Abstract
Although partially controlled with antiemetic drugs, postoperative nausea and vomiting (PONV) continues to be a problem for many patients. Clinical research suggests that opioid analgesics and volatile anesthetics are the main triggers of PONV. The aim of this study was to develop an animal model for post-anesthesia vomiting for future studies to further determine mechanisms and preclinical drug efficacy. Ferrets (N=34) were initially used because they have served as a gold standard for emesis research. Ferrets were tested with several doses of morphine, inhaled isoflurane, and a positive control injection of cisplatin (a chemotherapy agent) to induce emesis. Musk shrews (a small animal model; N=36) were also tested for emesis with isoflurane exposure. A control injection of cisplatin produced emesis in ferrets (ip, 129.8±22.0 retches; 13.7±2.3 vomits; mean ± SEM). Morphine produced a dose-response on emesis in ferrets, with maximal responses at 0.9 mg/kg (sc, 29.6±12.6 retches; 1.8±0.9, vomits). Isoflurane exposure (2–4% for 10 min to 6 h exposure) failed to induce vomiting, was not associated with an increased frequency in emesis when combined with a low dose of morphine (0.1 mg/kg, sc), and failed to produced consistent effects on food and water intake. In contrast to ferrets, musk shrews were very sensitive to isoflurane-induced emesis (0.5 to 3%, 10 min exposure; up to 11.8±2.4 emetic episodes). Overall, these results indicate that ferrets will not be useful for delineating mechanisms responsible for isoflurane-induced emesis; however, musk shrews may prove to be a model for vomiting after inhalation of volatile agents.
Keywords: Vomiting, Nausea, Models, Animal, Behavior
1. Introduction
About 30% of patients experience postoperative nausea and vomiting (PONV) following general anesthesia [1]. However, we currently lack an animal model that can be used (i) to evaluate antiemetic strategies; and (ii) to understand the pathophysiology of PONV. Clinical research suggests that opioid analgesics and volatile anesthetics are primary triggers of PONV [2, 3]. Volatile anesthetics are still used in patient care despite hyperalgesic [4] and emetogenic [2, 3] properties. It is well-known that morphine induces vomiting in ferrets, dogs, and cats [5–11], however, there has been limited work to model volatile agent-induced emesis in animals; developing a successful model would facilitate mechanistic research.
The current study focused on establishing an emetogenic effect of isoflurane exposure, in conjunction with the effects of morphine. Ferrets were the initial focus of these studies because they are a gold standard in emesis research, particularly in the development of antiemetics for the control of chemotherapy-induced emesis [12]. We exposed ferrets to isoflurane concentrations up to 4% for 30 min to 6 h to mimic the level of exposure occurring during short and long duration surgical procedures. Morphine was injected at 0.1 to 0.9 mg/kg because this dose range has substantial overlap with doses established to produce emesis in the ferret [7]. The hypothesis was that ferrets could serve as a model for anesthesia-induced emesis. Because we found no evidence that isoflurane exposure produced emesis in ferrets (data below), we then tested musk shrews, a small animal also used in emesis research [13–16].
2. Materials and methods
All experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care international-accredited animal care facility. Studies were designed to use 6 to 8 animals in each condition [4 to 8 animals per group is common in studies of emesis using ferrets and musk shrews, e.g., 7, 8, 15–17].
2.1. Animals
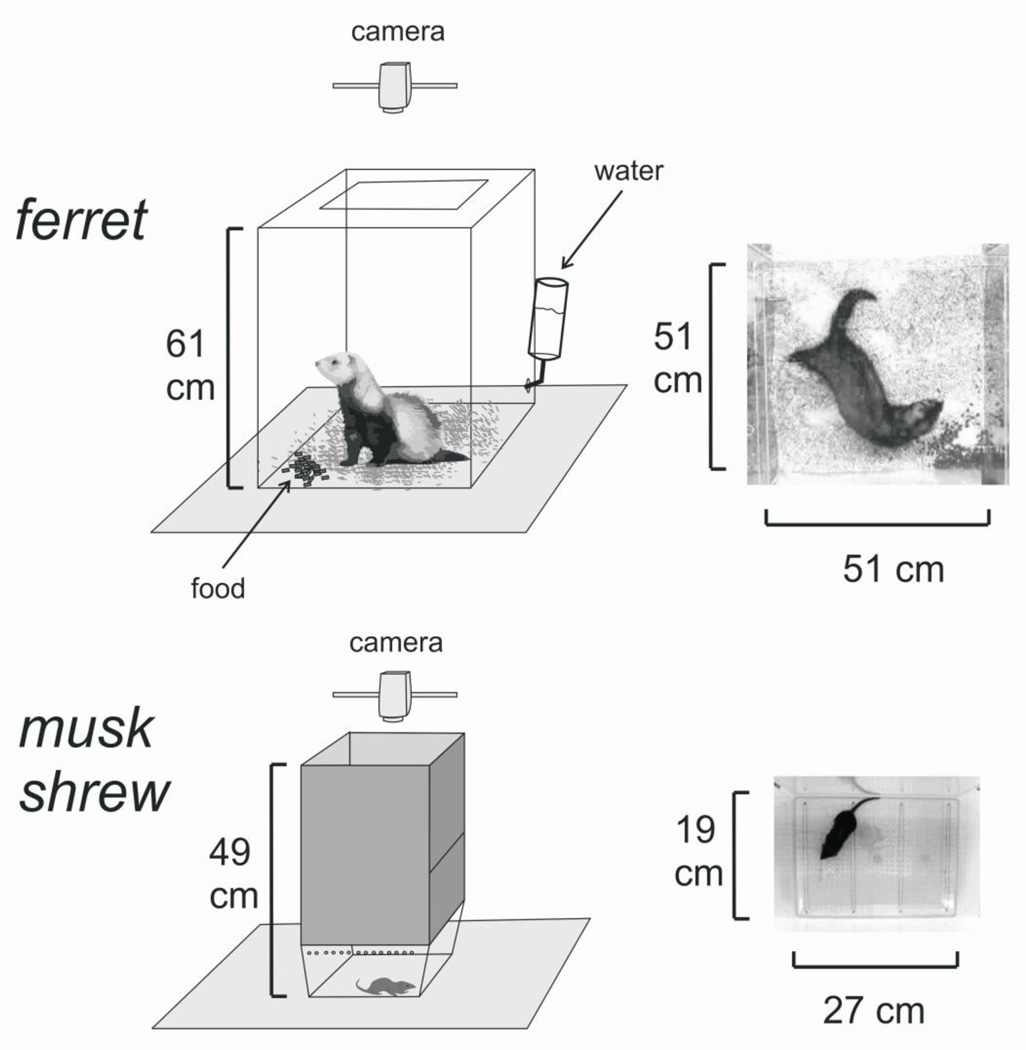
Subjects included 34 male Fitch ferrets (Mustela putorius furo; 16–19 weeks, 1–1.5 kg; Marshall BioResources, North Rose, NY, USA) and 30 female and 6 male musk shrews (Suncus murinus; 32–53 g, female, 69–84 g, male); musk shrews were derived from breeding stock originating from Taiwan (Chinese University of Hong Kong). Animals were housed individually in home cages (ferrets, 61 × 64 × 43 cm; musk shrews, 28 × 17 × 12 cm) with a 12:12 h light/dark cycle (0700–1900 light period), and water and food were available ad libitum. Ferrets were fed Mazuri Ferret Diet (PMI Nutrition, St. Louis, MO, USA) and musk shrews were provided a mixture of 75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets (Milk Specialty, New Holstein, WI, USA). Two weeks prior to emetic testing in ferrets, animals were allowed two acclimation periods of 2 hours each in an observation chamber (Fig. 1). Animals were not fasted prior to emetic testing. At the end of each study, ferrets were euthanized with 1 ml Beuthanasia-D solution (intracardiac injection) under isoflurane anesthesia (3%). Musk shrews were euthanized via CO2 exposure.
Figure 1.
Experimental observation chambers and camera position for ferret and musk shrew emetic testing. The musk shrew is a small animal model for emesis research [14–16, 18, 19, 29– 31].
2.2. Administration of emetogenic agents
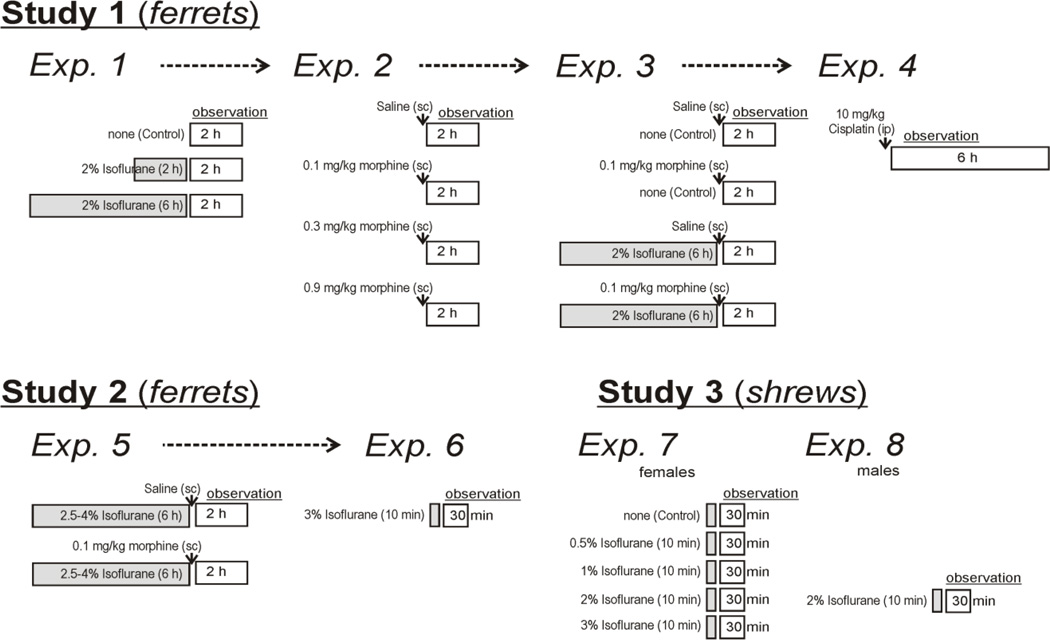
Isoflurane was provided via a mask (only in ferrets) or into enclosed chambers (allowed to fill for 2 min before use). Face masks completely covered the snout and contained a rubber seal around the head. Ferrets exposed to isoflurane from a face mask were placed on a warm water heating pad (Gaymar T/Pump) and body temperature was measured with an anal probe (TC-1000 Temperature monitor, Cwe Inc.; probe, YSI). Heart rate and blood pressure were recorded using pulse oximeter attached to the forepaw. Flow rates of gas (from 100% O2 compressed air canister flowing through an isoflurane vaporizer; Molecular Imaging Products Co., AS-01-0007, ferret studies, or Matrx, TEC-3, shrew studies) were 3 L/min and 6 L/min for ferret and shrew experiments, respectively. Percentage of isoflurane was set by the dial on the vaporizer. In Experiment 7 (see below), the 0% isoflurane condition consisted of running only 100% O2 into the induction chamber. The enclosed induction chambers (clear acrylic) in Experiment 6 (see below; ferrets) measured 29.2 (height) × 26.7 (width) × 45.7 (length) cm and Experiment 7 (shrews), 10 × 8.5 cm, height and diameter. Subcutaneous injections of saline (0.9% NaCl; 0.5 ml/kg) or morphine sulfate solution (Baxter Healthcare; prepared in 0.9% NaCl; 0.5 ml/kg) were given immediately before observation testing (Fig. 2).
Figure 2.
The schematic diagram shows the protocol for the three studies. Shaded boxes show isoflurane exposure and open boxes show observation times. Dashed arrow lines indicate that some of the same animals were used in subsequent experiments with at least one week of recovery time between experiments.
2.3. Measurement of Emesis, Locomotion, Food intake, Water intake, and Body weight
Emesis was characterized by rhythmic abdominal contractions that were either associated with the oral expulsion of solid or liquid material (i.e., vomits), or not associated with the passage of material (i.e., retches). In ferrets, vomits and retches are mutually exclusive events and were counted by observation with an observer sitting outside the clear acrylic test chambers in all experiments (up to 2 h tests) except in Experiment 4 (a 6 h test) when videos were collected and replayed for scoring. In musk shrews, emesis was visually scored as emetic episodes (a series of retches that ends with a large event, which could be a vomit or larger retch [18, 19]). The frequency of retching in musk shrews is too fast to directly observe, preventing the quantification of individual retches and vomits [18, 19].
Video cameras were positioned above each cage to record movement and emesis (except in Experiment 4 with two cameras, one above and one to the side, used to record 6 h after cisplatin treatment). Up to four ferrets were tested simultaneously, and two researchers (blinded to the treatment) observed and recorded emesis. Only one shrew was tested at each time point. For measuring locomotion (cm of movement), videos were imported into software to track the center point of the body (Ethovision 7.1; Noldus) [15].
For Studies 1 and 2 (ferrets), food and water was measured from the home cage one day before the start of experiments (baseline day) and two days after the emesis observation test. The water bottle and food hopper (corrected for spillage) were measured to the nearest 2 grams. During the observation test, ferrets were also given a water bottle (pre-weighed) and food (50 or 100 g) in the corner of the chamber (Fig.1); water and food intake were measured at the end of the experiment. Body weights were measured daily and before each test.
2.4. Study 1: 2% Isoflurane (2 or 6 h) and Morphine, Ferrets
In Study 1, twenty-four ferrets were used in a series of four experiments with at least 1 week of recovery between treatments (Fig.2). Isoflurane-treated animals were placed in an induction chamber (4% isoflurane) for 10 to 15 min, and were then transferred to a face mask (2% isoflurane) for 2 or 6 h. Following emetic stimuli, animals were observed for 2h (1500 to 1700 h). One ferret developed helicobacter gastric ulceration and was euthanized before Experiment 2.
The first three experiments included the following groups (Fig.2): 1) Experiment 1 (n = 8, per group), control no isoflurane, 2% isoflurane for 2 h, and 2% isoflurane for 6 h, 2) Experiment 2 (n = 5 to 6, per group), 0, 0.1, 0.3, and 0.9 mg/kg, morphine, and 3) Experiment 3 (n = 5 to 6, per group), saline, 0.1 mg/kg morphine, 2% isoflurane for 6 h plus saline, and 2% isoflurane for 6 h plus 0.1 mg/kg morphine. Animals were randomized to each condition for Experiment 1 (using a randomization function on a calculator), were blocked by previous treatment condition, and were then randomized for Experiments 2 and 3.
For Experiment 4 (Fig. 2), six animals were randomly selected to receive cisplatin (10 mg/kg, ip) and then video-recorded for 6 h (1000 to 1600 h) in the observation chambers. Cisplatin injection (a positive control) was used as the last treatment in this series because this agent produces nephrotoxicity and peripheral neuropathy several days after treatment.
2.5. Study 2: 2.5 to 4% Isoflurane and Morphine, Ferrets
In Experiment 5, to investigate the effects of a greater depth of anesthesia on emesis, we increased the concentration of isoflurane (animals were not responsive to toe pinch, fingertips, in this new experiment but were frequently responsive to this test in Study 1). A total of ten naïve ferrets were used. The procedures for administration of chemicals and testing (1500 to 1700 h) in Experiment 5 were the same as Experiment 3 (Fig.2). Animals received two treatments (isoflurane plus saline or isoflurane plus morphine, 0.1 mg/kg), separated by at least one week of rest (randomized order). All animals in this experiment also received subcutaneous fluid injections to reduce hypotension and hypoglycemia (25 ml of dextrose 25 mg/ml at 0, 2.5, and 5 h after start of isoflurane exposure). In Experiment 5, we also measured blood pressure (non-invasive tail cuff; DRE Waveline EZ), and respiration rate (by observation) every 15 min. In Experiment 6 (one week after Exp. 5; Fig.2), we used a short duration of 3% isoflurane exposure (10 min, in an induction chamber) and observation testing for 30 min (0900 – 1030 h).
2.6. Study 3: 0.5 to 3% Isoflurane (10 min), Musk shrews
In Experiment 7, female musk shrews were randomly assigned to 5 treatments (n = 6, each group; Fig.2): 0, 0.5, 1, 2, and 3% isoflurane. Animals were placed in a transparent induction chamber for 10 min of isoflurane exposure, and then transferred to a transparent observation chamber (Fig. 1). In Experiment 8 (Fig.2), we also tested 6 male musk shrews using an exposure of 2% isoflurane. Male musk shrews were tested as a control experiment to determine the effects of sex on these responses. All shrews were tested at 0900 – 1030 h.
2.7. Statistical Analysis
We conducted analyses on the number of retches, vomits, emetic episodes, locomotion, food intake, water intake, and body weight using analysis of variance (ANOVA) (Statistica 11.0; StatSoft). Daily food and water intakes were analyzed as a percentage of baseline intake. When an ANOVA was statistically significant, we conducted planned mean comparisons using the least significance difference test (LSD-test). For latency measures (i.e., latency to the first emetic response; using measures from animals showing emesis) we used the Kruskal-Wallis and Mann-Whitney U tests. Physiological parameters, including heart rate, percentage oxygen saturation of the blood, body temperature, respiration rate, and blood pressure, were compared using Student’s t-tests (two-tailed). These parameters remained relatively constant over the course of the isoflurane exposure; therefore, we used the average values from the entire exposure time (2 or 6 h) for these analyses. For all statistical tests, p<0.05 was used to detect statistical significance.
3. Results
Daily body weights for ferrets were not significantly affected (p>0.05, two-way repeated measures ANOVA) by any of the treatment conditions and are not shown.
3.1. Study 1: 2% Isoflurane (2 or 6 h) and Morphine, Ferrets
Experiment 1: 2% Isoflurane for 2 or 6 h
Maintenance of ferrets on a mask using 2% isoflurane failed to block the pedal withdrawal response; some ferrets were restless and would move out of the mask. Heart rate and percentage oxygen saturation of the blood were not significantly different between animals exposed to isoflurane for 2 or 6 h (p > 0.05, t-tests), however, body temperature was slightly higher in the 6 h condition compared to 2 h group [t(14) = 2.4, p< 0.5; Table 2]. Although some animals did not move, others moved frequently (e.g., every 10 min). When an animal moved out of the mask, it was returned to the mask in < 30 s. None of the animals retched or vomited during induction or maintenance of anesthesia. During the 2 h observation after anesthesia, none of the animals retched or vomited (Table 1). There were no statistically significant differences in locomotion (p > 0.05, one-way ANOVA; Table 3) or food and water intake in the observation chambers or home cages (p > 0.05, two-way repeated measures ANOVA; Table 4).
Table 2.
Physiological parameters for ferrets exposed to isoflurane
| Heart rate (min) |
% O2 Sat | Body temp (°C) |
||||
|---|---|---|---|---|---|---|
| Study 1, ferrets | ||||||
| Exp. 1 | ||||||
| 2% Isoflurane (2 h) | 230.3 (± 5.3) | 97.7 (± 0.7) | 35.8 (± 1.8) | |||
| 2% Isoflurane (6 h) | 231.0 (± 3.2) | 98.3 (± 0.6) | 38.9 (± 0.1)* | |||
| Exp. 3 | ||||||
| 2% Iso (6 h)/Saline | 213.1 (± 5.8) | 98.5 (± 0.2) | 38.5 (± 0.1) | |||
| 2% Iso(6 h)/0.1 mg/kg Morphine | 224.6 (± 5.1) | 98.4 (± 0.2) | 38.5 (± 0.2) | |||
| Study 2, ferrets |
Resp. (min) |
BP (systolic) |
BP (diastolic) |
|||
| Exp. 5 | ||||||
| 2.5–4% Iso(6 h)/Saline | 215.4 (± 5.6) | 97.8 (± 0.5) | 37.9 (± 0.1) | 35.1 (± 3.3) | 75.9 (± 5.2) | 32.7 (± 2.6) |
| 2.5–4% Iso(6 h)/0.1 mg/kg Morphine | 212.3 (± 5.5) | 97.8 (± 0.7) | 38.2 (± 0.2) | 34.3 (± 2.9) | 88.3 (± 5.7) | 40.3 (± 4.1) |
% O2 Sat = percentage oxygen saturation of the blood; Resp. = respiration rate; BP = blood pressure. Values represent the mean ± SEM.
p < 0.05, t-test.
Table 1.
Emetic testing in ferrets
| # animals with emesis/total |
Latency (min) |
Retches | Vomits | |
|---|---|---|---|---|
| Study 1 | ||||
| Exp. 1 | ||||
| Control | 0/8 | - | - | - |
| 2% Isoflurane (2 h) | 0/8 | - | - | - |
| 2% Isoflurane (6 h) | 0/8 | - | - | - |
| Exp. 2 | ||||
| Saline | 0/6 | - | - | - |
| 0.1 mg/kg Morphine | 2/6 | 20.8 | 5.2 (± 3.3) | - |
| 0.3 mg/kg Morphine | 6/6 | 6.6 | 29.3 (± 5.9)* | 2.8 (± 1.0)* |
| 0.9 mg/kg Morphine | 4/5 | 5.0 | 29.6 (± 12.6)* | 1.8 (± 0.9) |
| Exp. 3 | ||||
| Control/Saline | 0/5 | - | - | - |
| Control/0.1 mg/kg Morphine | 3/6 | 34.1 | 3.3 (± 2.1) | - |
| 2% Iso (6 h)/Saline | 0/6 | - | - | - |
| 2% Iso(6 h)/0.1 mg/kg Morphine | 3/6 | 35.4 | 3.3 (± 1.8) | 0.2 (± 0.2) |
| Exp. 4 | ||||
| 10 mg/kg Cisplatin | 6/6 | 85.6 | 129.8 (± 22.0) | 13.7 (± 2.3) |
| Study 2 | ||||
| Exp. 5 | ||||
| 2.5–4% Iso(6 h)/Saline | 0/6 | - | - | - |
| 2.5–4% Iso(6 h)/0.1 mg/kg Morphine | 1/6 | 3.0 | 0.2 (± 0.2) | 0.2 (± 0.2) |
| Exp. 6 | ||||
| 3% Isoflurane (10 min) | 1/4 | 4.6 | 0.5 (± 0.5) | - |
Latencies represent the median values of only animals demonstrating emesis.
Retches and vomits represent the mean ± SEM.
p < 0.05, planned comparison, LSD-test vs. saline/control.
Table 3.
Locomotion in ferrets and musk shrews
| # animals | Test time |
Total cm | |
|---|---|---|---|
| Study 1, ferrets | |||
| Exp. 1 | |||
| Control | 8 | 2 h | 25465 (± 3156) |
| 2% Isoflurane (2 h) | 8 | 2 h | 21440 (± 1872) |
| 2% Isoflurane (6 h) | 8 | 2 h | 21532 (± 3056) |
| Exp. 2 | |||
| Saline | 6 | 2 h | 29432 (± 3131) |
| 0.1 mg/kg Morphine | 6 | 2 h | 31819 (± 2118) |
| 0.3 mg/kg Morphine | 6 | 2 h | 47932 (± 2269)* |
| 0.9 mg/kg Morphine | 5 | 2 h | 50205 (± 9497)* |
| Exp. 3 | |||
| Control/Saline | 5 | 2 h | 24440 (± 1876) |
| Control/0.1 mg/kg Morphine | 6 | 2 h | 25472 (± 2118) |
| 2% Iso (6 h)/Saline | 6 | 2 h | 29279 (± 3001) |
| 2% Iso(6 h)/0.1 mg/kg Morphine | 6 | 2 h | 25169 (± 1621) |
| Study 2, ferrets | |||
| Exp. 5 | |||
| 2.5–4% Iso(6 h)/Saline | 6 | 2 h | 11655 (± 1240) |
| 2.5–4% Iso(6 h)/0.1 mg/kg Morphine | 6 | 2 h | 11008 (± 1316) |
| Exp. 6 | |||
| 3% Isoflurane (10 min) | 4 | 30 min | 7593 (± 1106) |
| Study 3, musk shrew | |||
| Exp. 7 (females) | |||
| Control | 6 | 30 min | 2674 (± 441) |
| 0.5% Isoflurane (10 min) | 6 | 30 min | 4170 (± 447)* |
| 1% Isoflurane (10 min) | 6 | 30 min | 3855 (± 367)* |
| 2% Isoflurane (10 min) | 6 | 30 min | 3160 (± 351) |
| 3% Isoflurane (10 min) | 6 | 30 min | 2092 (± 240) |
| Exp. 8 (males) | |||
| 2% Isoflurane (10 min) | 6 | 30 min | 5236 (± 363) |
Values represent the mean ± SEM.
p < 0.05, planned comparison, LSD-test vs. saline/control.
Table 4.
Food and water intake in ferrets
| Observation chamber | Home cages | ||||||
|---|---|---|---|---|---|---|---|
| Food (g) | Water (ml) | Food (% baseline) | Water (% baseline) | ||||
| Study 1 | n | 24 h | 48 h | 24 h | 48 h | ||
| Exp. 1 | |||||||
| Control | 8 | 7.5 (± 2.8) | 17.8 (± 4.3) | 87.2 (± 9.9) | 105.5 (± 8.8) | 94.6 (± 6.1) | 89.0 (± 2.8) |
| 2% Isoflurane (2 h) | 8 | 5.2 (± 2.5) | 9.0 (± 1.7 | 102.9 (± 9.9) | 107.4 (± 11.0) | 100.7 (± 7.4) | 106.7 (± 8.0) |
| 2% Isoflurane (6 h) | 8 | 6.8 (± 2.8) | 13.0 (± 5.1) | 84.4 (± 10.2) | 74.4 (± 6.3 | 94.5 (± 5.6) | 84.6 (± 5.3 |
| Exp. 2 | |||||||
| Saline | 6 | 3.6 (± 1.6) | 12.1 (± 2.1) | 106.2 (± 10.8) | 93.9 (± 11.2) | 102.5 (± 4.4) | 116.0 (± 11.0) |
| 0.1 mg/kg Morphine | 6 | 0.5 (± 0.3) | 14.1 (± 3.4) | 106.3 (± 12.1) | 93.3 (± 13.5) | 97.8 (± 9.8 | 91.4 (± 9.5 |
| 0.3 mg/kg Morphine | 6 | 5.1 (± 1.3) | 5.8 (± 1.5) | 107.6 (± 7.6) | 111.5 (± 12.0) | 94.5 (± 6.5) | 86.2 (± 7.6 |
| 0.9 mg/kg Morphine | 5 | 0.5 (± 0.1) | 4.1 (± 1.2)* | 118.6 (± 10.0) | 97.9 (± 4.2) | 102.4 (± 10.7) | 80.3 (± 15.6) |
| Exp. 3 | |||||||
| Control/Saline | 5 | 8.8 (± 2.3) | 17.6 (± 7.0) | 93.5 (± 7.5) | 138.4 (± 25.1) | 78.1 (± 6.3) | 113.8 (± 10.3) |
| Control/0.1 mg/kg Morphine | 6 | 7.1 (± 3.5) | 17.9 (± 3.3) | 101.1 (± 7.8) | 82.1 (± 5.5)* | 126.1 (± 17.8)* | 111.8 (± 12.3) |
| 2% Iso (6 h)/Saline | 6 | 8.0 (± 3.0) | 14.9 (± 5.4) | 91.8 (± 2.9) | 92.1 (± 1.9)* | 96.3 (± 9.5) | 91.1 (± 5.0) |
| 2% Iso(6 h)/0.1 mg/kg Morphine | 6 | 10.0 (± 2.5) | 16.3 (± 6.3 | 110.1 (± 16.2) | 102.2 (± 9.7)* | 133.0 (± 9.5)* | 97.2 (± 7.8) |
| Exp. 4 | |||||||
| 10 mg/kg Cisplatin | 6 | 2.9 (± 2.2) | 11.9 (± 4.7) | ||||
| Study 2 | |||||||
| Exp. 5 | |||||||
| 2.5–4% Iso(6 h)/Saline | 6 | 1.6 (± 0.3) | 2.1 (± 0.2) | 185.2 (± 39.9) | 75.3 (± 7.6) | 93.1 (± 17.1) | 98.5 (± 18.4) |
| 2.5–4% Iso(6 h)/0.1 mg/kg Morphine | 6 | 3.2 (± 2.6) | 1.7 (± 0.4) | 157.5 (± 33.8) | 90.8 (± 8.9) | 81.4 (± 8.4) | 98.4 (± 5.5) |
p < 0.5, planned comparison, LSD-test vs. saline/control. Values are the mean ± SEM.
Experiment 2: Morphine
Morphine injection produced a dose-related increase in emesis [retches and vomits, F(3,19) ≥ 4.7, p ≤ 0.02, one-way ANOVAs]. Morphine treatment increased the number of retches (0.3 and 0.9 mg/kg) and vomits (0.3 mg/kg, LSD-tests; Table 1). There were no significant differences in emesis latency (p>0.05, Kruskal-Wallis; Table 1). Locomotion in the observation chamber (2 h) was significantly increased by morphine treatment [F(3,19) = 5.2, p < 0.009, one-way ANOVA; Table 3]. Food and water intakes in the observation chambers were significantly changed by morphine treatment [F(3,19) ≥ 4.2, p ≤ 0.02, one-way ANOVAs; Table 4], and water intake was reduced with 0.9 mg/kg morphine compared to saline control (p > 0.05, LSDtest, Table 3). Home cage food and water intakes were not significantly affected by morphine treatment (p > 0.05, two-way repeated measures ANOVA; Table 4).
Experiment 3: Combined treatment with 2% isoflurane (6 h) and morphine (0.1 mg/kg)
Heart rate, percentage oxygen saturation of the blood, and body temperature were not significantly different between animals in the two groups (p > 0.05, t-tests; Table 2). None of the animals retched or vomited during induction anesthesia. There were no effects of isoflurane on the emetic response to morphine (p > 0.05, two-way ANOVA, interaction effects). However, morphine produced an increase in the number of retches [F(3,19) = 5.4, p < 0.05, two-way ANOVA, main effect of morphine; Table 1]. Comparison of the morphine-only and the isoflurane-plus-morphine treatments revealed no statistically significant difference in emetic latency (p > 0.05, Mann-Whitney U; Table 1). Food and water intake, and locomotion, in the observation chamber (2 h) were not significantly different across conditions (p > 0.05, two-way ANOVA; Tables 3 and 4). There were significant changes in 24 and 48 h food and water intake [F(1,19) ≥ 7.2, p ≤ 0.02, three-way repeated measures ANOVA; three-way interaction effect on food intake; two-way interaction effects on time by morphine and time by isoflurane]. Control food intake at 48 h was elevated and water intake at 24 h was elevated in the two morphine treatment groups (p < 0.05; LSD-test; Table 4).
Experiment 4: Cisplatin control treatment
Cisplatin injection (10 mg/kg, ip) produced retching and vomiting in all six animals during the 6 h (Table 1).
3.2. Study 2: 2.5 to 4% Isoflurane and Morphine, Ferrets
Experiment 5: 2.5 to 4% Isoflurane for 6 h
Even when closely monitoring cardiac rate, respiration, and blood pressure only 2 of 6 animals survived 4% isoflurane exposure on a mask for 6 h. In these two surviving animals, we reduced the isoflurane concentration to 3% to complete the testing. We continued the experiment with four additional animals using 2.5% isoflurane, and all four ferrets survived. We combined the data from these six animals because they all demonstrated a lack of the pedal withdrawal response to toe pinch, with no obvious differences between measures of emesis or other parameters. Heart rate, percentage oxygen saturation of the blood, body temperature, and blood pressure (systolic and diastolic) were not significantly different between animals in the two groups (p > 0.05, t-tests; Table 2). Isoflurane exposure produced no effects on retching or vomiting (Table 1). The low dose of morphine produced a low-intensity response for retching and vomiting in only one animal (Table 1). There were no differences between the two groups for food and water intake in the observation test or home cage (p > 0.05, t-tests; Table 4).
Experiment 6: 3% Isoflurane for 10 min
One ferret out of 4 exposed to 3% isoflurane for 10 min (Table 1) displayed 2 retches when sedated and lying on its side. There were no other retches or vomits in the induction chamber or during observation.
3.3. Study 3: 0.5 to 3% Isoflurane (10 min), Musk shrews
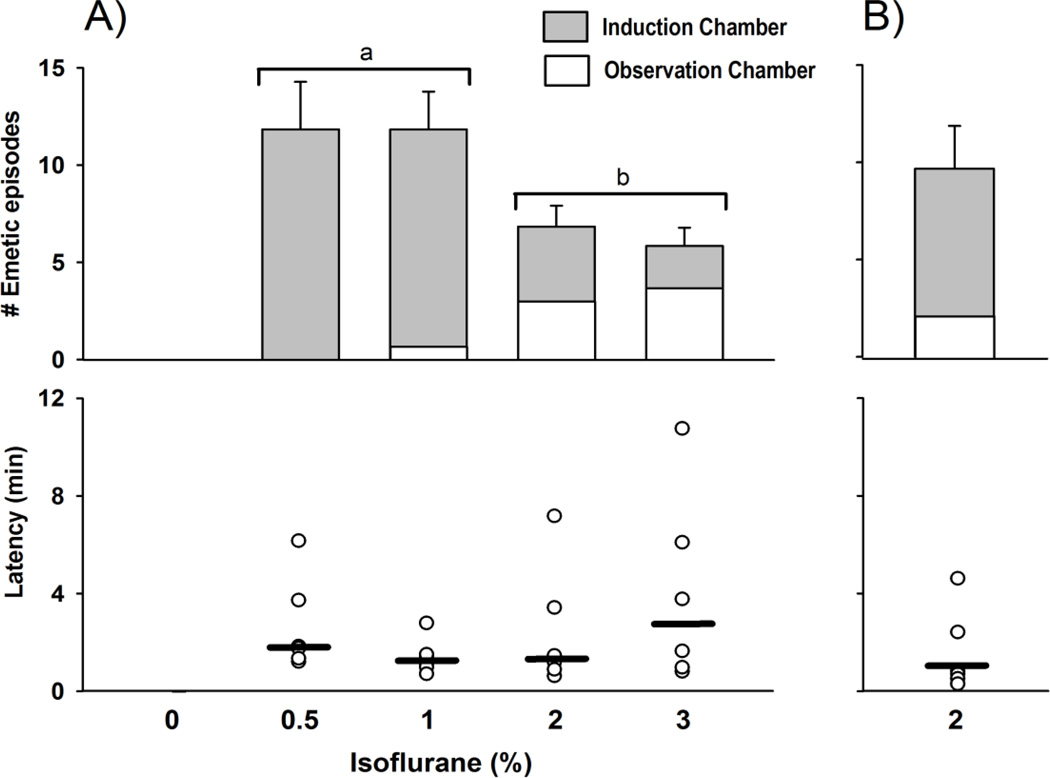
All musk shrews tested displayed emesis in response to isoflurane exposure. Animals were not sedated at 0.5%, partially at 1%, and fully at 2 and 3% isoflurane. Isoflurane exposure at 2 and 3% produced fewer emetic episodes when compared with 0.5% and 1%, with respect to responses in the induction chamber but more in the observation chamber [F(3,20) ≥ 10.2, p ≤ 0.005, one-way ANOVAs; and p < 0.05, LSD-tests, Fig. 3). There was no statistically significant effect of isoflurane concentration on emesis latency (p > 0.05, Kruskal-Wallis, Fig. 3). Locomotion in the observation chamber (30 min) was significantly increased by isoflurane treatment [F(4,25) = 5.1, p < 0.005, one-way ANOVA; Table 3]. In Experiment 8, using 6 male shrews, 2% isoflurane exposure induced emesis (7.5 ± 1.6 and 2.2 ± 0.9 emetic episodes, induction and observation chambers, respectively; Fig. 3). Isoflurane exposure produced full sedation in male shrews.
Figure 3.
Isoflurane-induced emesis in A) female and B) male musk shrews. Top: number of emetic episodes after exposure to isoflurane in the induction chamber (10 min) and observation chamber (30 min). The control condition of “0” isoflurane (%) was oxygen only. Results represent the mean ± SEM. Bars with a different letter are significantly different, p < 0.05, planned comparison, LSD-test. Bottom: median latency (heavy bar) and scatter plot of values to the first emetic episode after start of exposure to isoflurane.
4. Discussion
Isoflurane exposure (2 to 4%) for up to 6 h produced no vomiting in ferrets. Uniquely, one ferret out of 4 exposed to 3% isoflurane for 10 min (Table 1; Exp. 6) displayed 2 retches when sedated and lying on its side. In a prior study, 5% isoflurane (5 min, induction chamber) plus 2.5% isoflurane (5 min, face mask) produced no emesis in six ferrets over a 30 min observation [8]. An additional study suggests that halothane exposure could be antiemetic in decerebrated ferrets tested for emesis using electrical stimulation of the vagus [20]. We also assessed the potential interaction of isoflurane exposure with a low dose of morphine. A low dose was selected because we wanted to reduce any potential ceiling effect on emesis that could result from using a peak dose of morphine (i.e., 0.3 or 0.9 mg/kg [7]). Isoflurane exposure failed to produce additional emesis when combined with morphine. There were also no consistent adverse effects of isoflurane or morphine treatment on body weight, food intake, and water intake in ferrets; these findings suggest the absence of gastrointestinal malaise in the 48 h following treatment with these agents. Altogether, these data and a prior study [8] indicate that isoflurane-induced emesis is an uncommon effect in ferrets.
Although not responsive to isoflurane-induced emesis, our data indicate that these ferrets can produce retching and vomiting to other emetic stimuli. Anesthesia in ferrets (and musk shrews) reduces the speed of the emetic response produced by a variety of stimuli (e.g., electrical stimulation of the vagus, distension of the esophagus [21, 22]), i.e., the retching sequence is slower than in freely moving animals [19, 23]. This might be the reason why 2 and 3% isoflurane exposure produces less emesis than 0.5 and 1% isoflurane exposure in musk shrews. In ferrets exposed to isoflurane, locomotion, eating, and drinking behaviors had recovered during the test period, therefore, it is unlikely that isoflurane had produced a substantial suppressive effect on the emetic system. Indeed, several ferrets exposed to isoflurane for 6 h showed morphine-induced emesis (Table 1), indicating that ferrets were capable of emesis even after a long duration exposure to isoflurane. There are reports of variability in the emetic responses using different strains of ferrets [12]. Control injections of cisplatin and morphine show that ferrets in our study are clearly capable of producing emetic responses that are similar in number and latency to other reports [7, 8, 12].
The current investigation has several limitations. Emesis testing occurred for a short time (up to 2 h) after exposure with isoflurane. We chose this testing duration because 1) humans show nausea and vomiting within the first few hours after volatile anesthetics, including isoflurane [3], and 2) we wanted to evaluate emesis dose-response effects of isoflurane, beyond only several minutes, as reported previously [8]. Moreover, there were no consistent effects of isoflurane exposure on food or water intake over 48 h after exposure, which suggests that animals were not adversely affected by isoflurane for a longer period (Table 4). Isoflurane failed to produce emesis in male ferrets but both male and female musk shrews displayed isoflurane-induced emesis. The observed species differences do not appear to be related to sex, however, the responses of female ferrets to isoflurane exposure will need to be evaluated. Although the current study used only isoflurane, other volatile agents (e.g., sevoflurane) appear to produce similar PONV in patients [3]. Dogs were reported to regurgitate after exposure to halothane, isoflurane, or sevoflurarane [24].
In contrast to ferrets, musk shrews were very sensitive to isoflurane-induced emesis. Musk shrews showed emetic episodes in response to isoflurane at a low concentration of 0.5% -- a dose that did not produce sedation. Although there was no effect of isoflurane dosage on the median latency for the first emetic episode, these data suggest that increasing the concentration of isoflurane suppresses the total number of emetic episodes (Figure 3). A prior study reported that 1% halothane (in 80% nitrous oxide/20% oxygen) produces a comparable effect on emesis in musk shrews [17]. Furthermore, we have noticed that sodium pentobarbital maintenance anesthesia produces emesis in musk shrews (lead author, unpublished observations). Therefore, musk shrews appear to be generally sensitive to anesthesia-induced emesis. Although not tested here, musk shrews are reported to display more complicated responses to morphine. Although morphine alone appears not to produce emesis in musk shrews [25], naloxone pre-treatment can reveal morphine-induced emesis [26]. Opioid receptor agonists are well known to suppress emesis [27], usually at higher doses, and this inhibitory system is apparent in musk shrews [25, 26, 28].
In summary, the current studies suggest that ferrets will be of little use for modeling PONV after use of volatile anesthetics. Conversely, musk shrews might prove useful in modeling PONV after volatile agent use. However, the neural circuitry for the emetic effects of opioids in musk shrews requires more detailed study, so that opioid contributions to PONV (in isolation, or simultaneously) can also be evaluated in the same species. Recent advances in the detection of emesis in musk shrews using computer algorithms [19] and behavioral signatures for nausea [15] should greatly facilitate high throughput work to delineate new pharmacological targets for the control of PONV.
Highlights.
-
>
Opioid analgesics and volatile anesthetics are the main triggers of post-operative nausea and vomiting (PONV).
-
>
Morphine produced a dose-response effect on emesis in ferrets.
-
>
Isoflurane exposure failed to induce emesis in ferrets.
-
>
In contrast to ferrets, musk shrews were very sensitive to isoflurane-induced emesis.
-
>
These results indicate that musk shrews may prove to be a useful model for vomiting after inhalation of volatile agents.
Acknowledgements
The authors wish to thank Dr. Nathalie Percie du Sert for her help in the design of the experiments and for useful discussions. We also thank the University of Pittsburgh, Division of Laboratory Animal Research (DLAR) for care of the musk shrew colony and Mike Nakon, Surgical Research, who was instrumental in assisting with the isoflurane anesthesia equipment. This work was supported by the a Department of Anesthesiology, University of Pittsburgh School of Medicine, Seed Grant and an NIH grant to the University of Pittsburgh Cancer Institute, P30 CA047904 (Cancer Center Support Grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Apfel CC, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. 2004;350:2441–2451. doi: 10.1056/NEJMoa032196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apfel CC, Laara E, Koivuranta M, Greim CA, Roewer N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. doi: 10.1097/00000542-199909000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: a randomized controlled trial of factorial design. Br J Anaesth. 2002;88:659–668. doi: 10.1093/bja/88.5.659. [DOI] [PubMed] [Google Scholar]
- 4.Tan T, Bhinder R, Carey M, Briggs L. Day-surgery patients anesthetized with propofol have less postoperative pain than those anesthetized with sevoflurane. Anesthesia and analgesia. 2010;111:83–85. doi: 10.1213/ANE.0b013e3181c0ee9e. [DOI] [PubMed] [Google Scholar]
- 5.Foss JF, Bass AS, Goldberg LI. Dose-related antagonism of the emetic effect of morphine by methylnaltrexone in dogs. Journal of clinical pharmacology. 1993;33:747–751. doi: 10.1002/j.1552-4604.1993.tb05618.x. [DOI] [PubMed] [Google Scholar]
- 6.Barnes NM, Bunce KT, Naylor RJ, Rudd JA. The actions of fentanyl to inhibit drug-induced emesis. Neuropharmacology. 1991;30:1073–1083. doi: 10.1016/0028-3908(91)90136-y. [DOI] [PubMed] [Google Scholar]
- 7.Thompson PI, Bingham S, Andrews PL, Patel N, Joel SP, Slevin ML. Morphine 6-glucuronide: a metabolite of morphine with greater emetic potency than morphine in the ferret. Br J Pharmacol. 1992;106:3–8. doi: 10.1111/j.1476-5381.1992.tb14284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn RL, Essien E, Thut PD. The effects of different antiemetic agents on morphine-induced emesis in ferrets. Eur J Pharmacol. 1993;241:47–54. doi: 10.1016/0014-2999(93)90931-7. [DOI] [PubMed] [Google Scholar]
- 9.Lefebvre RA, Willems JL, Bogaert MG. Gastric relaxation and vomiting by apomorphine, morphine and fentanyl in the conscious dog. European journal of pharmacology. 1981;69:139–145. doi: 10.1016/0014-2999(81)90408-8. [DOI] [PubMed] [Google Scholar]
- 10.Burgess JW, Villablanca JR. Ontogenesis of morphine-induced behavior in the cat. Brain research. 2007;1134:53–61. doi: 10.1016/j.brainres.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 11.Robertson SA, Wegner K, Lascelles BD. Antinociceptive and side-effects of hydromorphone after subcutaneous administration in cats. J Feline Med Surg. 2009;11:76–81. doi: 10.1016/j.jfms.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Percie du Sert N, Rudd JA, Apfel CC, Andrews PL. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT(3) receptor antagonists. Cancer Chemother Pharmacol. 2010;67:667–686. doi: 10.1007/s00280-010-1339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews PL, Okada F, Woods AJ, Hagiwara H, Kakaimoto S, Toyoda M, et al. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br.J.Pharmacol. 2000;130:1247–1254. doi: 10.1038/sj.bjp.0703428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sam TS, Cheng JT, Johnston KD, Kan KK, Ngan MP, Rudd JA, et al. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew) Eur J Pharmacol. 2003;472:135–145. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- 15.Horn CC, Henry S, Meyers K, Magnusson MS. Behavioral patterns associated with chemotherapy-induced emesis: A potential signature for nausea in musk shrews. Frontiers in Neuroscience. 2011;5:88. doi: 10.3389/fnins.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horn CC, Still L, Fitzgerald C, Friedman MI. Food restriction, refeeding, and gastric fill fail to affect emesis in musk shrews. Am J Physiol Gastrointest Liver Physiol. 2010;298:G25–G30. doi: 10.1152/ajpgi.00366.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner C, Perren M. Inhibition of anaesthetic-induced emesis by a NK1 or 5-HT3 receptor antagonist in the house musk shrew, Suncus murinus. Neuropharmacology. 1998;37:1643–1644. doi: 10.1016/s0028-3908(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 18.Andrews P, Torii Y, Saito H, Matsuki N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. Eur.J.Pharmacol. 1996;307:305–313. doi: 10.1016/0014-2999(96)00275-0. [DOI] [PubMed] [Google Scholar]
- 19.Huang D, Meyers K, Henry S, De la Torre F, Horn CC. Computerized detection and analysis of cancer chemotherapy-induced emesis in a small animal model, musk shrew. Journal of neuroscience methods. 2011;197:249–258. doi: 10.1016/j.jneumeth.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zunini GS, Roth SH, Lucier GE. The inhibitory effect of halothane on the emetic response in the ferret. Canadian journal of physiology and pharmacology. 1990;68:374–378. doi: 10.1139/y90-052. [DOI] [PubMed] [Google Scholar]
- 21.Onishi T, Mori T, Yanagihara M, Furukawa N, Fukuda H. Similarities of the neuronal circuit for the induction of fictive vomiting between ferrets and dogs. Auton Neurosci. 2007;136:20–30. doi: 10.1016/j.autneu.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp.Physiol. 2002;87:563–574. doi: 10.1113/eph8702424. [DOI] [PubMed] [Google Scholar]
- 23.Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Reduced normogastric electrical activity associated with emesis: a telemetric study in ferrets. World J Gastroenterol. 2009;15:6034–6043. doi: 10.3748/wjg.15.6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson DV, Boruta DT, Evans AT. Influence of halothane, isoflurane, and sevoflurane on gastroesophageal reflux during anesthesia in dogs. American journal of veterinary research. 2006;67:1821–1825. doi: 10.2460/ajvr.67.11.1821. [DOI] [PubMed] [Google Scholar]
- 25.Selve N, Friderichs E, Reimann W, Reinartz S. Absence of emetic effects of morphine and loperamide in Suncus murinus. Eur J Pharmacol. 1994;256:287–293. doi: 10.1016/0014-2999(94)90554-1. [DOI] [PubMed] [Google Scholar]
- 26.Javid FA, Naylor RJ. Opioid receptor involvement in the adaptation to motion sickness in Suncus murinus. Pharmacol Biochem Behav. 2001;68:761–767. doi: 10.1016/s0091-3057(01)00470-1. [DOI] [PubMed] [Google Scholar]
- 27.Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton.Neurosci. 2006;129:3–16. doi: 10.1016/j.autneu.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Kakimoto S, Saito H, Matsuki N. Antiemetic effects of morphine on motion- and drug-induced emesis in Suncus murinus. Biol Pharm Bull. 1997;20:739–742. doi: 10.1248/bpb.20.739. [DOI] [PubMed] [Google Scholar]
- 29.Javid FA, Naylor RJ. Variables of movement amplitude and frequency in the development of motion sickness in Suncus murinus. Pharmacol.Biochem.Behav. 1999;64:115–122. doi: 10.1016/s0091-3057(99)00066-0. [DOI] [PubMed] [Google Scholar]
- 30.Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci. 1987;41:513–518. doi: 10.1016/0024-3205(87)90229-3. [DOI] [PubMed] [Google Scholar]
- 31.Matsuki N, Ueno S, Kaji T, Ishihara A, Wang CH, Saito H. Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn.J.Pharmacol. 1988;48:303–306. doi: 10.1254/jjp.48.303. [DOI] [PubMed] [Google Scholar]