artery pressure, and pulmonary vascular resistance similar to those of control subjects. However only 16 (48%) of the patients in that study had prenatal diagnosis of HLHS, and those who did not require postnatal urgent atrial decompression were not included, making their results difficult to compare directly with ours.
Additional somewhat better short-term survival for neonates who require left atrial decompression prior to S1P was described in 2007 by Vida et al.6 in a group of patients with HLHS and severely restrictive or intact atrial septum undergoing prenatal or postnatal atrial decompression followed by S1P. They reported an overall survival of 69% to hospital discharge. In this cohort, prenatal diagnosis alone was not associated with improved survival versus historical controls, but infants who had undergone prenatal intervention (some of whom also required postnatal atrial decompression) had improved hospital survival of 79% compared with 61% of infants treated only postnatally. Interstage mortality was reported at 23% and Kaplan-Meier survival estimate at 6 months of age was 49%. Importantly, fetuses and neonates with moderate restriction who did not undergo atrial decompression were not included in this report. This again limits comparison of this prior study with our cohort with diagnosis of restriction based on fetal echocardiographic characteristics but decision to intervene based on criteria related to severity of clinical presentation at birth.
Therefore, our patients' early outcomes in the face of severe restriction are in keeping with what has previously been published; further supporting that prenatal echocardiographic diagnosis of atrial septal restriction in the setting of HLHS is associated with a significant survival disadvantage5,6,15. Indeed, there was no observed difference in the perioperative morbidity and mortality between their cohorts and ours5,6,15. Our data additionally identify a new group of at-risk patients, however-- those diagnosed prenatally with some degree of restriction but who do not require atrial decompression on an urgent basis postnatally. ROC analysis for forward:reverse VTI ratio suggested a cutoff value of 5.1:1 was associated with the best positive predictive value for need for immediate intervention, similar to the previously published work by Michfelder et al10. Unfortunately pulmonary venous Doppler forward to reverse VTI ratio was less useful in predicting 2 year survival in our cohort.
It is well known that severe restriction at the atrial level in fetuses with HLHS causes increased arterial medial thickness with muscular extension to the smaller respiratory arterioles4,16 leading to lymphangiectasia, poor pulmonary mechanics and poor outcomes in the immediate postnatal period. We speculate that there may be differences in pulmonary vasculature at birth, which may in some cases be clinically important beyond the immediate perinatal period, and suggest further study. Whether prenatal intervention could potentially reduce the pathological pulmonary process and thus improve both neonatal and longer-term outcomes in patients with restrictive atrial septum diagnosed in-utero remains unknown. Recently, fetal intervention for HLHS with an intact septum or a highly restrictive septum 17has been preliminarily investigated by the group at Boston Children's Hospital with a hope that prenatal decompression of the left atrium will mitigate the development of adverse pulmonary venous remodeling4. In 2008, Marshall et al. reported results of a highly select group of patients with prenatal detection of intact or severely restrictive atrial septum and HLHS undergoing intervention at a median gestational age of 29 weeks (range, 23–34 weeks)17. Though a high rate of technical success was achieved, there were 2 fetal deaths associated with the procedure and a substantial number of patients needed additional atrial decompression at birth prior to S1P. Further, there was no overall improvement in survival associated with a prenatal procedure. Ongoing research in this area is suggested given the uniformly poor outcomes with postnatal intervention.
Our study has a number of limitations. We chose to analyze the two groups' S1P surgical variables and outcomes in an as-treated manner, excluding those that were intended to, but did not actually undergo S1P. An intent-to-treat analysis would have however only exaggerated the difference in “surgical” survival, while not contributing to the operative or postoperative variables analyzed (i.e. bypass times, ventilator days, length of stay). Low survival to the BDG precluded meaningful analysis of preoperative and postoperative characteristics in this cohort, which might have shed some light on the etiology of poorer survival. The retrospective design led to limited availability of echocardiographic information in some patients. There was however no clear difference in ascertainment of restriction on fetal echocardiograms related to era. In addition this single-center experience with a relatively rare disease was underpowered to detect differences in survival within sub-classifications of restriction, limiting this analysis to a descriptive one; furthermore, the 10-year span may have confounded morbidity and mortality data given improvements in operative and postoperative care during this time period. Larger multicenter studies will be necessary to determine whether the decreased survival in patients with left atrial hypertension in utero who are hemodynamically stable after birth (without urgent atrial decompression) truly have decreased long-term survival as suggested by our results. If the observation holds true, additional investigation may be warranted to determine whether prenatal intervention to decompress the left atrium with the intention of mitigating or reversing pulmonary vascular changes during fetal life will improve long-term prognosis.
What is already known on this topic
Prenatally diagnosed severe atrial level restriction in hypoplastic left heart syndrome confers a dismal outcome, with very few patients surviving the neonatal period.
Little is known about longer term outcomes in this population regardless of degree of atrial restriction.
What this study adds
The two main points that this study adds to the literature are:
Prenatal echocardiographic diagnosis of any degree of atrial septal restriction is associated with a significant survival disadvantage.
This survival disadvantage persists even after the neonatal period and out to two years of life.
Acknowledgement
This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH
Although none of the authors received funding directly, statistical support for this publication was supported by. NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
None of the authors have a conflict of interest.
References
- 1.Friedberg M, Silverman N, Moon-Grady A, et al. Prenatal detection of congenital heart disease. J Pediatr. 2009;155:26–31. 31.e1. doi: 10.1016/j.jpeds.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 2.Stasik C, Gelehrter S, Goldberg C, et al. Current outcomes and risk factors for the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:412–7. doi: 10.1016/j.jtcvs.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 3.Tweddell J, Hoffman G, Mussatto K, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–9. [PubMed] [Google Scholar]
- 4.Rychik J, Rome JJ, Collins MH, et al. The hypoplastic left heart syndrome with intact atrial septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999;34:554–60. doi: 10.1016/s0735-1097(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 5.Glatz J, Tabbutt S, Gaynor J, et al. Hypoplastic left heart syndrome with atrial level restriction in the era of prenatal diagnosis. Ann Thorac Surg. 2007;84:1633–8. doi: 10.1016/j.athoracsur.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Vida V, Bacha E, Larrazabal A, et al. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: surgical experience from a single center. Ann Thorac Surg. 2007;84:581–5. doi: 10.1016/j.athoracsur.2007.04.017. discussion 586. [DOI] [PubMed] [Google Scholar]
- 7.Better D, Apfel H, Zidere V, et al. Pattern of pulmonary venous blood flow in the hypoplastic left heart syndrome in the fetus. Heart. 1999;81:646–9. doi: 10.1136/hrt.81.6.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chintala K, Tian Z, Du W, et al. Fetal pulmonary venous Doppler patterns in hypoplastic left heart syndrome: relationship to atrial septal restriction. Heart. 2008;94:1446–9. doi: 10.1136/hrt.2007.123497. [DOI] [PubMed] [Google Scholar]
- 9.Crowe D, Allan L. Patterns of pulmonary venous flow in the fetus with disease of the left heart. Cardiol Young. 2001;11:369–74. doi: 10.1017/s1047951101000464. [DOI] [PubMed] [Google Scholar]
- 10.Michelfelder E, Gomez C, Border W, et al. Predictive value of fetal pulmonary venous flow patterns in identifying the need for atrial septoplasty in the newborn with hypoplastic left ventricle. Circulation. 2005;112:2974–9. doi: 10.1161/CIRCULATIONAHA.105.534180. [DOI] [PubMed] [Google Scholar]
- 11.Taketazu M, Barrea C, Smallhorn J, et al. Intrauterine pulmonary venous flow and restrictive foramen ovale in fetal hypoplastic left heart syndrome. J Am Coll Cardiol. 2004;43:1902–7. doi: 10.1016/j.jacc.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Vlahos A, Lock J, McElhinney D, et al. Hypoplastic left heart syndrome with intact or highly restrictive atrial septum: outcome after neonatal transcatheter atrial septostomy. Circulation. 2004;109:2326–30. doi: 10.1161/01.CIR.0000128690.35860.C5. [DOI] [PubMed] [Google Scholar]
- 13.Divanovic A, Hor K, Cnota J, et al. Prediction and perinatal management of severely restrictive atrial septum in fetuses with critical left heart obstruction: clinical experience using pulmonary venous Doppler analysis. J Thorac Cardiovasc Surg. 2011;141:988–94. doi: 10.1016/j.jtcvs.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Tabbutt S, Dominguez TE, Ravishankar C, et al. Outcomes after the stage I reconstruction comparing the right ventricular to pulmonary artery conduit with the modified Blalock Taussig shunt. Ann Thorac Surg. 2005;80:1582–90. doi: 10.1016/j.athoracsur.2005.04.046. discussion 1590-1. [DOI] [PubMed] [Google Scholar]
- 15.Rychik J, Szwast A, Natarajan S, et al. Perinatal and early surgical outcome for the fetus with hypoplastic left heart syndrome: a 5-year single institutional experience. Ultrasound Obstet Gynecol. 2010;36:465–70. doi: 10.1002/uog.7674. [DOI] [PubMed] [Google Scholar]
- 16.Graziano JN, Heidelberger KP, Ensing GJ, et al. The influence of a restrictive atrial septal defect on pulmonary vascular morphology in patients with hypoplastic left heart syndrome. Pediatr Cardiol. 2002;23:146–51. doi: 10.1007/s00246-001-0038-7. [DOI] [PubMed] [Google Scholar]
- 17.Marshall A, Levine J, Morash D, et al. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008;28:1023–8. doi: 10.1002/pd.2114. [DOI] [PubMed] [Google Scholar]