Abstract
Glucagon-like peptide-1 (GLP-1) is released in response to nutrient ingestion and is a regulator of energy metabolism and consummatory behaviors through both peripheral and central mechanisms. The GLP-1 receptor (GLP-1R) is widely distributed in the central nervous system, however little is known about how GLP-1Rs regulate ambulatory behavior. The abused psychostimulant amphetamine (AMPH) promotes behavioral locomotor activity primarily by inducing the release of the neurotransmitter dopamine. Here, we identify the GLP-1R agonist exendin-4 (Ex-4) as a modulator of behavioral activation by AMPH. We report that in rats a single acute administration of Ex-4 decreases both basal locomotor activity as well as AMPH-induced locomotor activity. Ex-4 did not induce behavioral responses reflecting anxiety or aversion. Our findings implicate GLP-1R signaling as a novel modulator of psychostimulant-induced behavior and therefore a potential therapeutic target for psychostimulant abuse.
Keywords: amphetamine, psychostimulant, Glucagon-like peptide-1, exenatide, dopamine
1. Introduction
Glucagon-like peptide-1 (GLP-1) is an endogenous peptide secreted from the gastrointestinal tract in response to nutrient ingestion and is a physiological regulator of glucose homeostasis and food intake [1, 2]. The effects of GLP-1 are best understood in the pancreas where it enhances glucose-dependent insulin secretion. GLP-1 receptor (GLP-1R) agonists are currently in clinical use for the treatment of type II diabetes [3]. The first GLP-1 analog to be approved for human clinical use was exenatide, a synthetic form of the naturally occurring Gila monster (Heloderma suspectum) peptide exendin-4 (Ex-4). This peptide exhibits about 50% amino acid identity with human GLP-1 and is a potent agonist of human and rodent GLP-1Rs [4, 5]. While GLP-1 is rapidly inactivated in circulation by cleavage by the proteolytic enzyme dipeptidyl peptidase IV [6], Ex-4 is resistant to this cleavage. Thus Ex-4 has a significantly longer half-life and pharmacokinetic efficacy in vivo than GLP-1. Furthermore, systemically administered Ex-4 readily crosses the blood brain barrier [7–9]. In this study, we used Ex-4 to investigate the effect of GLP-1R signaling on both basal locomotion and locomotor activation by AMPH. We also evaluated the behavioral effects of Ex-4 by using a conditioned place assay. This is a standard behavioral test for assessing the positive (“preference”) or negative (“aversion”) state that an animal associates with administration of a drug.
GLP-1Rs are broadly distributed throughout the central nervous system [10, 11]. In addition to release from the gut into circulation, GLP-1 is also produced in the brain by neurons in the nucleus tractus solatarius (NTS). NTS GLP-1 neurons project widely to brain regions including the ventral tegmental area (VTA) and the nucleus accumbens (NAc) [12–17]. GLP-1R signaling in the brain is known to regulate food intake as well as other behaviors [16, 18–20].
There are well established links between regulation of food intake and responses to drugs of abuse [21, 22]. Several neuropeptides regulating food intake have also been demonstrated to modulate the behavioral responses to drugs of abuse including psychostimulants [23–25]. It has also been shown that GLP-1R signaling in brain regulates ambulatory activity [20, 26]. However, whether GLP-1R signaling can modulate the locomotor behavioral response to the abused psychostimulant amphetamine (AMPH) is unknown. We hypothesized that GLP-1R activation may regulate the locomotor activating properties of AMPH. Here, we demonstrate that in rats a single acute administration of the GLP-1R agonist Ex-4 blunts the locomotor activating response to AMPH, implicating GLP-1R signaling as a novel modulator of psychostimulant induced behavior.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (275–300g, Charles River) were housed in a facility kept on a 12-hour light cycle. Subjects were acclimated for one week housed two per cage. After the week of acclimation, rats were handled and given an intraperitoneal (i.p.) saline injection (1 mL/kg) once a day for 3 days. All behavior tests were performed at the Vanderbilt Murine Neurobehavioral Lab core facility. Rats had continuous access to standard chow and water ad libidum in their home cages including on the day of experiments. All assays were initiated during the light cycle 3–4 h after lights on.
2.2. Experiment 1: Locomotor Activity Assay
This experiment tested whether i.p. injection with Ex-4 (compared with saline control) modified the subsequent locomotor response to AMPH during the same testing session.
Rats were handled and injected i.p. with saline for three days in order to minimize stress. On the fourth day, rats were given an i.p. saline injection and habituated to an open field locomotor activity chamber (San Diego Instruments) for 60 min. 24 h following the habituation session the experimental test of Ex-4 effects on locomotion was conducted. See Figure 1A for a schematic representation of the drug treatment groups and time course. There were two drug treatment times in the protocol. The first injection was at time 30 min (where time 0 is the placement of the rat in the chamber) and the second injection was at time 120 min. The rats were studied in the following drug groups of pairs of injections with the number of animals for each group indicated in parentheses: saline/AMPH (n=8), Ex-4/AMPH (n=8), saline/saline (n=8), Ex-4/saline (n=7), Statistical analysis across all 4 groups was performed by a 2 way ANOVA followed by Bonferroni post hoc tests. Drugs were administered i.p. at the following doses: saline (1 mL/kg), Ex-4 (30 μg/kg), AMPH i.p. (2.78 mg/kg). The Ex-4 dose was chosen to be consistent with previous reports of i.p. administration of Ex-4 modifying behavior [27–29]. The AMPH dose was chosen to be consistent with our previous studies of AMPH i.p. administration and within the common range in the literature ([30–32]). The 90 minute interval between injections was chosen based on the pharmacokinetics of Ex-4 [33] and the behavioral time course of Ex-4 on food intake [28]. At this time point, saline or Ex-4 injected rats also exhibited equal locomotor activity (p>0.05 Bonferroni posthoc test following 2 way ANOVA). Locomotor activity was recorded by an infrared photobeam array and analyzed by Photobeam Activity System (PAS) software (San Diego Instruments). Locomotor activity is expressed as distance traveled (cm).
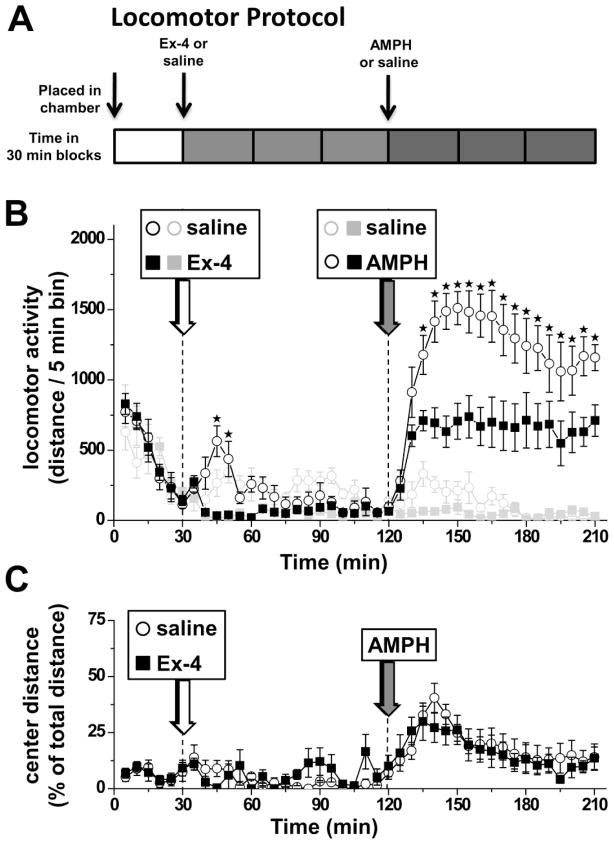
Figure 1. Ex-4 decreases basal and AMPH-induced locomotor activity.
A) The diagram depicts the timeline for the experimental protocol. Injection times are indicated by arrows. B) Locomotor activity is plotted in 5 min blocks for saline/AMPH (open black circles), Ex-4/AMPH (closed black squares), saline/saline (open gray circles), and Ex-4/saline (closed gray squares). Drugs were administered i.p. at the following doses: Ex-4 (30 μg/kg), AMPH (2.78 mg/kg). Statistical analysis across all 4 groups was performed by a 2 way ANOVA followed by Bonferroni post hoc tests. Stars indicate significant difference between saline/AMPH (open black circles) and EX-4/AMPH (closed black squares) groups (*p<0.05). C) Center distance was quantified throughout the locomotor experiment as an index of anxiety-like behavior. The relative distance traveled in the center of the chamber is expressed as a percentage of total distance. There was no significant difference between saline/AMPH (open black circles) and EX-4/AMPH (closed black squares) groups (2 way ANOVA p>0.05).
2.3. Experiment 2: Conditioned Place Assay
This experiment tested whether or not i.p. injection with Ex-4 (compared with saline control) paired with specific sensory cues induced a conditioned place aversion (CPA) to that context. CPA induction by Ex-4 would be consistent with a subjectively negative behavioral state following Ex-4 injection that may explain at least in part a decrease in locomotor activity.
The conditioned place apparatus (Med Associates) consisted of two chambers. One side had checkered walls and a smooth floor. The other side had striped walls and a textured floor. Thus, the chamber consisted of two distinct sides, both visually and texturally. The conditioned place paradigm consisted of: a) a pre-test; b) training days; and c) a post-test. Time in each chamber and locomotor activity were monitored with video tracking software (ANY-Maze, Stoelting Co., Wood Dale, IL). Rats were assigned to the following drug groups for two respective days of training drug injections. The total number of subjects for each group is indicated in parentheses: Ex-4/saline (n=8), saline/saline (n=8). Drugs were administered i.p. at the same doses as in experiment 1 saline (1 mL/kg), Ex-4 (30 μg/kg).
During the pre-test, animals were injected with saline then placed in the chamber and allowed to explore both sides freely. The relative time spent on each side was monitored and used to account for any unconditioned preference. There was no significant difference between the two groups in terms of locomotor activity during the pre-test (p>0.05 by Student’s t test; n=8). Two training association sessions were conducted on subsequent days in a randomized order. On “training day 1”, animals were restricted to one side of the chamber for 30 min following injection of either saline (control) or Ex-4. On “training day 2”, animals were restricted to the other side of the chamber and administered saline.
The experimental (post-test) day consisted of the rats having free access to both sides of the chamber for 30 min following a saline injection. There was no difference between groups in locomotor activity on the post-test day (p>0.05 by Student’s t test; n=8). The difference score index of place preference or aversion was calculated as post-test time minus pre-test time for the drug paired (training day 1) side of the chamber.
In an independent cohort of rats, the aversive stimulus LiCl was tested as a positive control for the conditioned place aversion assay. The experiment design was identical with the exception that the two groups for drug injection were LiCl/saline (n = 8) and saline/saline (n = 6). Drugs were administered i.p. at the following doses: saline (1 mL/kg), LiCl (127 mg/kg). This dose of LiCl has been demonstrated to induce conditioned place aversion [34–36].
2.4 Statistical analysis
Data are presented as mean ± s.e.m. Statistical analysis was performed in Prism software (Graphpad).
3. Results
3.1. Ex-4 decreases basal and AMPH-induced locomotion
In Experiment 1, Ex-4 was used to investigate the effect of GLP-1R signaling on both basal locomotion and locomotor activation by AMPH. The schematic of the protocol is illustrated in Figure 1A. Locomotor activity was monitored in 5 minute intervals and analyzed across all drug treatment groups over time by 2 way ANOVA followed by Bonferroni posthoc tests. Rats were placed in a locomotor activity chamber for 30 min and then given an i.p. injection of either Ex-4 or saline (Fig. 1B, white arrow). Within 15 minutes following drug treatment, rats injected with Ex-4 (Fig. 1B, filled black squares) exhibited reduced basal locomotor activity compared with saline controls (Fig. 1B, open black circles). However, 90 min after the Ex-4 or saline injection rats displayed no significant difference in locomotor activity. At this time point, rats were injected i.p. with AMPH (Fig. 1B, gray arrow). As expected, the saline/AMPH group rats increased locomotor activity dramatically in response to AMPH (Fig. 1B, open black circles). Importantly, Ex-4/AMPH rats displayed a blunted locomotor response to AMPH (Fig. 1B, filled black squares). Notably, this difference remained significant up to the longest time point monitored after AMPH.
To control for an effect of Ex-4 on the general reactivity of the animals to an injection, we tested a saline injection instead of AMPH (gray symbols). The animals received first Ex-4 (filled gray squares) or saline (open gray circles) at time 30 min (Fig. 1B, white arrow). 90 min later, in the same session, both groups received an injection of saline (Fig. 1B, gray arrow). There was no significant difference in the locomotor activity between saline/saline (open gray circles) and Ex-4/saline (filled gray squares) groups at any time point (p>0.05; 2 way ANOVA followed by Bonferroni posthoc tests).
GLP-1 has been suggested to be associated with the satiety response following food consumption [20, 37] and activating GLP-1 receptors may induce a resting behavior that is similar to that observed during satiety after feeding [38]. Alternatively, the decrease in ambulatory activity following Ex-4 could result from inducing anxiety-like behavior [39] and/or a negative subjective or affective state [40]. We addressed both of these issues experimentally.
3.2. Ex-4 does not induce an anxiety-like behavior
In Experiment 1, we also quantified the relative distance traveled in the center of the chamber expressed as a percentage of total distance (Fig. 1C). The center distance-to-total distance ratio (thigmotaxis) can be used as an index of anxiety-related responses [41]. Ex-4/AMPH rats displayed no difference from saline/AMPH rats in center distance following AMPH injection (Fig. 1C gray arrow). These data are consistent with the hypothesis that Ex-4 inhibits basal and AMPH-induced locomotion without inducing an anxiety-related response.
3.3. Ex-4 does not induce conditioned place aversion
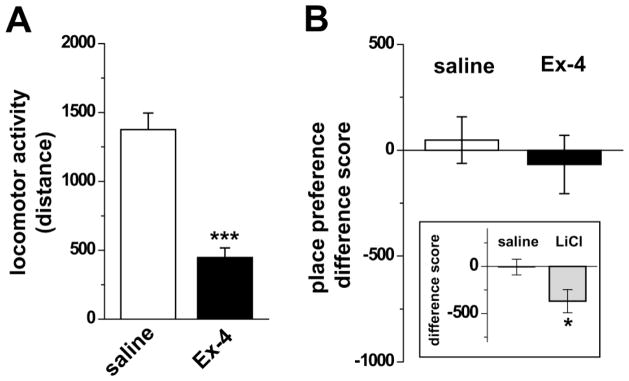
In Experiment 2, we determined whether the reduction in locomotion was associated with aversive effects of Ex-4 by using a conditioned place assay (Fig. 2). Rats were assigned into two groups for drug injection on alternate training days for each rat: Ex-4/saline and saline/saline. The training (conditioning) days paired drug injection with sensory cues on a specific chamber side. Similar to the open field paradigm in Experiment 1 (Fig. 1), during the drug training Ex-4 decreased locomotor activity in the conditioned place chamber compared to saline controls (Fig. 2A), The preference or aversion was quantified by the “difference score” of time spent on the test drug paired side induced by drug association. Ex-4 induced no significant change in the difference score compared to the saline control rats (Fig. 2B, compare Ex-4 (open bar) to saline (filled bar); p>0.05; n=8), demonstrating that Ex-4 did not induce a conditioned place aversion in our paradigm.
Figure 2. Ex-4 does not induce conditioned place aversion.
A) Ex-4 (30 μg/kg, i.p.) decreases locomotor activity in the chamber during the training session compared with control saline treated animals. (***p<0.001; Student’s t-test). B) The difference score quantifies the change in the amount of time (s) spent on the drug associated side of the chamber induced by the drug exposure training. Ex-4 did not significantly alter the difference score compared to saline controls and was therefore not aversive. (p>0.05; Student’s t-test). Inset: As a positive control for the CPA assay, LiCl (127 mg/kg i.p.) treated rats displayed a significantly altered difference score compared to saline controls. (*p<0.01; Student’s t-test). The negative difference score indicates that rats spent less time on the chamber side associated with LiCl, reflecting CPA to LiCl administration.
3.4. LiCl does induce a conditioned place aversion
As a positive control for the conditioned place assay, we tested the well characterized aversive stimulus LiCl [34–36]. In a parallel experiment we applied to LiCl the same behavioral paradigm tested above for Ex-4. LiCl did induce a conditioned place aversion as LiCl exposure resulted in a significant shift in the difference score for time on the drug associated chamber side (Fig. 2B inset, compare LiCl (gray bar) to the saline bar). This result validates the assay as capable of resolving conditioned place aversion by the control known aversive drug LiCl. Therefore, in our paradigm, a single exposure to Ex-4 is not aversive. These data suggest that Ex-4 inhibits ambulatory activity by a mechanism not dependent on inducing an aversive behavioral state.
4. Discussion
4.1. Ex-4 regulates AMPH-induced locomotion
The major conclusion of this study is that systemic administration of the GLP1-R agonist Ex-4 decreases both basal and AMPH-induced locomotor activity (Fig. 1B). This observation may be specific to the drug dosage and timing of our protocol. Future studies characterizing the full dosage and time dependence of both Ex-4 and AMPH may be important in elucidating details of this effect. GLP-1 has previously been reported to decrease basal locomotor activity when injected (i.c.v.) directly into the brain [20]. Consistently, a GLP-1 receptor antagonist administered i.c.v. increases locomotor activity [26]. Given that Ex-4 readily crosses the blood brain barrier [7–9], it is likely that, at least in part, Ex-4 decreases basal ambulatory activity by targeting GLP-1Rs in brain. Notably, we show that Ex-4 blunts the locomotor activating response to AMPH. Since the NAc is a key brain region for AMPH-induced locomotion [42, 43], it is possible that activation of GLP-1Rs in the NAc [16, 17] may decrease AMPH-induced locomotion. This is a logical but still putative working hypothesis based on the observations reported here but will require future evaluation by detailed mechanistic investigations, including probing the role of specific neurotransmitters. Certainly, mechanisms other than GLP-1R activation in the NAc remain plausible. For example, activation GLP-1Rs in other brain regions such as the hypothalamus [44, 45] as well as peripheral GLP-1Rs [46] may contribute to blunting of AMPH-induced locomotor activity.
4.2. Does Ex-4 regulation of locomotion reflect anxiety?
There are a wide range of effects reported in response to activating GLP-1Rs in the brain including regulating food intake, glucose homeostasis, the hypothalamic-pituitary-adrenal axis, the sympathetic nervous system, and the parasympathetic nervous system [20, 39, 47–49]. While GLP1-Rs have been reported to modulate the hypothalamic-pituitary-adrenal axis [39, 50, 51], it is unclear whether activation of GLP-1Rs directly induces anxiety-like behavior. However, in humans intravenously administered GLP-1 does not have anxiogenic properties, even in subjects with panic disorder at highest risk for such reactions [52]. Exenatide (Ex-4) therapy has been reported to decrease a clinical index of anxiety [53]. Consistently, we observe no effect of Ex-4 on relative center distance in rats (a behavioral correlate of an anxiety-like phenotype) concurrent with alterations in locomotor behavior (Fig. 1C,D).
4.3. Does Ex-4 regulation of locomotion reflect aversion?
Another alternative explanation for the decrease in locomotor activity would be induction of a visceral illness or malaise similar to that observed in response to LiCl [36]. In contrast to LiCl, we did not observe behavioral aversion with Ex-4 (Fig. 2). This suggests that, in our experimental paradigm, activation of GLP-1Rs alone is insufficient to induce an aversive behavioral state and that reduction in locomotor activity occurs in the absence of such a process.
There is mixed evidence concerning the question of whether behavioral responses to GLP-1R activation are due to satiety or to an aversive-like state or malaise [54–56]. However, either acute or chronic administration of a GLP-1R antagonist in the brain results in hyperphagia without evidence of visceral illness [46, 55], suggesting an important role of endogenous GLP-1R signaling in regulating behavior independent of any potential role in mediating visceral responses to aversive stimuli. Furthermore, studies in both humans and non-human primates suggest GLP-1 reduces food intake through a satiety effect and not food aversion [57–60]. For example, monkeys administered Ex-4, even at high doses that essentially prevented food intake, “did not exhibit any signs of nausea, vomiting, nor malaise…”, but “…simply seemed uninterested in acquiring food” [60]. Our finding in rats of decreased locomotion in the absence of aversion is also consistent with a satiety response to Ex-4 and with Turton et al who noted anecdotally that the behavior of GLP-1 treated rats “could not be distinguished, by observation, from those fed a palatable meal” [20].
AMPH acts primarily by increasing the extracellular concentration of dopamine (DA), a monoamine considered a key neurotransmitter for the rewarding properties of drugs of abuse [61–64]. Interestingly, GLP-1 analogs decrease preference for “candy” (palatable food) intake in rats [65] and GLP-1Rs in the NAc mediate a component of food reward [16]. These data suggest that GLP-1R signaling may regulate feeding, in part, by modulating reward systems including DA. Future studies will be necessary to evaluate GLP-1R signaling as a possible modulator of psychostimulant reward and therefore potential therapeutic target for psychostimulant abuse. Notably, the absence of Ex-4 induced conditioned place preference in our data (Figure 2C) is consistent with Ex-4 not having inherent rewarding properties that could potentially compromise the utility of GLP-1R agonists as possible treatments for drug abuse.
4.4. Conclusions
The key observation reported in this study is that administration of Ex-4 blunts the locomotor response to the psychostimulant AMPH. Although the pharmacology of AMPH-like psychostimulants is well understood, there are still no efficacious therapeutics for the treatment of abuse of this class of drugs [66]. In this study, we demonstrate that administration of the GLP-1R agonist Ex-4 blunts the locomotor response to AMPH, implicating GLP-1R signaling as a potentially novel modulator of behaviors associated with the DA system and also as a potential therapeutic target for psychostimulant abuse.
Acknowledgments
We thank Nicole Bibus Christianson for excellent technical support and Lotte Bjerre Knudsen for constructive comments on the manuscript.
Funding
NIH K99DA025716 (KE); NARSAD Young Investigator (KE); NIH R01DK085712 (AG)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kevin Erreger, Email: kevin.erreger@vanderbilt.edu.
Adeola R. Davis, Email: adeola.davis@vanderbilt.edu.
Amanda M. Poe, Email: amanda.poe@vanderbilt.edu.
Nigel H. Greig, Email: greign@grc.nia.nih.gov.
Gregg D. Stanwood, Email: gregg.stanwood@Vanderbilt.Edu.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–57. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Holst JJ, Deacon CF, Vilsboll T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14:161–8. doi: 10.1016/j.molmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–5. [PubMed] [Google Scholar]
- 5.Goke R, Fehmann HC, Linn T, Schmidt H, Krause M, Eng J, et al. Exendin-4 is a high potency agonist and truncated exendin-(9–39)-amide an antagonist at the glucagon-like peptide 1-(7–36)-amide receptor of insulin-secreting beta-cells. J Biol Chem. 1993;268:19650–5. [PubMed] [Google Scholar]
- 6.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–96. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 7.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes Relat Metab Disord. 2003;27:313–8. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 8.Kastin AJ, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 9.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–12. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez E, Roncero I, Chowen JA, Thorens B, Blazquez E. Expression of the glucagon-like peptide-1 receptor gene in rat brain. J Neurochem. 1996;66:920–7. doi: 10.1046/j.1471-4159.1996.66030920.x. [DOI] [PubMed] [Google Scholar]
- 11.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271:519–32. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 13.Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience. 1997;77:257–70. doi: 10.1016/s0306-4522(96)00434-4. [DOI] [PubMed] [Google Scholar]
- 14.Llewellyn-Smith IJ, Reimann F, Gribble FM, Trapp S. Preproglucagon neurons project widely to autonomic control areas in the mouse brain. Neuroscience. 180:111–21. doi: 10.1016/j.neuroscience.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinaman L. Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res. 1350:18–34. doi: 10.1016/j.brainres.2010.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 Neurons in the Nucleus of the Solitary Tract Project Directly to the Ventral Tegmental Area and Nucleus Accumbens to Control for Food Intake. Endocrinology. 2011 doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Silva A, Salem V, Long CJ, Makwana A, Newbould RD, Rabiner EA, et al. The Gut Hormones PYY(3–36) and GLP-1(7–36 amide) Reduce Food Intake and Modulate Brain Activity in Appetite Centers in Humans. Cell Metab. 2011;14:700–6. doi: 10.1016/j.cmet.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003;9:1173–9. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- 20.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 21.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and Drug Reward: Overlapping Circuits in Human Obesity and Addiction. Curr Top Behav Neurosci. 2011 doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 22.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci. 2011;12:638–51. doi: 10.1038/nrn3105. [DOI] [PubMed] [Google Scholar]
- 23.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and Behavioral Sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Cummings DE, Naleid AM, Lattemann DPF. Ghrelin: a link between energy homeostasis and drug abuse? Addiction Biology. 2007;12:1–5. doi: 10.1111/j.1369-1600.2007.00053.x. [DOI] [PubMed] [Google Scholar]
- 25.Opland DM, Leinninger GM, Myers MG. Modulation of the mesolimbic dopamine system by leptin. Brain Research. 2010;1350:65–70. doi: 10.1016/j.brainres.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knauf C, Cani PD, Ait-Belgnaoui A, Benani A, Dray C, Cabou C, et al. Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology. 2008;149:4768–77. doi: 10.1210/en.2008-0180. [DOI] [PubMed] [Google Scholar]
- 27.Mack CM, Moore CX, Jodka CM, Bhavsar S, Wilson JK, Hoyt JA, et al. Antiobesity action of peripheral exenatide (exendin-4) in rodents: effects on food intake, body weight, metabolic status and side-effect measures. Int J Obes (Lond) 2006;30:1332–40. doi: 10.1038/sj.ijo.0803284. [DOI] [PubMed] [Google Scholar]
- 28.Peters CT, Choi YH, Brubaker PL, Anderson GH. A glucagon-like peptide-1 receptor agonist and an antagonist modify macronutrient selection by rats. J Nutr. 2001;131:2164–70. doi: 10.1093/jn/131.8.2164. [DOI] [PubMed] [Google Scholar]
- 29.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48:1026–34. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 30.Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, et al. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5:e274. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pijnenburg AJ, Honig WM, Van Rossum JM. Inhibition of d-amphetamine-induced locomotor activity by injection of haloperidol into the nucleus accumbens of the rat. Psychopharmacologia. 1975;41:87–95. doi: 10.1007/BF00421062. [DOI] [PubMed] [Google Scholar]
- 32.Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165:359–69. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- 33.Parkes D, Jodka C, Smith P, Nayak S, Rinehart L, Gingerich R, et al. Pharmacokinetic actions of exendin-4 in the rat: Comparison with glucagon-like peptide-1. Drug Development Research. 2001;53:260–7. [Google Scholar]
- 34.Meachum CL, Bernstein IL. Behavioral conditioned responses to contextual and odor stimuli paired with LiCl administration. Physiol Behav. 1992;52:895–9. doi: 10.1016/0031-9384(92)90368-c. [DOI] [PubMed] [Google Scholar]
- 35.Parker LA. Place conditioning in a three- or four-choice apparatus: role of stimulus novelty in drug-induced place conditioning. Behav Neurosci. 1992;106:294–306. doi: 10.1037//0735-7044.106.2.294. [DOI] [PubMed] [Google Scholar]
- 36.Tenk CM, Kavaliers M, Ossenkopp KP. Dose response effects of lithium chloride on conditioned place aversions and locomotor activity in rats. Eur J Pharmacol. 2005;515:117–27. doi: 10.1016/j.ejphar.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Flint A, Raben A, Astrup A, Holst JJ. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101:515–20. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halford JC, Wanninayake SC, Blundell JE. Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav. 1998;61:159–68. doi: 10.1016/s0091-3057(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 39.Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, et al. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–70. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, et al. The role of CNS glucagon-like peptide-1 (7–36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci. 2000;20:1616–21. doi: 10.1523/JNEUROSCI.20-04-01616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treit D, Fundytus M. Thigmotaxis as a test for anxiolytic activity in rats. Pharmacol Biochem Behav. 1988;31:959–62. doi: 10.1016/0091-3057(88)90413-3. [DOI] [PubMed] [Google Scholar]
- 42.Heusner CL, Hnasko TS, Szczypka MS, Liu Y, During MJ, Palmiter RD. Viral restoration of dopamine to the nucleus accumbens is sufficient to induce a locomotor response to amphetamine. Brain Res. 2003;980:266–74. doi: 10.1016/s0006-8993(03)02986-x. [DOI] [PubMed] [Google Scholar]
- 43.Chartoff EH, Heusner CL, Palmiter RD. Dopamine is not required for the hyperlocomotor response to NMDA receptor antagonists. Neuropsychopharmacology. 2005;30:1324–33. doi: 10.1038/sj.npp.1300678. [DOI] [PubMed] [Google Scholar]
- 44.Rodriquez de Fonseca F, Navarro M, Alvarez E, Roncero I, Chowen JA, Maestre O, et al. Peripheral versus central effects of glucagon-like peptide-1 receptor agonists on satiety and body weight loss in Zucker obese rats. Metabolism. 2000;49:709–17. doi: 10.1053/meta.2000.6251. [DOI] [PubMed] [Google Scholar]
- 45.Tachibana T, Tanaka S, Furuse M, Hasegawa S, Kato H, Sugahara K. Intracerebroventricular injection of glucagon-like peptide-1 decreases monoamine concentrations in the hypothalamus of chicks. Br Poult Sci. 2002;43:122–6. doi: 10.1080/00071660120109971. [DOI] [PubMed] [Google Scholar]
- 46.Barrera JG, Sandoval DA, D’Alessio DA, Seeley RJ. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nature Reviews Endocrinology. 2011;7:507–16. doi: 10.1038/nrendo.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knauf C, Cani PD, Perrin C, Iglesias MA, Maury JF, Bernard E, et al. Brain glucagon-like peptide-1 increases insulin secretion and muscle insulin resistance to favor hepatic glycogen storage. J Clin Invest. 2005;115:3554–63. doi: 10.1172/JCI25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–68. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gil-Lozano M, Perez-Tilve D, Alvarez-Crespo M, Martis A, Fernandez AM, Catalina PA, et al. GLP-1(7–36)-amide and Exendin-4 stimulate the HPA axis in rodents and humans. Endocrinology. 151:2629–40. doi: 10.1210/en.2009-0915. [DOI] [PubMed] [Google Scholar]
- 51.Tauchi M, Zhang R, D’Alessio DA, Seeley RJ, Herman JP. Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp Neurol. 2008;210:458–66. doi: 10.1016/j.expneurol.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strawn JR, D’Alessio DA, Keck PE, Jr, Seeley RJ. Failure of glucagon-like peptide-1 to induce panic attacks or anxiety in patients with panic disorder. J Psychiatr Res. 2008;42:787–9. doi: 10.1016/j.jpsychires.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Grant P, Lipscomb D, Quin J. Psychological and quality of life changes in patients using GLP-1 analogues. J Diabetes Complications. 25:244–6. doi: 10.1016/j.jdiacomp.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Tang-Christensen M, Cowley MA. GLP-1 analogs: satiety without malaise? Am J Physiol Regul Integr Comp Physiol. 2007;293:R981–2. doi: 10.1152/ajpregu.00449.2007. [DOI] [PubMed] [Google Scholar]
- 55.McMahon LR, Wellman PJ. PVN infusion of GLP-1-(7–36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol. 1998;274:R23–9. doi: 10.1152/ajpregu.1998.274.1.R23. [DOI] [PubMed] [Google Scholar]
- 56.Thiele TE, Van Dijk G, Campfield LA, Smith FJ, Burn P, Woods SC, et al. Central infusion of GLP-1, but not leptin, produces conditioned taste aversions in rats. Am J Physiol. 1997;272:R726–30. doi: 10.1152/ajpregu.1997.272.2.R726. [DOI] [PubMed] [Google Scholar]
- 57.Toft-Nielsen MB, Madsbad S, Holst JJ. Continuous subcutaneous infusion of glucagon-like peptide 1 lowers plasma glucose and reduces appetite in type 2 diabetic patients. Diabetes Care. 1999;22:1137–43. doi: 10.2337/diacare.22.7.1137. [DOI] [PubMed] [Google Scholar]
- 58.Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541–4. doi: 10.1152/ajpregu.1999.276.5.R1541. [DOI] [PubMed] [Google Scholar]
- 59.Naslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–11. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 60.Scott KA, Moran TH. The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol. 2007;293:R983–7. doi: 10.1152/ajpregu.00323.2007. [DOI] [PubMed] [Google Scholar]
- 61.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 62.Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 63.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- 64.Everitt BJ, Wolf ME. Psychomotor stimulant addiction: a neural systems perspective. J Neurosci. 2002;22:3312–20. doi: 10.1523/JNEUROSCI.22-09-03312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 66.Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007;9:389–97. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]