Abstract
The objective of this study was to examine the influence of anthropometric measurements of pregnant women, gestational weight gain, fundal height, and maternal factors, namely age, education, family income, parity along with maternal hemoglobin, on birth weight of neonates. A cross sectional study was performed in Khoy City in north west of Iran. Four hundred and fifty healthy pregnant women in the age between 16-40 years were selected for this study from seven health urban centers and one referral hospital. Findings showed that the mean age, height, fundal height, maternal weight, and gestational weight gain during pregnancy were 26.1 years, 159.1 cm, 32.9 cm, 72.0 kg, 11.8 kg respectively. The mean birth weight of neonates was 3.2 kg and 11% of neonates showed low birth weight. Age, family income, maternal height, weight, gestational weight gain and fundal height were significantly associated with birth weight of neonates. Using binary logistic regression analysis, fundal height, maternal hemoglobin, family income and gestational weight gain of pregnant women could be considered as predictive factors of birth weight of neonates.
Keywords: Maternal weight, gestational weight gain, birth weight, maternal factors
Introduction
More than 20 million infants are born each year weighing less than 2500 g, accounting for 17 percent of all births in the developing world [1]. Birth weight plays an important role in infant mortality and morbidity, development, and future health of the child [2]. Low birth weight (LBW) is a significant risk factor for adverse health outcomes including many childhood diseases [3]. The associations between LBW and a greatly elevated risk of infant mortality [4] and other physical and neurologic impairments [5], are well established. Thus, prevention of LBW is a major public health priority.
Risk factors for LBW are many and varied [6]. Demographic risk factors include young maternal age [7], primiparity [8], and low education [9]. Maternal nutritional status both before and during pregnancy is a well-recognized determinant of birth outcomes [10]. Only two indicators, namely maternal pre pregnancy body mass index and weight gain during pregnancy have shown consistent positive associations with infant birth weight [11]. Reports from developed and developing countries show that maternal anthropometric measurements are associated with birth outcome [12].
Gestational weight gain during pregnancy influence infant birth weight. A strong relationship between maternal pregnancy weight gain and birth weight has been consistently demonstrated, and low maternal weight gain is considered a preventable risk factor for LBW [13]. However, weight gain in most pregnant women is not within the range as recommended by IOM, and is considered too low or too high compared with current standards [14].
Therefore, understanding association maternal anthropometrics, maternal factors and fetal growth is critical. Various maternal anthropometric criteria (pre-pregnancy weight, height, weight gain during pregnancy period) has been significantly associated with intrauterine growth. These parameters should be viewed as "predictors" of low birth weight to be used for risk detection [15].
Very few studies have been done regarding the nutritional status of pregnant women and the relationship between maternal anthropometrics, maternal factors and birth weight in Khoy city in north west Iran. Therefore, this study was designed to assay the influence of maternal anthropometrics, maternal factors and birth weight of expectant women in pregnancy and to relate the birth weight as the outcome of these parameters in pregnancy.
Subjects and Methods
Selection of area, hospital and subjects
Khoy, a city of Western Azarbayjan province located in the North West of Iran, was selected for the study. Seven urban health centers and one hospital were selected in this city. With the help of a statistician and the statistics of annual deliveries in this hospital, 450 pregnant women were selected as subjects of the study. All subjects were invited by the head midwife of the health centers to take part in this study. The inclusive criteria were the age group (16 to 40 years) and continuous visits for health check up during the three trimesters of pregnancy in selected urban health care centers in Khoy city. Eligible subjects were referred for delivery to the hospital. Pregnant women with diabetes mellitus or cardio vascular disease (CVD), multiple pregnancy, mothers with placenta previa and placenta abruptia were excluded from this study. The study was approved by the Human Ethical Committee of the University of Mysore since the researcher has registered in the University of Mysore for Ph.D. Permission was obtained from Urmia Medical University which was affiliated with all the health centers and hospitals in Khoy city, where the study was conducted. Informed written consent from all of the subjects was obtained and they also accepted to be part of the study until birth of the babies.
Questionnaires : general characteristics of the subjects
A Questionnaire is a tool or device for securing answers to a set of questions by the respondent who fills in the Questionnaire. The Questionnaire method was selected for the present study, as it is a frequently used method of data collection [16]. Information about the maternal demographic characteristics was obtained using a structured questionnaire.
Anthropometric measurements
Anthropometry provides a simple, reliable and low-cost method of assessing maternal nutrition status which can be universally applied at the primary care. Maternal anthropometry indicates the risk of intrauterine growth retardation and low birth weight [17]. The measurements were made on the participants wearing a minimum amount of clothing. The weights of pregnant women were recorded at the early first trimester during their first visit and continued in every trimester by using a digital weighing balance with a sensitivity of 100 g. Total gestational weight gain was estimated by subtracting the early first trimester weight from the last measured weight before delivery. Height was measured in cm using a locally made anthrop-meter. The pregnant women were asked to maintain an upright and erect posture with her feet together and the back of her heels touching the pole of the anthrop-meter. The height was measured when the horizontal headpiece was lowered onto the women's head. Fundal height was measured by a midwife as the distance between the symphysis pubis and the highest point of the uterine fundus, defined with a gentle pressure on a plain at a right angle of the abdominal wall. Gestational weight gain in relation to pregnancy Birth weights of neonates were taken within 24 hours after birth using a standard procedure. A beam balance with an accuracy of 50 g was employed for weighing the infants. The infants were weighed with minimum clothing while the child was restful.
Statistical analysis
Statistical analysis was performed using SPSS software, version 16.0(SPSS, Chicago, IL, USA). Quantitative variables were analyzed by student's t-test and One way- ANOVA. When the One way- ANOVA results were significant, the Bonferroni test was used to determine the significance between different variables means. Binary logistic regression analysis was done to find out the association among body mass index, gestational weight gain, some maternal factors such as age, height, education, income and parity.
Results
Information on pregnant women & their family background
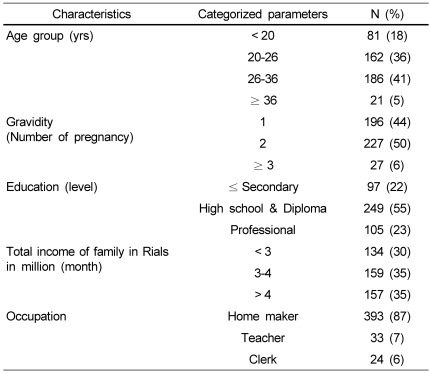
Details of all selected pregnant women under examination are presented in Table 1. The mean age of pregnant women was 26.1 ± 5.8 years and the age range was 18-40 years. Majority (41%) of pregnant women were in the age group of 26-36 years. The highest percent (50%) of pregnant women were expecting a second child. Sixty three percent of pregnant women had secondary and/or diploma level of education and the majority (30%) of subjects had income of less than 3 million Iranian rials per month. Eighty seven percent of subjects were not employed. Other subjects details are presented in Table 1.
Table 1.
Information about pregnant women & their family background (n = 450)
Anthropometric measurement, hemoglobin levels and gestational wight gain of pregnant women
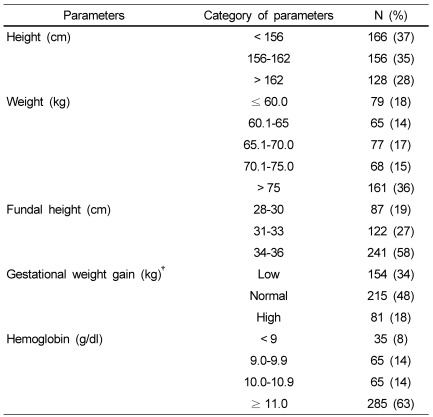
Anthropometric measurements of subjects at the end of the third trimester, namely height, weight along with fundal height and hemoglobin levels are given in Table 2. The finding showed that 43% of subjects' heights were in the range between 156-162 cm, fifty eight percent of subjects had fundal heights between -34-36 cm, the majority of pregnant women (68%) weighed more than 65 kg, and 48% had normal gestational weight gain with reference to IOM guidelines [13]. The majority of subjects (63%) had hemoglobin levels greater than 11 g/dl, which is considered normal as per WHO standards [18].
Table 2.
Anthropometric measurements, gestational wight gain and hemoglobin levels of pregnant women (n = 450)
†Recommendations for gestational weight gain are for BMI < 19.8 kg/m2 total weight gain between 12.5 to 18 kg; BMI = 19.8 to 26.0 kg/m2 total weight gain between 11.5 to 16 kg; BMI > 26.0 to 29.0 kg/m2 total weight gain between 7.0 to 11.5 kg. and for BMI > 29.0 kg/m2 total weight gain of 7.0 kg [37].
Neonate information
Table 3 shows that the majority (89%) of neonates had normal birth weight and eleven percent of them belonged to the low birth weight category. Forty six percent males and forty three percent females had normal birth weight, whereas five percent males and six percent females had low birth weight.
Table 3.
Prevalence of low birth weight and normal birth weight (n = 450)
Birth weight of neonates: maternal anthropometric and other maternal parameters
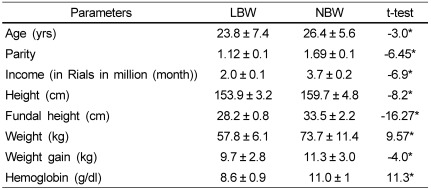
As a first challenge to find out the association between birth weight and maternal and anthropometric factors of pregnant women, birth weights of neonates were classified into two categories LBW and NBW and subjected to student's t-test. The results are presented in Table 4.
Table 4.
LBW and NBW of neonates: age, income, hemoglobin anthropometric and maternal factors of pregnant women
*Significance at the 0.05 level, LBW (Birth weight ≤ 2.5 kg), NBW (Birth weight > 2.5 kg)
As shown in the Table, pregnant women who gave birth to LBW babies had significantly lower age, parity, family income, height, weight, fundal height, gestational weight and hemoglobin levels than women who gave birth to neonates with normal birth weight.
Birth weight vs maternal factors and maternal anthropometric
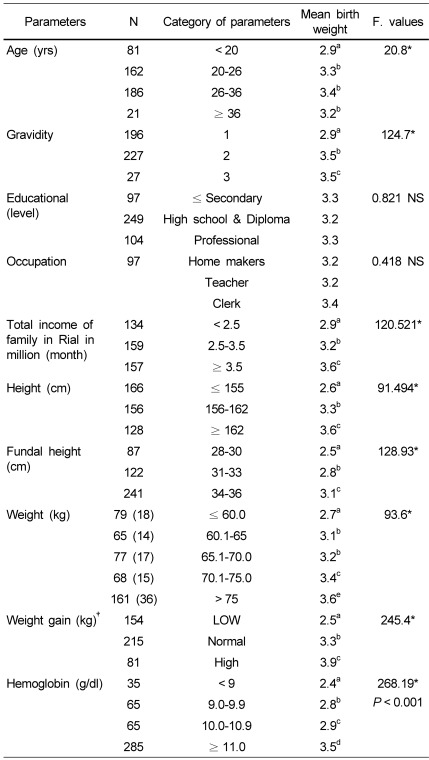
It was interesting to analyze the results of different categories of maternal age, gravidity, education levels and other mentioned parameters of pregnant women with reference to variations in birth weight of neonates. The data was subjected to one-way ANOVA and the findings are presented in Table 5.
Table 5.
Age, education, family income and body measurements of pregnant women vs. birth weight of neonates (N = 450)
*P < 0.05 Different superscripts indicate significant difference at 5% level as shown by Post hoc Bonferroni.
†IOM recommendation for gestational weight gain [38].
It is evident from the Table that as age increased from 20 to 36, the birth weight increased from 2.9 to 3.4 kg. Pregnant women who were pregnant for the second and third time gave birth to neonates with the mean birth weight of 3.5 kg, while women with first gravida gave birth to neonates with 2.9 kg. Subjects who had an income of less than 2.5 million in Rials per month gave birth to neonates with the mean birth weight of 2.9 kg, while pregnant women with > 3.5 million in Rials per month gave birth to neonates with the mean birth weight of 3.6 kg. It is clear from the Table that taller pregnant women (taller than 162 cm) gave birth to significantly heavier babies (3.6 kg) than shorter women. Pregnant women with higher levels of fundal height (34-36 cm) at the end of the third trimester gave birth to neonates with significantly heavier birth weights. Pregnant women who weighed less than 60 kg gave birth to neonates with the mean birth weight of 2.7 kg, while subjects who weighed more than 75 kg gave birth to heavier neonates (3.6 kg). Women with normal weight gain gave birth to babies with the mean birth weight of 3.3 kg, while pregnant women with low gestational weight gain gave birth to babies with the mean birth weight of 2.5 kg. Pregnant women with hemoglobin levels less than 9 g/dl, which is considered anemic, gave birth to neonates with low birth weights, while pregnant women with higher hemoglobin levels (> 11 g/dl), who were considered normal, gave birth to heavier and normal babies (3.5 kg). Different levels of education in pregnant women showed no significant influence on the birth weight of babies.
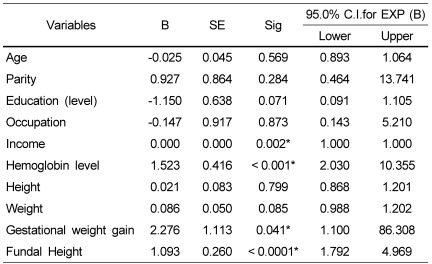
Thus the results of the study revealed that age, parity, family income, height, fundal height, weight, gestational weight gain and hemoglobin levels influenced the birth weight of the neonates. It was interesting to find out among these parameters, which factors could be considered as major predictable factors for birth weight of neonates. Therefore, the binary logistic regression was carried out to investigate the possible factors associated with birth weight (results are presented in Table 6).
Table 6.
Results of binary logistic regression- predictive factors in relation to birth weight
Variable(s) entered on step 1: Age, Parity, Education Statues, Occupation, Income, Height, Fundal Height, Weight, Gestational Weight gain and Hemoglobin Status.
B, Binary; SE, standard error; Sig, Significant
Discussion
In the current study, pregnant women who gave birth to LBW babies had significantly lower body measurements than women who gave birth to neonates with normal birth weight. It was also observed that these women who gave birth to babies with normal weight had significantly higher fundal height, gestational weight gain and total family income than women gave birth to neonates with LBW. The present findings were similar with other reports from New York [19], Brazil [20], India [21] and Yemen [22]. They also reported that there were associations between maternal factors, gestational weight gain, body mass index and fundal height with birth weight of neonates.
As age increased from 20 to 36, the birth weight increased from 2.9 to 3.4 kg. There was a significant progression of birth weight with advancing age. Our findings are consistent with those reported by Li and Chang [23] in Eastern Taiwan; MacLeod and Kiely [19] in New York, which revealed that low-birth weight was significantly related to mother's age.
MacLeod abd Kiely [19] in New York demonstrated that birth weight increased from parity 1 to 3. Celik and Younis [24] in Turkey found out that birth order was one of the major factors affecting birth weight. Similar results were also observed in the current study. Women who were pregnant for the second and third time gave birth to neonates with higher birth weights (3.5 kg), while women with first gravid gave birth to neonates with lower birth weights (2.8 kg).
It is clear from table 6 that taller pregnant women (more than 155 cm) gave birth to significantly heavier and normal babies (3.6 kg) when compared to shorter women (mean birth weight 2.6 kg). The present findings were similar with findings reported by Morrison et al. [25] from Sudan and Zhang et al. [26] from Swede reported that maternal height had a significant effect on birth weight.
Increase in fundal height of pregnant women showed a significant increase in birth weight. Rondó et al. [20] in Braziland and Jeffery et al. [27] in South Africa reported about the similar findings. Dare et al. [28] found that symphysis-fundal measurement in labor had a correlation with birth mass of 0.742.
It is clear from Table 4 that pregnant women with normal weight gain gave birth to 3.3 kg weight babies, while pregnant women with low gestational weight gain gave birth to neonates with a lower mean birth weight of 2.5 kg. Merchant et al. [29] from South Carolina claimed that pregnancy weight gain within the IOMs recommended range is associated with the best outcome for both mothers and infants.
Pregnant women with hemoglobin levels greater than 11 g/dl, which is considered normal, gave birth to neonates with normal weight, while pregnant women with lower hemoglobin levels (< 9 g/dl), who were considered anaemic gave birth to low birth weight babies. Steer et al. [30], Malhotra et al. [31] from India and Yazdani et al. [32] in Iran also indicated in their study the importance of normal haemoglobin level on pregnancy outcome, and their results agree with current findings. Their findings revealed that normal concentration of hemoglobin significantly influenced birth weight.
Yekta et al. [33] in Iran found that level of education did not have a significant effect on birth weight but illiterate subjects were more at risk for poor weight gain. Similarly, in the present study, different levels of education in pregnant women showed no significant influence on birth weight of babies. While it is true that healthcare providers cannot alter the mother's education, these conditions may provide valuable clues regarding the likelihood of babies being born with low birth weight and assist in designing a comprehensive care plan.
Varying degrees of total family income showed significant influence on birth weight of babies. It is evident from the present study (Table 6) that pregnant women who had a family income of less than 2.5 million in Rials per month gave birth to neonates with the mean birth weight of 2.9 kg, while pregnant women with > 3.5 million in Rials per month gave birth to neonates with the mean birth weight of 3.6 kg. Similar results were reported by Elshibly and Schmalisch [34] in Sudan and Jafari et al. [35] in Iran who reported that there was a significant association between income and birth weight of neonates.
The findings showed that the fundal height, hemoglobin levels, family income and gestational weight gain of women could be considered as "predictor factors" for birth weight of neonates. In agreement with our results; Neggers and Goldenberg in Bringham [36]; Jeffery et al. [27] in South Africa; Joseph and Kramer [4] in Canada have reported the same results.
Acknowledgments
We are indebted to the administrators of hospitals, and laboratory; Dr. Mohammad Reza Frootani and Mr. Samadi, for their support and cooperation. Cooperation of the staff in the selected health care centers is highly acknowledged. We are sincerely indebted to all the participants who made this study possible.
Footnotes
This study was supported by Urmia Medical University.
References
- 1.UNICEF. Progress for Children: A Report Card on Nutrition. Other Nutrition Indicators, Low Birthweight. New York: UNICEF; 2006. [Google Scholar]
- 2.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 3.Boucher BJ. Determinants of size at birth. QJM. 2002;95:331–333. doi: 10.1093/qjmed/95.5.331. [DOI] [PubMed] [Google Scholar]
- 4.Joseph KS, Kramer MS. Recent trends in infant mortality rates and proportions of low-birth-weight live births in Canada. CMAJ. 1997;157:535–541. [PMC free article] [PubMed] [Google Scholar]
- 5.Whitaker AH, Van Rossem R, Feldman JF, Schonfeld IS, Pinto-Martin JA, Tore C, Shaffer D, Paneth N. Psychiatric outcomes in low-birth-weight children at age 6 years: relation to neonatal cranial ultrasound abnormalities. Arch Gen Psychiatry. 1997;54:847–856. doi: 10.1001/archpsyc.1997.01830210091012. [DOI] [PubMed] [Google Scholar]
- 6.Fraser AM, Brockert JE, Ward RH. Association of young maternal age with adverse reproductive outcomes. N Engl J Med. 1995;332:1113–1117. doi: 10.1056/NEJM199504273321701. [DOI] [PubMed] [Google Scholar]
- 7.Lee KS, Ferguson RM, Corpuz M, Gartner LM. Maternal age and incidence of low birth weight at term: a population study. Am J Obstet Gynecol. 1988;158:84–89. doi: 10.1016/0002-9378(88)90783-1. [DOI] [PubMed] [Google Scholar]
- 8.Abrams B, Newman V. Small-for-gestational-age birth: maternal predictors and comparison with risk factors of spontaneous preterm delivery in the same cohort. Am J Obstet Gynecol. 1991;164:785–790. doi: 10.1016/0002-9378(91)90516-t. [DOI] [PubMed] [Google Scholar]
- 9.Karim E, Mascie-Taylor CG. The association between birthweight, sociodemographic variables and maternal anthropometry in an urban sample from Dhaka, Bangladesh. Ann Hum Biol. 1997;24:387–401. doi: 10.1080/03014469700005152. [DOI] [PubMed] [Google Scholar]
- 10.Osrin D, de L Costello AM. Maternal nutrition and fetal growth: practical issues in international health. Semin Neonatol. 2000;5:209–219. doi: 10.1053/siny.2000.0024. [DOI] [PubMed] [Google Scholar]
- 11.Neggers Y, Goldenberg RL, Cliver SP, Hoffman HJ, Cutter GR. The relationship between maternal and neonatal anthropometric measurements in term newborns. Obstet Gynecol. 1995;85:192–196. doi: 10.1016/0029-7844(94)00364-J. [DOI] [PubMed] [Google Scholar]
- 12.Mavalankar DV, Trivedi CC, Gray RH. Maternal weight, height and risk of poor pregnancy outcome in Ahmedabad, India. Indian Pediatr. 1994;31:1205–1212. [PubMed] [Google Scholar]
- 13.Mathews F, Youngman L, Neil A. Maternal circulating nutrient concentrations in pregnancy: implications for birth and placental weights of term infants. Am J Clin Nutr. 2004;79:103–110. doi: 10.1093/ajcn/79.1.103. [DOI] [PubMed] [Google Scholar]
- 14.Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. Am J Clin Nutr. 2000;71:1233S–1241S. doi: 10.1093/ajcn/71.5.1233s. [DOI] [PubMed] [Google Scholar]
- 15.Sachdev HP. Low birth weight in South Asia. Int J Diabetes Dev Ctries. 2001;21:13–33. [Google Scholar]
- 16.Young PV. Scientific Social Surveys and Research. Englewood Cliffs, NJ: Prentice-Hall; 1987. p. 186. [Google Scholar]
- 17.Report of a WHO Expert Committee. WHO Technical Report Series, No. 854. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 18.Gammon A, Baker SJ. Studies in methods of haemoglobin estimation suitable for use in public health programmes. Indian J Med Res. 1977;65:150–156. [PubMed] [Google Scholar]
- 19.MacLeod S, Kiely JL. The effects of maternal age and parity on birthweight: a population-based study in New York City. Int J Gynaecol Obstet. 1988;26:11–19. doi: 10.1016/0020-7292(88)90191-9. [DOI] [PubMed] [Google Scholar]
- 20.Rondó PH, Maia Filho NL, Valverde KK. Symphysis-fundal height and size at birth. Int J Gynaecol Obstet. 2003;81:53–54. doi: 10.1016/s0020-7292(02)00407-1. [DOI] [PubMed] [Google Scholar]
- 21.Deshmukh JS, Motghare DD, Zodpey SP, Wadhva SK. Low birth weight and associated maternal factors in an urban area. Indian Pediatr. 1998;35:33–36. [PubMed] [Google Scholar]
- 22.Heude B, Thiébaugeorges O, Goua V, Forhan A, Kaminski M, Foliguet B, Schweitzer M, Magnin G, Charles MA EDEN Mother-Child Cohort Study Group. Pre-pregnancy body mass index and weight gain during pregnancy: relations with gestational diabetes and hypertension, and birth outcomes. Matern Child Health J. 2012;16:355–363. doi: 10.1007/s10995-011-0741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YM, Chang TK. Maternal demographic and psyhosocial factors associated with low birth weight in eastern Taiwan. Kaohsiung J Med Sci. 2005;21:502–510. doi: 10.1016/s1607-551x(09)70158-5. [DOI] [PubMed] [Google Scholar]
- 24.Celik Y, Younis MZ. Effects of antenatal care services on birthweight: importance of model specification and empirical procedure used in estimating the marginal productivity of health inputs. J Med Syst. 2007;31:197–204. doi: 10.1007/s10916-007-9055-2. [DOI] [PubMed] [Google Scholar]
- 25.Morrison J, Williams GM, Najman JM, Andersen MJ. The influence of paternal height and weight on birth-weight. Aust N Z J Obstet Gynaecol. 1991;31:114–116. doi: 10.1111/j.1479-828x.1991.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Cnattingius S, Platt RW, Joseph KS, Kramer MS. Are babies born to short, primiparous, or thin mothers "normally" or "abnormally" small? J Pediatr. 2007;150:603–607. doi: 10.1016/j.jpeds.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 27.Jeffery BS, Pattinson RC, Makin J. Symphysis-fundal measurement as a predictor of low birthweight. Early Hum Dev. 2001;63:97–102. doi: 10.1016/s0378-3782(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 28.Dare FO, Ademowore AS, Ifaturoti OO, Nganwuchu A. The value of symphysio-fundal height/abdominal girth measurements in predicting fetal weight. Int J Gynaecol Obstet. 1990;31:243–248. doi: 10.1016/0020-7292(90)91018-l. [DOI] [PubMed] [Google Scholar]
- 29.Merchant SS, Momin IA, Sewani AA, Zuberi NF. Effect of prepregnancy body mass index and gestational weight gain on birth weight. J Pak Med Assoc. 1999;49:23–25. [PubMed] [Google Scholar]
- 30.Steer P, Alam MA, Wadsworth J, Welch A. Relation between maternal haemoglobin concentration and birth weight in different ethnic groups. BMJ. 1995;310:489–491. doi: 10.1136/bmj.310.6978.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malhotra M, Sharma JB, Batra S, Sharma S, Murthy NS, Arora R. Maternal and perinatal outcome in varying degrees of anemia. Int J Gynaecol Obstet. 2002;79:93–100. doi: 10.1016/s0020-7292(02)00225-4. [DOI] [PubMed] [Google Scholar]
- 32.Yazdani M, Tadbiri M, Shakeri S. Maternal hemoglobin level, prematurity, and low birth weight. Int J Gynaecol Obstet. 2004;85:163–164. doi: 10.1016/j.ijgo.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Yekta Z, Ayatollahi H, Porali R, Farzin A. The effect of pre-pregnancy body mass index and gestational weight gain on pregnancy outcomes in urban care settings in Urmia-Iran. BMC Pregnancy Childbirth. 2006;6:15. doi: 10.1186/1471-2393-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elshibly EM, Schmalisch G. The effect of maternal anthropometric characteristics and social factors on gestational age and birth weight in Sudanese newborn infants. BMC Public Health. 2008;8:244. doi: 10.1186/1471-2458-8-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jafari F, Eftekhar H, Pourreza A, Mousavi J. Socio-economic and medical determinants of low birth weight in Iran: 20 years after establishment of a primary healthcare network. Public Health. 2010;124:153–158. doi: 10.1016/j.puhe.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Neggers Y, Goldenberg RL. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J Nutr. 2003;133:1737S–1740S. doi: 10.1093/jn/133.5.1737S. [DOI] [PubMed] [Google Scholar]
- 37.Abrams B, Newman V, Key T, Parker J. Maternal weight gain and preterm delivery. Obstet Gynecol. 1989;74:577–583. [PubMed] [Google Scholar]
- 38.Institute of Medicine. Nutrition During Pregnancy. Washington, D.C.: National Academy Press; 1990. [Google Scholar]