Abstract
Objectives
We propose a theoretical framework for the behavioral modulation of pain based on constructivism, positing that task engagement, such as listening for errors in a musical passage, can establish a construction of reality that effectively replaces pain as a competing construction. Graded engagement produces graded reductions in pain as indicated by reduced psychophysiological arousal and subjective pain report.
Methods
Fifty-three healthy volunteers having normal hearing participated in four music listening conditions consisting of passive listening (no task) or performing an error detection task varying in signal complexity and task difficulty. During all conditions, participants received normally painful fingertip shocks varying in intensity while stimulus evoked potentials (SEP), pupil dilation responses (PDR), and retrospective pain reports (PR) were obtained.
Results
SEP and PDR increased with increasing stimulus intensity. Task performance decreased with increasing task difficulty. Mixed model analyses, adjusted for habituation/sensitization and repeated measures within person, revealed significant quadratic trends for SEP and PR (Pchange<0.001) with large reductions from no task to easy task and smaller graded reductions corresponding to increasing task difficulty/complexity. PDR decreased linearly (Pchange<0.001) with graded task condition. We infer that these graded reductions in indicators of central and peripheral arousal and in reported pain correspond to graded increases in engagement in the music listening task.
Discussion
Engaging activities may prevent pain by creating competing constructions of reality that draw on the same processing resources as pain. Better understanding of these processes will advance the development of more effective pain modulation through improved manipulation of engagement strategies.
Keywords: music, analgesia, distraction, psychophysiology, experimental
Introduction
Pain is a complex, unpleasant sensory and emotional somatic awareness normally associated with tissue trauma. Behavioral methods for modulating pain such as distraction have proven successful in both clinical and experimental settings but often with limited effect and with significant differences in benefit from individual to individual. The mechanisms by which distraction reduces pain are poorly understood. In this study we employed a theoretical framework for cognitive modulation of pain using the concept of engagement, positing that performing a highly engaging task can markedly alter the cognitive-emotional aspects of pain, and that graded engagement will produce graded reductions in pain as reflected in diminished psychophysiological arousal and subjective report.
Studies on Cognitive Processing of Pain and Distraction
Studies of distraction for pain relief have a long history marked by inconsistent methods and findings. Lack of a guiding theory for distraction limits this literature, and attempts to grade distraction effects have been crude. Results vary depending on whether pain is acute, chronic, or experimental, the type of distractor used, and the outcomes measured. In 3 studies using cold pressor pain (CP), one found no pain reduction using a reaction time task (1), another using immersive video goggles reported decreased pain and unpleasantness ratings (2), and a third using mental arithmetic had mixed results (3). Burn patients demonstrated pain reduction with virtual reality immersion (4) and sensory focusing (5). Studies evaluating distraction for pain reduction using functional brain imaging (fMRI), magnetoencephalography (MEG), and sensory evoked potentials (SEP) have found increased cerebral blood flow in anterior cingulate cortex (ACC) during distraction (6) and decreases in other limbic areas (7), but conflicting SEP findings (8). Fear of pain (9), catastrophizing (10), and motivation for task performance (11) also may play an important role in determining the effectiveness of distraction for pain reduction, potentially interfering with pain reduction effects by influencing the ability to perform distracting tasks (12; 13).
Recent studies investigating the bi-directional effect of attention demanding tasks on pain and pain on task performance support the idea that pain and cognitive processing share the same brain resources (14; 15). Other evidence suggests pain shares resources with emotional processes as well (16). Taken together, this literature suggests that attentional and/or emotional demands compete with the same resources that noxious stimulation requires for producing awareness of pain.
Development of a Theoretical Framework for Engagement
We propose that highly engaging activities may prevent pain by creating competing constructions of reality that draw on the same processing resources. We define engagement in the framework of constructivism, an area of consciousness research, as a dynamical, complex process of constructing models of reality in consciousness, with pain being one such model (17). Constructivism, originating largely with the work of Piaget (18), has become a viable perspective in the study of consciousness. In the constructivist view, consciousness does not passively represent the external environment and the body; rather, it actively and continuously constructs and revises a model of the body and the external world. This construction process draws upon pre-consciously processed sensory, memory and emotional input. The brain continuously forms and reforms short-lived perceptual wholes from arrays of incoming information.
The process of constructing consciousness is dynamic and complex. The available construction materials include schemata- normally non-conscious patterns of concepts or affects and associations that reflect a person’s past experience and influence as well as expectations for the present and future (19). A schema may be associated with immediate sources of physiological stimulation (sensory experience), immediate cognitive and affective productions, other schemata produced in imagination, and other schemata stored in memory. Most schemata are short lived because the constant changes of dynamic perception over-write the neural representations before they have a chance to consolidate. However, some are learned; that is, they survive indefinitely in memory. Learned schemata, such as memory for familiar tunes, are readily available for recall, and certain sensory events or other schemata can act as triggers for a particular schema.
Distraction models derive from the construct of attentional resource allocation (20). Essentially, distraction comprises the shifting allocation of attentional resources from one focus to another. Distraction studies typically report observations of behavior (with some brain imaging evidence of corresponding changes in regions of neuroanatomical activation) without offering critical insights about underlying psychological mechanisms. At the end of the day, pain researchers need to understand the nature of distraction in order to manipulate it effectively. We view distraction as one component within an overarching constructivist framework. Constructivism offers a richer basis for understanding, allowing us to introduce the construct of engagement as an organizing principle.
In the constructivist framework, how can a contextually rich, dynamic and complex experience (i.e., an engaging activity) influence the perception of pain? A key concept is that processing limitations constrain the conditions for the construction of momentary consciousness. Using the metaphor of a theater stage for the focus of consciousness: only a few actors (schemata) can occupy the stage at a given moment, and their interaction against the background determines the meaning of the play (21). The brain samples selectively and self-organizes what it will use to construct an immediate reality. Attention is a part of this process, directing the focus of ongoing construction and governing the rate at which the brain either shifts or fixates the reality that it constructs. The presence of an elaborate construction on the stage of awareness, and the active sustaining of this construction, prevents the formation of competing patterns of awareness. Because pain, like every other aspect of awareness, is a construction, the brain can modulate the entry of nociceptive signals from tissue trauma into consciousness (strictly speaking, it modulates the schemata related to those signals). It will do this because it is engaged fully in constructing some immediate alternate “scene” that requires the assembling and sustaining of other, elaborate schemata, such as those necessary for processing music.
The constructivist position on non-pharmacological analgesia thus differs substantially from models of attention resource allocation and distraction. The distraction model assumes that pain is a fully formed perception and that a competing perception or task pulls the subject away from pain. The subject directs limited attention resources to a competing perception or task, putting pain in the background. The constructivist position asserts that pain cannot form as a complete perception because there are ongoing constraints on the construction of pain when the subject is fully engaged in a competing construction. Pain becomes at best an adumbration, not a vivid awareness. The key to successful pain control is not the “pull” of the distractor, but rather the degree of engagement the subject is able to produce. Engagement, then, is the ability of the subject to construct a vivid arena of focused awareness and to sustain that construction through task performance for a prolonged period of time.
Given that successful engagement requires a “contextually rich, dynamic and complex experience,” what activities might meet the requisite conditions? Listening to music offers a rich environment for cognitive, emotional, and even spiritual satisfaction for many people (22). The history of the use of music for pain relief is long, and reveals mixed results. Music is effective for reducing pain, but the effect is small, and varies considerably depending on many factors (23). Although research on music analgesia almost uniformly fails to identify a mechanism for effect, most investigators assume that music acts as a distraction, thereby reducing perceived pain (24). Other research has shown that pleasant emotions evoked by music reduces pain (25). We hold that listening to music can have analgesic benefit for listeners if they can fully construct and sustain the musical experience, and thus impede the construction of pain. Potential determinants of degree of music engagement may include the complexity of the musical structure, the difficulty of identifying and tracking musical features such as melody, familiarity and preference and other stable features unique to the individual listener.
The constructivist framework bridges pain research to research on music cognition and emotion. A constructivist framework has been used to describe the way in which emotional responses to complex and familiar music might arise from processing schemata of musical structure and expectancy generation (26). In this framework, music listening consists of detecting and interpreting patterns in sensory input on many levels: unique frequency discriminations, event durations, and complex acoustical characteristics, which activate networks of learned associations. These associations, or schemata, allow the listener to construct musical images, process musical structure, and generate expectancies. Numerous studies have addressed components of this process including the acquisition of tonality (27), melody (28), rhythmic structure (29), and expectancy formation (30). This body of research suggests that music provides an optimal medium for developing a constructivist approach to pain control.
Study Objectives
The purpose of the present study was to demonstrate rigorously that engagement in a music listening task alters the psychophysiological arousal that normally accompanies the perception of painful events in a graded fashion depending on level of engagement. The music listening task required subjects to construct an auditory experience, fit sensory input to musical schemata, and identify subtle aberrations in goodness of fit, such as pitch variants, as they occur. This requires processing of complex auditory input and sustained attention in the task. When the music task is complex, subjects must effectively organize their perceptual and cognitive resources to activate and integrate the relevant schemata and perform the task successfully. Noxious signaling occurring during task performance, requiring the activation and integration of schemata relevant to pain, will fail to penetrate consciousness when the brain is fully engaged in constructing and sustaining the music task. However, if the auditory input lacks complexity, fewer resources are required to activate the necessary musical schemata. Engagement theory would predict that such a condition provides little interference to the entry of noxious signaling into consciousness. We predicted that performing a highly engaging listening task would reduce the psychophysiological arousal associated with painful stimulation, and that the greater the signal complexity and task difficulty, the greater the reduction in that arousal. The ability to engage in listening tasks, and thus the degree to which the task may interfere with the arousal response to noxious stimulation, will vary from person to person depending on ability to absorb oneself in an activity. Emotion has been shown to affect attentional mechanisms regarding pain and may play a role in how well a person can engage in the task (31). Specifically, anxiety as a persistent characteristic of the person, or state anxiety related to the test environment may influence the level of engagement (32).
Materials and Methods
Participants
We recruited 53 participants from the University of Utah campus and surrounding community, 21 females and 32 males. Participants were 18 years or older, healthy, not using psychoactive or blood pressure medication, and had normal hearing as confirmed by audiometric testing (Earscan Audiometer). Volunteers consented to participate according to the University of Utah Institutional Review Board approved protocol and in accordance with the ethical standards established in the 1964 Declaration of Helsinki. Participants were compensated for their time spent in the study. Four participants failed to complete the study, three due to equipment and software failure and one due to inability to perform the task.
Design
This study employed a multivariate repeated measures design with two within subjects manipulated factors. Subjects experienced four conditions, No Task, Easy Task, Moderate Task, and Hard Task. The conditions increased in a graded fashion in task difficulty and complexity from simple/easy to complex/hard. Condition order was counterbalanced across subjects.
Experimental Conditions
Subjects experienced a total of 9 blocks of 24 trials each. Task condition blocks were repeated and No Task blocks occurred 3 times. To provide a control for habituation effects, No Task blocks were always presented in positions 1, 5, and 9, with Task blocks presented at positions 2-4 and 6-8 with orders randomized across subjects. Each block consisted of a test window during which music played while the subject received a total of 24 stimulus trials. Noxious fingertip stimuli occurred at three intensity levels varying in random order with equal numbers of trials delivered over each block. The inter-trial interval for stimuli varied randomly from 8 – 11 sec.
Music Listening Task
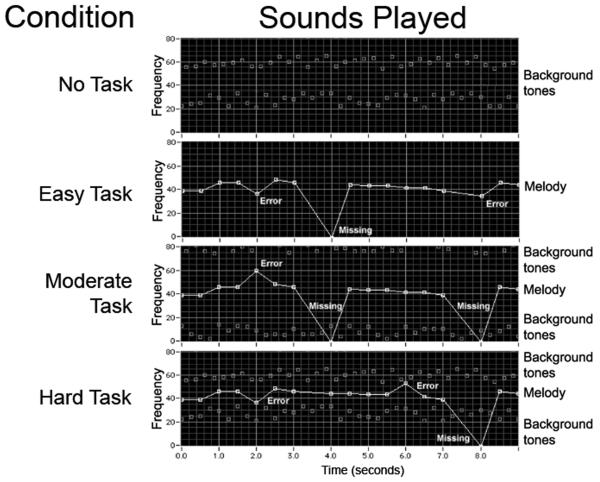
We operationalized the manipulation of engagement using a music listening task that varied on two dimensions: structural complexity of the auditory signal and difficulty of the listening task. The music listening tasks were constructed to produce a linear variation from minimum engagement to maximum engagement. The music listening conditions consisted of an auditory analog to the familiar visual figure-ground perceptual challenge (Figure 1). The auditory figure comprised a familiar melody (e.g., “Twinkle, twinkle, little star”) presented at a rate of one tone per second. The background comprised two series of random tones varying over a 6 semitone range and sounding either above or below the frequency range of the melody. Background tones occurred at twice the rate of the melody tones. Melody tones were selected at random to either deviate by 12 semitones (one octave) from the normally occurring pitch frequency or to be omitted. The listening task was to track the melody and identify deviant tones while ignoring omitted tones.
Figure 1.
Auditory sequences used for test conditions. Melodic example shown is “Twinkle, twinkle, little star.”
Adjusting the frequency distance between the melody and background varied the task difficulty. Pilot tests found that positioning the background ±12 semitones from the melody or adjacent to the frequency range of the melody provided the most consistent contrast between task conditions. Thus, we defined three task conditions. In the Easy Task condition, subjects heard the melody alone (without background). This condition had low signal complexity and the listening task was easy to perform since the melody was easy to track without a background and deviant tones could be clearly distinguished as occurring in a pitch range higher or lower than the melody. In the Moderate and Hard Task conditions subjects heard the melody against the background of random tones sounding ±12 semitones (Moderate Task) or adjacent (Hard Task) to the melody. These conditions represented moderate and high signal complexity, respectively. These tasks became more difficult since they required that subjects track the melody against a background and deviant tones occurred either in a separate pitch range (Moderate Task) or were embedded in the same pitch range as the background (Hard Task). During the No Task control condition, the background tones were played without a melody and subjects did not perform a task.
Manipulating the loudness of the melody relative to the background provided an effective way to adjust for individual differences in task performance ability. During pre-test training, we optimized the loudness adjustments so that subjects achieved 95% task performance in the moderate (background ±12 semitones from the melody) condition. We applied Fletcher-Munson corrections to the frequency amplitudes to assure equal loudness across the entire frequency range (similar to pressing the loudness toggle on a stereo system). Auditory signals were delivered free-field over a loudspeaker positioned in front of the subject. To assure that subjects were familiar with the test melody, could easily recall and track it, and had an expressed preference for it, we asked each subject to select a single favored tune from a set of simple children’s songs.
Dolorimetry
We delivered safe but noxious stimuli to laboratory stimulation of subjects using a modified version of a standard laboratory pain stimulation technology (33). As a fingertip electrode we use a standard prick lancetter (Bayer No. 170400 B03) having a 1mm triangular tip, which we inserted in the stratum corneum of a fingertip on the subject’s non-dominant hand. A stimulus trial consisted of a 5 msec square wave constant current pulse delivered to the fingertip by a Grass S-44 stimulator and a stimulus isolation unit connected in series. Stimulus levels for testing were established for each subject, based on the subject’s pain threshold and tolerance. To establish pain threshold and tolerance, we stimulated the fingertip with gradually increasing stimulus intensity and asked subjects to indicate the point at which they first felt slight pain and the point at which they did not wish to receive higher intensity stimulation. This procedure was repeated twice or more, as needed, to achieve consistent reports from subjects. We identified pain threshold as the intensity at which the subject first consistently reported slight pain, and pain tolerance as the maximum intensity the subject was willing to tolerate. Using the range in current values obtained from threshold to tolerance, we set 3 stimulus levels: 20%, 50%, 80% of pain tolerance for low, medium and high stimulus, respectively.
Psychophysiological Data Collection
Changes in electroencephalographic (EEG) and pupil diameter data streams time-locked with stimulus onsets provided indicators of psychophysiological responses to noxious events. During an experimental session, EEG data were collected continuously from a single high impedance electrode placed at vertex (Cz) using an ActiveTwo high resolution biopotential EEG acquisition system (BioSemi Instrumentation, Amsterdam, Netherlands) recorded EEG. This system provides 24-bit analog-to-digital conversion per channel with exceptional common-mode rejection and dynamic range. The conditioned signal was sampled by 1024 Hz. Single trial epochs were selected from the data stream at 100ms pre- and 500ms post-stimulus intervals and low-pass filtered at 20 HZ using zero phase shift inverse fast Fourier transformations (FFT) digital filtering. For each subject, a grand average of single trials was inspected to locate pilot latencies for identifying negative (N150) and positive (P250) EEG peaks. We manually inspected each single trial to identify the local minimum negative and maximum positive amplitude peaks proximal to the corresponding pilot latencies. The peak-to-peak amplitudes provided single trial stimulus evoked potential (SEP) values for subsequent analysis.
Single trial pupil dilation response (PDR) amplitudes were obtained from the continuous pupil diameter data collected using an iView X pupillometry system (SensoMotoric Instruments, Needham, MA) sampled at 256 Hz. An in-house software system built in LabView (National Instruments, Austin, TX) identified and removed eye blinks with interpolation. Identifying single trials as the segment beginning 500ms before and continuing 2 sec after a stimulus event, we obtained grand averages for each subject and identified pilot peak latencies corresponding to the peak (usually around 1250 ms) and a baseline latency prior to the response (typically within 500ms after the stimulus). PDR amplitude was calculated from the difference between amplitudes taken at the peak and baseline latency measurements.
Outcome Measures
Psychophysiological arousal indicators served as the primary outcome measures of response to painful stimulation. SEP and PDR provided indicators of central nervous system (CNS) and peripheral sympathetic nervous system (SNS) responses to noxious stimulation, respectively. Subjects reported worst, least, and average pain experienced during the immediately preceding set of 8 trials using a 0 to 10 scale (0= no pain, 10= pain equal to predetermined pain tolerance). For each report type in each block, subjects provided three reports. Because individual sub-blocks of 8 trials did not have equal numbers of trials for each of the three stimulus levels (although equal within task), we aggregated reports across blocks within task condition. This yielded three pain reports per task condition for each pain report type.
The Tellegen Absorption Scale (TAS), and the State-Trait Anxiety Inventory (STAI-State and STAI-Trait) yielded secondary outcomes that could potentially clarify individual differences in ability to engage in a task. The TAS is a validated measure assessing openness to absorbing experiences as a personality trait (34). The STAI scales are well validated measures of present anxiety and persistent anxiety as a personality characteristic, respectively (35). As a manipulation check for task difficulty, we recorded accuracy (number of correct detections – number of false alarms) and response time measures in each block.
Study Procedures
Prior to testing, subjects practiced the easy music listening task until they achieved 100% performance. They then practiced the moderate and hard tasks, while adjusting the melody and background sound levels, until achieving 85% and 70% performance criteria, respectively. We then established pain threshold and tolerance and set stimulus intensity levels. During testing, subjects experienced 9 blocks of test trials varying by condition. Each block comprised 24 stimulus trials. In task conditions, subjects verbally reported deviant tones as quickly and accurately as possible while ignoring missing tones. During the control condition blocks, subjects heard background tones playing but did nothing. A pause in stimulations and sounds occurred after every 8 trials during which subjects were prompted to report their worst, least, and average level of pain. Within each test block, 24 fingertip shocks occurred with 8 shocks delivered at each current intensity (low, medium, and high) in random order, providing a total of 48 trials per task condition and 72 trials for the control condition. Subjects rated their engagement at the end of each test block.
Data Analysis
To investigate the effects of the music task manipulation, we initially modeled, for each measure, music complexity as a categorical factor with four levels, along with stimulus intensity, a categorical factor with three levels. As a control for possible habituation or sensitization through the course of the experiment, we included trial index as a quantitative covariate, and allowed individual variation in this temporal relationship as a random effect. The coefficient of linear trial represents the expected change in the dependent variable per trial, conditional on all other explanatory variables. Individuals with negative trial coefficients display habituation, while individuals with positive coefficients display sensitization. To control for lack of independence in repeated measures on the same individuals, we also included a random subject intercept effect, which was allowed to correlate with the habituation/sensitization effect. The resulting mixed effects models effects models can be expressed as
where
μ is the population intercept,
b0i is the intercept for subject i,
Xij is the fixed effect of the jth Task condition,
Zik is the fixed effect of the kth Stimulus level,
t is the tth trial,
bi is the habituation/sensitization regression coefficient for subject i,
β is the vector of population mean regression coefficients,
Ψ is the unstructured covariance matrix of the b0i and b1i random effects, and εijkt is random error, with mean zero and variance σ2 .
Although the factor categorical model provides the best possible fit to the data, it does not allow direct evaluation of the key hypothesis concerning the rank ordering of the music complexity conditions. For this purpose we conducted a trend analysis within the mixed effects framework similar to the model above, but treating Task as a quantitative covariate. The best- fitting model is categorical, equivalent to a cubic polynomial fit for task complexity. Linear and quadratic models necessarily fit more poorly, but may well approximate the cubic fit and also yield interpretable tests of order. To evaluate the trend components, we examined two indices of fit. P(approximation) is the p-value for the null hypothesis that the reduced trend fits as well as the categorical model (cubic trend). We regard a high p-value for this statistic as evidence that the reduced trend adequately approximates the categorical model and the observed pattern of means. P(change) is the p-value for the null hypothesis that the fit of the higher trend is no better than that of the lower trend. The most appropriate model is therefore one with a significant P(change) and nonsignificant P(approximation). Parameter estimates for the most appropriate model for each measure can then be interpreted in terms of order predictions. In linear models, negative slope coefficients correspond to predicted ordering with task complexity. In quadratic models, the linear coefficient corresponds to the instantaneous slope at the zero point of the no task condition, with the quadratic coefficient modifying how the slope changes at other task levels according to the first derivative b1 + 2b2X, where X is the coded task complexity and b1 and b2 are the linear and quadratic coefficients, respectively. Model comparisons were conducted with likelihood ratio tests of the maximum-likelihood deviance statistics for the nested models. All models were analyzed with SPSS 18 (IBM Corporation, Somers, NY) and SAS 9.2 Proc Mixed (SAS Institute, Inc., Cary, NC). Significance of factor and covariate effects were evaluated under maximum likelihood using F-tests and Satterthwaite denominator degrees of freedom.
Results
The average age of participants was 26.5 (±1.33) (Table 1). With the exception of two participants for STAI-Trait and three for STAI-State, all scores were below a reported threshold for low anxiety of 45 (36). With one exception, TAS scores fell within reported ranges (37; 38).
Table 1.
Description of participants by age, state and trait anxiety, and absorption.
| Mean | Minimum | Maximum | S.D. | |
|---|---|---|---|---|
| Age | 26.5 | 18 | 61 | 1.33 |
| STAI-State | 30.3 | 21 | 53 | 7.0 |
| STAI-Trait | 34.5 | 23 | 54 | 7.2 |
| TAS | 20.6 | 5 | 33 | 6.9 |
Abbreviations: S.D.= standard deviation; STAI= Spielberger State-Trait Anxiety Inventory; TAS= Tellegen Absorption Scale.
Table 2 provides raw means and standard deviations for each psychophysiological measure by stimulus level and task condition. All measures increased with increasing stimulus level, and generally decreased with increasing order of task. PRs showed similar patterns, increasing with stimulus level and generally decreasing with increasing order of task condition. Task performance measures showed a linear decrease with increasing task difficulty, indicating the manipulation of engagement in terms of task difficulty varied consistently in a graded manner across condition.
Table 2.
Results for physiological measures, pain reports and task difficulty.
| Measure | Stimulus Level | Condition | |||||
|---|---|---|---|---|---|---|---|
| Low | Medium | High | No Task | Easy | Moderate | Hard | |
| SEP (μVolts) Mean (s.d.) |
38.38 (16.93) |
40.46 (16.48) |
41.42 (17.31) |
45.51 (17.29) |
39.38 (17.20) |
38.59 (16.54) |
38.66 (16.21) |
| PDR (mm) Mean (s.d.) |
0.173 (0.442) |
0.239 (0.475) |
0.340 (0.555) |
0.277 (0.502) |
0.256 (0.494) |
0.234 (0.506) |
0.225 (0.489) |
| Pain Rating Mean (s.d.) |
|||||||
| Worst Pain | 7.49 (1.53) |
6.36 (2.10) |
6.51 (1.91) |
6.29 (1.80) |
|||
| Least Pain | 1.98 (1.24) |
1.81 (1.10) |
1.98 (1.21) |
1.85 (1.29) |
|||
| Average Pain | 4.94 (1.12) |
4.50 (1.34) |
4.57 (1.34) |
4.58 (1.43) |
|||
| Task Difficulty Mean (95% CI) |
|||||||
| Accuracya (%) | 91.3 (87.5- 95.1) |
89.2 (85.4- 93.0) |
86.3 (82.5- 90.1) |
||||
| Response time (ms) |
0.59 (0.57- 0.61 |
0.65 (0.63- 0.66) |
0.67 (0.65- 0.69) |
||||
Task accuracy= (number correct = number false alarms)/total pitch errors
Abbreviations: SEP= stimulus evoked potential; mm= millimeters; PDR= pupil dilation response; S.D.= standard deviation; CI= confidence interval; ms= milliseconds.
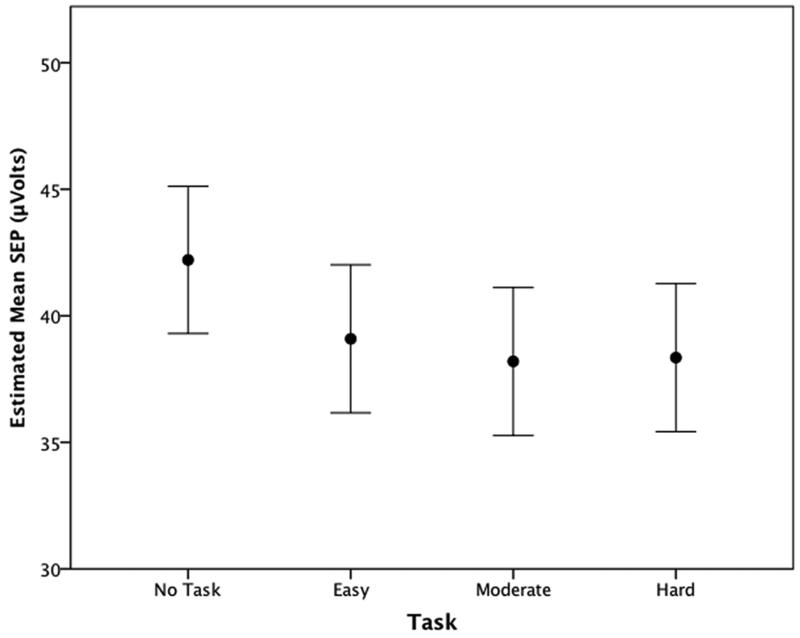
The full model test of SEP yielded significant differences for stimulus level (p<0.001) and task (p<0.001), as well as the habituation/sensitization effect (p<0.001). The test of the linear and quadratic trends showed significant reduction in SEP with increasing task difficulty/complexity, with the quadratic trend providing a better fit to the full model. The likelihood ratio test of approximation to the full model yielded a p approximation value of 0.322 for the quadratic compared to p< 0.001 for the linear trend (larger p is better) (Table 3). As indicated by the size and sign of the slope coefficients β1 and β2, SEP decreases rapidly from No Task to Easy Task then changes more gradually with each successive task level (Figure 2).
Table 3.
Mixed model trend analysis of stimulus response and pain report measures.
| Model | Devianceb | P (change)c | P (approximate)d | β 1 | β 2 |
|---|---|---|---|---|---|
| SEP | |||||
| Fulla | 75228.28 | - | - | - | - |
| Linear | 75269.13 | < 0.001 | <0.001 | −1.40*** | - |
| Quadratice | 75229.26 | < 0.001 | 0.322 | −3.89*** | 0.860*** |
| PDR | |||||
| Fulla | 13139.28 | - | - | - | - |
| Linear | 13139.74 | < 0.001 | 0.795 | −0.018*** | - |
| Quadratic | 13139.36 | 0.538 | 0.777 | −0.027*** | 0.0029 |
| Worst Pain | |||||
| Fulla | 1856.39 | - | - | - | - |
| Linear | 1903.21 | < 0.001 | <0.001 | −.35*** | - |
| Quadratic | 1857.38 | < 0.001 | 0.320 | −1.04*** | 0.23*** |
Task difficulty/complexity modeled as a categorical factor (equivalent to a cubic trend).
−2 times the log likelihood of maximum likelihood solution.
P-value for the null hypothesis that the fit of the higher-order model is not better than (i.e., that the deviance is equal to) that of the lower-order model.
P-value for the null hypothesis that the fit of the reduced model is as good as (i.e., that the deviance is equal to) that of the full model. Higher values are better.
Best fitting models are indicated in bold typeface.
Value of the coefficient is significant at p<0.001.
Figure 2.
Meana stimulus evoked potential changes due to varying task conditions.
a Estimated marginal means and 95% confidence intervals adjusted to allow for repeated measures within subjects.
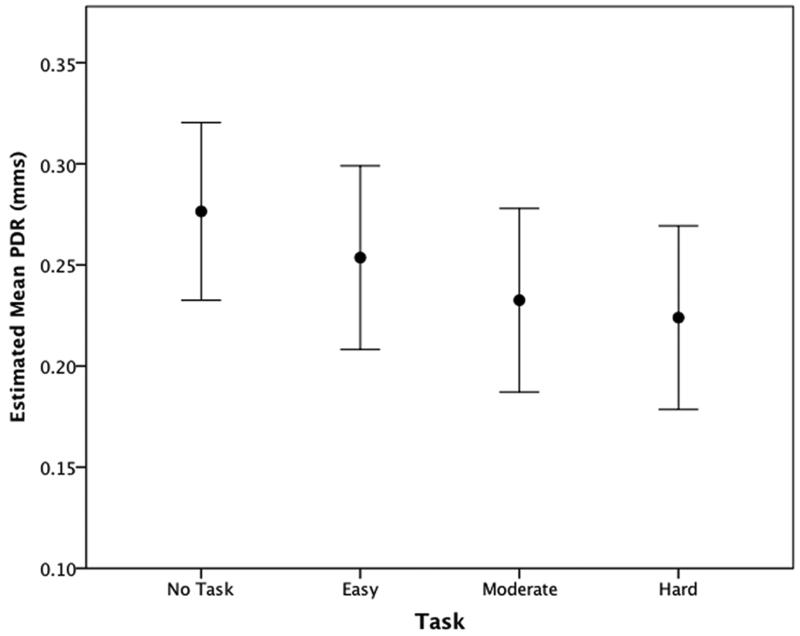
We evaluated variation in PDR with a similar full mixed effects model to test fixed effects of stimulus level, task, and their interaction, adjusting for habituation/sensitization over trials and repeated measures over trials while allowing for individual differences in the intercepts and rate of habituation/sensitization. Significant differences emerged for stimulus level (p<0.001) and task (p<0.001). Habituation/sensitization also proved significant (p=0.01). The test of the linear and quadratic trends showed the linear trend provided the best fit to the full model (p approximation = 0.795 vs 0.777) (Table 3). The slope coefficient indicates a linear reduction in PDR with increasing task difficulty/complexity (Figure 3).
Figure 3.
Meana pupil dilation response changes due to varying task conditions.
a Estimated marginal means and 95% confidence intervals adjusted to allow for repeated measures within subjects.
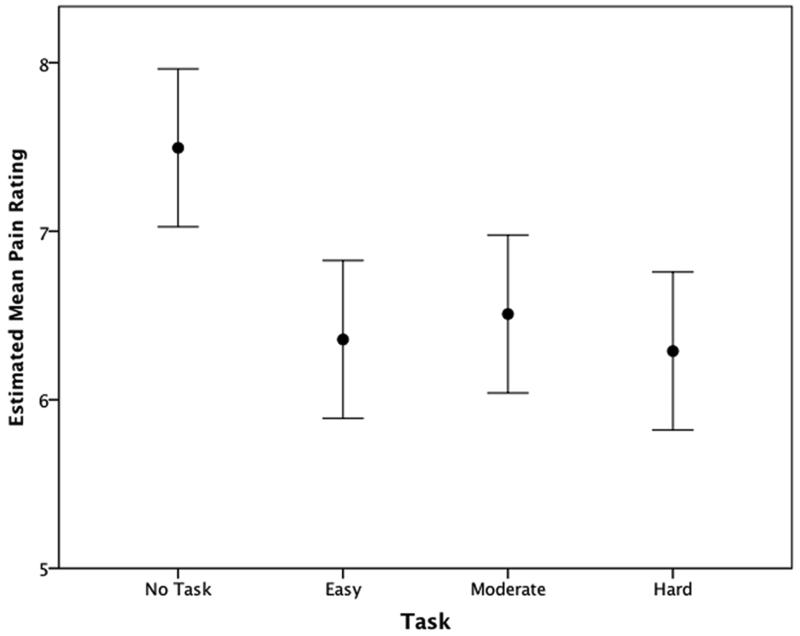
Of the three subjective pain reports, worst pain proved most responsive to manipulations of task and therefore we only evaluated the full model and trends for worst pain. The full mixed effects model tested the fixed effects of mean stimulus current, serving as proxy for stimulus level since the pain ratings measured aggregated responses to multiple stimulus levels. Task but not stimulus current proved significant (p< 0.001 and p=.348, respectively). The quadratic trend provided a better fit to the full model (p approximation = 0.320 vs <0.001) (Table 3). The coefficients indicate a reduction in worst pain that begins sharply from the No Task to Easy Task condition and continues more gradually thereafter (Figure 4).
Figure 4.
Meana worst pain report changes due to varying task conditions.
a Estimated marginal means and 95% confidence intervals adjusted to allow for repeated measures within subjects.
The measures of absorption and state and trait anxiety failed to improve model fits. This indicates that these indicators of individual differences did not contribute significantly to the variation in responses across task conditions. Thus, we did not analyze these measures further.
Discussion
We investigated whether performing a music listening task of increasing difficulty and complexity produces graded reductions in indicators of arousal and pain report accompanying noxious stimulation. Performing the listening task resulted in less reported pain and smaller SEP and PDR, with the size of reductions increasing with increasing level of task difficulty and complexity. Although we predicted linear reductions according to ordered task levels, this prediction held only for PDR. For both SEP and PR, we found a curvilinear relationship, with the largest reduction in responses occurring between no task and easy task and reductions of decreasing size as task levels increased from Easy to Moderate to Hard. On reflection, this finding does not seem particularly surprising. Task performance measures indicated that the task became progressively more difficult corresponding to the designed ordering of task conditions. Nevertheless, the magnitude of the distances from one level to the next could not be carefully equilibrated. It seems reasonable to expect that the largest difference might occur from performing no task while hearing random musical stimuli played, and both hearing a structurally organized melodic phrase and attempting to detect deviant tones in the phrase. Perhaps levels might be found that would subdivide the distance from no task to easy task and thus define a more linear progression in the response vectors. For instance, passively listening to a melody played without performing the detection task might result in smaller response reductions than those found for the easy task.
The finding that the PDR had a linear relationship with task whereas SEP was quadratic is of interest. Pupil dilation has proven to be a reliable indicator of attentional load (39). As such, it might more closely track changes in attention to the stimulus. The changes in response to the single dimension of attention may be more equivalent from one task level to the next. Changes in the SEP, an indicator of overall central nervous system arousal, tend to reflect a summation of cortical activity. Thus, as SEP reflects the change in total activity from one condition to the next, the change from no task to easy task represents a larger change in total activity than the change from easy to moderate to hard tasks.
Interestingly, the pattern of change in PR corresponds more closely to that of the SEP than to the PDR in its relation to ordered task level change. Similar to SEP, PR may represent a summary response to the brain’s overall activity during noxious stimulation and during changes in activity level due to task performance. Thus, as with SEP, PR reflects the larger additive effects of no task compared to task.
Alternative explanations for these results could be made. A noxious stimulus produces an orienting response (40). Perhaps the deviant tones heard during task conditions produced an orienting response as well that could compete with that produced by the noxious stimulus. This explanation is unlikely since deviant tones would be most salient, producing the strongest orienting response and the largest interference with stimulus responses, during the easy task that had no background tones. This effect should be strongest for PDR as an indicator of attention. Rather, PDR was largest for easy compared to moderate and hard task conditions. Although research on effects of music on pain has suggested that positive emotional responses generated by music listening could be responsible for pain reduction (25), we doubt this explanation holds for our task. In fact, some participants reported they found the task somewhat unpleasant or boring. Nevertheless, task performance can elicit a strong desire to do well in some persons. Motivation to pursue a goal, particularly an important one, has been shown to reduce capture of attention by painful events (41). Appetitive motivation may activate descending pain inhibitory pathways (42). Evidence suggests that cognitive distraction tasks during noxious stimulation increase activity in the periacqueductal gray region of the midbrain, an area associated with inhibition of sensory processing of noxious signals (43). Taken together, these studies suggest a mechanism that combines task attention and motivation towards a goal with activation of the descending pain inhibitory pathway (44). We have conceptualized engagement as incorporating motivation and attention as factors that contribute to the constructivist framework but that they are not sufficient in themselves to account for the effects. We suggest that it is the experience of performing the task, i.e, task Engagement, that provides the alternative to and competes with the experience of pain. Motivation may intensify the Engagement experience, and maintaining focus of course requires attentional resources. But motivation and attention are modifiers of the experience itself, which varies autonomously. It is the ongoing dynamic construction of musical schemata that defines this experience, and which disrupts the very formation of the competing pain schemata.
We have proposed engagement as a concept that incorporates the multidimensional activity responsible for constructing reality schemata. Central nervous system arousal as measured by SEP, and attentional load as measured by PDR, provide two possible indicators of the levels of activity required to generate these schemas. In this study, we have shown that changes in these measures reflect change in activity related to central representations of pain. Reductions in these indicators are inversely related to increased engagement in alternative representations, and the degree of reduction provides an indicator of degree of change in engagement. In the study, subjects first engage in experiencing noxious stimulation without competing processing (the No Task control), producing elevated values on these indicators. They then engage in performing music listening tasks of varying difficulty and complexity, with corresponding reductions in the indicators. As the measures are time-locked with stimulus events, they provide indication of level of engagement in pain schema production, rather than production of the competing task schema. It would be of interest to devise a reverse paradigm that evaluated change in task performance resulting from noxious stimulation. Crombez and colleagues (45; 46) performed a series of behavioral studies investigating the disruptive effects of painful stimuli on task performance, attributing these effects to change in attentional demands. A psychophysiological study paradigm that provides for multi-dimensional tasks having graded levels and that can evaluate the effects of task on pain and pain on task may prove useful for revealing in more detail how engagement relates to the cognitive processes involved in pain.
An emerging view of brain activity networks suggests that areas active during the experiencing of pain overlap significantly with areas involved in cognitive processing (e.g., ACC, orbitofrontal and prefrontal cortex) (14; 15) and emotional arousal (e.g., insula, pregenual ACC, amygdala) (15). As our study shows, arousal due to noxious stimulation decreases with ordered increases on the dimensions of task difficulty and complexity. We suggest that increasing the dimensionality of a competing activity will increase the cognitive and emotional resources allocated to that activity relative to those allocated to processing pain. Thus increasing engagement in a competing activity can reduce engagement in the production of pain. Admittedly, the auditory stimuli used in the present study were of very limited dimensionality. As this study represented a first step towards demonstrating graded effects corresponding to graded conditions in a music listening task, we limited the musical selections to very simple melodies that were highly over-learned by participants. This assured us that participants could easily call up the schemata required to readily perform the task. The contextual manipulation of random background tones gave us the ability to carefully control the task difficulty within reasonably tight constraints. However, the resulting listening experience was contrived and did not represent the high dimensionality afforded in music listening experiences that might normally be described as enjoyable and engaging. Creating an activity in which persons can immerse themselves in emotionally arousing music in combination with an increasingly challenging listening effort would certainly involve more cognitive and emotional processing than required in the present study, and thus provide greater engagement and greater reduction in pain-related arousal. Future studies can address the proposed multidimensional activities required to evaluate this conjecture.
Acknowledgements
Funding for this study was provided by the National Center for Complementary and Alternative Medicine (NCCAM) grant 1R21AT001586. The authors have no conflicts of interest to disclose.
Funding for this study was provided by the National Center for Complementary and Alternative Medicine (NCCAM) grant 1R21AT001586.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCaul KD, Monson N, Maki RH. Does distraction reduce pain-produced distress among college students? Health Psychol. 1992;11(4):210–7. doi: 10.1037//0278-6133.11.4.210. [DOI] [PubMed] [Google Scholar]
- 2.Bentsen B, Svensson P, Wenzel A. The effect of a new type of video glasses on the perceived intensity of pain and unpleasantness evoked by a cold pressor test. Anesth Prog. 1999;46(4):113–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Hodes RL, Howland EW, Lightfoot N, Cleeland CS. The effects of distraction on responses to cold pressor pain. Pain. 1990;41(1):109–14. doi: 10.1016/0304-3959(90)91115-Y. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman HG, Patterson DR, Carrougher GJ, Sharar SR. Effectiveness of virtual reality-based pain control with multiple treatments. Clin J Pain. 2001;17(3):229–35. doi: 10.1097/00002508-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Haythornthwaite JA, Lawrence JW, Fauerbach JA. Brief cognitive interventions for burn pain. Ann Behav Med. 2001;23(1):42–9. doi: 10.1207/S15324796ABM2301_7. [DOI] [PubMed] [Google Scholar]
- 6.Frankenstein UN, Richter W, McIntyre MC, Remy F. Distraction modulates anterior cingulate gyrus activations during the cold pressor test. Neuroimage. 2001;14(4):827–36. doi: 10.1006/nimg.2001.0883. [DOI] [PubMed] [Google Scholar]
- 7.Yamasaki H, Kakigi R, Watanabe S, Hoshiyama M. Effects of distraction on pain-related somatosensory evoked magnetic fields and potentials following painful electrical stimulation. Brain Res Cogn Brain Res. 2000;9(2):165–75. doi: 10.1016/s0926-6410(99)00056-7. [DOI] [PubMed] [Google Scholar]
- 8.Friederich M, Trippe RH, Ozcan M, Weiss T, Hecht H, Miltner WH. Laser-evoked potentials to noxious stimulation during hypnotic analgesia and distraction of attention suggest different brain mechanisms of pain control. Psychophysiology. 2001;38(5):768–76. [PubMed] [Google Scholar]
- 9.Van Damme S, Crombez G, Van Nieuwenborgh-De Wever K, Goubert L. Is distraction less effective when pain is threatening? An experimental investigation with the cold pressor task. Eur J Pain. 2008;12(1):60–7. doi: 10.1016/j.ejpain.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Campbell CM, Witmer K, Simango M, Carteret A, Loggia ML, Campbell JN, Haythornthwaite JA, Edwards RR. Catastrophizing delays the analgesic effect of distraction. Pain. 2010;149(2):202–7. doi: 10.1016/j.pain.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verhoeven K, Crombez G, Eccleston C, Van Ryckeghem DM, Morley S, Van Damme S. The role of motivation in distracting attention away from pain: an experimental study. Pain. 2010;149(2):229–34. doi: 10.1016/j.pain.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Goubert L, Crombez G, Eccleston C, Devulder J. Distraction from chronic pain during a pain-inducing activity is associated with greater post-activity pain. Pain. 2004;110(1-2):220–7. doi: 10.1016/j.pain.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 13.Quartana PJ, Burns JW, Lofland KR. Attentional strategy moderates effects of pain catastrophizing on symptom-specific physiological responses in chronic low back pain patients. J Behav Med. 2007;30(3):221–31. doi: 10.1007/s10865-007-9101-z. [DOI] [PubMed] [Google Scholar]
- 14.Buhle J, Wager TD. Performance-dependent inhibition of pain by an executive working memory task. Pain. 2010;149(1):19–26. doi: 10.1016/j.pain.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiech K, Seymour B, Kalisch R, Stephan KE, Koltzenburg M, Driver J, Dolan RJ. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27(1):59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 16.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288(5472):1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 17.Chapman CR. Psychological Aspects of Pain: A Consciousness Studies Perspective. In: Pappagallo M, editor. The Neurological Basis of Pain. MacGraw-Hill; New York: 2005. pp. 157–70. [Google Scholar]
- 18.Wadsworth BJ. Piaget’s Theory of Cognitive and Affective Development: Foundations of Constructivism. Longman Publishing; New York: 1995. [Google Scholar]
- 19.Rumelhart DE, Hinton GE, Williams RJ. Learning internal representations by error propagation. In: Rumelhart DR, McClelland JD, editors. Parallel Distributed Processing. Vol. 1. MIT Press; 1986. pp. 318–62. [Google Scholar]
- 20.Johnson MH. How does distraction work in the management of pain? Curr Pain Headache Rep. 2005;9(2):90–5. doi: 10.1007/s11916-005-0044-1. [DOI] [PubMed] [Google Scholar]
- 21.Baars BJ. In the Theater of Consciousness: the Workspace of the Mind. Oxford University Press; New York: 1996. [Google Scholar]
- 22.Kohut H, Levarie S. On the enjoyment of listening to music. The Psychoanalytic Quarterly. 1950;19:64–87. [Google Scholar]
- 23.Cepeda M, Carr D, Lau J, Alvarez H. Music for pain relief. Cochrane Database Syst Rev. 2006;(2):CD004843. doi: 10.1002/14651858.CD004843.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell LA, MacDonald RA, Brodie EE. A comparison of the effects of preferred music, arithmetic and humour on cold pressor pain. Eur J Pain. 2006;10(4):343–51. doi: 10.1016/j.ejpain.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Roy M, Peretz I, Rainville P. Emotional valence contributes to music-induced analgesia. Pain. 2008 doi: 10.1016/j.pain.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Gaver WW, Mandler G. Play it again, Sam: On liking music. Cognition and Emotion. 1987;1(3):259–82. [Google Scholar]
- 27.Abe J, Hoshino E. Schema driven properties in melody cognition: Experiments on final tone extrapolation by music experts. Psychomusicology. 1990;9(2):161–72. [Google Scholar]
- 28.Deliege I. Cue abstraction as a component of categorisation processes in music listening. Psychology of Music. 1996;24(2):131–56. [Google Scholar]
- 29.Povel DJ, Jansen E. Perceptual mechanisms in music processing. Music Perception. 2001;19(2):169–97. [Google Scholar]
- 30.Narmour E. Music expectation by cognitive rule-mapping. Music Perception. 2000;17(3):329–98. [Google Scholar]
- 31.Huber C, Kunz M, Artelt C, Lautenbacher S. Attentional and emotional mechanisms of pain processing and their related factors: a structural equations approach. Pain Res Manag. 2010;15(4):229–37. doi: 10.1155/2010/516176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews G, Campbell SE, Falconer S, Joyner LA, Huggins J, Gilliland K, Grier R, Warm JS. Fundamental dimensions of subjective state in performance settings: task engagement, distress, and worry. Emotion. 2002;2(4):315–40. doi: 10.1037/1528-3542.2.4.315. [DOI] [PubMed] [Google Scholar]
- 33.Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods and findings in experimental and clinical pharmacology. 1984;6(7):405–10. [PubMed] [Google Scholar]
- 34.Tellegen A, Atkinson G. Openness to absorbing and self-altering experiences ("absorption"), a trait related to hypnotic susceptibility. Journal of abnormal psychology. 1974;83(3):268–77. doi: 10.1037/h0036681. [DOI] [PubMed] [Google Scholar]
- 35.Spielberger CD. Manual for the state-trait anxiety inventory (Form Y) Consulting Psychologists Press, Inc.; Palo Alto, CA: 1970. [Google Scholar]
- 36.Kajimura N, Kato M, Sekimoto M, Watanabe T, Takahashi K, Okuma T, Mizuki Y, Yamada M. A polysomnographic study of sleep patterns in normal humans with low- or high-anxiety personality traits. Psychiatry and clinical neurosciences. 1998;52(3):317–20. doi: 10.1046/j.1440-1819.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 37.Carleton RN, Abrams MP, Asmundson GJ. The Attentional Resource Allocation Scale (ARAS): psychometric properties of a composite measure for dissociation and absorption. Depress Anxiety. 2010;27(8):775–86. doi: 10.1002/da.20656. [DOI] [PubMed] [Google Scholar]
- 38.Platt RD, Lacey SC, Iobst AD, Finkelman D. Absorption, dissociation, and fantasy-proneness as predictors of memory distortion in autobiographical and laboratory-generated memories. Applied Cognitive Psychology. 1998;12:S77–S89. [Google Scholar]
- 39.Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154(756):1583–5. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- 40.Dowman R. Attentional set effects on spinal and supraspinal responses to pain. Psychophysiology. 2001;38(3):451–64. [PubMed] [Google Scholar]
- 41.Van Damme S, Legrain V, Vogt J, Crombez G. Keeping pain in mind: a motivational account of attention to pain. Neurosci Biobehav Rev. 2010;34(2):204–13. doi: 10.1016/j.neubiorev.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5(7):565–75. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 43.Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85(1-2):19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- 44.Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144(3):230–2. doi: 10.1016/j.pain.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 45.Crombez G, Eccleston C, Baeyens F, Eelen P. Habituation and the interference of pain with task performance. Pain. 1997;70(2-3):149–54. doi: 10.1016/s0304-3959(96)03304-0. [DOI] [PubMed] [Google Scholar]
- 46.Crombez G, Eccleston C, Baeyens F, Eelen P. The disruptive nature of pain: an experimental investigation. Behav Res Ther. 1996;34(11-12):911–8. doi: 10.1016/s0005-7967(96)00058-7. [DOI] [PubMed] [Google Scholar]