Abstract
This study was conducted to investigate the effects of Sasa quelpaertensis bamboo and green tea on plasma and liver lipids, platelet aggregation, and erythrocyte membrane Na channels in ovariectomized (OVX) rats. Thirty female rats were OVX, and ten female rats were sham-operated at the age of 6 weeks. The rats were divided into four groups at the age of 10 weeks and fed the experiment diets: sham-control, OVX-control, OVX-bamboo leaves (10%), or OVX-green tea leaves (10%) for four weeks. Final body weight increased significantly in the OVX groups compared with that in the sham-control, whereas body weight in the OVX-green tea group decreased significantly compared with that in the OVX-control (P < 0.01). High density lipoprotein (HDL)-cholesterol level decreased in all OVX groups compared with that in the sham-control rats (P < 0.05) but without a difference in plasma total cholesterol. Plasma triglycerides in the OVX-green tea group were significantly lower than those in the sham-control or OVX-control group (P < 0.05). Liver triglycerides increased significantly in the OVX-control compared with those in the sham-control (P < 0.01) but decreased significantly in the OVX-green tea group compared with those in the OVX-control or OVX-bamboo group (P < 0.01). Platelet aggregation in both maximum and initial slope tended to be lower in all OVX rats compared with that in the sham-control rats but was not significantly different. Na-K ATPase tended to increase and Na-K cotransport tended to decrease following ovariectomy. Na-K ATPase decreased significantly in the OVX-green tea group compared with that in the OVX-control group (P < 0.01), and Na-K cotransport increased significantly in the OVX-bamboo and OVX-green tea groups compared with that in the OVX-control (P < 0.05). Femoral bone mineral density tended to be lower in OVX rats than that in the sham-control, whereas the green tea and bamboo leaves groups recovered bone density to some extent. The results show that ovariectomy caused an increase in body weight and liver triglycerides, and that green tea was effective for lowering body weight and triglycerides in OVX rats. Ovariectomy induced an increase in Na efflux via Na-K ATPase and a decrease in Na efflux via Na-K cotransport. Furthermore, consumption of green tea and bamboo leaves affected Na efflux channels, controlling electrolyte and body water balance.
Keywords: Green tea, Sasa quelpaertensis bamboo, Na efflux channel, platelet aggregation, ovariectomized rats
Introduction
Metabolic disorders following menopause are manifested by cardiovascular complications as well as weight gain and osteoporosis. American woman tend to start weight gain around the time of menopause and begin or accelerate change in body composition including loss of bone minerals and body cell mass and increase in total body fat, visceral fat, and extracellular fluid during menopausal period [1,2]. Hypertension, dislipidemia, obesity, and insulin resistance are the most common high-risk metabolic symptoms in menopausal woman [3,4]. Estrogen deficiency may cause an increase in cardiovascular risks by affecting lipoprotein metabolism, platelet aggregability, electrolyte disturbance and vessel resistance [5-7]. Significant increases in total cholesterol, low density lipoprotein (LDL)-cholesterol and triglycerides are observed in perimenopausal to postmenopausal woman [7], and treatment with estrogen and/or 3-hydroxy-3-methylglutaryl-Coenzyme A (HMG-CoA) reductase inhibitor, statin caused decreases in plasma total cholesterol, LDL-cholesterol and triglycerides in menopausal woman [8]. Platelets from hypercholesterolemic patients were more reactive to aggregating reagents such as epinephrine and adenosine diphosphate (ADP) than platelets from normal individuals [9], and ex vivo platelets from hypercholesterolemic patients showed hyperactivity and shortened platelet survival [10]. Estrogen deficiency in OVX animals and menopausal woman can cause an increased platelet aggregation and thrombosis by affecting platelet agonist hydrolysis [11] and blood vessel nitric oxide production [6]. Hormonal changes in menopausal woman may affect electrolyte disturbance, water retention and blood pressure. Female sex hormones influence the systemic and renal response to salt by increasing salt sensitivity and depressing natriuresis. Increased salt sensitivity in hypertensive menopausal woman is associated with vascular tone and membrane transport of cation [12]. Platelet activating factor administration suppresses Na-K pump activity in Wistar rats, causing decreases in excretion of urinary water and electrolytes [13]. Musselman et al. [14] reported that ovarian hormone treatment of OVX rats causes changes in Na-K cotransport, thereby affecting sodium and water balance. Estrogen deficiency following ovariectomy causes Na-K ATPase activation in rat hippocampus [15]. Ethinyl estradiol caused suppression of Na-K ATPase in the basolateral membrane of rabbit intestine by modulating membrane lipids, controlling sodium and water absorption [16].
Bamboo leaves have been used as oriental medicament with effects of antifebrile, diuresis, antihypertensive, and hypoglycemic for thousands of years. Orientin, isoorientin, and isovitexin extracted from Phyllostachys nigra bamboo leaves caused Ca2+ channel-mediated vasorelaxation, increasing coronary blood flow, and preventing myocardial ischemia in rabbits [17]. P. pubescens bamboo leave extract had antihypertensive effects by suppressing angiotensin converting enzyme and relaxing arterial blood vessels [18]. The dwarf bamboo, Sasa quelpaertensis Nakai, grows on Halla mountain and has been used as tea for therapeutic purposes with antidiabetic, diuretic and antiinflammatory effects. Sultana and Lee [19] reported that phenylpropanoids such as 3-O-p-coumaronyl-1-(4-hydroxy-3,5-dimethoxy-phenyl)-1-propanone and N-p-coumaronylserotonin extracted from S. quelpaertensis Nakai have inhibitory effects on tyrosine hydroxylase, which catalyzes the conversion of L-tyrosine to dihydroxyphenylalanine in biosynthesis of catecholamines. Hormone epinephrine and sympathetic neurotransmitter norepinephrine play roles in heart and blood vessels, exerting adverse effects on blood pressure. Green tea consumption is inversely related with the incidence of cardiovascular diseases in the general population [20,21]. Green tea extract in diabetic rats has protective effects on the cardiovascular system by affecting lipid peroxidation, protein glycation and Na-K ATPase [22], and the green tea polyphenol, epigallocatechin gallate (EGCG) exerts hypolipidemic and antiobesity actions by suppressing fatty acid synthetase at the gene level [23]. In this study, we compared the preventive effects of S. quelpaertensis bamboo leaves and green tea on cardiovascular complications using related parameters such as erythrocyte membrane Na channels, platelet aggregation, plasma, and liver lipids and bone density in OVX rats.
Materials and Methods
Animals and diets
This study was approved by the Laboratory Animal Care Committee of Jeju National University, and rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of the University.
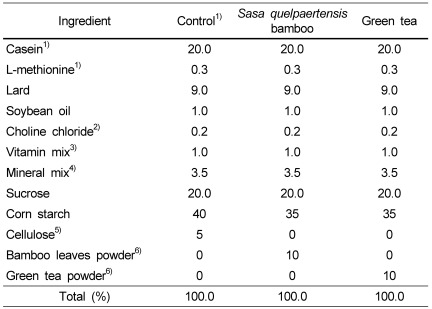
Thirty of 40 6-week-old female Sprague-Dawley rats (Orient Bio Co., Ltd, Seoul, Korea) were ovariectomized and ten rats were sham operated. The rats were raised for 4 weeks with pellet diet. At the age of 10 weeks, the OVX rats were divided into three groups of OVX-control, OVX-bamboo and OVX-green tea and fed the following diets with sham-control rats (Table 1). S. quelpaertensis bamboo leaves and green tea powders for the diet mix were provided by the Jeju Agricultural Development and Technology Extension Center.
Table 1.
Composition of the experimental diets (%)
1)Teklad, Harlan Madison WI, USA
2)Junsei Chemical Co., Ltd.
3)Teklad, Harlan Madison WI, USA; AIN 76A vitamin mix,
4)Mineral mixture (g/100 g): CaHPO4 50.0, NaCl 7.4, K3C6H5O7·H2O 22.0, K2SO4 5.2, MgO 2.4, Manganous carbonate (43-48%Mn) 0.35, Ferric citrate (16.7%Fe) 0.6, Zinc carbonate (70%Zn) 0.16, Cupric carbonate (53-55%Cu) 0.03, KIO3 0.001, Na2SeO3·5H2O 0.001, CrK(SO4)2·12H2O 0.055, sucrose 11.804
5)Sigma Chemical Co.
6)Jeju Agricultural Development and Technology Extension Center
Rats had free access to water and were housed in individual cages in a room maintained at 20-25℃ with a 12-hour dark-light cycle. After 4 weeks of ad libitum feeding, the rats were anesthetized with ether and blood samples were obtained by cardiac puncture into heparinized vacuum tubes. Platelet aggregation and erythrocyte Na efflux were assessed with fresh blood, and plasma and liver samples were stored at -70℃ for later assays. Femurs were obtained after removing fat, muscle, and tendons and stored at 4℃ to determine bone mineral density (BMD).
Platelet aggregation
Platelet aggregation was measured using a Chronolog Whole Blood Aggregometor (model 500-Ca, Havertown, PA, USA). Fresh whole blood was diluted with isotonic saline (1:4) to give platelet concentration of approximately 200,000 platelets/µl. ADP (2 µM; Chronolog) was added to initiate aggregation, and three readings of impedance changes were averaged for each rat to determine the maximum aggregation and the initial slope. The instrumental principle is based on the increase in impedance (Ω) across two platinum electrodes as platelet aggregation proceeds.
Plasma and liver lipid assays
Plasma total cholesterol, HDL-cholesterol, triglycerides, and glucose were assayed using enzymatic kits (Asan Pharmaceuticals, Seoul, Korea). Ten µl of plasma was used for the total cholesterol, triglyceride, and glucose assays. For the HDL cholesterol assay, 200 µl of plasma was incubated with dextran sulfate to precipitate apo B-containing lipoprotein, and 50 µl of the supernatant was used.
Solvents for the liver extraction were supplied by Merck (Darmstadt, Germany). Liver lipids were extracted by a modified Folch method [24]. One gram of liver tissue was homogenized for 5 min in 6 ml of Folch solution (2:1, chloroform: methanol) and 2 ml H2O. After centrifugation for 10 min, the lower phase containing the liver lipids was separated. The lower phase of the lipid fraction was assayed after treatment with Triton X-100:chloroform (25:475 µl) for total cholesterol or with methanol for triglycerides using enzymatic kits (Asan Pharmaceuticals).
Na efflux channels
Red blood cell (RBC) preparation
Chemicals for the mediums including ouabain and furosemide were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Blood was centrifuged at 1,000 × g for 10 min, and the plasma and buffy coat were removed. RBCs were washed five times with cold isotonic washing solution [150 mM choline chloride, 10 mM Tris-4 morpholinopropane sulfonic acid (MOPS), pH 7.4 at 4℃] and centrifuged at 1,000 × g for 5 minutes after each wash. The RBC pellet was resuspended in choline chloride to give a hematocrit of 40-50%, which was also measured. A 50 µl aliquot of the RBC suspension was added to 5 ml of 0.02% acationox (a metal free detergent, Scientific products, McGraw Park, IL, USA) to determine intracellular Na concentrations.
Na efflux
Four ml each of the RBC suspension were added to 40 ml MgCl2 medium with and without ouabain (1 mM ouabain in 70 mM MgCl2, 10 mM KCl, 85 mM sucrose, 10 mM glucose, 10 mM Tris MOPS pH 7.4 at 37℃) to determine Na efflux via Na-K ATPase. Two ml of the RBC suspension was added to 40 ml of choline chloride medium with and without furosemide (150 mM choline chloride, 10 mM glucose, 1 mM ouabain, 10m Tris-MOPS pH 7.4 at 37℃, 1 mM furosemide) to determine Na efflux via Na-K cotransport. The RBCs in each media were mixed and aliquoted into 12 tubes. Duplicate tubes were transferred to an ice bath after a 37℃ incubation in a shaking water bath for 0, 2, 4, 6, 8, and 10 min for Na-K ATPase and for 0, 10, 20, 30, 40, and 50 min for Na-K cotransport determinations. Tubes were centrifuged at 1,000 × g for 5 minutes, the supernatant was removed, and Na concentrations were measured using an atomic absorption spectrophotometer (Shimadzu model AA6701F) [25].
Calculations
Na efflux: Na (µg)/(ml × min) × 60 min × µmole/23 µg × [44 ml - (4 ml × Hct)/(4 ml × Hct)] = mmol/ℓrbc/hr
Intracellular Na: Na (µg)/(ml × min) × 60 min × µmol/23 µg × 101/Hct = mmol/ℓrbc
Femur BMD
Bone mineral content and bone width were measured in femurs by dual energy X-ray absorptiometry (model pDEXA Sabre, Norland Cooper Surgical Co., Trumbull, CT, USA) designed for small animal bone and body composition research. BMD was calculated from bone mineral content (g)/bone width (cm2).
Statistical analysis
Values were statistically analyzed using SAS software (SAS Institute, Cary, NC, USA). Analysis of variance was conducted in a completely randomized block design. Duncan's multiple range test was applied to compare individual means when the F-value was significant (P < 0.05).
Results
Weight gain and food efficiency
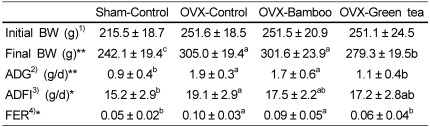
Four weeks after ovariectomy when the experimental diet began, the average weights of the OVX and sham operated rats were 251.5 and 215.5 g, respectively (Table 2). The final weights after 4 weeks of the experimental diet increased significantly in the OVX-control compared with that in the sham-control group (P < 0.05), whereas the OVX-green tea (GT) group showed significantly lower body weight compared with that in the three OVX groups (P < 0.05). Daily weight gains (ADG) of the sham-control and OVX-GT groups were significantly higher than those in the OVX-control and OVX-bamboo groups (P < 0.05). Average daily feed intake (ADFI) of the sham-control was significantly lower than that of the OVX-control (P < 0.05). Food efficiency ratio (FER = ADG/ADFI) was significantly higher in the OVX-control and OVX-bamboo groups than that in the other groups (P < 0.05)
Table 2.
Effects of Sasa quelpaertensis bamboo and green tea on growth rate and feed intake in ovariectomized rats
1)Initial BW: Body weight at four weeks after ovariectomy (OVX) and before 4 weeks of the experimental diets
2)ADG, average daily gain
3)ADFI, average daily feed intake
4)FER, feed efficiency ratio
*Values in the same row not sharing the same superscript differ (P < 0.05)
**Values in the same row not sharing the same superscript differ (P < 0.01) Values are means ± SDs of 10 rats.
Plasma and liver cholesterol and triglycerides
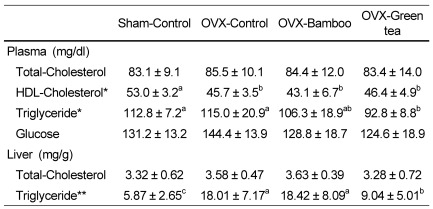
No difference was observed in plasma total cholesterol among the groups, but HDL cholesterol decreased significantly in the OVX groups compared with that in the sham-control (P < 0.05) (Table 3). Plasma triglyceride levels were significantly lower in the OVX-green tea group compared with those in the OVX-control (P < 0.05). Liver total cholesterol was not different among the groups, but liver triglyceride level was significantly lower in the sham-control and OVX-green tea groups than that in the OVX-control and OVX-bamboo groups (P < 0.01). No differences in plasma glucose were observed.
Table 3.
Effects of Sasa quelpaertensis bamboo and green tea on plasma cholesterol, triglyceride and glucose levels and liver lipids in ovariectomized rats
OVX, ovariectomized; HDL, high-density lipoprotein
Values are means ± SDs of 10 rats.
*Values in the same row not sharing the same superscript differ (P < 0.05)
**Values in the same row not sharing the same superscript differ (P < 0.01)
Whole blood platelet aggregation
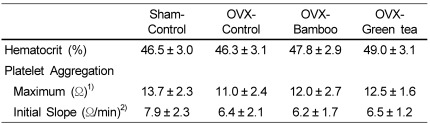
No difference in hematocrit was observed among groups (Table 4). The maximum aggregation was not different between any two groups, but initial slope tended to decrease in the OVX groups compared to that in the sham-control.
Table 4.
Effects of Sasa quelpaertensis bamboo and green tea on hematocrit and platelet aggregation in ovariectomized rats
OVX, ovariectomized
1)Maximum aggregation is ohm at the point where the aggregate dissociated.
2)Initial slope ohm change for the first one minute of aggregation
Values are means ± SDs of 10 rats.
Na efflux channels
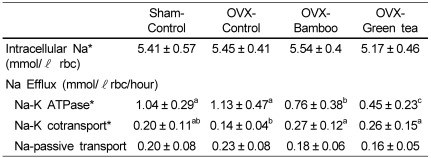
Intracellular Na was not different among the groups (Table 5). Na-K ATPase decreased significantly in the OVX-bamboo and OVX-green tea groups compared with that in the sham-control and OVX-control groups (P < 0.05). Na-K cotransport increased significantly in the OVX bamboo and OVX-green tea groups compared with that in the OVX-control (P < 0.05). Na passive transport was somewhat decreased in the OVX-bamboo and OVX-green tea group but was not significantly different between any two groups.
Table 5.
Effects of Sasa quelpaertensis bamboo and green tea on erythrocyte sodium efflux in ovariectomized rats
OVX, ovariectomized
Values are means ± SD of 10 rats.
*Values in the same row not sharing the same superscript differ (P < 0.05)
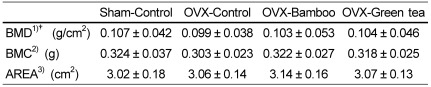
Femur BMD
BMD tended to decrease in the OVX-control compared with that in the sham-control group but recovered to some extent in the OVX-bamboo and OVX-green tea groups. The sham-control group had the highest bone mineral content with the smallest bone width, thereby the highest BMD (Table 6).
Table 6.
Effects of Sasa quelpaertensis bamboo and green tea on bone mineral density in ovariectomized rats
1)BMD, bone mineral density
2)BMC, bone mineral concentration
3)AREA, bone width
Values are means ± SDs of 10 rats.
† Rats were ages 14 weeks at 8 weeks after ovariectomy (OVX).
Discussion
The present study showed that OVX rats gained much more weight than that of the sham operated female rats during 4 weeks recovery before being fed the experimental diets. Weight gain of OVX rats fed the control diet exceeded that of the sham control rats with a twice higher FER. Oh et al. [26] reported that FER of OVX rats was far higher than that of normal female rats, while no difference was observed in the FER between orchidectomized rats and normal male rats, inferring that female sex hormones may be involved in weight gain and energy metabolism. Ovariectomy in rats causes an increase in body weight and food efficiency with elevated plasma leptin, whereas 17β-estradiol treatment reverses weight gain and food efficiency without changing plasma leptin, where ovariectomy and 17β-estradiol treatment does not affect thyroid stimulating hormone, triiodothyronine, or thyroxine levels [27]. Ferrara et al. [2] reported that lower lipolysis and higher adipose tissue lipoprotein lipase activity are responsible for the increased abdominal and gluteal fat depot in menopausal woman. Considering our result that FER of OVX rats far exceeded the increased food consumption, depressed adipose fat utilization in OVX rats may be another factor involved in the weight gain and body composition in the present study. The bamboo diet did not affect weight gain or food efficiency, whereas the green tea leaf diet suppressed weight gain and food efficiency in OVX rats. Kang et al. [28] reported that green tea has antiobesity effects in pair-fed male rats, and Diepvens et al. [29] suggested that green tea catechins may affect resting energy expenditures and fat oxidation during weight loss in overweight woman. Considering that green tea leaves diet did not suppress appetite in OVX rats, the antiobesity effects of green tea may be associated with its action controlling fat synthesis and utilization. Tea polyphenols, EGCG, and theaflavin suppress the fatty acid synthetase gene in breast cancer cells [23], and green tea extract stimulates fat oxidation in mice [30].
Increases in plasma total and LDL cholesterol, triglycerides, and decreases in HDL cholesterol are common metabolic symptoms in menopausal woman [3]. OVX rats fed a high cholesterol diet have significantly elevated plasma and total liver cholesterol [26]. In the present study, OVX rats fed the cholesterol-free diet did not have elevated plasma or liver total cholesterol. HDL cholesterol was lower in all OVX rats as seen in menopausal woman, but bamboo leaves or green tea rich in polyphenols did not affect HDL cholesterol levels. Increased intra-abdominal fat may lower HDL levels by increasing the fractional catabolic rate of Lp A-I in postmenopausal woman, suggesting central adiposity may be proatherogenic [31].
Plasma triglyceride levels remained unchanged, whereas liver triglycerides increased significantly following OVX in the present study. Decreased plasma triglycerides and elevated liver triglycerides are observed in analbuminaemic OVX rats [32] and cholesterol fed rats [26,33]. Liu et al. [33] suggested that plasma triglycerides below normal or control levels with severely high liver levels are associated with disturbances in the secretion of newly synthesized hepatic triglycerides, causing fat accumulation in the liver. Unlike the bamboo leaf diet, green tea lowered elevated plasma and liver triglycerides in the OVX rats in the present study. Green tea consumption is inversely correlated with serum lipids and lipoprotein levels in Japanese men [21] and green tea and green tea extract decreases plasma and liver triglyceride in rats [28]. The hypolipidemic effect of green tea extract is associated with increased fat oxidation [30], and green tea polyphenol EGCG exerts its hypolipidemic effects by suppressing intestinal lipid absorption [34].
High platelet aggregability or platelet sensitivity is associated with a high incidence of thrombosis and stroke. Estrogen deficiency in OVX animals and menopausal woman may affect platelet reactivity and thrombosis. Reduced estrogen in OVX Wistar rats causes a decrease in NO production in venules, resulting in thrombus formation, and estrogen treatment restores NO production and endothelium relaxation by increasing platelet velocity and decreasing platelet adhesion [6]. Estrogen replacement in OVX rats induces vascular enzyme ADPase activity, thereby eliminating the ADP platelet activator [11]. In the present study, whole blood platelet aggregation in OVX rats tended to decrease from the maximum and initial slope. Estrogen deficiency may dampen platelet reactivity, showing a slow or weak response to aggregating agent and no dissociation after aggregation. Healthy platelets are continuously dissociated from their aggregates under certain physiological conditions, and are expressed as platelet survival in relation to platelet aggregation and disaggregation kinetics [35]. Hubbard et al. [36] reported that ingesting onion soup inhibits the generation of essential components for collagen-stimulated platelet activation and aggregation in humans. Resveratrol, at concentrations attainable with moderate wine consumption, inhibits human platelet aggregation by activating platelet endothelial NO synthase, and platelet NO inhibits platelet recruitment and reactive oxygen species (ROS) formation [37]. Tea catechins and bamboo leaf polyphenols did not affect platelet aggregation in OVX rats in the present study. Antiplatelet compounds such as herbal extracts may suppress platelet activity in vitro at high concentrations, but they may stimulate or have no effect on platelet aggregation at low concentrations or in vivo.
Estrogen deficiency in menopausal woman causes increased salt sensitivity and depresses natriuresis. Increased salt sensitivity affects vascular tone and membrane transport of cations [12]. Ethinyl estradiol treatment causes a suppression of Na-K ATPase in the basolateral membrane of rabbit intestines by modulating membrane lipid fluidity and controlling sodium and water absorption [16]. Schwarz et al. [16] suggested that an increased cholesterol to phospholipid molar ratio induces high membrane fluidity, resulting in increased Na-K ATPase. 17-β Estradiol treatment of OVX Wistar rats causes an increase in the Na-K-Cl cotransporter, thereby controlling renal sodium and water reabsorption [17]. In the present study, increased Na-K ATPase in OVX rats was not correlated with plasma cholesterol, which may affect membrane fluidity. However the increased Na-K ATPase and decreased Na-K cotransport in OVX rats observed agreed with other reports that intact or sham animals have lower Na-K ATPase and higher Na-K cotransport activity than those of OVX rats [16,17]. Insulin resistance, water retention, and hypertension are clustered symptoms in menopausal woman in whom hyperinsulinemia is a key factor for metabolic syndrome [4]. Previous studies have suggested that hyperinsulinemia and insulin resistance may be involved in altering sodium channels and affecting body fluid and blood pressure. Unlike insulin independent Na-K ATPase in the liver, insulin dependent Na-K ATPase activity in rat muscle and adipocytes is correlated with plasma insulin level [38]. Banday et al. [39] observed that continuous exposure to insulin diminishes dopamine-mediated inhibition of renal Na-K ATPase in Sprague-Dawley rat kidneys. Although it was unclear whether plasma insulin levels were elevated OVX rats, Na-K ATPase and plasma glucose in OVX rats was elevated to some extent in the present study. A review on herbal tea and diuretics [40] reported that many herbal teas are useful to treat urinary retention and hypertension. P. nigra and P. pubescens bamboo leaves exert their hypertensive effects by affecting ion channels [17] and angiotensin converting enzyme levels [18]. Phenylpropanoids from S. quelpaertensis bamboo has inhibitory activity against tyrosine hydroxylase (tyrosinase) in in vitro experiments [19], suggesting that S. quelpaertensis bamboo may play a favorable role in the cardiovascular system by decreasing catecholamine production. The present study showed that both green tea and bamboo leaves suppressed elevated Na-K ATPase and stimulated the depressed Na-K cotransporter in OVX rats. Theoretically, Na-K ATPase and Na-K cotransport play roles in Na reabsorption in the renal tubules and collecting duct. However, increased erythrocyte Na-K ATPase and Na-K cotransport do not necessarily indicate a hypertensive condition. Na channels function differently in different cells; Na-K ATPase in neuronal cells contribute to membrane gradient and Na-K ATPase in the intestine stimulates glucose absorption.
Decreased BMD is the most common symptom in OVX animals and menopausal woman [41,42]. García-Moreno et al. [42] reported that BMD in lumbar vertebra measured with DEXA is 226 mg/cm2 in OVX versus 262 mg/cm 2 in sham operated Wistar rats aged 9 months with a 6 month OVX duration. Considering OVX rats in the present study were 14 weeks of age and used 8 weeks after OVX, which is a comparatively young and short OVX duration, some differences in femoral BMD were observed between the OVX and sham operated rats. The OVX-bamboo and OVX-green tea groups recovered the decreased BMD, suggesting that the antioxidants in green tea and bamboo leaves may play some role preserving BMD in OVX rats. OVX induces oxidative stress and impairs bone antioxidants, which may modulate osteoblastic differentiation in bone cells [43]. Epidemiological studies of tea drinking and bone health [44] have shown that green tea drinking increases hip, femoral, and lumbar spine BMD compared with not drinking tea. Although there are no supportive reports on bamboo leaves and bone health, we found that bamboo leaves recovered BMD in OVX rats with the highest bone width, promising large and strong bones.
In conclusion, ovariectomy caused increased body weight and body fat, whereas green tea consumption had favorable effects on these complications in rats. Green tea and bamboo leaves may reverse or at least affect the increased Na efflux via Na-K ATPase and the decreased Na efflux via Na-K cotransport in OVX rats, thereby controlling electrolyte and body water balance. However, it is difficult to consider whether this is a good or bad effect, as Na-K ATPase and the Na-K cotransporter measured by erythrocyte Na efflux does not represent all channels from various types of cells such as intestinal, neuronal, renal, and other cells. Discrepancies in the interpretation of ion channel activity have been reported; thus, further studies are necessary.
References
- 1.Heymsfield SB, Gallagher D, Poehlman ET, Wolper C, Nonas K, Nelson D, Wang ZM. Menopausal changes in body composition and energy expenditure. Exp Gerontol. 1994;29:377–389. doi: 10.1016/0531-5565(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara CM, Lynch NA, Nicklas BJ, Ryan AS, Berman DM. Differences in adipose tissue metabolism between postmenopausal and perimenopausal women. J Clin Endocrinol Metab. 2002;87:4166–4170. doi: 10.1210/jc.2001-012034. [DOI] [PubMed] [Google Scholar]
- 3.Creatsas G, Christodoulakos G, Lambrinoudaki I. Cardiovascular disease: screening and management of the a-symptomatic high-risk post-menopausal woman. Maturitas. 2005;52(Suppl 1):S32–S37. doi: 10.1016/j.maturitas.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Gaspard U. Hyperinsulinaemia, a key factor of the metabolic syndrome in postmenopausal women. Maturitas. 2009;62:362–365. doi: 10.1016/j.maturitas.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Gorodeski EZ, Gorodeski GI. Epidemiology and risk factors of cardiovascular disease in post menopausal woman. In: Lobo RA, editor. Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. 3rd ed. New York: Elsevier; 2007. pp. 405–452. [Google Scholar]
- 6.Uematsu K, Katayama T, Katayama H, Hiratsuka M, Kiyomura M, Ito M. Nitric oxide production and blood corpuscle dynamics in response to the endocrine status of female rats. Thromb Res. 2010;126:504–510. doi: 10.1016/j.thromres.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 7.de Aloysio D, Gambacciani M, Meschia M, Pansini F, Bacchi Modena A, Bolis PF, Massobrio M, Maiocchi G, Peruzzi E The Icarus Study Group. The effect of menopause on blood lipid and lipoprotein levels. Atherosclerosis. 1999;147:147–153. doi: 10.1016/s0021-9150(99)00315-9. [DOI] [PubMed] [Google Scholar]
- 8.Lemay A, Dodin S, Turcot L, Déchêne F, Forest JC. Estrogen/progesterone replacement versus pravastatin and their sequential association in hypercholesterolemic postmenopausal women. Maturitas. 2001;40:247–257. doi: 10.1016/s0378-5122(01)00244-4. [DOI] [PubMed] [Google Scholar]
- 9.Aviram M, Brook GJ. The effect of human plasma on platelet function in familial hypercholesterolemia. Thromb Res. 1982;26:101–109. doi: 10.1016/0049-3848(82)90019-6. [DOI] [PubMed] [Google Scholar]
- 10.Sinzinger H, Pirich C, Bednar J, O'Grady J. Ex-vivo and in-vivo platelet function in patients with severe hypercholesterolemia undergoing LDL-apheresis. Thromb Res. 1996;82:291–301. doi: 10.1016/0049-3848(96)00079-5. [DOI] [PubMed] [Google Scholar]
- 11.Pochmann D, Rücker B, Battastini AM, Sarkis JJ. Ovariectomy and estradiol replacement therapy alters the adenine nucleotide hydrolysis in rat blood serum. Thromb Res. 2004;114:275–281. doi: 10.1016/j.thromres.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Pechère-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens. 2004;17:994–1001. doi: 10.1016/j.amjhyper.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Handa RK, Strandhoy JW, Giammattei CE, Handa SE. Platelet-activating factor and solute transport processes in the kidney. Am J Physiol Renal Physiol. 2003;284:F274–F281. doi: 10.1152/ajprenal.00117.2002. [DOI] [PubMed] [Google Scholar]
- 14.Musselman TM, Zhang Z, Masilamani SM. Differential regulation of the bumetanide-sensitive cotransporter (NKCC2) by ovarian hormones. Steroids. 2010;75:760–765. doi: 10.1016/j.steroids.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteiro SC, Matté C, Delwing D, Wyse AT. Ovariectomy increases Na+, K+-ATPase, acetylcholinesterase and catalase in rat hippocampus. Mol Cell Endocrinol. 2005;236:9–16. doi: 10.1016/j.mce.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz SM, Bostwick HE, Medow MS. Estrogen modulates ileal basolateral membrane lipid dynamics and Na+-K+-ATPase activity. Am J Physiol. 1988;254:G687–G694. doi: 10.1152/ajpgi.1988.254.5.G687. [DOI] [PubMed] [Google Scholar]
- 17.Fu XC, Wang MW, Li SP, Zhang Y, Wang HL. Vasodilatation produced by orientin and its mechanism study. Biol Pharm Bull. 2005;28:37–41. doi: 10.1248/bpb.28.37. [DOI] [PubMed] [Google Scholar]
- 18.Lee HS, Park MH, Kim JS, Lim BO, Moon GS, Shin HM. Anti-hypertensive effects of ethanol extract of Phyllostachys pubescens via antioxidant activity. Korean J Orient Physiol Pathol. 2007;21:658–665. [Google Scholar]
- 19.Sultana N, Lee NH. New phenylpropanoids from Sasa quelpaertensis Nakai with tyrosinase inhibition activities. Bull Korean Chem Soc. 2009;30:1729–1732. [Google Scholar]
- 20.Kuriyama S. The relation between green tea consumption and cardiovascular disease as evidenced by epidemiological studies. J Nutr. 2008;138:1548S–1553S. doi: 10.1093/jn/138.8.1548S. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki E, Yorifuji T, Takao S, Komatsu H, Sugiyama M, Ohta T, Ishikawa-Takata K, Doi H. Green tea consumption and mortality among Japanese elderly people: the prospective Shizuoka elderly cohort. Ann Epidemiol. 2009;19:732–739. doi: 10.1016/j.annepidem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Babu PV, Sabitha KE, Shyamaladevi CS. Green tea impedes dyslipidemia, lipid peroxidation, protein glycation and ameliorates Ca2+ -ATPase and Na+/K+ -ATPase activity in the heart of streptozotocin-diabetic rats. Chem Biol Interact. 2006;162:157–164. doi: 10.1016/j.cbi.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Lin JK, Lin-Shiau SY. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol Nutr Food Res. 2006;50:211–217. doi: 10.1002/mnfr.200500138. [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Kang JS, Cregor MD, Smith JB. Effect of calcium on blood pressure, platelet aggregation and erythrocyte sodium transport in Dahl salt-sensitive rats. J Hypertens. 1990;8:245–250. [PubMed] [Google Scholar]
- 26.Oh IS, Kang JA, Kang JS. Gender difference in the effects of gonadectomy and hypercholesterol diet on plasma and liver cholesterol and Triglyceride levels, platelet aggregation and liver tissue in Sprague Dawley rats. Korean J Nutr. 2002;35:15–23. [Google Scholar]
- 27.Pantaleão TU, Mousovich F, Rosenthal D, Padrón AS, Carvalho DP, da Costa VM. Effect of serum estradiol and leptin levels on thyroid function, food intake and body weight gain in female Wistar rats. Steroids. 2010;75:638–642. doi: 10.1016/j.steroids.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Kang JA, Chae IS, Song YB, Kang JS. Effects of green tea on weight gain, plasma and liver lipids and lipid peroxidation in pair fed rats. Korean J Nutr. 2008;41:602–611. [Google Scholar]
- 29.Diepvens K, Kovacs EM, Nijs IM, Vogels N, Westerterp-Plantenga MS. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. Br J Nutr. 2005;94:1026–1034. doi: 10.1079/bjn20051580. [DOI] [PubMed] [Google Scholar]
- 30.Shimotoyodome A, Haramizu S, Inaba M, Murase T, Tokimitsu I. Exercise and green tea extract stimulate fat oxidation and prevent obesity in mice. Med Sci Sports Exerc. 2005;37:1884–1892. doi: 10.1249/01.mss.0000178062.66981.a8. [DOI] [PubMed] [Google Scholar]
- 31.Vajo Z, Terry JG, Brinton EA. Increased intra-abdominal fat may lower HDL levels by increasing the fractional catabolic rate of Lp A-I in postmenopausal women. Atherosclerosis. 2002;160:495–501. doi: 10.1016/s0021-9150(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 32.Joles JA, Bijleveld C, van Tol A, Geelen MJ, Koomans HA. Ovariectomy decreases plasma triglyceride levels in analbuminaemic rats by lowering hepatic triglyceride secretion. Atherosclerosis. 1995;117:51–59. doi: 10.1016/0021-9150(95)05557-d. [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Huang MT, Huang PC. Sources of triacylglycerol accumulation in livers of rats fed a cholesterol-supplemented diet. Lipids. 1995;30:527–531. doi: 10.1007/BF02537027. [DOI] [PubMed] [Google Scholar]
- 34.Löest HB, Noh SK, Koo SI. Green tea extract inhibits the lymphatic absorption of cholesterol and alpha-tocopherol in ovariectomized rats. J Nutr. 2002;132:1282–1288. doi: 10.1093/jn/132.6.1282. [DOI] [PubMed] [Google Scholar]
- 35.Huang PY, Hellums JD. Aggregation and disaggregation kinetics of human blood platelets: Part II. Shear-induced platelet aggregation. Biophys J. 1993;65:344–353. doi: 10.1016/S0006-3495(93)81079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard GP, Wolffram S, de Vos R, Bovy A, Gibbins JM, Lovegrove JA. Ingestion of onion soup high in quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in man: a pilot study. Br J Nutr. 2006;96:482–488. [PubMed] [Google Scholar]
- 37.Gresele P, Pignatelli P, Guglielmini G, Carnevale R, Mezzasoma AM, Ghiselli A, Momi S, Violi F. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. J Nutr. 2008;138:1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 38.Lytton J, Lin JC, Guidotti G. Identification of two molecular forms of (Na+,K+)-ATPase in rat adipocytes. Relation to insulin stimulation of the enzyme. J Biol Chem. 1985;260:1177–1184. [PubMed] [Google Scholar]
- 39.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopaminemediated inhibition of renal Na,K-ATPase is reduced by insulin. Hypertension. 2003;41:1353–1358. doi: 10.1161/01.HYP.0000069260.11830.CD. [DOI] [PubMed] [Google Scholar]
- 40.Wright CI, Van-Buren L, Kroner CI, Koning MM. Herbal medicines as diuretics: a review of the scientific evidence. J Ethnopharmacol. 2007;114:1–31. doi: 10.1016/j.jep.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 41.Ohta H, Makita K, Komukai S, Nozawa S. Bone resorption versus estrogen loss following oophorectomy and menopause. Maturitas. 2002;43:27–33. doi: 10.1016/s0378-5122(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 42.García-Moreno C, Calvo OM, Herrero S, Martín E, Suquía B, San Román JI, Martín M, García-Talavera JR, Calvo JJ, del Pino J. Heterogeneous decrease of bone mineral density in the vertebral column of ovariectomized rats. Bone. 1995;16:295S–300S. doi: 10.1016/8756-3282(95)00023-7. [DOI] [PubMed] [Google Scholar]
- 43.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 44.Shen CL, Yeh JK, Cao JJ, Wang JS. Green tea and bone metabolism. Nutr Res. 2009;29:437–456. doi: 10.1016/j.nutres.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]