Abstract
Objectives
Affect is neurobiologically based, influences emotions, contributes to temperamental characteristics, and can be evaluated from both a state and trait perspective. Associations between state-related positive affect (PA), negative affect (NA), and chronic pain have been investigated. However, little is known about the relationship between trait affect patterns and pain-related experiences. Affect balance style (ABS) provides a framework to assess the combined contribution of trait PA and NA. Psychological factors and experimental pain sensitivity are indicated as predictors of chronic pain onset. The current study investigated the relationship between ABS, pain sensitivity, and pain-related measures in healthy adults.
Methods
Subjects (n=372) completed quantitative sensory testing, pain-related questionnaires, and the Positive and Negative Affect Scale (PANAS). ABS groups were categorized as Healthy (high PA, low NA), Low (low PA, low NA), Depressive (low PA, high NA), and Reactive (high PA, high NA). Z-scores were computed for three experimental pain measures: ischemic, pressure, and heat.
Results
ABS groups significantly differed on ischemic pain sensitivity and pain-related measures. Specifically, the Healthy group demonstrated lower ischemic pain sensitivity compared to the Reactive group (p=0.02); the Depressive and Reactive groups endorsed higher somatic symptoms compared to the Healthy group (p<0.02); the Low and Depressive groups reported more physical stimuli sensitivity than the Healthy group (p<0.02); and the Reactive group indicated more passive coping strategies then the Low and Healthy groups (p=0.001).
Discussion
Findings from the study suggest that among healthy adults, trait affect patterns are associated with ischemic experimental pain sensitivity and other pain-related measures.
Keywords: Affect, Temperament, Experimental Pain Sensitivity, Pain Coping
Introduction
Studies across multiple fields have demonstrated distinct neurophysiological and biological patterns associated with the activation of positive affect (PA) and negative affect (NA) and the experience of emotion (1-4). As described in the literature, high PA is characterized by pleasant and inspiring emotions while high NA is associated with unpleasant and threat-related emotions (2;3;5;6). In contrast, in the case of low affect intensity, low PA is experienced as unpleasant emotions and decreased enthusiasm (e.g. disengaged and apathetic), while low NA is characterized by pleasant feelings such as relaxation and contentment (5;6). Affect can be assessed from both a state and trait perspective. “State” affect is temporally dynamic and occurs in response to internal (e.g., thoughts) or external (e.g., environmental) stimuli. “Trait” affect reflects a pattern of affect response, temperamental features, contributes to dispositional mood, and provides the foundation of personality characteristics (6-12). High trait NA has been associated with anxiety and depression whereas low trait PA has been associated with depression (12-15).
Relationships between state measures of PA, NA, and chronic pain have been investigated (16-20). Numerous studies indicate that high levels of state PA can be protective, decreasing the NA and pain relationship and reducing the level of distress experienced in response to increased pain intensity in individuals with chronic pain (17;21;22). Interestingly, the association between NA and pain has been less consistent (23). In general, state NA is associated with increased pain report, passive coping skills, and greater functional disability (24;25). Poor state affect regulation has been associated with increased pain and distress (18;26;27), while persistent dysregulated affect patterns (i.e., high NA and/or low PA), have been identified as diatheses to psychopathology (7-9;13;15;28).
Limited research has addressed associations of temperament and trait affect with pain responses. One investigation found trait PA and NA were associated with health status satisfaction and with functional impairment in individuals with rheumatoid arthritis (29). While cross-sectional and short-term prospective clinical studies demonstrate a relationship between state PA, NA, and symptoms in patients with existing chronic pain conditions, whether a person’s trait affect style represents a characteristic that could increase the risk of developing chronic pain and/or is influenced by experiences of persistent pain remains to be elucidated.
Psychological factors, including measures of affect and mood, have been proposed as one of the intermediate phenotypes that represent proximal risk factors for the development of chronic pain (30). Empirical support for this hypothesis is growing. For example, several prospective studies have demonstrated that pre-existing psychological factors (e.g. somatization, psychological distress, depression) predict the development of new onset chronic pain conditions, including chronic widespread pain (31), regional musculoskeletal pain (32), low back pain (32-34), and temporomandibular disorders (35;36). One mechanism whereby psychological processes, such as NA, might influence risk for chronic pain is via their effects on pain processing. Indeed, another intermediate phenotype proposed as a risk factor for chronic pain development is pain amplification (30), a tenet supported by several recent studies demonstrating that heightened sensitivity to experimental pain predicts future development of chronic pain (36-38). Therefore, whether trait measures of affect are associated with experimental pain sensitivity in healthy adults represents an important yet unanswered question.
In a previous study of healthy subjects, higher trait PA predicted thermal and ischemic pain tolerance (r=0.33 and 0.41, p <0.05) in men and NA was associated with thermal pain and ischemic pain ratings in women (r=-0.31 and 0.32, p<0.05) and ischemic pain tolerance in women (r=-0.29) and men (r=-0.39, p<0.05) (39). However, like the majority of pain-related research on affect, this study evaluated PA and NA separately without consideration for the combined influence (40), which has been supported theoretically and neurobiologically (4;6;41).
Investigating the association between the combined trait patterns of PA and NA, experimental pain sensitivity, and pain-related psychosocial measures may enhance our understanding of the role of affect patterns in the onset and maintenance of chronic pain experiences. Based on self-reported responses from the Positive and Negative Affect Schedule (PANAS), affect balance style (ABS) is an integrated model of PA and NA that provides a general, non-pathological framework in which to understand affective patterns (6;40). Four affect balance style patterns are identified in the construct (Low, Depressive, Healthy, and Reactive) and are depicted in Figure 1. In a recent study of ABS groups among individuals with fibromyalgia and other medical conditions (controls), a greater proportion of individuals with fibromyalgia were in the Depressive (5.60 OR/95% CI) and Reactive (3.81 OR/95% CI) groups rather than the Healthy group compared to controls (40). Whether affect patterns, measured by ABS, are related to laboratory pain sensitivity and other pain-related measures in healthy subjects has not been determined.
Figure 1.
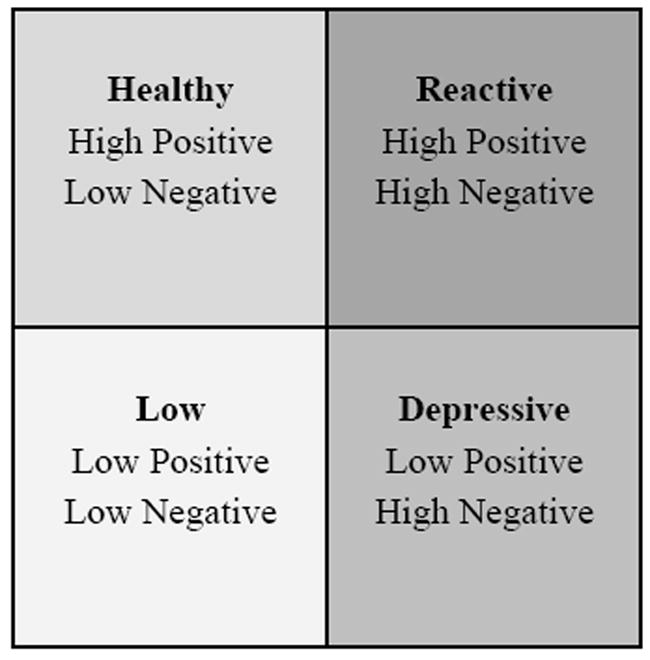
Affect Balance Style Groups by Positive and Negative Affect
This investigation was completed on data collected from healthy subjects involved in two studies examining baseline pain sensitivity and analgesic response to exogenous opioids. We hypothesized that 1) the Healthy ABS group would report the lowest levels of pain sensitivity, somatic symptoms, physical stimuli sensitivity, and passive coping; and the highest level of active coping; and 2) based on prior findings, the Depressive ABS group would report the highest levels of pain sensitivity, somatic symptoms, physical stimuli sensitivity, and passive coping; and the lowest level of active coping (39;40;42). Additionally, we were interested in evaluating the possible pain-related buffering effects of high PA or low NA in the Reactive and Low ABS groups.
Materials and Methods
Subjects
A total of 372 healthy adults, 167 males and 205 females, were recruited through Institutional Review Board (IRB) approved posted advertisements. Participants were between the ages of 18 and 45 without report of clinical pain, psychiatric disturbance, a substance disorder, or use of tobacco products or centrally acting medications. The research was conducted at the Clinical Research Unit at the University of Florida. All participants were screened by the study physician and reimbursed $25 an hour for their involvement in the study. The data presented herein were derived from more extensive protocols in our lab evaluating baseline pain sensitivity as well as responses to opioid analgesics. The procedures described below will be specific to the current analysis. The protocol and all procedures were approved by the University of Florida’s IRB. Informed consent was obtained from each subject.
General Experimental Procedures
All participants underwent experimental sessions, minimum two and a maximum of three based on the protocol, involving quantitative sensory testing before and after drug administration, which were identical except for the drug administered (which could include morphine, butorphanol, and/or saline). Only baseline (i.e. pre-drug) pain responses are considered in this analysis. Quantitative sensory testing procedures implemented in this study followed the general protocol from prior research (43;44). Specifically, two experimenters and a registered nurse conducted the testing sessions. One experimenter was responsible for the sensory testing component by the bedside and the other experimenter operated the equipment and recorded the data. The gender of the bedside experimenter was documented and remained consistent for the three experimental sessions completed. The clinical research nurse was responsible for monitoring vital signs, administering drug, and completing blood draws. Subjects maintained a semi-recumbent position in a hospital bed during all study procedures. An intravenous (IV) cannula was inserted at the beginning of each experimental session followed by a 10-minute rest period. Ten minutes following IV placement, the pre-drug sensory testing protocol was completed including thermal pain, pressure pain, and ischemic pain measures (described below). The order of thermal and pressure pain was randomly determined for each subject and maintained for all sessions. The ischemic pain procedure was always completed last to reduce carry over effects.
Pain Testing Procedures
Pressure Pain Threshold
Pressure pain threshold (PPT) was assessed with a handheld algometer (Pain Diagnostics and Therapeutics, Great Neck, NY). Mechanical pressure was applied with a 1-cm2 probe with a constant rate of pressure of 1kg/second, which helps reduce artifact related to reaction time. Subjects were instructed to report (verbally or by raising their hand) their first feeling of pain as a result of the pressure. PPTs were assessed at three sites on the right side of the body: the center of the upper trapezius (posterior to the clavicle), the upper masseter (approximately midway between the ear opening and the corner of the mouth) and the ulna (dorsal forearm, approximately 8 cm distal to the elbow). The site order was randomly counterbalanced and a minimum of three trials (with readings within 1 kg) were recorded at each position. The average of the three assessments for each site was calculated and used in subsequent analysis.
Thermal Pain Procedures
Threshold and tolerance
The first thermal procedure involved assessment of heat pain threshold and tolerance. Contact heat stimuli were delivered using a computer-controlled Medoc Thermal Sensory Analyzer (Pathway Pain & Sensory evaluation System, Ramat Yishai, Israel), which includes a Peltier-element-based stimulator. Temperature levels were monitored by a contactor-contained thermistor, and returned to a preset baseline of 32°C by active cooling at a rate of 10°C/s. The 3 cm × 3 cm contact probe was applied to the right ventral forearm. In separate series of trials heat pain threshold (HPTh) and heat pain tolerance (HPTo) were assessed using an ascending method of limits. From a baseline of 32°C, probe temperature increased at a rate of 0.5°C/s until the subject responded by pressing a button to indicate when they first felt pain (HPTh), and when no longer able to tolerate the pain (HPTo). This slow rise-time was selected as a test of pain evoked mainly by stimulation of C-nociceptive afferents, as has been previously demonstrated (45;46). Four trials of HPTh and HPTo were presented to each subject. The position of the thermode was altered slightly between trials (though it remained on the ventral forearm) in order to avoid either sensitization or response suppression of cutaneous heat nociceptors. For each measure, the average of all four trials was computed for use in subsequent analyses.
Temporal summation of thermal pain
The second thermal procedure involved administration of brief, repetitive, suprathreshold heat pulses to assess temporal summation of heat pain (47). Series of ten repetitive pulses were applied to the right dorsal forearm using the Contact Heat Evoked Potential Stimulator (CHEPS), which combines heat-foil technology with a Peltier element, thereby achieving heating and cooling rates of at least 40 °C /sec. Three series of ten stimuli were applied at target temperatures: 46, 48, and 50°C. For each series, the baseline temperature was 35°C, the target temperature was delivered for 700 msec, and the inter-stimulus interval (at the baseline temperature) was 2.5 seconds. Subjects rated the peak pain for each of the ten heat pulses using a numerical rating scale (0 represented no sensation and 100 represented the most intense pain imaginable). The average rating across all 10 trials for each temperature was used in subsequent analyses. To replace missing values created by subjects terminating the procedure before the tenth trial, the last rating provided was carried forward.
Modified Submaximal Tourniquet Procedure
Following completion of the pressure and thermal pain procedures, a rest period of 5-minutes was implemented prior to beginning the tourniquet procedure (48;49). The right arm was exsanguinated by elevating it above heart level for 30 seconds, after which the blood flow to the arm was occluded with a standard blood pressure cuff positioned proximal to the elbow and inflated to 240 mm Hg using a Hokanson E20 Rapid Cuff Inflator (D.E. Hokanson, Bellevue, WA, USA). In response to recorded instructions, subjects performed 20 hand grip exercises of two second duration at four second intervals at 50% of their maximum grip strength. Subjects were instructed to report when they first felt pain, ischemic pain threshold (IPTh), then to continue until the pain became intolerable, ischemic pain tolerance (IPTo), at which point the procedure was stopped. The IPTh and IPTo time points were recorded. Every 30 seconds during the procedure, subjects were prompted to rate either the “intensity” or “unpleasantness” of pain using a combined numerical (0-20) and verbal descriptor box scales (50). Additionally, cardiovascular measures: systolic, diastolic, mean arterial blood pressure, and heart rate were recorded every 60 seconds. An uninformed 15-minute time limit was observed. In addition to IPTh and IPTo, two total pain scores were created by summing all ratings obtained during the procedure: ischemic pain intensity (IPInt) and ischemic pain unpleasantness (IPUnpl). To replace missing values created by subjects terminating the procedure before the time limit, the last rating provided was carried forward.
Psychological measures
Coping Strategies Questionnaire – Revised (CSQ-R)
The CSQ measures cognitive and behavioral coping responses to pain (51). The CSQ-R is a revised version of the CSQ, retaining 27 items (52). Responses range across a 7-point scale (0-6) from “Never do that” to “Always do that.” Based on previous factor analyses in healthy subjects (52), the six subscales of the CSQ were combined into two categories and defined as active and passive coping. The subscales combined under active coping include: Diverting Attention, Coping Self –Statements, Ignoring Sensations, and Reinterpreting Pain Sensations. The factors defined as passive coping include Catastrophizing and Praying-Hoping. A mean is calculated for each category. Analyses of the CSQ-R have demonstrated strong psychometric properties (52-54).
Kohn Reactivity Scale (KRS)
The KRS consists of 24 items that assess sensitivity to physical stimuli (55). Subjects are asked to indicate the extent to which they agree with particular statements (e.g., I could never bath or shower in ice cold water or I’ve often had motion sickness) on a five point scale (1=disagree strongly; 5=agree strongly). The measure has demonstrated adequate reliability and validity (55), is correlated negatively with pain tolerance (56), and has been used to assess the construct of hypervigilance (57).
Pennebaker Inventory of Limbic Languidness (PILL)
The PILL assesses the frequency of 54 common physical symptoms and sensations and has been associated with the tendency to endorse physical symptoms or somatization (58). Frequency of experience are conveyed on a five point scale (1 = have never or almost never experienced this symptom; 5 = experience this symptom more than once every week). The PILL has demonstrated high internal consistency and adequate test-retest reliability (58).
Positive and Negative Affect Scale (PANAS)
The PANAS is a 20 item scale that assesses positive and negative affect (5;14). Items are rated on a 5-point scale (1 = very slightly or not at all; 5 = extremely) resulting in scale scores for positive and negative affect ranging from 10 to 50 (2;59). The PANAS has demonstrated adequate reliability, validity, and the stability of mood measures over time (5;60). The PANAS can capture both state and trait responses. Instructions regarding the time frame may vary from moment to days, weeks, month, year, or in general. For this study, subjects were requested to provide “trait” information by responding to items from an “in general” time frame. Importantly, the PANAS has demonstrated predictive utility in affective neuroscience studies (2;59).
Affect Balance Style
Individual PA and NA scale scores were categorized as high or low based on the published adult normative means for the PANAS, PA = 35.0 ± 6.4 and NA = 18.1± 5.9 (5), and consistent with the ranges implemented in the clinical study by Hassett and colleagues (40). Thus, high PA was >35 and low PA ≤35. Likewise, high NA was categorized as >18.1 and low NA ≤18.1. PA and NA categories were combined and designated into one of four groups as presented in Figure 1: Healthy, Depressive, Low, or Reactive. The integrated relationship between high and low PA and NA has been characterized in different models (4;6). In general, approach-related emotions (PA) are associated with motivation and withdrawal-related emotions (NA) are associated with fear or disgust (3). Based on these conceptualizations, ABS groups can be described as:
Low – decreased affect intensity and reactivity, reduced experience of withdrawal-related and approach-related emotions, mixed experience of pleasant (low NA) and unpleasant (low PA) emotions
Depressive – affect arousal associated with withdrawal-related and unpleasant emotions (high NA and low PA);
Healthy – affect arousal associated with approach-related and pleasant emotions (high PA and low NA);
Reactive – heightened affect intensity and reactivity, increased experience of withdrawal-related/unpleasant emotions (high NA) and approach-related/pleasant emotions (high PA) (3;4;6).
Data Analysis
SPSS software, PASW Statistics 19.0 was used in the completion of the statistical analyses (61). A Chi-square test for independence and ANOVA were used to compare demographic characteristics. Baseline pain sensitivity was computed by averaging data from the number of quantitative sessions completed. For purposes of variable reduction, Z-scores were computed for each of the pain modalities, consistent with the findings of a previous factor analysis in our laboratory (62). A positive z-score represents lower pain sensitivity while a negative z-score represents greater pain sensitivity. Univariate General Linear Models were used to compare psychophysical and questionnaire findings across ABS groups. Sex was included as a covariate based on prior findings of significant sex differences in laboratory pain testing (63). Pairwise comparisons were completed with a Bonferroni correction.
Results
Demographic and Affect Balance Style Characteristics
A total of 372 subjects, 167 males and 205 females participated in the study, the mean age was 23.7. The ethnic/racial distribution for the sample was: 72% non-Hispanic whites, 8% African American, 9% Hispanic, 5 % Asian, and 6% other or not identified. There was no significant difference in sex or age distribution across ABS groups, (p> 0.05). ABS calculations resulted in the following representations: Low 24.7%, Depressive 15.3%, Healthy 43.5%, and Reactive 16.4%. Table 1 provides information for each ABS group including sample size, sex distribution, mean age, and the mean and standard deviation for PA and NA.
Table 1.
ABS Group Demographics and PANAS PA and NA by Normative Means
| ABS Group | Group N % | % Sex Age Mean | Positive Affect M±SD | Negative Affect M±SD |
|---|---|---|---|---|
| Low Low PA, Low NA | 92 (24.7%) | 39%M, 61%W 23.4 years | 31.4±4.2 | 14.2±2.2 |
| Depressive Low PA, High NA | 57 (15.3%) | 49%M, 51%W 22.9 years | 31.5±3.5 | 24.0±4.8 |
| Healthy High PA, Low NA | 162 (43.5%) | 46%M, 54%W 24.4 years | 40.0±3.4 | 13.9±2.4 |
| Reactive High PA, High NA | 61 (16.4%) | 46%M, 54%W 23.1 years | 39.5±3.2 | 22.4±4.1 |
ABS = Affect balance style
PA = Positive affect, NA = Negative affect
M = Men, W = Women
High PA defined as > 35 on the PA scale
Low PA defined as ≤ 35 on the PA scale
High NA defined as >18.1 on the NA scale
Low NA defined as ≤ 18.1 on the NA scale
Experimental Pain Measures
A main effect was found for ABS group on ischemic pain F (3, 358) = 4.09, p = 0.007, partial eta squared = 0.033, but not for heat pain or pressure pain after controlling for sex. Post hoc analysis with a Bonferroni adjustment for multiple comparisons revealed that the Reactive ABS group reported significantly greater ischemic pain sensitivity compared to the Healthy ABS group (p = 0.02; Figure 2).
Figure 2.
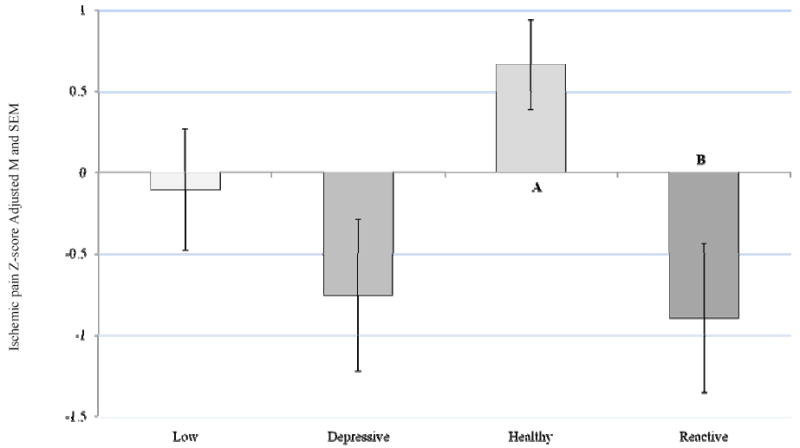
Ischemic Pain Z-Score by ABS
Covariate in the model: Sex = 1.55
Bonferroni adjustment for multiple comparisons
Healthy - Reactive group difference, p = 0.023
Somatic Symptoms and Level of Reactivity
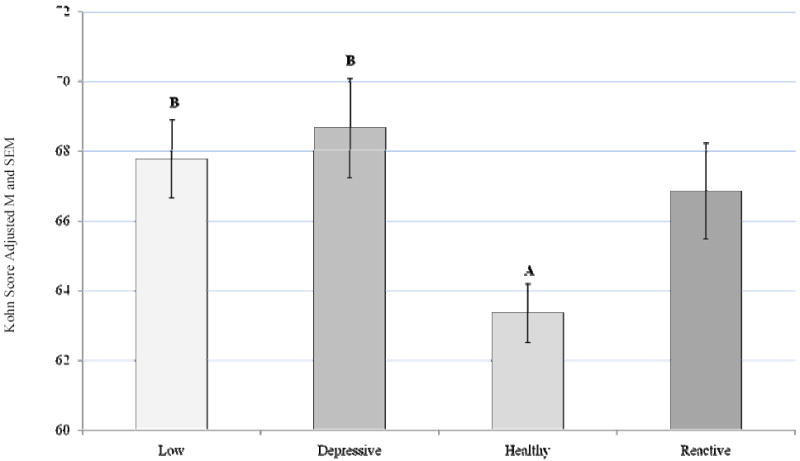
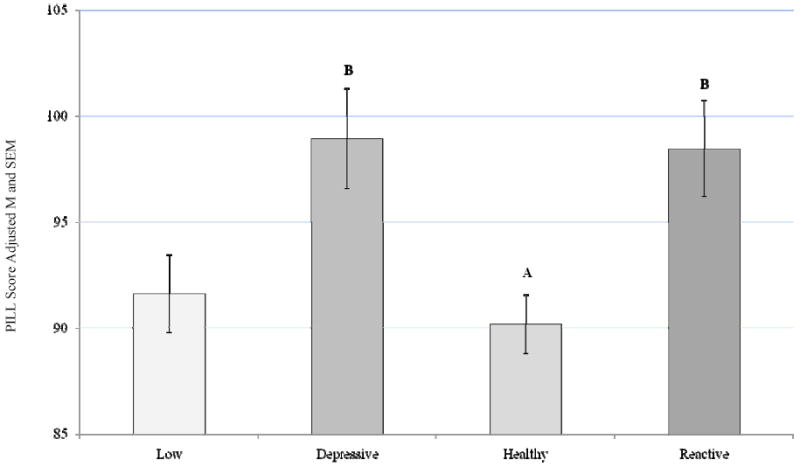
A main effect for ABS group emerged on the PILL, a measure of somatic symptoms F (3, 341) = 5.57, p = 0.001, partial eta squared = 0.047, after controlling for sex. Post hoc analysis with a Bonferroni adjustment for multiple comparisons revealed that the Depressive and Reactive ABS groups reported significantly more somatic symptoms compared to the Healthy group (p = 0.009 and p = 0.012 respectively; Figure 3). A main effect for ABS group was also demonstrated on a measure of physical stimuli sensitivity (KRS), F (3, 367) = 5.33, p = 0.001, partial eta squared = 0.042, after controlling for sex. Post hoc analysis with a Bonferroni adjustment for multiple comparisons revealed that the Low and Depressive groups reported significantly more sensitivity to physical sensations compared to the Healthy group (p = 0.011 and p = 0.009 respectively; Figure 4).
Figure 3.
PILL Score by ABS
Covariate in the model: Sex = 1.55
Bonferroni adjustment for multiple comparisons
Depressive-Healthy group difference, p = 0.009
Reactive-Healthy group difference, p= 0.012
Figure 4.
Kohn (KRS) Score by ABS
Covariate in the model: Sex = 1.55
Bonferroni adjustment for multiple comparisons
Depressive-Healthy group difference, p = 0.009
Low-Healthy group difference, p= 0.011
Active and Passive Pain Coping
A main effect for ABS group was not demonstrated on active pain coping, (CSQ-R) F (3, 366) = 2.09, p = 0.101, partial eta squared = 0.017 (Figure 5). However, a main effect for ABS group was indicated for passive pain coping (CSQ-R) F (3, 366) = 6.45, p < 0.0005, partial eta squared = 0.05, after controlling for sex. Post hoc analysis with a Bonferroni correction for multiple comparisons indicated that the Reactive ABS group endorsed more passive pain coping strategies compared to the Low (p = 0.001) and Healthy (p = 0.001) groups (Figure 6).
Figure 5.
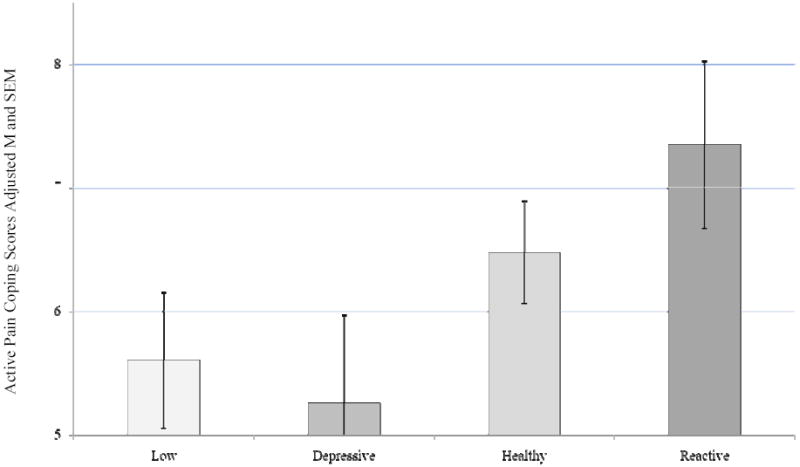
Active Coping (CSQ-R) Score by ABS
Covariate in the model: Sex = 1.55
ABS group differences were not significant, p = 0.101
Figure 6.
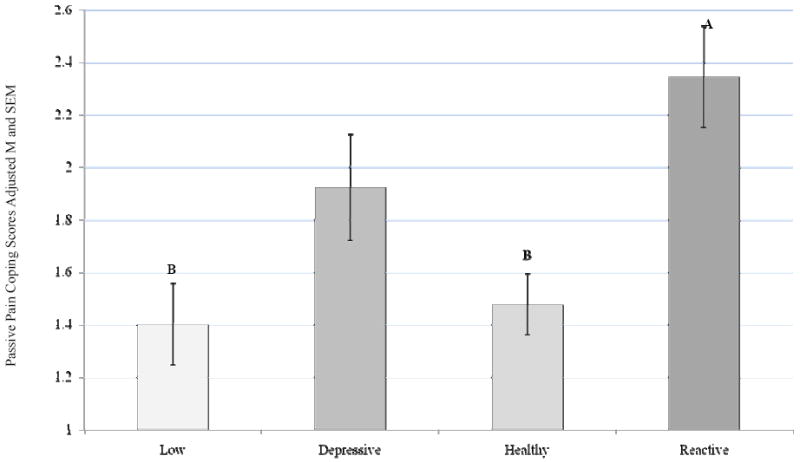
Passive Coping (CSQ-R) Score by ABS
Covariate in the model: Sex = 1.55
Bonferroni adjustment for multiple comparisons
Reactive-Low group difference, p = 0.001
Reactive-Healthy group difference, p= 0.001
Discussion
The purpose of this laboratory pain study was to investigate the relationships among experimental pain sensitivity, pain–related measures, and trait affect patterns (ABS) in healthy subjects. Significant group differences were found for ischemic pain and pain-related psychosocial measures. Specifically, the Healthy group demonstrated lower ischemic pain sensitivity compared to the Reactive group; the Depressive and Reactive group endorsed higher somatic symptoms compared to the Healthy group; the Low and Depressive group reported more physical stimuli sensitivity than the Healthy group; and the Reactive group indicated more passive coping strategies then the Low and Healthy groups.
As previously noted, a number of studies have provided evidence that psychological factors and elevated levels of experimental pain sensitivity are predictive of chronic pain onset. Although significant associations between ABS groups and experimental pain sensitivity was limited to measures of ischemic pain, of the experimental pain measures incorporated into the design, ischemic pain is longer in duration than the other measures (thermal and pressure) and may tap into qualities more consistent with chronic pain. Indeed, it was previously shown that ischemic (but not thermal) pain was associated with clinical pain severity among patients with temporomanibular disorders (64). Moreover, individuals with depressive disorders have shown greater sensitivity to ischemic but not thermal pain (65). These previous findings combined with the current results suggest that ischemic pain responses may be more particularly sensitive to associations with NA.
In review of the first and second hypotheses, a number of the anticipated patterns were demonstrated for the Healthy and Depressive ABS groups. An additional goal of the investigation was to better understand pain-related response patterns manifested by individuals endorsing trait-related high PA and high NA or low PA and low NA. Based on prior research findings indicating that state-related high PA can counter NA (21;22), we were curious if similar patterns would be observed for trait affect patterns in the Reactive group. Interestingly, one clinical study described the experiences of chronic pain patients endorsing high state affect intensity for both PA and NA as “ambiguous” and a “double-edged sword”(27). Our findings of individuals in the Reactive ABS group suggested minimal protective effects of PA, as this group showed significantly greater ischemic pain sensitivity, passive coping, and somatic symptoms compared to the Healthy ABS group.
In the case of individuals endorsing low trait PA and NA, Low ABS, one clinical study indicated that the profile may reflect a possible resilience pattern (40). Our results suggest that the Low ABS group demonstrated a somewhat mixed response pattern characterized by lower levels of passive coping compared to the Reactive group but a higher level of physical stimuli sensitivity compared to the Healthy group. Trait patterns of low PA and low NA would result in a tendency toward an affect style of low affect intensity, arousability and reactivity. Interestingly, the KRS is an instrument that essentially captures tolerance of or sensitivity to physical sensations. Thus, low affect intensity does not necessarily align with low physical sensitivity. Further investigation is needed to explore trait affect patterns and association with other pain-related psychosocial and physical measures.
Clinical Significance
A simple comparison between the current study of healthy subjects and participants in a prior study of patients with chronic pain and other medical conditions reflects differences in the distribution of ABS groups, particularly for the Healthy and Depressive profiles (40):
Healthy - 43.5% current study, 10.1% FM patients, and 34.8% medical controls;
Depressive - 15.3% current study, 54.4% FM patients, and 31.5% medical controls;
Low - 24.7% current study, 17.7% FM patients, and 18.5% medical controls; and
Reactive - 16.4% current study, 17.7% FM patients, and 15.2% medical controls.
While it is unclear if the differences precede or follow the development of chronic pain, or a combination of both, the disparate proportions in ABS groups between the two studies is compelling. One might speculate that the ABS profiles examined in the present study represent foundational psychological phenotypes (i.e. traits) that contribute to both psychological symptomatology and pain sensitivity, thereby influencing risk for chronic pain development.
It is possible that the disproportionate representation of subjects in the Depressive ABS group with chronic pain may result from a combination of predisposing affective features as well as neuroplastic changes due the persistent presence of chronic pain and resulting NA activation (41). Research has demonstrated left prefrontal cortex (PFC) activation with PA and right PFC activation with NA. Importantly, hemispheric activation patterns are associated with trait related temperamental features and “affective style” (1;3). Interestingly, brain areas associated with pain and NA processing have been functionally and anatomically linked (66). Additionally, factors identified as increasing pain-related vulnerability such as anxiety sensitivity, pain avoidance, and catastrophizing are associated with perceptions of threat and would likely contribute to further activation of NA (67). Likewise, neural substrates could also be influenced in the other direction with PA activation resulting from pain-related protective factors such as optimism, hope, and benefit finding. In fact, left PFC activation in response to PA activation, inhibits amygdala responses, decreasing NA activation (2).
Limitations and future directions
In addition to the need to replicate findings, there are several limitations in the present study which illuminate a number of opportunities for future research. First, the ABS grouping is a new method of interpretation of the PANAS, further evaluation and validation of the ABS model is needed. Second, this study was completed in a population of young healthy, adult subjects. A follow-up study of ABS and quantitative sensory testing in healthy controls and chronic pain patients would help clarify ABS group proportion differences, associations with pain sensitivity, and pain-related measures. Additionally, a prospective study of ABS would allow for the opportunity to evaluate the role of trait affect in individuals who do and do not transition into a chronic pain condition. Third, the addition of other measures including neuroendocrine, inflammatory, and genetic markers would help extend our understanding of the biological interface of psychosocial factors and the chronic pain experience.
Conclusions
Previous research efforts have enhanced our understanding of the influence of state affect on pain and functioning. Trait affect patterns have been identified as predisposing to psychopathology, whether trait affect represents a characteristic that can increase the risk of developing chronic pain and/or is influenced by experiences of persistent pain remains to be elucidated. Results from the current study extend previous findings from a clinical study (40) by demonstrating significant trait affect differences, measured by ABS, in healthy subjects on ischemic pain sensitivity and other pain-related measures that have been associated with the onset of chronic pain. Current results emphasize the importance of evaluating the combined experience of trait PA and NA for a comprehensive understanding of individual differences in response to pain-related experiences and measures.
Acknowledgments
This work was supported by NIH/NINDS grant NS041670, NINDS training grant NS045551, CTSA grant RR029890, and the North Florida/South Georgia Veterans Health System, Gainesville, FL. Roger Fillingim, Ph.D. is a stockholder in Algynomics. A portion of this work was presented at the 2010 American Pain Society Annual Scientific Meeting (68). Appreciation is extended to Burel Goodin, Ph.D. for reviewing the manuscript.
Reference List
- 1.Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychol Sci. 1992;3:39–43. [Google Scholar]
- 2.Davidson RJ. Toward a biology of personality and emotion. Ann N Y Acad Sci. 2001;935:191–207. doi: 10.1111/j.1749-6632.2001.tb03481.x. [DOI] [PubMed] [Google Scholar]
- 3.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 4.Posner J, Russell JA, Peterson BS. The circumplex model of affect: An integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. 2005;17:715–34. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 6.Watson D, Tellegen A. Toward a consensual structure of mood. Psych Bull. 1985;98:219–35. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- 7.Bradley SJ. Affect Regulation and the development of psychopathology. New York: The Guilford Press; 2000. [Google Scholar]
- 8.Whittle S, Allen NB, Lubman DI, et al. The neurobiological basis of temperament. Toward a better understanding of psychopathology. Neuroscience and Biobehavioral Reviews. 2006;30:511–25. doi: 10.1016/j.neubiorev.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Lonigan CJ, Vasey MW, Phillips BM, et al. Temperament, anxiety, and the processing of threat-relevant stimuli. J Clin Child Adolesc Psychol. 2010;33:8–20. doi: 10.1207/S15374424JCCP3301_2. [DOI] [PubMed] [Google Scholar]
- 10.Watson D, Clark LA. On traits and temperament: general and specific factors of emotional experience and their relation to the five-factor model. J Pers. 1992;60:441–76. doi: 10.1111/j.1467-6494.1992.tb00980.x. [DOI] [PubMed] [Google Scholar]
- 11.Watson D. Intraindividual and interindividual analyses of positive and negative affect: their relation to health complaints, perceived stress, and daily activities. J Pers Soc Psychol. 1988;54:1020–30. doi: 10.1037//0022-3514.54.6.1020. [DOI] [PubMed] [Google Scholar]
- 12.Rettew DC, McKee L. Temperament and its role in developmental psychopathology. Harv Rev Psychiatry. 2005;13:14–27. doi: 10.1080/10673220590923146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark LA, Watson D, Mineka S. Temperament, personality, and the mood and anxiety disorders. J Abn Psychol. 1994;103:103–16. [PubMed] [Google Scholar]
- 14.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43:245–65. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 15.Clark L, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. J Abn Psychol. 1991;100:316–36. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 16.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–71. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 17.Zautra AJ, Smith H, Affleck G, et al. Examinations of chronic pain and affect relationships: Application of a dynamic model of affect. J Consult Clin Psychol. 2001;69:786–95. doi: 10.1037//0022-006x.69.5.786. [DOI] [PubMed] [Google Scholar]
- 18.Connelly M, Keefe F, Affleck G, et al. Effects of day-to-day affect regulation on the pain experience of patients with rheumatoid arthritis. Pain. 2007;131:162–70. doi: 10.1016/j.pain.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Doyle WJ, Skoner DP, et al. State and trait negative affect as predictors of objective and subjective symptoms of respiratory viral infections. Journal of Personality & Social Psychology. 1995;68:159–69. doi: 10.1037//0022-3514.68.1.159. [DOI] [PubMed] [Google Scholar]
- 20.Smith BW, Zautra AJ. The role of personality in exposure and reactivity to interpersonal stress in relation to arthritis disease activity and negative affect in women. Health Psychol. 2002;21:81–8. [PubMed] [Google Scholar]
- 21.Strand EB, Zautra AJ, Thoresen M, et al. Positive affect as a factor of resilience in the pain-negative affecrt relationship in patients with rheumatiod arthritis. J Psychosom Res. 2006;60:477–84. doi: 10.1016/j.jpsychores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–20. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatchel R, Peng Y, Fuchs P, et al. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psych Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 24.Zautra AJ, Burleson MH, Smith CA, et al. Arthritis and perception of quality of life: An examination of positive and negative affect in rheumatoid arthritis patients. Health Psychol. 1995;14:399–408. doi: 10.1037//0278-6133.14.5.399. [DOI] [PubMed] [Google Scholar]
- 25.Smith JA, Lumley MA, Longo DJ. Contrasting emotional approach coping with passive coping for chronic myofascial pain. Ann Behav Med. 2002;24:326–35. doi: 10.1207/S15324796ABM2404_09. [DOI] [PubMed] [Google Scholar]
- 26.Keefe FJ, Lumley M, Anderson T, et al. Pain and emotion: new research directions. J Clin Psychol. 2001;57:587–607. doi: 10.1002/jclp.1030. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton NA, Zautra AJ, Reich J. Affect and pain in rheumatoid arthritis: Do individual differences in affective regulation and affective intensity predict emotional recovery from pain? Ann Behav Med. 2005;29:216–24. doi: 10.1207/s15324796abm2903_8. [DOI] [PubMed] [Google Scholar]
- 28.Schore A. Affect dysregulation and disorders of the self. NY: W W Norton & Company Inc; 2003. [Google Scholar]
- 29.Chou C, Brauer J. Temperament and satisfaction with health status among persons with rheumatoid arthritis. Clinical Nurse Specialist. 2005;19:94–100. doi: 10.1097/00002800-200503000-00070. [DOI] [PubMed] [Google Scholar]
- 30.Diatchenko L, Nackley AG, Slade GD, et al. Idiopathic pain disorders--pathways of vulnerability. Pain. 2006;123:226–30. doi: 10.1016/j.pain.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 31.McBeth J, Macfarlane GJ, Benjamin S, et al. Features of somatization predict the onset of chronic widespread pain: results of a large population-based study. Arthritis Rheum. 2001;44:940–6. doi: 10.1002/1529-0131(200104)44:4<940::AID-ANR151>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Nahit ES, Pritchard CM, Cherry NM, et al. The influence of work related psychosocial factors and psychological distress on regional musculoskeletal pain: a study of newly employed workers. J Rheumatol. 2001;28:1378–84. [PubMed] [Google Scholar]
- 33.Linton SJ. A review of psychological risk factors in back and neck pain. Spine. 2000;25:1148–56. doi: 10.1097/00007632-200005010-00017. [DOI] [PubMed] [Google Scholar]
- 34.Linton SJ. Do psychological factors increase the risk for back pain in the general population in both a cross-sectional and prospective analysis? Eur J Pain. 2005;9:355–61. doi: 10.1016/j.ejpain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal VR, Macfarlane GJ, Farrar JT, et al. Risk factors for onset of chronic oro-facial pain, results of the North Cheshire oro-facial pain prospective population study. Pain. 2010;149:354–9. doi: 10.1016/j.pain.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slade GD, Diatchenko L, Bhalang K, et al. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 2007;86:1120–5. doi: 10.1177/154405910708601119. [DOI] [PubMed] [Google Scholar]
- 37.Werner MU, M HN, Nielsen PR, Rudin A. Prediction of postoperative pain. Anesthesiology. 2010;112:1494–502. doi: 10.1097/ALN.0b013e3181dcd5a0. [DOI] [PubMed] [Google Scholar]
- 38.Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–8. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 39.Fillingim RB, Hastie BA, Ness TJ, et al. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol. 2005;69:97–112. doi: 10.1016/j.biopsycho.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 40.Hassett AL, Simonelli LE, Radvanski DC, et al. The relationship between affect balance style and clinical outcomes in fibromyalgia. Arth Rheum. 2008;59:833–40. doi: 10.1002/art.23708. [DOI] [PubMed] [Google Scholar]
- 41.Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: Perspectives from affective neuroscience. Psych Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- 42.Watson D, Pennebaker JW. Health complaints, stress, and distress: exploring the central role of negative affectivity. Psychol Rev. 1989;96:234–54. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- 43.Fillingim RB, Ness TJ, Glover TL, et al. Experimental pain models reveal no sex differences in pentazocine analgesia in humans. Anesthesiology. 2004;100:1263–70. doi: 10.1097/00000542-200405000-00031. [DOI] [PubMed] [Google Scholar]
- 44.Fillingim RB, Ness TJ, Glover TL, et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–24. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 45.Yeomans DC, Pirec V, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: behavioral evidence. Pain. 1996;68:133–40. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 46.Yeomans DC, Cooper BY, Vierck CJ., Jr Effects of systemic morphine on responses of primates to first or second pain sensations. Pain. 1996;66:253–63. doi: 10.1016/0304-3959(96)03082-5. [DOI] [PubMed] [Google Scholar]
- 47.Price DD, Hu JW, Dubner R, et al. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 48.Maixner W, Gracely RH, Zuniga JR, et al. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol. 1990;259:R1156–R1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- 49.Moore PA, Duncan GH, Scott DS, et al. The submaximal effort tourniquet test: its use in evaluating experimental and chronic pain. Pain. 1979;6:375–82. doi: 10.1016/0304-3959(79)90055-1. [DOI] [PubMed] [Google Scholar]
- 50.Sternberg WF, Bailin D, Grant M, et al. Competition alters the perception of noxious stimuli in male and female athletes. Pain. 1998;76:231–8. doi: 10.1016/s0304-3959(98)00050-5. [DOI] [PubMed] [Google Scholar]
- 51.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 52.Riley JL, Robinson ME. CSQ: Five factors or fiction. Clin J Pain. 1997;13:156–62. doi: 10.1097/00002508-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Robinson ME, Riley JL, III, Myers CD, et al. The Coping Strategies Questionnaire: a large sample, item level factor analysis. Clin J Pain. 1997;13:43–9. doi: 10.1097/00002508-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Riley JL, III, Robinson ME, Geisser ME. Empirical subgroups of the Coping Strategies Questionnaire-Revised: a multisample study. Clin J Pain. 1999;15:111–6. doi: 10.1097/00002508-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Kohn PM. Sensation-seeking, augmenting-reducing, and strength of the nervous system. In: Spence JT, Izard DE, editors. Motivation, Emotion, and Personality. Amsterdam: Elsevier; 1985. pp. 167–73. [Google Scholar]
- 56.Dubreuil DL, Kohn PM. Reactivity and response to pain. Pers Indiv Diff. 1986;7:907–9. [Google Scholar]
- 57.McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain. 1996;66:133–44. doi: 10.1016/0304-3959(96)03059-x. [DOI] [PubMed] [Google Scholar]
- 58.Pennebaker JW. The psychology of physical symptoms. New York: Springer-Verlag; 1982. [Google Scholar]
- 59.Abercrombie HC, Schaefer SM, Larson CL, et al. Metabolic rate in the right amygdala predicts negative affect in depressed patients. Neuroreport. 1998;9:3301–7. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- 60.Watson D, Walker LM. The long-term stability and predictive validity of trait measures of affect. J Pers Soc Psychol. 1996;70:567–77. doi: 10.1037//0022-3514.70.3.567. [DOI] [PubMed] [Google Scholar]
- 61.SPSS software, PASW Statistics 17.0. Chicago: 2010. [Google Scholar]
- 62.Hastie BA, Riley JL, III, Robinson ME, et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116:227–37. doi: 10.1016/j.pain.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Fillingim R, King C, Ribeiro-Dasilva M, et al. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fillingim RB, Maixner W, Kincaid S, et al. Pain sensitivity in patients with temporomandibular disorders: relationship to clinical and psychosocial factors. Clin J Pain. 1996;12:260–9. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Bar KJ, Brehm S, Boettger MK, et al. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 66.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–72. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- 68.Sibille KT, Kindler L, Glover T, et al. Affect balance style predicts ischemic pain sensitivity, somatic symptoms, and pain coping. J Pain. 2010;11:S9. [Google Scholar]


