Abstract
Large, osseous, segmental defects heal poorly. Muscle has a propensity to form bone when exposed to an osteogenic stimulus such as that provided by transfer and expression of cDNA encoding bone morphogenetic protein-2 (BMP-2). The present study evaluated the ability of genetically modified, autologous muscle to heal large cranial defects in rats. Autologous grafts (8mm × 2mm) were punched from the biceps femoris muscle and transduced intraoperatively with recombinant adenovirus vector containing human BMP-2 or green fluorescent protein cDNA. While the muscle biopsies were incubating with the vector, a central parietal 8mm defect was surgically created in the calvarium of the same animal. The gene-activated muscle graft was then implanted into the cranial defect. After 8 weeks, crania were examined radiographically, histologically, and by micro-computed tomography and dual energy X-ray absorptiometry. Although none of the defects were completely healed in this time, muscle grafts expressing BMP-2 deposited more than twice as much new bone as controls. Histology confirmed the anatomical integrity of the newly formed bone, which was comparable in thickness and mineral density to the original cranial bone. This study confirms the in vivo osteogenic properties of genetically modified muscle and suggests novel strategies for healing bone.
Keywords: Gene Therapy, Bone Healing, Muscle, BMP-2, Osteogenesis
Introduction
As reviewed recently1, gene transfer holds much potential as an enhancing technology for bone healing, and there are several strategies for achieving this. Here we report data from a rat study in which a recombinant adenovirus (Ad.BMP-2) was used to transfer cDNA encoding human bone morphogenetic protein-2 (BMP-2) into skeletal muscle biopsies that were then inserted into 8mm cranial defects in the same animals during a single operative session. The ability to perform ex vivo gene therapy in an expedited fashion during one surgery brings great advantages of economy, safety and utility.2
Skeletal muscle has an enormous propensity to form bone. This is seen most dramatically in the genetic disease fibrodysplasia ossificans progressiva (FOP) where patients develop a second skeleton as muscle spontaneously ossifies.3 Heterotopic ossification of muscle is also common after joint replacement surgery4 and following blast injuries.5 Although these are troubling medical conditions, they suggest novel opportunities for regenerative medicine to address circumstances where it is necessary to grow large amounts of new bone.
In considering muscle as a basis for repairing large osseous lesions, it needs to be compared to the present clinical practice of using bone graft and alternative future strategies such as artificial biomaterials. Autologous bone grafting is the present method of choice, but supplies of autologous bone are limited and graft harvesting is associated with morbidity at the donor site. Artificial biomaterials hold much promise, but do not, by themselves, supply the osteoprogenitor cells needed for bone formation. Moreover, there are often issues with resorption of the scaffold used in the biomaterial. As noted, muscle is a rich source of osteoprogenitor cells and has the advantage of being intrinsically osteogenic. Moreover, because of the phenomenon of heterotopic ossification, we know that muscle is highly osteogenic in vivo in humans. The relative merits of the various different approaches to bone healing will only become clear with more research.
FOP results from an activating mutation in ALK2, a BMP receptor6, suggesting that a sustained BMP signal drives the massive amount of new bone formation that occurs in this disease. Moreover, it is known that muscle contains osteo-7 and chondro-8 progenitor cells, suggesting that the formation of bone within muscle need not rely on outside progenitors. In view of this, we have suggested that the implantation of muscle biopsies expressing BMP-2 can be used to heal bone in vivo. Proof of principle has been demonstrated in a rat, critical-size, femoral, segmental defect model, using Ad.BMP-2 as the vector for gene transfer. Implantation of gene-activated muscle grafts led to rapid, reliable and uniform healing of the defects via endochondral ossification.9
Cranial defects offer an additional opportunity to evaluate this technology. Calvarial healing differs in several important ways from that of long bones. Although young children and immature animals heal large calvarial defects, this ability is lost in adult animals and humans older than 24 months.10, 11 The reason for this is unknown, but it has been attributed to poor blood supply, lack of mechanical stimulation, deficiency of bone marrow and insufficient presence of progenitor cells. Unlike the long bones, which form endochondrally during development, those of the cranium are formed via intramembranous ossification. Studies on the repair of cranial defects in animal models have noted both endochondral and intramembranous routes of regeneration, depending upon the experimental model.12-14
Here we describe studies in rats aimed at evaluating the ability of gene-activated muscle grafts to heal critical-sized defects in the cranium.
Materials and Methods
Animals
Fischer F344 male rats (Charles River Laboratory, Wilmington, MA) weighing 200 to 250g and 8 weeks of age were used for all experiments. Animal care and experimental protocols were followed in accordance with National Institutes of Health guidelines and approved by the Brigham and Women's Hospital Institutional Animal Care and Use Committee.
Preparation of Adenovirus Vectors
First generation adenovirus (ΔE1, ΔE3), serotype 5, carrying human BMP-2 cDNA (Ad.BMP-2) or green fluorescent protein cDNA (Ad.GFP) under the transcriptional control of the human cytomegalovirus early promoter were constructed by cre-lox recombination15 as we have described previously.16, 17 Viruses were propagated in 293 cells (ATCC, Manassas, VA), purified on cesium-chloride gradients, and dialyzed against 10 mM Tris-HCl (pH 7.8), 150 mM NaCl, 10 mM MgCl2, and 4% sucrose buffer. Viral titers were estimated as 1012 to 1013 viral particles (vp)/ml by optical density and 1010 to 1011 plaque forming units (pfu)/ml by standard plaque assay.16, 17
These vectors and the rats were used in a series of in vitro and in vivo studies (figure 1).
Figure 1. Experimental design.
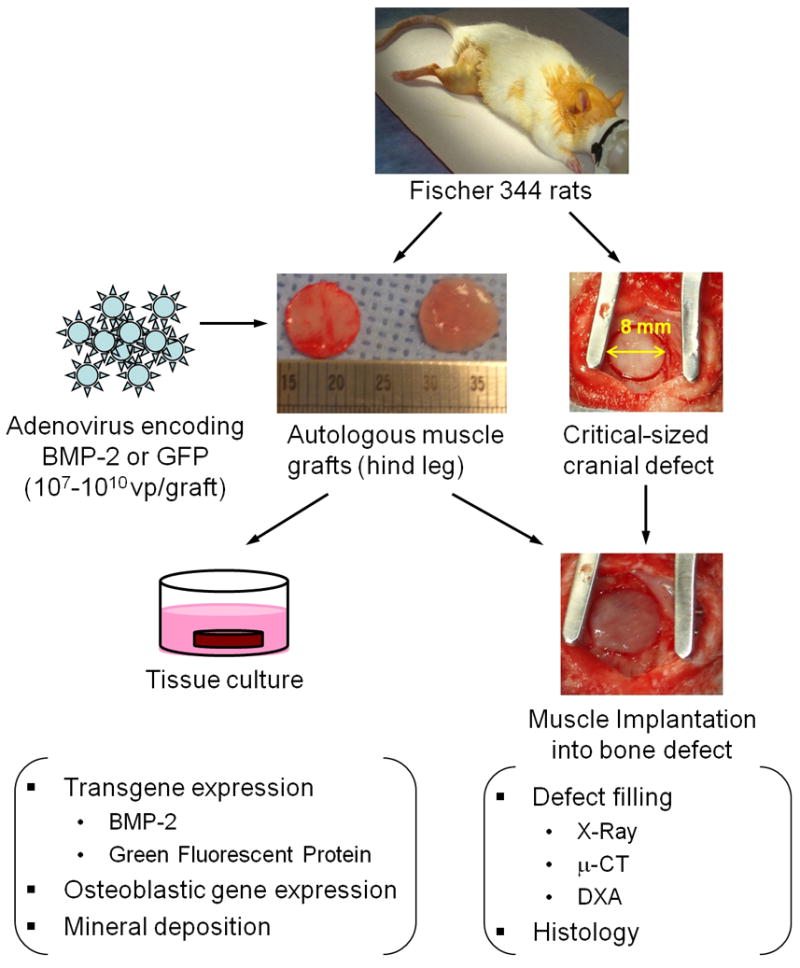
Skeletal muscle was harvested from the hind limbs of Fischer F344 rats, and 8 mm discs were made using a biopsy punch. Discs were transduced with adenoviral vectors encoding human BMP-2 or GFP and cultured in vitro to evaluate transgene expression and consequent osteogenic response. To determine whether gene activated muscle could improve cranial bone healing, critical size defects were generated in rat skulls. Test groups received autologous muscle graft with or without intraoperative adenoviral modification. After 8 weeks, defect healing was evaluated qualitatively by X-Ray imaging and histology as well as quantitatively by μ-CT and DXA.
In Vitro Experiments
Genetic modification and osteogenesis of muscle biopsies
Skeletal muscle tissue was harvested aseptically from donor animals and cut into 2mm thick slices. Standard 8mm × 2mm muscle discs were produced with an 8mm skin biopsy punch. These were placed individually into 24-well plates and genetically transduced by dropping a 50μl aliquot of virus suspension onto the surface of the disc. The plates were returned to the incubator (37°; 5% CO2) for 30min. The muscle discs were then turned over and virus suspension dropped onto the other side of the tissue. Plates were again returned to the incubator for 30 min. Discs were then washed with phosphate buffered saline (PBS), and 1ml basal osteogenic medium (Dulbecco's Modified Essential Medium (DMEM), 10% fetal calf serum, 50μg/ml ascorbic acid, 10 mM β-glycerol phosphate) added to each well; this medium was replaced every 48h. All experiments were run in triplicate, using three pieces of muscle taken from a single donor rat in each case.
One week after transduction, 48 h conditioned media from muscle discs modified with 107, 108, 109, or 1010 vp/disc of Ad.BMP-2 were collected and their BMP-2 content measured using a commercial ELISA kit (R&D Systems, Minneapolis, MN, USA). To assess the temporal expression of BMP-2 by these modified muscle discs, 48 h conditioned media were collected at 3, 7, 21 and 42 days after transduction with 1010 vp Ad.BMP-2, and their BMP-2 contents were measured. Additional muscle discs were collected at the 2 week timepoint for measurement of BMP-2 message levels, as detailed below.
To determine whether BMP-2 over-expression within the muscle graft could stimulate osteoblastic differentiation of endogenous cells, discs were cultured for up to 8 weeks in vitro in basal osteogenic medium. Osteogenesis was quantitatively assessed on the basis of alkaline phosphatase activity on Day 10; transcript levels for the bone matrix markers were measured after 14 and 28 days by quantitative, real time RT-PCR. To determine the deposition of extracellular mineral by the genetically modified muscle discs, certain samples were processed for histology, as described below, and stained with alizarin red.
Alkaline phosphatase assay
Alkaline phosphatase enzyme activity was determined kinetically by monitoring the conversion of p-nitrophenol phosphate to p-nitrophenol using a commercially available kit (Sigma Chemical Co., St Louis, MO, USA). Briefly, cultures were washed twice with PBS and 250 μl of the substrate solution added to each well. After 15 minutes incubation, the resulting solution was transferred to tubes containing the same volume of 1 M sodium hydroxide to stop the reaction. The discs were immediately washed twice with PBS and then digested with collagenase (Sigma) to release cells for DNA assay. The absorbance was read at 405 nm on an UVmax Kinetic Microplate Reader (Molecular Devices, Sunnyvale, CA). Alkaline phosphatase activity was expressed as nitrophenol product/minute using a standard curve and normalized to total DNA content from each muscle disc, which was analyzed using Quant-iT™ PicoGreen® dsDNA Assay Kit (Invitrogen). All experiments were performed in triplicate.
Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Muscle discs were homogenized in TRIzol® (Invitrogen, Carlsbad) and total RNA was extracted according to the manufacturer's protocol. The concentration of total RNA was assessed spectrophotometrically at 260nm and its purity from the 260:280nm absorption ratio; its integrity was evaluated by agarose gel electrophoresis. Total RNA (1μg) was transcribed into cDNA using the SuperScript® First-Strand Synthesis System and oligo-dT primers (50μM) (Invitrogen), according to manufacturer instructions.
A highly sensitive quantitative PCR method was performed on an M×3000P™ QPCR System (Stratagene, USA), using the SYBR Green ® Dye detection system (Applied Biosystems), using rat GAPDH as an endogenous reference gene.18 According to the information provided on the database and with the aid of the Primer3 software, pairs of gene specific primers for human BMP-2 and rat bone matrix markers were designed as described in Table 1. The 10 μl reaction mix contained 2× Power SYBR® Green PCR Master Mix, 10-fold diluted cDNA and 0.2μM of each primer. The thermal protocol consisted of 10 min polymerase activation at 95°C, followed by 40 cycles of denaturation at 95°C for 30 sec, primer annealing at 55°C for 1 min and extension at 72°C for 30 sec. During expression analysis, each sample was amplified in duplicate, the average Ct value was calculated and a dissociation curve was generated by plotting each of the PCR products against its specific melting temperature (Tm) for verification. The mRNA expression levels were normalized to those of the endogenous reference gene GAPDH and reported as relative values (ΔΔCT) to those obtained from the control group.
Table 1. Primer Pairs for target and housekeeping genes used in qRT-PCR assays.
| Gene | Species | Sequence (5′-3′) |
Size (bp) |
Accession genea |
|---|---|---|---|---|
| BMP 2 | Human | CCAGAAACGAGTGGGAAAAC ACCAACCTGGTGTCCAAAAG | 227 | NM_001200 |
| Col1a1 | Rat | TCCAGTTCGAGTATGGAAGC AAGCGTGCTGTAGGTGAATC | 233 | NM_053304 |
| BSP | Rat | GAAGCAGGTGCAGAAGGAAC ACTCAACCGTGCTGCTCTTT | 157 | AB001383.1 |
| OCN | Rat | GAGGGCAGTAAGGTGGTGAA GTCCGCTAGCTCGTCACAAT | 135 | NM_013414 |
| GAPDH | Rat | TTCCTACCCCCAATGTATCC TGCTGTTGAAGTCACAGGAG | 162 | NM_017008 |
BMP2: bone morphogenetic protein 2; Col1a1: type I collagen, alpha 1 chain; BSP: bone sialoprotein; OCN: Osteocalcin; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
The accession number is the GenBank™ accession number.
In Vivo Experiments
Preparation of modified muscle grafts for insertion into craniotomy defects
Before making craniotomy defects, each rat was anesthetized and its right hind leg shaved and disinfected. A 15 mm skin incision was made to expose the hind limb muscle. A 1×1 cm2 muscle sheet was harvested from the biceps femoris and the harvest site sutured with 3-0 Vicryl sutures. The overlying skin was closed with Autoclip Wound clips. Standard 8 mm × 2 mm muscle discs for implantation were created and transduced with adenovirus, as described above. During this period, the critical-sized cranial defect was created in the same animal as described above. After craniotomy, the genetically-modified muscle autograft was washed with PBS and transplanted.
Surgical Procedure
Animals were divided randomly into three surgical groups, with 4 animals per group. A central parietal critical-size defect of 8 mm in diameter was created in the animals according to the method of Schmitz and Hollinger.19 Briefly, the rats were placed under general anesthesia by administration of isoflurane with a small animal anesthesia machine. A 3 cm midline sagittal incision over the cranium was made and the periosteum carefully elevated. A circular, full-thickness bone defect was trephined in the center of parietal bone using a 8 mm trephine bur with low-speed rotation (Torque Plus - AEU-12C, Aseptico, US) under constant irrigation with sterile saline to prevent overheating of the bone margins. Care was taken not to injure the underlying dura mater. The cranial defect was flushed with saline solution and then carefully filled using a sterile metal spatula with either Ad.BMP-2 transduced muscle implants or control implants including Ad.GFP transduced muscle and unmodified muscle, as well as a sham-operated control. The periosteum was subsequently repositioned, sutured with 3-0 Vicryl sutures and the incision site of the scalp closed using Autoclip wound clips.
Animals were euthanized 8 weeks after craniotomy. The cranial defect sites were harvested, along with surrounding bones, soft tissues, and brain, and rinsed with saline solution. After harvesting, radiographs were taken using a dental X-ray unit (Sirona Dental System GmbH, Bensheim, Germany) and the defect site fixed for subsequent micro-computed tomography (μCT) scanning and histology.
Three-Dimensional μCT and Dual Energy X-ray Absorptiometry (DXA)
We used 3-dimensional μCT in accordance with previous reports.20, 21 Briefly, the architecture of newly formed bone in the calvarial defect was examined using a desktop microtomographic imaging system (μCT40; Scanco Medical AG, Bassersdorf, Switzerland) equipped with a 10 mm focal spot microfocus x-ray tube. The calvarial defects were scanned with a 16 mm isotropic voxel size at 55 keV energy, 200 ms integration time with approximately 150 to 200 μCT slices per specimen. By combining the slice images taken at 8 μm, the three dimensional architecture of the newly formed bone was reconstructed. The newly formed bone volume fraction in the defect (BV/TV) was calculated by the μCT system software package. Statistical analysis of the data was carried out using ANOVA. Values of P <0.05 were considered significant.
DXA measurements were made with a PIXImus 2 apparatus (GE-Lunar, Madison, WI, USA). Specimens were placed on a Lucite block during scanning to simulate soft tissue. The scans were acquired using small animal high resolution mode. All excised crania were evaluated after 8 weeks of treatment in the area corresponding to the region of the critical-sized bone defect.
Histology
Repair tissue from euthanized animals and in vitro cultured muscle discs were fixed in 4% ice cold paraformaldehyde for 24h at 4°C before decalcification in 10% EDTA in 0.1M phosphate buffer (pH 7.4). The specimens subsequently underwent dehydration in graded ethanol and embedding in paraffin. Serial 5μm paraffin sections were placed on poly-L-lysine-coated slides, dried overnight, stained with hematoxylin and eosin, and examined under light microscopy. Specimens from in vitro cultured muscle discs were also processed in this fashion, but without decalcification, and the paraffin sections were stained with 0.5% alizarin red (Sigma-Aldrich) to detect mineralized deposits.
Statistical Analysis
Comparisons of continuous variables between two treatment groups were performed using the two-tailed Student's t-test, and between three groups by analysis of variance (One Way-ANOVA). If the difference between the control and the treatment groups was significant, a post-hoc test (Tukey) was performed. All in vitro experiment was performed triplicate. Data are expressed as means ± standard deviation (SD). The results were taken to be statistically significant at a probability level of p<0.05, unless otherwise noted as p<0.01.
Results
In vitro transgene expression by adenovirus-modified muscle discs
Muscle discs were incubated with recombinant adenovirus vectors, washed, and placed into organ culture. Within three days of applying 1010 vp of Ad.GFP to 8 mm disks, GFP-positive cells were clearly visible within the discs (figure 2A). GFP expression remained high for 2 weeks (figure 2C) and persisted within the muscle disks for at least 6 weeks (data not shown). When the Ad.BMP-2 vector was used under the same conditions, BMP-2 message expression was increased nearly 500-fold (figure 2D). After 1 week in culture, BMP-2 secretion from muscle discs increased in a dose-dependent fashion in response to transduction with Ad.BMP-2 (figure 2E). At the highest dose (1010 vp), muscle discs produced approximately 8 ng BMP-2/disc over a 48-hour period. A dose of 1010 vp Ad.BMP-2/disc was used for all subsequent experiments. Using this amount of vector, the transduced muscle discs continued to produce BMP-2 at a declining rate for at least 6 weeks (figure 2F).
Figure 2. Transgene expression within genetically-modified muscle discs.
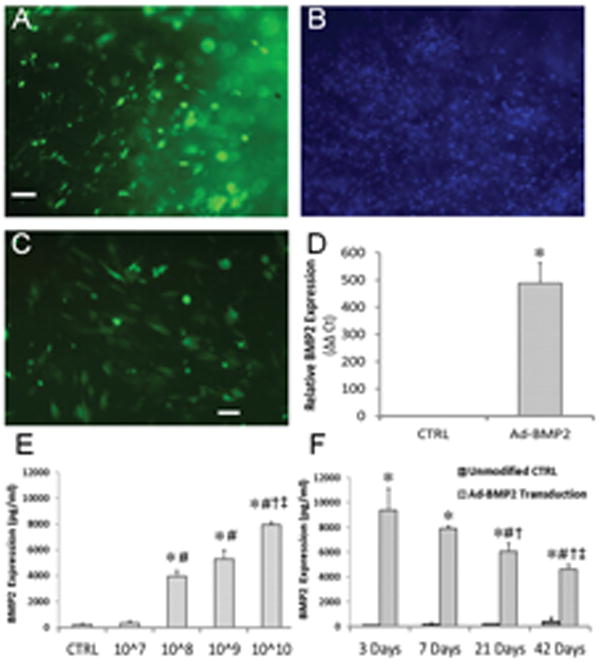
(A-C) Fluorescence microscopic evaluation of muscle discs modified with Ad.GFP. The number of green fluorescent cells near the disc surface after 3 days (A) represents a considerable fraction of the total cell population, as indicated by DAPI staining (B). (C) Additional discs imaged 2 weeks post-modification demonstrate persistent GFP expression within the discs. Scale bars = 100 μm. (D) Discs modified with Ad.BMP-2 (1010 vp/disc) were cultured for 2 weeks, at which point BMP-2 message was detected by qRT-PCR. * indicates a significant increase (p<0.01) over unmodified muscle. (E) Conditioned media were collected from muscle discs transduced with 107 – 1010 vp Ad.BMP-2 after 1 week of culture, and BMP-2 protein levels were measured by ELISA. * and # indicate significant increases (p<0.01) over the control and 107 vp groups, respectively; † and ‡ represent significant increases (p<0.05) relative to the 108 and 109 vp groups, respectively. (F) BMP-2 levels within muscle-conditioned media at different time points following transduction with 1010 vp/disc Ad.BMP-2. * indicates a significant increase (p<0.01) over the control group; #, †, and ‡ represent significant decreases (p<0.05) relative to 3, 7, and 21 days, respectively.
Osteoblastic differentiation within BMP-2 gene-activated muscle discs
Alkaline phosphatase activity, an early marker of osteogenesis, increased within the discs approximately 6-fold (p<0.01) 10 days after transduction with Ad.BMP-2 (figure 3A). Collagen type I expression increased in response to BMP-2 overexpression by the 2 week time point (p<0.05), rising to approximately 6 times control levels by week 4 (p<0.05). Osteocalcin message was also slightly elevated by week 2 and increased to 10-fold of control levels by week 4 (p<0.05). In contrast, transcript levels for bone sialoprotein, a relatively late osteogenic marker, were unchanged at 2 weeks but increased 6-fold over controls (p<0.05) by 4 weeks (figure 3B).
Figure 3. Expression of osteoblastic markers by genetically-modified muscle discs.
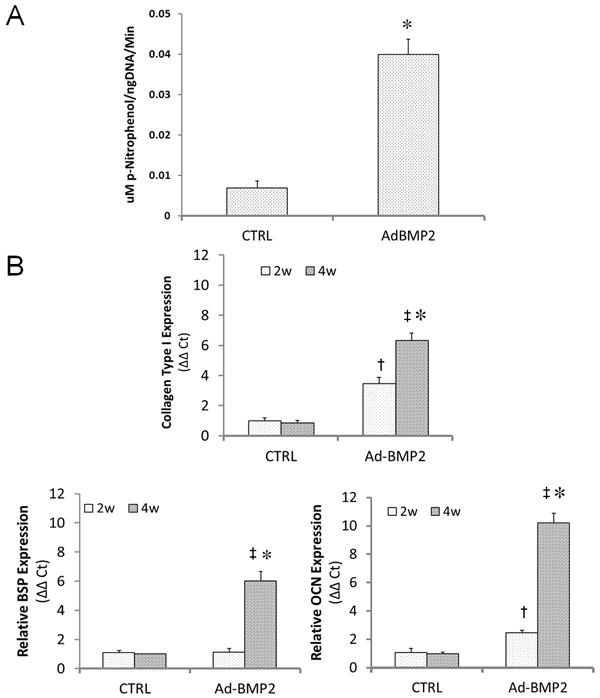
Discs were modified by 1010 vp Ad.BMP-2 and cultured for up to 4 weeks in basal osteogenic medium. (A) After 10 days in culture, alkaline phosphatase activity within muscle discs was measured and normalized by total DNA content. * indicates a significant increase (p<0.01) over the control group. (B) Additional discs were analyzed after 2 and 4 weeks of culture for expression of collagen type I, bone sialoprotein (BSP), and osteocalcin (OCN) by quantitative RT-PCR. † and ‡ denote significant increases (p<0.05) for the Ad.BMP-2 group relative to unmodified controls 2 and 4 weeks, respectively, while * indicates a significant difference (p<0.05) between the two time points for Ad.BMP-2-modified discs.
When cultured for the extended period of 8 weeks in the presence of β-glycerol phosphate and ascorbic acid, Ad.BMP-2-modified discs developed a visibly mineralized crust over their surface (figure 4A). Alizarin red staining of disc cross-sections revealed the deposition of calcified mineral on the superficial layers of the discs (figure 4B). The thickness of this layer increased with the dose of Ad.BMP-2.
Figure 4. Mineral deposition within muscle grafts cultured in vitro.
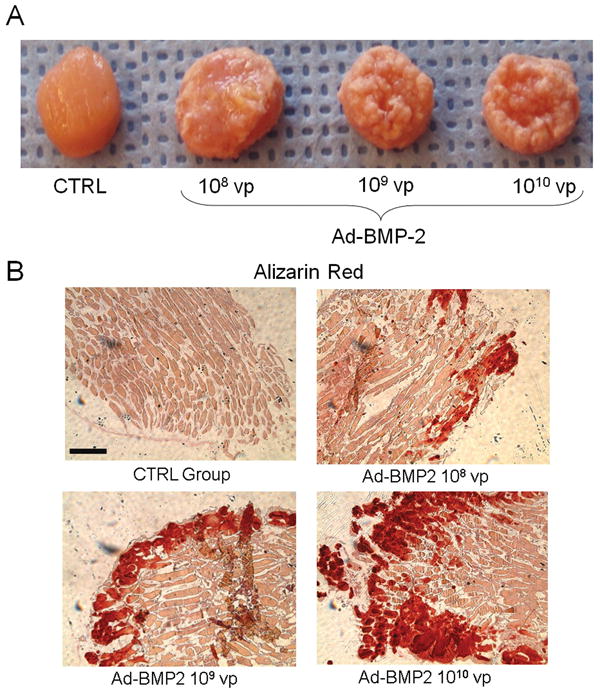
Muscle discs were modified with graded amounts of Ad.BMP-2 and cultured in basal osteogenic medium. (A) Gross appearance of muscle discs after 8 weeks. As the dose of Ad.BMP-2 and consequent BMP-2 secretion (see figure 2) increased, the discs became more extensively encased in a mineralized crust. (B) Alizarin red staining of disc cross sections revealed an increasingly thick layer of calcified mineral on the disc surface with increasing viral dose. Scale bar = 500 μm.
Enhanced cranial defect repair by BMP-2 gene-activated muscle grafts
To determine whether the responses demonstrated above could enhance the repair of a critical-sized cranial defect, autologous muscle was harvested, transduced intraoperatively during the generation of an 8 mm defect in the rat, and subsequently implanted. After 8 weeks, the skulls were harvested for evaluation of defect repair. Radiographs taken immediately after harvest demonstrated only a small degree of filling of defects with new bone for the sham-operated, unmodified muscle groups and Ad.GFP modified muscle groups (figure 5A).
Figure 5. Cranial defect healing using gene-activated muscle grafts.
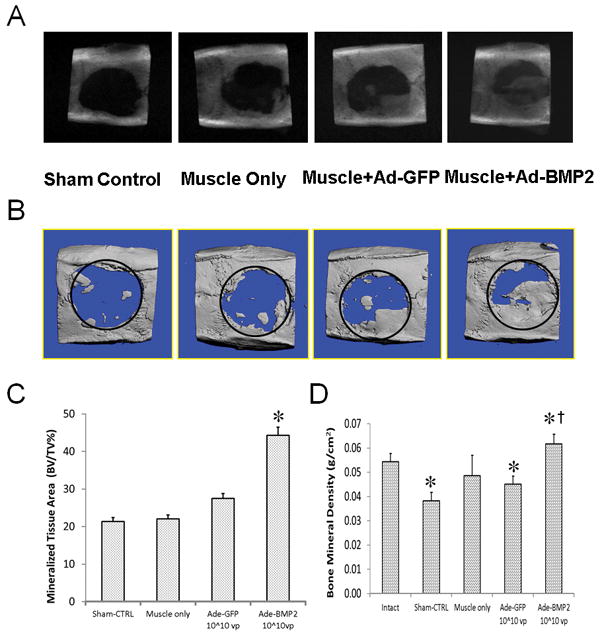
Critical size defects were left empty (sham control), were filled with autologous, unmodified muscle (muscle control), or were filled with muscle modified intraoperatively with Ad.GFP or Ad.BMP-2 (1010 vp/disc). (A) Representative radiographs of skulls taken after 8 weeks. (B) Micro-computed tomography (μCT) scans reinforced observations from X-Ray imaging. (C) Ratios of bone volume to total volume were quantified from CT scans. * indicates a significant increase (p<0.05) for the Ad.BMP-2 group compared to Ad.GFP control. (D) Bone mineral density of the repair tissue was determined by DXA for the treatment groups as well as intact crania. * denotes a significant difference from the intact cranial bone (p<0.05); † indicates a significant difference compared to the Ad.BMP-2-muscle group.
Defect filling increased considerably for rats given muscle graft modified with 1010 vp Ad.BMP-2, although filling was typically incomplete at the 8 week endpoint. Images from μCT scans of cranial explants were consistent with the X-Ray images (figure 5B). Measurement of relative bone volume within the defects revealed that treatment with BMP-2 expressing grafts contained twice as much new bone as control groups (figure 5C). DXA measurements of bone formed within the defects confirmed that, regardless of the amount of bone that was deposited, the new bone had normal mineral density (figure 5D).
Transverse sections of cranial specimens were stained with H&E to evaluate the quality of repair (figure 6). The new bone deposited within Ad.BMP-2 defects, while not yet remodeled into a continuous structure, was comparable in thickness to the original cranial bone. In contrast, defects for both control groups were filled primarily with fibrous tissue. Small remnants of muscle tissue could be seen within some of the defects given unmodified muscle, but not Ad.BMP-2 muscle defects.
Figure 6. Histological evaluation of filling within cranial defects.
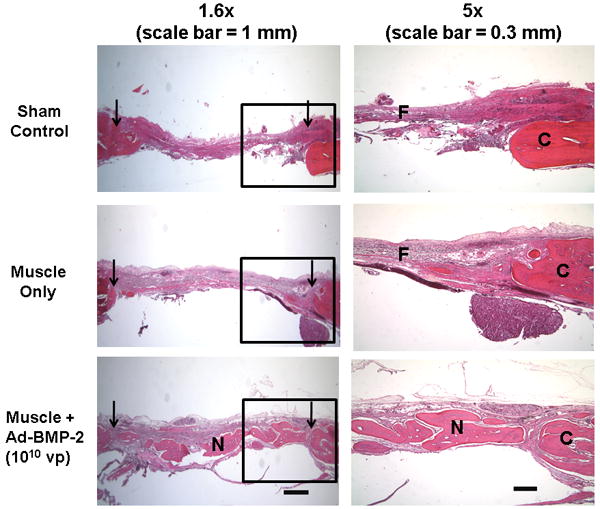
Transverse sections of decalcified rat crania were stained with hematoxylin and eosin (H&E). Left-hand column: images from representative sections of each experimental group are shown over the entire length of the defect. Arrowheads indicate the original defect boundaries. Scalebar = 1 mm. Right-hand column: higher-magnification regions (boxed areas from the left) are shown to better display one edge of the defect. “C” marks the original cranial bone, “N” denotes new bone formation within the defects, and “F” indicates fibrous repair tissue. Scale bar = 300 μm.
Discussion
These data confirm the ability of genetic transduction with Ad.BMP-2 to induce osteogenic activities in biopsies of skeletal muscle. These include the expression of alkaline phosphatase, type I collagen, bone sialoprotein, and osteocalcin, and the deposition of a mineralized matrix noticed both histologically and, remarkably, by the formation of an obvious crust on the surface of the muscle discs.
Transduced muscle discs conditioned their media to about 8ng/ml BMP-2, which is far below the concentration of recombinant, human BMP-2 that is normally needed to promote in vitro osteogenesis of precursor cells. Moreover, we have noted that rat muscle discs cultured in the presence of 100ng/ml BMP-2 produce far less mineral than those transduced with Ad.BMP-2 (data not shown). This indicates that transfer of the BMP-2 cDNA is more osteogenic than addition of recombinant BMP-2. The reason for this requires further research, but possible explanations include specific post-translational modifications undergone by the endogenously synthesized molecules, a potent paracrine or autocrine response, and a potentiating effect of infection with adenovirus.
The possible potentiation of BMP-2 signaling by adenovirus infection is intriguing because adenovirus infection activates mitogen activated protein kinases (MAPK).22 The MAPK pathway is known to be involved in BMP-2 signaling23, raising the possibility that infection with adenovirus reinforces or amplifies this signal. This may be relevant to FOP, where the heterotopic formation of bone in muscle occurs in response to injury and inflammation.3
Implantation of gene-activated muscle discs into calvarial defects increased the amount of bone deposited during an 8-week period. Although this is promising, it is less impressive than the dramatic healing seen in long bone defects. In the latter study9, similar gene-activated muscle discs rapidly and reliably healed 5 mm segmental defects in the femora of rats. Bridging of these critical-size defects was noted radiologically within 2 weeks and full boney union, with the reformation of marrow, was confirmed at 8 weeks. The possible reasons for greater efficacy in the long bone model are many, and include lack of mechanical stimulation in the cranium and issues related to blood supply and oxygen tension. The amount of muscle and surface area: volume ratio differences may also exert an influence. Four stacked discs of genetically modified muscle, each 4 mm × 1 mm, were implanted into the femoral defects, whereas in the calvarial defects, only a single disc measuring 8 mm × 2 mm was implanted. This may not have provided sufficient numbers of progenitor cells or endogenous BMP-2 production. Finally, there may be differences related to the periosteum. In the long bone defects, the periosteum was surgically removed when forming the segmental defect and histology suggested that the periosteum of the adjacent cut ends of the bone was not involved in the healing. In the cranial defects, the periosteum was deliberately preserved, but was not evident histologically at 8 weeks. The significance of this finding is unknown.
This study does not identify the origin of the cells that form the osteoblasts of the newly deposited bone within the defect. Possible sources include the grafted muscle, which has a rich population of osteoprogenitor cells,7 the periosteum and the dura mater. Further research will clarify this matter, and also determine to what degree the muscle graft merely serves as an endogenous source of BMP-2.
The mode of healing may also be different in the cranium and femur. Although transduced muscle discs undergo osteogenic differentiation in vitro, they rapidly form cartilage within large femoral defects and presumably heal the defect via endochondral ossification.9 Hypertrophic chondrocytes secrete angiogenic factors during endochondral ossification, thereby satisfying the need for new blood vessels.24 The present study provides no evidence of endochondral ossification; it may be that bone was formed intramembranously and the extent of new bone formation was limited by lack of sufficient angiogenesis. In agreement with this conclusion, Peng et al25 have confirmed that the repair of calvarial defects in mice by muscle-derived osteoprogenitor cells engineered to express BMP-4 is greatly enhanced by the co-expression of vascular endothelial growth factor.
Overall, these data illustrate the advantages of using an expedited, ex vivo gene transfer approach to bone healing, and indicate the possible need for different strategies to apply in different clinical settings. Ideally, clinical protocols based upon the technology discussed in this paper could occur in a single operative session without the need for expensive, time consuming ex vivo procedures in GMP (Good Manufacturing Practice) facilities2. As demonstrated in this rat study, muscle can be biopsied, genetically modified and implanted within the framework of a single surgery. Because the cranial area does not have much muscle for transplantation, muscle from another site, such as the latissimus dorsi or rectus abdominus, would be a better source. This could be harvested and transduced while other aspects of the surgery are taking place.
Clinical translation of orthopaedic gene therapy applications such as this is subject to several levels of scrutiny, including safety.26 Safety of the present protocol is enhanced because no viral particles are introduced directly into the body. Moreover, adenoviral vectors are non-replicating, non-integrating and their genomes do not normally persist in vivo. Despite continuing concerns about the safety of gene therapy, the experience of over 1,700 clinical trials involving tens of thousands of subjects reveals few serious adverse events.26 Gene therapy is beginning show clinical success in certain areas of medicine27, which, if substantiated, will facilitate applications in the orthopaedic field, including bone healing.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (R01 050243) and the AO Foundation.
References
- 1.Evans CH. Gene therapy for bone healing. Expert Rev Mol Med. 2010 Jun;12:e18. doi: 10.1017/S1462399410001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans CH, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13(8):1987–93. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 3.Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP) Bone. 2008;43(3):427–33. doi: 10.1016/j.bone.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoltny T, et al. Heterotopic ossification in patients after total hip replacement. Ortop Traumatol Rehabil. 2007;9(3):264–72. [PubMed] [Google Scholar]
- 5.Covey DC. Combat orthopaedics: a view from the trenches. J Am Acad Orthop Surg. 2006;14(10 Spec No):S10–7. doi: 10.5435/00124635-200600001-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shore EM, et al. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38(5):525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 7.Bosch P, et al. Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18(6):933–44. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 8.Kuroda R, et al. Cartilage repair using bone morphogenetic protein 4 and muscle-derived stem cells. Arthritis Rheum. 2006;54(2):433–42. doi: 10.1002/art.21632. [DOI] [PubMed] [Google Scholar]
- 9.Evans CH, et al. Use of genetically modified muscle and fat grafts to repair defects in bone and cartilage. Eur Cell Mater. 2009;18:96–111. doi: 10.22203/ecm.v018a09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Velardi F, et al. Osteogenesis induced by autologous bone marrow cells transplant in the pediatric skull. Childs Nerv Syst. 2006;22(9):1158–66. doi: 10.1007/s00381-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 11.Szpalski C, et al. Cranial bone defects: current and future strategies. Neurosurg Focus. 29(6):E8. doi: 10.3171/2010.9.FOCUS10201. [DOI] [PubMed] [Google Scholar]
- 12.Lemperle SM, et al. Bony healing of large cranial and mandibular defects protected from soft-tissue interposition: A comparative study of spontaneous bone regeneration, osteoconduction, and cancellous autografting in dogs. Plast Reconstr Surg. 1998;101(3):660–72. doi: 10.1097/00006534-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Ripamonti U, et al. Limited chondro-osteogenesis by recombinant human transforming growth factor-beta 1 in calvarial defects of adult baboons (Papio ursinus) J Bone Miner Res. 1996;11(7):938–45. doi: 10.1002/jbmr.5650110710. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka T, et al. Morphological study of recombinant human transforming growth factor beta 1-induced intramembranous ossification in neonatal rat parietal bone. Bone. 1993;14(2):117–23. doi: 10.1016/8756-3282(93)90237-5. [DOI] [PubMed] [Google Scholar]
- 15.Hardy S, et al. Construction of adenovirus vectors through Cre-lox recombination. J Virol. 1997;71(3):1842–9. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gouze JN, et al. In vitro gene transfer to chondrocytes and synovial fibroblasts by adenoviral vectors. Methods Mol Med. 2004;100:147–64. doi: 10.1385/1-59259-810-2:147. [DOI] [PubMed] [Google Scholar]
- 17.Palmer GD, et al. Gene transfer to articular chondrocytes with recombinant adenovirus. Methods Mol Biol. 2003;215:235–46. doi: 10.1385/1-59259-345-3:235. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Schmitz JP, Hollinger JO. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;(205):299–308. [PubMed] [Google Scholar]
- 20.Ueno T, et al. Pathological change of articular cartilage in the mandibular head treated with immunosuppressant FK 506. Histol Histopathol. 2004;19(1):15–21. doi: 10.14670/HH-19.15. [DOI] [PubMed] [Google Scholar]
- 21.Kawana F, et al. Porcine enamel matrix derivative enhances trabecular bone regeneration during wound healing of injured rat femur. Anat Rec. 2001;264(4):438–46. doi: 10.1002/ar.10016. [DOI] [PubMed] [Google Scholar]
- 22.Crofford LJ, et al. Adenovirus binding to cultured synoviocytes triggers signaling through MAPK pathways and induces expression of cyclooxygenase-2. J Gene Med. 2005;7(3):288–96. doi: 10.1002/jgm.661. [DOI] [PubMed] [Google Scholar]
- 23.Hassel S, et al. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am. 2003;85-A(3):44–51. doi: 10.2106/00004623-200300003-00009. [DOI] [PubMed] [Google Scholar]
- 24.Gerber HP, et al. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5(6):623–8. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 25.Peng H, et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110(6):751–9. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy - lost in translation? J Cellul Physiol. 2011;227:416–420. doi: 10.1002/jcp.23031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 29(2):121–8. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]


