Abstract
Purpose
The purpose of this study was to (1) develop a high resolution 3T MRA technique with in-plane resolution approximate to that of MDCT and a voxel size of 0.35 × 0.35 × 1.5 mm3 and to (2) investigate the image quality of this technique in healthy subjects and preliminarily in patients with known coronary artery disease (CAD).
Materials and Methods
A 3T coronary MRA technique optimized for an image acquisition voxel as small as 0.35 × 0.35 × 1.5mm3 (HRC) was implemented and the coronary arteries of twenty two subjects were imaged. These included 11 healthy subjects (average age 28.5 years old, five males) and 11 subjects (average age 52.9 years old, five females) with CAD as identified on multidetector coronary computed tomography (MDCT). Additionally, the 11 healthy subjects were imaged using a method with a more common spatial resolution of 0.7×1×3 mm3 (RRC). Qualitative and quantitative comparisons were made between the two MRA techniques.
Results
Normal vessels and CAD lesions were successfully depicted at 350×350μm2 in-plane resolution with adequate signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR). The CAD findings were consistent among MDCT and HRC. The HRC showed a 47% improvement in sharpness despite a reduction in SNR (reduced by 72%) and CNR (reduced by 86%) compared to the RRC.
Conclusion
This study, as a first step towards substantial improvement in the resolution of coronary MRA, demonstrates the feasibility of obtaining at 3T a spatial resolution that approximates that of MDCT. The acquisition in-plane pixel dimensions are as small as 350μm × 350μm with a 1.5 mm slice thickness. While SNR is lower, the images have improved sharpness resulting in image quality that allowed qualitative identification of disease sites on MRA consistent with MDCT.
Introduction
In this evolving age of multi detector coronary CT (MDCT), coronary magnetic resonance angiography (MRA) remains a fundamentally appealing non invasive tool for the evaluation of coronary artery disease (CAD) because it does not expose patients to radiation or contrast agents associated with potential nephrotoxicity 1–3. However, to achieve maximum utility in the evaluation of CAD, magnetic resonance imaging (MRI) methods require a higher spatial resolution to approach that of the current reference standard of x-ray coronary angiography (~0.2mm). To date, the commonly used and multi-center trial tested in-plane spatial resolution of coronary MRA at 1.5T using gradient echo sequences is ~0.7×1×3mm3 1. A smaller voxel size of 0.4×0.4×2mm3 has been reported using a black-blood fast spin-echo imaging technique at 1.5T 4. However, while this black blood technique may be better suited for vessel wall imaging, a comparable resolution for angiographic imaging has not been investigated in a clinical setting, to our knowledge 4.
With the growing availability of 3T MRI scanners, the question that merits investigation is whether the higher field strength, and its associated higher signal-to-noise (SNR), confers the anticipated benefits of significantly higher spatial resolution. The availability of 32 element coils for the thorax may further improve SNR. An early study comparing coronary MRA at 3T and 1.5T demonstrated a significantly higher SNR and CNR at 3T but without a significant improvement in diagnostic accuracy for detecting significant proximal CAD 5. However, the authors in that study compared relatively lower spatial resolution techniques at both 3T and 1.5T using an acquired voxel size of 0.9 × 0.9 × 3mm3 without using any higher spatial resolution at 3T. Other studies in healthy subjects compared whole heart coronary MRA using contrast enhanced techniques at 3T to balanced steady-state free precession (SSFP) at 1.5T. These showed an improvement in CNR and depiction of the distal coronary segments at 3T6,7. However, the impact of substantially improving spatial resolution was not studied. Yang et al8 investigated the value of contrast enhanced whole heart coronary MRA at 3T in 69 patients utilizing a spatial resolution of 1.3 × 1.3 × 1.3 mm3. This study did show some improvement in diagnostic parameters compared to 1.5T, yet it did not demonstrate improvement in spatial resolution. In another early 3T preliminary report, investigators demonstrated that a higher angiographic spatial resolution with a voxel size of 0.6 × 0.6 × 3mm3 could be obtained in selected healthy adult volunteers 9. However, maximizing the spatial resolution was not an aim of that early study. In addition, image quality characteristics (SNR, CNR and vessel sharpness) and the potential utility of high resolution methods in patients have not been investigated to our knowledge.
The purpose of this study was to (1) develop a high resolution 3T MRA technique with in-plane resolution approximate to that of MDCT and a voxel size of 0.35 × 0.35 × 1.5 mm3 and to (2) investigate the image quality of this technique in healthy subjects and preliminarily in patients with known CAD. For the second goal, we particularly sought to determine whether the image quality obtained in normal volunteers is also obtained in diseased vessels where flow based contrast may be compromised. It should be noted that the voxel size in this study, versus that used in conventional coronary MRA, is reduced by a factor of at least two in each of the dimensions. Thus the voxel in the technique under study has a volume which is ~1/11 that of the voxel used in prior (0.7 × 1 × 3 mm3) coronary MRA studies. The large reduction in voxel volume results in a concomitant reduction in intrinsic SNR and thus imposes substantial technical challenges that must be met to realize the intended clinical benefit.
Materials and Methods
Subjects
Twenty two subjects provided written informed consent for participation in this study, which was approved by the Institutional Review Board and was HIPAA-compliant. Eleven subjects were healthy adult volunteers without history or risk factors for CAD (<1% Framingham score). Their ages ranged from 18 to 42 years old (mean 28.5 years old) and five were males. The healthy subjects were scanned over a period of 5 months. The other subgroup included 11 patients with at least one CAD risk factors and/or known CAD based on MDCT scans previously interpreted as positive for coronary lesions (table 1). These CAD risk factors include: hypertension, diabetes, dyslipidemia, family history of coronary artery disease (early onset atherosclerosis <50 year old in males and <60 year old in females who is first degree relative) or smoking history. The patients’ ages ranged form 33 to 65 years old (mean age 52.9 years old) and five were females. Their Framingham scores ranged from less than 1% to 20% with an average score of 9%. The patients were scanned over a period of 5 months. The patients gave informed written consent to participate in this technical development study. They were included for high resolution coronary MR imaging if they also underwent MDCT and/or conventional angiography and were found on these studies to have coronary artery disease. Thus entry into this technical development study as a patient required a positive MDCT or conventional angiographic examination for coronary artery disease.
Table 1.
Demographics of the patients with risk factors and cardiac CT findings. Risk factors included hypertension (HTN), family history (FH), Dyslipidemia (DL), smoking (SM), prior myocardial infarction (MI) and diabetes (DM)
| Age | Gender | Risk factors | All diseased coronary segments on CTA | Segments on CTA with >50% stenosis | Agatston calcium score |
|---|---|---|---|---|---|
| 44 | female | HTN, DL, FH | 1,2,3,5,6,7 | none | 709 |
| 52 | female | HTN, DM, DL | 5,2,5,6,8,9 | none | 0 |
| 48 | female | SM, FH, DL | none | none | 0 |
| 59 | male | FH, DL | 2,5,11 | none | 192 |
| 76 | male | HTN, prior MI | 1,2,3,5,6,7,9,11,17 | 5,6,7,9 | 1710 |
| 65 | male | HTN, SM | 1,2,5,6,7,9 | 5,6,2 | 417 |
| 33 | male | HTN | 6,7 | none | 43 |
| 49 | female | HTN, DM, DL | 1,2,3,5,6,7,8,9,10 | none | 366 |
| 63 | female | DL | 1,2,6,7,8,9,11,12 | 8,9,12 | 469 |
| 37 | male | FH, DL | 7,10,11 | none | 0 |
| 56 | male | FH, DL | 7 | none | 0 |
Correlative Imaging
All eleven patients had at least one risk factor for CAD and/or known CAD based on MDCT scans previously interpreted as positive for coronary lesions. Prior to MR imaging, the patients had MDCT scans using a 320-detector scanner (Aquilion ONE; Toshiba Medical Systems, Tochigiken, Japan) in 9 patients and 64-detector scanner (DEFINITION, Siemens Health Care, Forchheim, Germany) in the remaining 2 patients. The MR scans were obtained within an average of 19 days of the MDCT scans. Two patients had an additional conventional X-ray angiogram.
The MDCT protocol was similar to previously described techniques using identical equipment 10–12. Briefly, 50–100mg of oral metoprolol was given 30 to 60 minutes prior to MDCT when the patients’ heart rate needed to be lowered below 65 beats per minute 13. All subjects were in sinus rhythm and none had atrial fibrillation. MDCT was performed using a tube voltage of 120 kV and current of 400–580 mAs with a 220mm field of view, 512 matrix and retrospective ECG gating for the 64 detectors scanner and for 3 of 9 patients using the 320 detectors scanner. Retrospective gating was needed when the patients’ heart rate was above 65bpm. Prospective gating was used for the remaining patients imaged on the 320 detector scanner. The average (and standard deviation) radiation dose was CTDI(vol): 29.7±17.2mGy and DLP: 481.1±300.9mGycm. For contrast, 70–80 ml of non-ionic contrast (Isovue, Bracco Diagnostic Inc, Princeton, NJ) was injected through an 18–20 gauge peripheral venous access at a rate of 4–5 ml/sec followed by 50 ml of normal saline at the same injection rate.
MR Angiography Acquisition
All twenty two participants were imaged on a Philips 3T system (Philips Medical Systems, Best, NL). For the normal healthy volunteers, a 6-element cardiac phased-array receiver coil was used. A 32-element cardiac phased-array receiver coil became available for the patients and was used in all of those studies. Vector electrocardiographic (VCG) gating 14 was employed in each case; this was used to overcome enhanced electrocardiographic (ECG) changes attributable to the amplified magneto-hydrodynamic effect at 3T. Arrhythmia rejection methods were not used for any of the imaging techniques to avoid further prolongation of scan times. For all MRI sequences, repetition times and radiofrequency excitation angles were set to operate within the manufacturer’s (Philips Medical Systems) prescribed limits set to stay within the maximum allowable Specific Absorption Rate (SAR).
Scout Scanning
A multi-slice gradient echo (TR= 11ms; TE= 2.4ms; α= 20°) scout scan was acquired in 3 orthogonal orientations for localization of the volume for whole-heart imaging and for navigator positioning at the dome of the right hemidiaphragm. A 3D scout scan, an axial VCG triggered, segmented k-space steady-state free precession (SSFP) cine image series (TR = 3.8 ms, TE= 1.8 ms, α = 45°, and temporal resolution of 39.6 ms) at the level of the proximal to mid right coronary artery (RCA) was obtained during free breathing. This was done for visual determination of the most quiescent patient specific rest period in the cardiac cycle. The patient specific time delay between the R-wave of the ECG and this rest period (TD) was used for the subsequent high and regular spatial resolution coronary MRA. This was followed by a 3D segmented k-space gradient-echo low resolution, navigator and VCG gated whole-heart scan for localization of the coronaries. A 2D selective RF pulse with 12 revolutions in k-space and a beam radius of 15 mm was used for gating and tracking of respiratory motion 15. The navigator beam was positioned at the dome of the right hemidiaphragm with an acceptance window of 8 mm.
Regular Resolution Coronary MRA Scans (RRC)
This sequence was performed for the 11 normal volunteers only and was used for qualitative and quantitative comparison with the high resolution (350μm) images. Both the left (n=11) and right (n=11) coronary arteries were imaged for each of these healthy adults. A 6-element coil which was available at that phase of the study was used. After the scout and preparatory scans, 3D volume-targeted navigator gated 3D segmented k-space gradient echo coronary MRA were acquired using the visually identified TD. The 3D volume-targeted coronary MRA was oriented in parallel to the major axes of the left main and left anterior descending (LM/LAD) and right coronary arterial (RCA) systems defined from coronaries seen in the transaxial and/or oblique sagittal planes (TR = 8 ms, TE = 2.1 ms, α = 20°, field of view (FOV) = 270mm×270mm, matrix = 384×268, bandwidth = 362Hz/pixel, 10 partitions of 3mm thickness interpolated to 20 partitions of 1.5mm thickness each during reconstruction). The left circumflex coronary artery (LCX) was included in the FOV of one or both of the 3D volumes. An acquired voxel dimension of 0.7×1×3 mm3 was obtained for this 3D volume-targeted acquisition. Real-time navigator respiratory gating (5 mm gating window, slice tracking) was used for respiratory motion suppression for the 3D volume-targeted sequences 16,17. The respiratory navigator efficiency was 35%–50%. The temporal acquisition window for the 3D segmented gradient echo sequences was approximately 75 ms. To allow for comparative measurement of SNR, CNR and vessel sharpness parallel imaging (SENSE) was not used for coronary MRA in the healthy volunteers. Contrast was enhanced by using an adiabatic T2-Prep pulse that takes advantage of natural T2 differences between blood and myocardium at the higher magnetic field strength18. Spectrally selective fat saturation was also utilized for additional endogenous contrast enhancement between the coronary blood-pool and the epicardial fat. Volumetric shimming was used for all 3D acquisitions.
High Resolution (350μm) coronary MRA scans (HRC)
To achieve a factor of eleven reduction in voxel size routinely obtained at 1.5T, several acquisition parameters were modified and optimized on an experimental and iterative basis. The improvement in spatial resolution by a factor of at least 2 in all three dimensions resulted in the voxel reduction by approximately a factor of 11 (an order of magnitude). Thus the SNR obtained at 3T is approximately 1/5 of that at 1.5T, when the SNR gain from doubling the field strength is considered. Therefore, a T2-Prep pulse was not used at 3T as this would cause a further reduction in SNR 19. To recoup some of the SNR loss, a slightly longer TE = 2.4ms (vs. 2.1ms) was used which supports a reduced signal-readout bandwidth and therefore an improved SNR. The combination of higher field strength, prolonged TE, smaller signal-readout bandwidth and the removal of the T2-Prep was then used to image the coronaries with the target resolution of 0.35mm x 0.35mm × 1.5mm.
In all 11 normal volunteers, the coronary arteries were imaged using the HRC method with the 6-element coil which was available at that phase of the study, and without the use of parallel imaging (SENSE). This was to allow for direct comparison of the RRC and HRC techniques.
For the 11 patients with established CAD, a 32-element cardiac coil was used (which became available during the patient phase). To reduce overall scan time for the patients, parallel imaging with a SENSE factor of 2 was used despite a further reduction in SNR. A free-breathing navigator gated segmented k-space 3D gradient echo sequence (TR=7.2ms, TE=2.4ms, α = 20°, FOV = 270mm×202mm, matrix = 800×764, bandwidth = 173.6 Hz/pixel) was employed with fat saturation for all scans. These 3D volumes had an acquired slice thickness of 1.5mm (20 slices) and an acquired in-plane resolution of ~350 × 350μm2. The temporal acquisition window for the HRC scan was 86 ms. Scan plane localization and respiratory navigator settings were identical to those of the RRC scans.
Image Post Processing and Analysis
For the MDCT studies, image post processing, analysis and interpretation were performed using a three dimensional software tool (AZE, Tokyo, Japan). Images were evaluated for the presence of disease with a binary grading of its severity (greater or less than 50% stenosis) for subsequent comparison to HRC images in order to determine the impact of disease severity on the MR image quality. For this grading, MDCT coronary artery images were assessed in consensus by two blinded readers (Reader A and Reader B) for the presence of CAD and a categorical degree of coronary artery stenosis. The degree of stenosis was classified as mild (<50% stenosis) or moderate to severe (>50% stenosis)20. The degree of stenosis on MDCT and MRA was assessed by area reduction of the coronary artery lumen.
For the MR images, the Soapbubble tool21 was used to reformat the RCA and LM/LAD and quantify SNR, CNR, vessel length, and vessel diameter. Vessel sharpness was assessed based on the vessel edge-slope steepness. This was measured across the vessel lumen as the ratio of (1) the change in intensity from 15% peak to 85% peak and (2) the distance (mm) over which this intensity change occurs. The sharpness index therefore has units of (1/mm). These measurements were obtained for both the HRCand RRC images. Although the LCX was visible on the FOV in the acquired 3D volumes for LM/LAD and/or RCA, these quantitative figures were not obtained for that artery. This approach was chosen to prevent redundant measurements on the same sequence which may not be appropriate for statistical analysis. The MR images for the patients and normal volunteers were anonymized; the order was randomized and evaluated by the two blinded readers (Reader A and Reader B) for assessment of the image quality and any CAD. The blinded readers, therefore, did not know if a given scan came from a healthy volunteer or patient. Scan parameters were also removed from images so that the method or coil used for image acquisition could not be identified. A previously described score of 1–4 22 was assigned to each image by the two readers as a consensus read. A score of 1 indicated poor image quality where the coronary artery is visible but with markedly blurredborders or edges; 2, good image quality where the coronary artery is visible with moderatelyblurred borders or edges; 3, very good image quality where the coronary artery is visible with mildly blurred borders or edges; and 4, excellent image quality where the coronaryartery is visible with sharply defined borders or edges. The presence or absence of disease was also recorded. No evidence of disease was scored as normal. If disease was present it was graded as either mild (<50% stenosis) or moderate to severe (>50% stenosis)20. The location of the stenosis was also noted. The findings for the diseased vessels were then compared to the MDCT and any conventional x-ray coronary angiography findings, to assess any impact of CAD and its severity on MR image quality and potential clinical utility. The readers had 6 years (Reader A) and 15 years (Reader B) of experience in interpreting cardiac and coronary MR images.
Statistical Analysis
The mean and standard deviation for quantitative figures of merit (SNR, CNR, scan time, vessel sharpness, vessel length and vessel diameter) were obtained for the two MR imaging methods (RRC and HRC) using the images of all 11 normal volunteers. Comparative measurements could not be obtained for the patients in whom practical imaging times allowed only HRC studies. However, the mean image quality score was recorded for the HRC images obtained in patients and compared to the score for HRC images from the healthy volunteers without CAD using the Mann-Whitney test. A paired student’s t-test was used to compare the quantitative measurements, while a Wilcoxon test was used for statistical comparison of the image quality scores. MedCalc for Windows, version 9.3.9 (MedCalc Software, Mariakerke, Belgium) was used for statistical analysis. A P value ≤0.05 was considered statistically significant for single measurement comparisons. However, when repeated (LM/LAD and RCA) measurements were used from the same subject a Bonferroni correction was performed, therefore, a P value 0.025 was considered statistically significant.
Results
All twenty-two subjects were successfully imaged using the HRC technique. Only one of the vessels (the LM/LAD) was not successfully imaged in one of the patients due to significant motion during the acquisition of this one targeted 3D volume. Otherwise, the remaining coronaries were successfully imaged in this patient. Examples of HRC images of the LM/LAD and RCA are shown in figure 1 in comparison with RRC images. The results of the qualitative and quantitative comparison are shown in table 2. As expected, there was a significant reduction in SNR and CNR for HRC when compared to RRC. However, the measured sharpness improved in all but one examination where uncompensated motion effects were also observed. This resulted in a significantly (p=0.01) sharper image for the HRC (0.28mm−1 ± 0.11) compared to RRC (0.19mm−1 ± 0.06) scans. The visualized vessel length LM/LAD was not significantly different between the HRC and RRC methods. However, on average a shorter length of the RCA was visualized by the HRC sequence compared to the RRC method, which is attributable to the slightly reduced volumetric coverage of HRC. The overall (combined LM/LAD and RCA) image quality score was significantly lower for HRC compared to RRC. However, only the RCA showed a significantly lower image quality score when the score analysis is performed separately for each vessel; the image quality for LM/LAD was not significantly different among the two techniques.
Figure 1.
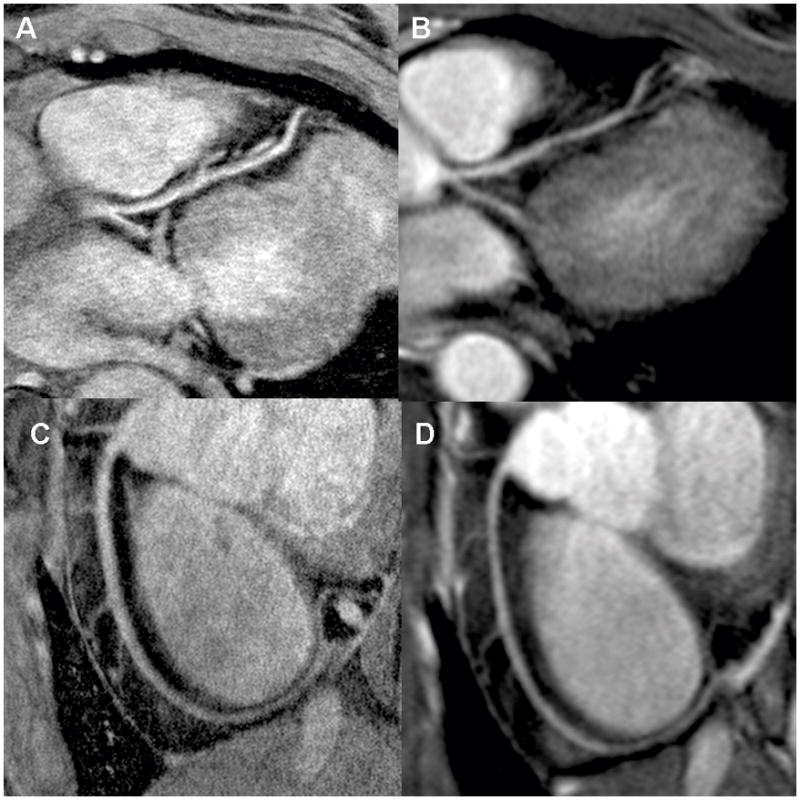
Multiplanar reformatted image of HRC (A and C) and RRC (B and D) images of the LM/LAD (A and B) and RCA (C and D) in a 28 year old female healthy volunteer. The HRC images have lower SNR but qualitatively sharper detail.
Table 2.
Results of average ± standard deviation results of the qualitative and quantitative image parameters. Table 2A demonstrates quantitative parameters, which include the prescribed scan time, comparing RRC and HRC in healthy volunteers. Note prescribed scan times generally differ from the actual times and are presented for theoretical technique comparison. Table 2B displays the results of image quality score in healthy volunteers. Table 2C compares the quality score values for HRC in patients and healthy volunteers. Table 2D shows the increase in sharpness and drop in SNR, CNR values of HRC in relation to RRC in healthy volunteers. P values less than 0.05 or 0.025 (when Bonferroni correction is applied) are statistically significant and displayed in red. (Myo= myocardium; Bld = Blood)
| Table 2A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Healthy volunteers | Scan time (min) | Diameter (mm) | SNR Bld | SNR Myo | CNR | Sharpness | All length (mm) | RCA (mm) | LM/LAD (mm) |
| HRC | 8.55 ± 1.7 | 4.05 ± 0.48 | 12.13 ± 3.42 | 8.88 ± 1.94 | 3.25 ± 2.45 | 0.28mm−1 ± 0.11 | 77.16 ± 24.6 | 91.98 ± 23.92 | 62.34 ± 14.65 |
| RRC | 4.06 ± 0.36 | 4.06 ± 0.47 | 45.1 ± 15.5 | 19.81 ± 7.5 | 25.26 ± 10.75 | 0.19mm−1 ± 0.06 | 86.98 ± 30.25 | 108.58 ± 24.33 | 65.38 ± 17.41 |
| P value | <0.01 | 0.784 | <0.01 | <0.01 | <0.01 | 0.01 | 0.125 | 0.013 | 0.336 |
| Table 2B | |||
|---|---|---|---|
| Quality Score | All Vessels | RCA | LM/LAD |
| HRC | 2.9 ± 0.9 | 3.1 ± 0.7 | 2.7 ± 1.1 |
| RRC | 3.4 ± 0.7 | 3.6 ± 0.5 | 3.1 ± 0.9 |
| P value | 0.002 | 0.031 | 0.125 |
| Table 2C | |
|---|---|
| Quality Score | HRC |
| Patients | 2.8 ± 0.8 |
| Healthy Volunteers | 2.9 ± 0.9 |
| P value | 0.868 |
| Table 2D | |
|---|---|
| % increase sharpness | 47% |
| % Drop Bld-SNR | 72% |
| % Drop CNR | 86% |
Assessments for both the presence and severity of CAD were consistent among the MDCT findings and HRC interpretations in all visualized vessels. Using MDCT as the reference, the presence and binary severity assessment of CAD (< or > 50%stenosis) was correspondingly identified on HRC imaging in all 11 patients except for the LM/LAD in the one patient with severe motion. MDCT image quality was excellent to good for all patients23. Prior to review the abnormal MR studies were anonymized and randomly distributed within the complete set of all HRC images inclusive of the healthy volunteers. Thus the blinded readers did not know if a given scan came from a healthy volunteer or patient. All of the findings of stenotic lesions on the HRC images were in agreement with MDCT as to the presence or absence and the binary severity category. In particular, the 3 patients with moderate to severe stenotic lesions and thus compromised flow had HRC image quality that was sufficient to render a blinded interpretation equivalent to that obtained on MDCT as shown in figure 2. The two patients with a conventional X-ray angiogram also had findings that confirmed the appearance and findings on the HRC images. Example of mild CAD affecting the RCA is shown in figure 3. The patient demographics, calcium score, and MDCT findings are displayed in table 1 using a modified 17-segment American Heart Association model of the coronary vessels24. The HRC image quality scores for all the patients was not significantly different from that of the HRC images obtained in healthy adult subjects (table 2C).
Figure 2.
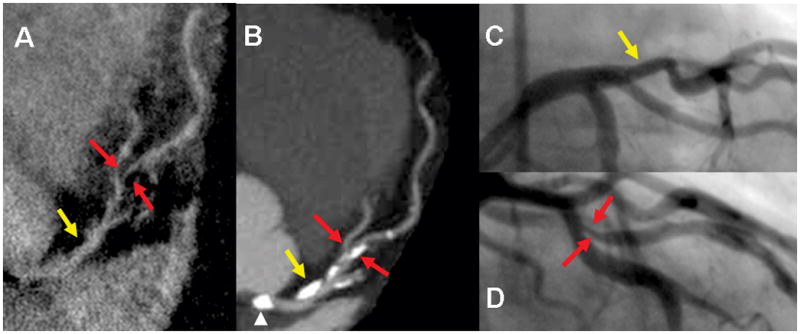
Demonstrates none-significant stenosis in the proximal LAD (yellow arrow) and significant stenosis in mid LAD (red arrows) in a 76 year old male seen both on HRC (A) 320-detectors CT (B). and conventional angiogram (C and D). Despite compromised flow in high grade stenosis, signal is adequate for visualization on the HRC image. A. Multiplanar reformatted image of HRC MRA. B. maximum intensity projection (MIP) in left anterior view of the LM/LAD. C and D. Conventional angiogram of LAD in left anterior oblique views. Note calcification (arrow head) in the left cusp partially projecting over the origin of the LM on the MIP due to image plane (B) is not causing severe narrowing as seen on the conventional angiogram (C) or HRC (A)
Figure 3.
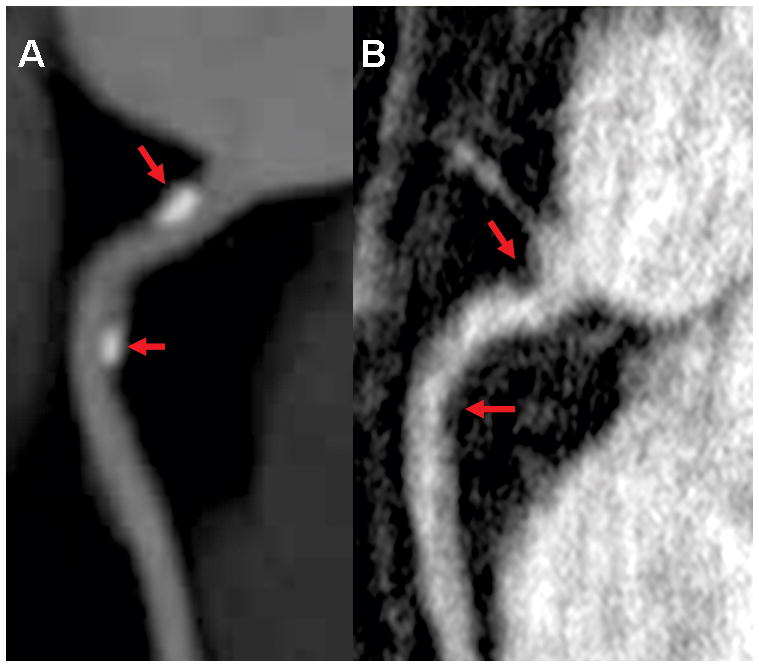
Images with mild stenosis red arrows involving RCA. A. maximum intensity projection (MIP) in right anterior oblique view of the RCA reformatted from 64-detectors CT. B. Multiplanar reformatted image of HRC MRA in the same view.
Using a 32-channel coil in the patient group allowed for the utilization of a SENSE factor of 2. This resulted in significant (P<0.025) reduction in prescribed scan time from 8.55 ±1.7 min (using a 6-channel coil without SENSE) to 3.48 ± 0.38 min (using a 32-channel coil with SENSE) without affecting the image quality score. With a respiratory navigator efficiency of 35–50% this resulted in an actual scan time of 9.3 ± 2.7 min for each vessel, allowing the successful acquisition of all but one vessel in the patient group. The actual scan time for the healthy subjects scanned using the 6-channel coil without SENSE with 35–50% navigator efficiency was 22.8 ± 4.5 min.
Discussion
Due to the small size of the coronary arteries and the comparatively large motion of the heart, non-invasive imaging of the coronary arteries and thereby accurate detection of CAD is challenging. The recent and ongoing success of CT coronary angiography is attributed to technical advances that have overcome some of these challenges and allow obtaining high resolution images (0.4mm3 voxel size) rapidly in a single breath hold 25. Coronary CT can also visualize atheroma and is not limited to lumenographic images only, as is characteristic of conventional angiography. As such, it does provide the additional benefit of some visualization of the vessel wall when it is thickened and remodeled with atheromatous plaque. However, the obvious drawbacks include the need for using ionizing radiation, substantial doses of potentially nephrotoxic contrast agents, and the frequent need for beta blockers to slow the heart rate. These limitations may inhibit CT coronary angiography from being used as an imaging tool to identify atherosclerotic changes in patients with relatively few risk factors or in the young with a higher lifetime risk of cancer induction. These may also limit the use of CT to follow changes in atherosclerotic plaque over time in response to different interventions or lifestyle changes 26,27. However, there remains a clear need for non-invasive imaging in these clinical settings. In particular, longitudinal studies with imaging assessment may be critical to better identify and improve treatment strategies. Coronary MRA, as a potentially complementary technique to CT, is well known to not use radiation but also does not require the use of beta blockers or necessarily contrast agents. Additionally, coronary MRA in conjunction with MRI can visualize the thickened wall and may have the potential of identifying none-calcified or calcified plaque that does not cause significant stenosis. However; the value of this approach for characterizing the vulnerability of such plaque is yet to be determined. Therefore, high field coronary MRA may provide the platform for better monitoring of the evolution of early to moderate atherosclerosis and changes in response to therapy or various risk factor modifications. Achieving these goals, however, requires significant improvement in the spatial resolution that is routinely achieved with conventional MR at 1.5T. Rather, spatial resolution that approaches that of MDCT seems vital. While coronary MRA scanning is unlikely to become as fast as MDCT, MR does appear to offer sufficient complementary advantages to accord it a unique clinical role should adequate spatial resolution with diagnostic image quality be achieved.
This study demonstrates the feasibility of obtaining coronary MRA at 3T with an in-plane voxel size as low as 350μm2. This in-plane resolution approximates that of coronary CT angiography 25. The substantially higher spatial resolution is a consequence of the higher intrinsic SNR at 3T and optimization of data acquisition parameters to salvage SNR which is necessarily lost due to the voxel size reduction. As expected, the high resolution and dramatic reduction in voxel size comes at a cost of significant reduction of both qualitative and quantitative figures of merit related to SNR. However, qualitatively the images appear sharper and this is supported by an improved quantitative measure of vessel sharpness. Nonetheless, there is a significant increase in scanning time which can be reduced to clinically practical levels by using a 32-channel coil. Utilizing a 32-channel coil without using parallel imaging at 1.5T may also offer an approach to provide higher SNR for finer resolution MRA. Whether this gain in SNR would be adequate in practice to achieve MRA voxels as small as those obtained in this study has not been determined. However, the absence of parallel imaging will result in prolonged scan times, thereby making the technique difficult for patients and less practical for routine clinical studies. Additionally, in some cases this could make the overall imaging more compromised by motion artifacts due to a lower level of patient tolerance. In sum, these challenges may explain the lack of published reports, to date, of this level of spatial resolution (0.35 mm × 0.35 mm × 1.5 mm) at 1.5T using 32-channel coils for coronary MRA. In this preliminary study, the HRC technique produced clinically interpretable images in both the presumptive normal subjects and in the patients with mild and moderate to severe stenosis. Although an early study, normal coronary arteries and coronary vessels with mild CAD (figure 1 and 3) and severe CAD (figure 2), as defined by MDCT, were similarly identified on HRC images with MR. This addresses the pre-study concern that MR images with such fine resolution might have too poor SNR and image quality to have any potential diagnostic value. This concern was greatest for patients with moderate to severe coronary stenosis where flow related signal may be further reduced. Rather, the findings suggest that the ~350 × 350μm2 resolution image quality at 3T may be generally adequate for use in a clinical setting even at present, while technical improvements and better image quality are still very likely to come.
It is worth noting, however, that the image quality scores and observed vessel length for the RCA were significantly lower for HRC than for RRC, though this was not seen for the LM/LAD. This may be attributable to the reduced volumetric coverage for HRC. For a fair comparison, the standard parameters shown to give highest quality images were used for RRC images i.e. the shortest acquisition window possible without the use of SENSE that yields a practically tolerable scan duration. This methodology allowed for a comparison between the HRC and the highest quality of the more traditional RCC images in order to determine the comparative capabilities of HRC at 3T. Despite, the better temporal resolution (75ms) used for the RRC compared to that used for HRC (86ms) scans, the latter produced quantitatively sharper images.
Limitations
The goal of this study was not to determine the sensitivity, specificity, and accuracy of HRC compared to RRC methods for the detection of CAD, and therefore they were not assessed. However, we did seek to advance the practical resolution limit at 3T by optimizing prior techniques for resolution at high field strength and comparing the image quality between this method and a more conventional standard MR methodology. Additionally, we evaluated the use of HRC imaging preliminarily in a clinical setting to assess its potential value in investigating CAD. Accordingly, the study demonstrated that coronary MRA images can be obtained with a 2 fold improvement in resolution at 3T in healthy subjects and patients with CAD where compromised flow might further decrease SNR and image quality. This work also demonstrates HRC potential utility in identifying and following up CAD development without concerns imposed about radiation dose. One concern that was not fully addressed in this study is the impact of bulk cardiac motion during the imaging window on the recovered resolution. From previous angiographic studies, the motion of the coronaries during the 86ms acquisition window can be greater than 350μm28. Thus, a limiting factor in actual spatial resolution may in fact be bulk cardiac motion related to the inherent speed of imaging acquisition. This problem, however, is similar for MDCT where acquisition windows are on the order of 80–160ms. At present, cardiac motion remains a challenge for coronary MRA. Another limitation is the prolonged scan time of HRC compared to that of the more commonly used RRC. Nevertheless, this study serves as a first successful demonstration of obtaining this level of spatial resolution with image quality sufficient to visualize both normal and diseased coronary arteries. We acknowledge that further work in this area is needed to shorten scan time for more routine clinical use and to determine its relative and absolute effectiveness in accurately detecting significant coronary artery stenosis.
In conclusion, to the best of our knowledge, this is the first study to demonstrate the feasibility of obtaining 350 micron spatial resolution coronary MRA images in patients with CAD. As such, it is an initial step towards much needed improvement in spatial resolution for coronary MRA. We anticipate that future studies using 32 channel coils with multi-coil transmission, faster imaging techniques such as compressed sensing and contrast agents may achieve further advances in image quality, reduction in scanning time and overall clinical utility. Recent studies using whole heart techniques at both 1.5T and 3T have demonstrated improved depiction of the distal coronary segments by utilizing a blood-pool contrast agent such as gadofosveset trisodium 7,29. This should also be of value for high resolution MRA techniques and warrants future investigation. If accomplished we also anticipate that routine coronary MRA will be performed with spatial resolution comparable to that of current MDCT without the concerns associated with radiation and x-ray contrast media. This should be of particular value in young patients (with higher lifetime risk of cancer induction) and those for whom serial examinations are needed.
Footnotes
Diclosures:
One author (M.S) is compensated as a consultant to Philips Research North America, which is part of Philips Medical Systems, Netherlands, the manufacturer of the equipment used in this study. All other authors who are not consultants or employees of Philips had full control of the inclusion of any data or information that might have presented a conflict of interest for the author who is a consultants of Philips. The opinions or assertions contained herein are the private views of the authors (V.B.H) and not to be construed as official or reflecting the views of the Uniformed Services University of the Health Sciences or the Department of Defense.
References
- 1.Kim WY, Danias PG, Stuber M, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345(26):1863–1869. doi: 10.1056/NEJMoa010866. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma H, Ichikawa Y, Chino S, Hirano T, Makino K, Takeda K. Detection of coronary artery stenosis with whole-heart coronary magnetic resonance angiography. J Am Coll Cardiol. 2006;48(10):1946–1950. doi: 10.1016/j.jacc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Sakuma H, Ichikawa Y, Suzawa N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237(1):316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 4.Stuber M, Botnar RM, Spuentrup E, Kissinger KV, Manning WJ. Three-dimensional high-resolution fast spin-echo coronary magnetic resonance angiography. Magn Reson Med. 2001;45(2):206–211. doi: 10.1002/1522-2594(200102)45:2<206::aid-mrm1028>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Sommer T, Hackenbroch M, Hofer U, et al. Coronary MR angiography at 3.0 T versus that at 1. 5 T: initial results in patients suspected of having coronary artery disease. Radiology. 2005;234(3):718–725. doi: 10.1148/radiol.2343031784. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Bi X, Huang J, Jerecic R, Carr J, Li D. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0 T: comparison with steady-state free precession technique at 1. 5 T. Investigative radiology. 2008;43(9):663–668. doi: 10.1097/RLI.0b013e31817ed1ff. [DOI] [PubMed] [Google Scholar]
- 7.Prompona M, Cyran C, Nikolaou K, Bauner K, Reiser M, Huber A. Contrast-enhanced whole-heart MR coronary angiography at 3. 0 T using the intravascular contrast agent gadofosveset. Investigative radiology. 2009;44(7):369–374. doi: 10.1097/rli.0b013e3181a40d1d. [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Li K, Liu X, et al. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3. 0-T: a comparative study with X-ray angiography in a single center. J Am Coll Cardiol. 2009;54(1):69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuber M, Botnar RM, Fischer SE, et al. Preliminary report on in vivo coronary MRA at 3 Tesla in humans. Magn Reson Med. 2002;48(3):425–429. doi: 10.1002/mrm.10240. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach S, Ropers D, Kuettner A, et al. Contrast-enhanced coronary artery visualization by dual-source computed tomography--initial experience. Eur J Radiol. 2006;57(3):331–335. doi: 10.1016/j.ejrad.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 11.Rybicki FJ, Otero HJ, Steigner ML, et al. Initial evaluation of coronary images from 320-detector row computed tomography. Int J Cardiovasc Imaging. 2008;24(5):535–546. doi: 10.1007/s10554-008-9308-2. [DOI] [PubMed] [Google Scholar]
- 12.de Graaf FR, Schuijf JD, van Velzen JE, et al. Diagnostic accuracy of 320-row multidetector computed tomography coronary angiography to noninvasively assess in-stent restenosis. Investigative radiology. 2010;45(6):331–340. doi: 10.1097/RLI.0b013e3181dfa312. [DOI] [PubMed] [Google Scholar]
- 13.Schoepf UJ, Zwerner PL, Savino G, Herzog C, Kerl JM, Costello P. Coronary CT angiography. Radiology. 2007;244(1):48–63. doi: 10.1148/radiol.2441052145. [DOI] [PubMed] [Google Scholar]
- 14.Fischer SE, Wickline SA, Lorenz CH. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magn Reson Med. 1999;42(2):361–370. doi: 10.1002/(sici)1522-2594(199908)42:2<361::aid-mrm18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Stuber M, Botnar RM, Danias PG, et al. Double-oblique free-breathing high resolution three-dimensional coronary magnetic resonance angiography. J Am Coll Cardiol. 1999;34(2):524–531. doi: 10.1016/s0735-1097(99)00223-5. [DOI] [PubMed] [Google Scholar]
- 16.Stuber M, Botnar RM, Danias PG, Kissinger KV, Manning WJ. Submillimeter three-dimensional coronary MR angiography with real-time navigator correction: comparison of navigator locations. Radiology. 1999;212(2):579–587. doi: 10.1148/radiology.212.2.r99au50579. [DOI] [PubMed] [Google Scholar]
- 17.Danias PG, McConnell MV, Khasgiwala VC, Chuang ML, Edelman RR, Manning WJ. Prospective navigator correction of image position for coronary MR angiography. Radiology. 1997;203(3):733–736. doi: 10.1148/radiology.203.3.9169696. [DOI] [PubMed] [Google Scholar]
- 18.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magnetic Resonance in Medicine. 1995;33(5):689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 19.Botnar RM, Stuber M, Danias PG, Kissinger KV, Manning WJ. Improved coronary artery definition with T2-weighted, free-breathing, three-dimensional coronary MRA. Circulation. 1999;99(24):3139–3148. doi: 10.1161/01.cir.99.24.3139. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Zhao X, Huang J, et al. Comparison of 3D free-breathing coronary MR angiography and 64-MDCT angiography for detection of coronary stenosis in patients with high calcium scores. AJR Am J Roentgenol. 2007;189(6):1326–1332. doi: 10.2214/AJR.07.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Etienne A, Botnar RM, Van Muiswinkel AM, Boesiger P, Manning WJ, Stuber M. “Soap-Bubble” visualization and quantitative analysis of 3D coronary magnetic resonance angiograms. Magn Reson Med. 2002;48(4):658–666. doi: 10.1002/mrm.10253. [DOI] [PubMed] [Google Scholar]
- 22.McConnell MV, Khasgiwala VC, Savord BJ, et al. Comparison of respiratory suppression methods and navigator locations for MR coronary angiography. AJR Am J Roentgenol. 1997;168(5):1369–1375. doi: 10.2214/ajr.168.5.9129447. [DOI] [PubMed] [Google Scholar]
- 23.Herzog C, Arning-Erb M, Zangos S, et al. Multi-detector row CT coronary angiography: influence of reconstruction technique and heart rate on image quality. Radiology. 2006;238(1):75–86. doi: 10.1148/radiol.2381041595. [DOI] [PubMed] [Google Scholar]
- 24.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51(4 Suppl):5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 25.Achenbach S. Computed tomography coronary angiography. J Am Coll Cardiol. 2006;48(10):1919–1928. doi: 10.1016/j.jacc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Garcia MJ, Lessick J, Hoffmann MH. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. Jama. 2006;296(4):403–411. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 27.Herzka DA, Gharib AM. Should all patients with suspected coronary artery disease undergo coronary angiography with 16-row MDCT? Nat Clin Pract Cardiovasc Med. 2007;4(2):74–75. doi: 10.1038/ncpcardio0777. [DOI] [PubMed] [Google Scholar]
- 28.Johnson KR, Patel SJ, Whigham A, Hakim A, Pettigrew RI, Oshinski JN. Three-dimensional, time-resolved motion of the coronary arteries. J Cardiovasc Magn Reson. 2004;6(3):663–673. doi: 10.1081/jcmr-120038086. [DOI] [PubMed] [Google Scholar]
- 29.Wagner M, Rosler R, Lembcke A, et al. Whole-heart coronary magnetic resonance angiography at 1.5 Tesla: does a blood-pool contrast agent improve diagnostic accuracy? Investigative radiology. 2011;46(3):152–159. doi: 10.1097/RLI.0b013e3181fac6ef. [DOI] [PubMed] [Google Scholar]


