Abstract
Purpose
The goal of this study was to evaluate the clinical anitplaque and antigingivitis effects of a mouthrinse containing cetylpyridinium chloride (CPC), triclosan and dipotassium glycyrrhizinate (DPZ) in patients with gingivitis and mild periodontitis.
Methods
Thirty-two subjects were randomized into 2 groups. The test group used a mouthrinse containing 0.05% CPC, 0.02% triclosan and 0.02% DPZ, while the control group used a placebo mouthrinse. At baseline, 2 weeks and 4 weeks, the papillary bleeding index (PBI), Turesky-Quigley-Hein plaque index (PI) and Löe-Silness gingival index (GI) were assessed. During the experimental period, the patients used the mouthrinse for 30 seconds, 4 to 5 times/day (10 mL/time) within 30 minutes after toothbrushing.
Results
No adverse effects appeared in either the experimental or the control group. Regarding PBI, PI and GI values, statistical significance was detected between values at baseline and 2 weeks for both groups (P<0.05). In the experimental group, statistically significantly lower values were detected at 4 weeks compared to at 2 weeks. However, in the control group, no statistically significant difference was detected between the values at 2 weeks and 4 weeks. Additionally, the mean value after 4 weeks for the control group was slightly higher than the mean value after 2 weeks for the control group.
Conclusions
This study for 4 weeks demonstrated that mouthrinses containing CPC, triclosan and DPZ may contribute to the reduction of supragingival plaque and gingivitis.
Keywords: Cetylpyridinium, Dental plaque index, Glycyrrhizic acid, Prevention mouthrinse, Triclosan
INTRODUCTION
The concept of bacterial specificity in periodontal infections is now widely accepted [1,2]. Three factors are currently considered to play a role in establishing an active periodontal infection; 1) susceptible host, 2) presence of periodonto-pathogens, and 3) absence of beneficial species [3]. The reduction of pathogenic microbes and plaque is needed for successful treatment because a susceptible host is unlikely to be change into an invulnerable one. Previous studies have demonstrated that long-term stability of the clinical benefits obtained via periodontal therapy can be maintained only when cause-related treatment is followed by effective supportive periodontal care (SPC) [4]. Within this SPC program, self-performed plaque control is crucial in attaining the best long-term results after periodontal therapy [5].
As patient compliances with mechanical oral hygiene practices are not always as good as desired, chemical agents have been used to improve plaque control and to reduce gingivitis [6]. The use of mouthrinse containing antiseptic agents is an effective and feasible way to reduce viable bacteria in the oral cavity [7-9]. The antimicrobial and antiplaque effects of various mouthrinses have been studied as well [10-12]. Chlorhexidine (CHX) is considered as the gold standard among oral antiseptics due to its superior clinical and microbiological effects [13]. However, CHX is known to have undesirable side effects, such as tooth staining, burning sensation and soft tissue irritation [10]. As such, a variety of mouthrinse substitutes with similar formulas to that of CHX have been studied.
In the present study, mouthrinse containing cetylpyrydinium chloride (CPC), triclosan, and dipotassium glycyrrhizinate (DPZ) was used. CPC, a cationic surface-active agent, has a broad antimicrobial spectrum and can rapidly kill particular gram-positive pathogens and yeast [14]. Several studies have reported that a mouthrinse containing 0.05% CPC is efficacious in controlling established dental plaque and gingivitis [15]. Triclosan, a broad spectrum antimicrobial agent, has been widely used in mouthrinses and dentifrices. It acts as an antibacterial agent by inhibiting the enoylreductase activity of the type II fatty acid synthase in bacteria [16]. There have been studies which reported triclosan mouthrinse to have a continuous disinfecting effect and to be helpful in controlling dental plaque formation and adhesion [17,18]. DPZ, a component of licorice root, has been known to have detoxicant, anti-ulcerative and anti-inflammatory effects. It is already being used as a component of mouthrinses in Japan. There is a published article that demonstrated mouthrinses containing 0.05% CPC and 0.015% DPZ were useful in preventing periodontal disease [19]. Previous studies have assessed each of 3 components (CPC, triclosan, DPZ) independently, however there is not any study that has reported the effect of a mouthrinse containing a combination of all 3 components.
The goal of this study was to evaluate the clinical antiplaque and antigingivitis effects of a mouthrinse containing CPC, triclosan and DPZ in patients with gingivitis and mild periodontitis.
MATERIALS AND METHODS
Patient selection
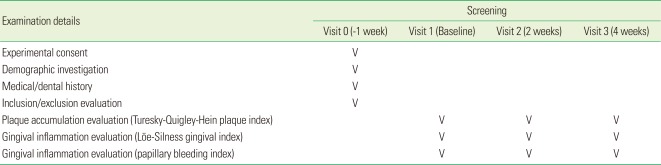
This study was performed at the Department of Periodontology, Dankook University School of Dentistry, Korea. Thirty-two subjects who met the following inclusion criteria were entered into the study: 1) a minimum of 20 sound, natural teeth, 2) healthy and without any history of allergies to experimental mouthrinses, 3) a mean Turesky-Quigley-Hein plaque index (PI) of at least 1.5, and 4) a mean Löe-Silness gingival index (GI) of at least 1.0. Subjects with orthodontic appliances, removable partial dentures, intraoral lesions, and severe periodontal disease, as well as those currently using other mouthrinses were all excluded.
In this clinical study, 3 patients who did not visit our clinic on the appropriate dates were excluded. Written informed consent was obtained from all subjects. The protocol for human subjects was reviewed and approved by the Institutional Review Board of Dankook University in 2011 (H-1102-001-002). The study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. Inclusion and exclusion criteria are as follows.
Experimental material
The test group used GUM Dental Rinse GNN (GUM, Sunstar Inc., Osaka, Japan) while the control group used a placebo mouthrinse. GUM Dental Rinse GNN is a mouthrinse containing 0.05% CPC, 0.02% triclosan and 0.02% DPZ. The placebo mouthrinse did not contain CPC, triclosan or DPZ. A disclosing solution was used to evaluate the PI. During the experimental period, the patients used a smooth tooth brush and a custom-made toothpaste without sodium lauryl sulfate.
Clinical parameter
Patients were evaluated using the PBI, Turesky-Quigley-Hein PI and Löe-Silness GI. Plaque accumulation was evaluated via the Turesky-Quigley-Hein PI. This index emphasizes differences in plaque accumulation in the gingival one-third of the tooth and tends to overestimate the incisal one-half of the crown, at the expense of the gingival margin. The scoring system was as follows:
0= no plaque;
1= separate flecks of plaque at the cervical margin of the tooth;
2= a thin continuous band of plaque (up to 1 mm) at the cervical margin of the tooth;
3= a band of plaque wider than 1 mm but covering less than one-third of the crown of the tooth;
4= plaque covering at least one-third but less than two-thirds of the crown of the tooth;
5= plaque covering two-thirds or more of the crown of the tooth.
Gingivitis was evaluated using the PBI and GI.
The PBI was based on bleeding following gentle probing of the interdental papilla:
0=no bleeding;
1=a single discrete bleeding point appears;
2=several isolated bleeding points on a small area of blood;
3=interdental triangle filled with blood;
4=profuse bleeding spreading toward the gingival margin.
The GI was designed to estimate different degrees of inflammation in the marginal gingiva. The severity of inflammatory change was scored from 0 to 3, as follows:
0=absence of inflammation;
1= mild inflammation; slight change in color and little change in texture;
2= moderate inflammation; moderate glazing, redness, edema, and hypertrophy; bleeding on pressure;
3= severe inflammation; marked redness and hypertrophy; tendency to spontaneous bleeding; ulceration.
Experimental design
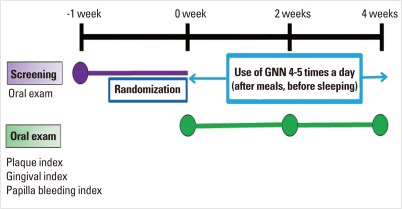
Before the experiment, the patients in both the test group and the control group received an oral prophylaxis and toothbrushing instruction. The clinical parameters (GI, PBI, and PI) were measured at baseline, 2 weeks and 4 weeks. During the experimental period, patients used the mouthrinse 4 to 5 times a day for 30 seconds with 10 mL. They used the mouthrinse within 30 minutes after toothbrushing. The experimental protocol is described in Fig. 1 and Table 1.
Figure 1.
Experimental protocol.
Table 1.
Experimental protocol.
Statistical analysis
The differences in clinical parameters among the values at baseline, 2 weeks and 4 weeks for each group were evaluated using the Wilcoxon signed rank test. The differences between the test group and control group were evaluated using the Mann-Whitney test. The value of α was 0.05. These analyses were conducted using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Discoloration of the teeth and tongue, taste alteration, mucosal irritation, hypersensitivity, discomfort during use, and a feeling of irritation did not appear in either the experimental or the control group.
PBI (Table 2)
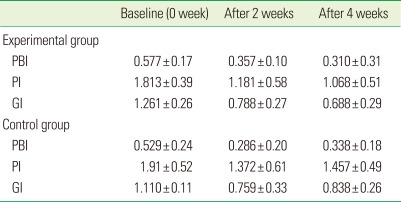
Table 2.
Mean values of the clinical parameters.
Values are presented as mean±SD.
PBI: papillary bleeding index, PI: Turesky-Quigley-Hein plaque index, GI: Löe-Silness gingival index.
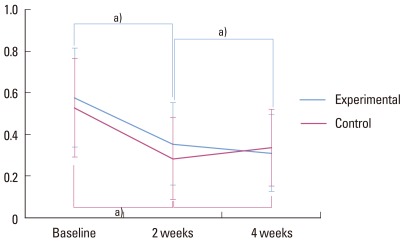
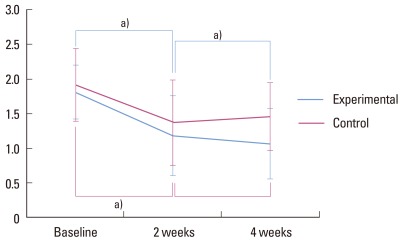
The mean PBI value was 0.577 in the experimental group and 0.529 in the control group, both at baseline. Statistical significances were detected between values at baseline and 2 weeks in both groups (P<0.05). Between 2 weeks and 4 weeks, changes in the PBI values displayed different patterns between the experimental and control groups. Statistically significant difference was found between values at 2 weeks and 4 weeks for the experimental group, as values were lower at 4 weeks (P<0.05). However, for the control group, no statistically significant difference was detected between values at 2 weeks and 4 weeks. The mean PBI value after 4 weeks for the control group was slightly higher than the mean PBI value after 2 weeks for the control group (Fig. 2).
Figure 2.
Papillary bleeding index. a)Statistically significant (P<0.05).
PI (Table 2)
The mean PI was 1.813 for the experimental group and 1.917 for the control group, both at baseline. Statistical significance was shown between values at baseline and 2 weeks in both groups (P<0.05). A statistically significant difference was found between the values at 2 weeks and 4 weeks for the experimental group, as values were lower at 4 weeks (P<0.05). After 4 weeks, the mean PI value in the control group was slightly higher than at 2 weeks, without any statistical significance (Fig. 3).
Figure 3.
Plaque index. a)Statistically significant (P<0.05).
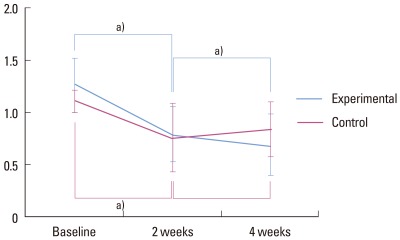
GI (Table 2)
The mean GI was 1.261 in the experimental group and 1.110 in the control group, both at baseline.
Statistical significance was observed between the values at baseline and 2 weeks for both groups (P<0.05). A statistically significant difference was found between the values at 2 weeks and 4 weeks for the experimental group, as values were lower at 4 weeks (P<0.05). However, in the control group, no statistically significant difference was detected between the values at 2 weeks and 4 weeks. Additionally, for the control group, slightly higher GI values were detected between 2 weeks and 4 weeks (Fig. 4).
Figure 4.
Gingival index. a)Statistically significant (P<0.05).
DISCUSSION
The present study demonstrated that mouthrinses containing CPC, triclosan, and DPZ produced significant reductions in supragingival plaque and gingivitis.
Previous studies have compared the efficacy of various mouthrinses using the Turesky-Quigley-Hein PI for evaluation of plaque accumulation and the Löe-Silness GI for gingivitis evaluation [20-23]. In other previous studies, PBI was an excellent parameter for the measurement of the severity of gingival inflammation [24]. Therefore, in this study, plaque accumulation was measured using the Turesky-Quigley-Hein PI while gingivitis was measured using the Löe-Silness and papilla bleeding indexes.
Statistical significance was detected between the values at baseline and 2 weeks for both groups with regard to PBI, PI and GI (P<0.05). It seems that both the experimental group and the control group displayed noticeable differences because the effects of scaling and polishing were sustained. However, between 2 weeks and 4 weeks, the change in the PBI, PI and GI values showed significant differences only in the experimental groups. This observation is in agreement with other previous studies. Zimmer et al. [25] reported that CPC containing mouthrinses decreased PBI scores over the course of 8 weeks. Mankodi et al. [26] and Allen et al. [27] reported in a 6-month clinical study that an increased PI score decreased when CPC-containing mouthrinses were as used, compared with results when placebo mouthrinses were used. Other authors have reported that the GI score decreased when CPC-containing mouthrinses were used. [27,28]
When changes in the experimental group and in the control group were compared, , the experimental group showed a continuous decrease while the control group showed a slight increase following an initial decrease until 2 weeks. Charles et al. [29] compared a CHX group, an essential oil group, and a control group. The authors found that the CHX and essential oil groups showed decreasing GI and PI scores, but the control group showed an initial decreasing trend before a subsequent increase of the scores. This indicates that scaling and polishing were effective only at the initial stage. Therefore, applying mouthrinses after such procedures can contribute to prevention of plaque accumulation and gingivitis.
In previous studies using CPC-containing mouthrinse, there was not any significant difference between the group using CPC mouthrinse and a control group [25-29]. In contrast, within the present study, clinical indexes in the group using a mouthrinse containing a combination of CPC, triclosan, and DPZ was significantly different from the control group. This may not serve as evidence of synergistic effects in these 3 components, but we can assume that two other antimicrobial actions and an anti-inflammatory effect would maximize the contribution for periodontal health. In order to more comparatively evaluate efficacy between a group using a CPC-only mouthrinse and another group using a mouthrinse containing a combination of all 3 components, a more precise experimental design will be needed. In a longer period of trial, since anti-inflammatory reaction may bring reduced probing depth and gained attachment level, these factors should also be considered in the future study.
Nevertheless, in this study, mouthrinses containing CPC, triclosan, and DPZ showed positive effect on clinical indexes with statistical significance. However, to draw more definite conclusions regarding the efficacy of mouthrinses containing all 3 agents, future studies should include longer observation periods at least 6 months and larger sample sizes, as well as a microbiological focus.
Footnotes
The clinical studies were supported through funding from the Sunstar Inc. (Osaka, Japan).
References
- 1.Slots J, Rams TE. New views on periodontal microbiota in special patient categories. J Clin Periodontol. 1991;18:411–420. doi: 10.1111/j.1600-051x.1991.tb02309.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63(4 Suppl):322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 3.Quirynen M, Bollen CM, Vandekerckhove BN, Dekeyser C, Papaioannou W, Eyssen H. Full- vs. partial-mouth disinfection in the treatment of periodontal infections: short-term clinical and microbiological observations. J Dent Res. 1995;74:1459–1467. doi: 10.1177/00220345950740080501. [DOI] [PubMed] [Google Scholar]
- 4.Becker W, Becker BE, Berg LE. Periodontal treatment without maintenance. A retrospective study in 44 patients. J Periodontol. 1984;55:505–509. doi: 10.1902/jop.1984.55.9.505. [DOI] [PubMed] [Google Scholar]
- 5.Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD. Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol. 1984;11:448–458. doi: 10.1111/j.1600-051x.1984.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 6.Escribano M, Herrera D, Morante S, Teughels W, Quirynen M, Sanz M. Efficacy of a low-concentration chlorhexidine mouth rinse in non-compliant periodontitis patients attending a supportive periodontal care programme: a randomized clinical trial. J Clin Periodontol. 2010;37:266–275. doi: 10.1111/j.1600-051X.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- 7.DePaola LG, Minah GE, Overholser CD, Meiller TF, Charles CH, Harper DS, et al. Effect of an antiseptic mouthrinse on salivary microbiota. Am J Dent. 1996;9:93–95. [PubMed] [Google Scholar]
- 8.Herrera D, Santos S, Ferrus J, Barbieri G, Trombelli L, Sanz M. Efficacy of a 0.15% benzydamine hydrochloride and 0.05% cetylpyridinium chloride mouth rinse on 4-day de novo plaque formation. J Clin Periodontol. 2005;32:595–603. doi: 10.1111/j.1600-051X.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 9.Quirynen M, Soers C, Desnyder M, Dekeyser C, Pauwels M, van Steenberghe D. A 0.05% cetyl pyridinium chloride/0.05% chlorhexidine mouth rinse during maintenance phase after initial periodontal therapy. J Clin Periodontol. 2005;32:390–400. doi: 10.1111/j.1600-051X.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 10.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15:488–498. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen MG, Slots J. Antimicrobials in periodontal maintenance. J Dent Hyg. 2001;75:233–239. [PubMed] [Google Scholar]
- 12.Wu CD, Savitt ED. Evaluation of the safety and efficacy of over-the-counter oral hygiene products for the reduction and control of plaque and gingivitis. Periodontol 2000. 2002;28:91–105. doi: 10.1034/j.1600-0757.2002.280105.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang NP, Catalanotto FA, Knopfli RU, Antczak AA. Quality-specific taste impairment following the application of chlorhexidine digluconate mouthrinses. J Clin Periodontol. 1988;15:43–48. doi: 10.1111/j.1600-051x.1988.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 14.Pitten FA, Kramer A. Efficacy of cetylpyridinium chloride used as oropharyngeal antiseptic. Arzneimittelforschung. 2001;51:588–595. doi: 10.1055/s-0031-1300084. [DOI] [PubMed] [Google Scholar]
- 15.Silva MF, dos Santos NB, Stewart B, DeVizio W, Proskin HM. A clinical investigation of the efficacy of a commercial mouthrinse containing 0.05% cetylpyridinium chloride to control established dental plaque and gingivitis. J Clin Dent. 2009;20:55–61. [PubMed] [Google Scholar]
- 16.Gaffar A, Afflitto J, Nabi N, Herles S, Kruger I, Olsen S. Recent advances in plaque, gingivitis, tartar and caries prevention technology. Int Dent J. 1994;44(1 Suppl 1):63–70. [PubMed] [Google Scholar]
- 17.Wu X, Zhang T, Zhang Y. Effect of a new triclosan-containing mouth rinse on oral infection. Zhonghua Kou Qiang Yi Xue Za Zhi. 2001;36:301–303. [PubMed] [Google Scholar]
- 18.Zuckerbraun HL, Babich H, May R, Sinensky MC. Triclosan: cytotoxicity, mode of action, and induction of apoptosis in human gingival cells in vitro. Eur J Oral Sci. 1998;106(2 Pt 1):628–636. doi: 10.1046/j.0909-8836.1998.eos106204.x. [DOI] [PubMed] [Google Scholar]
- 19.Masatake T, Hidekazu K, Yasuhiko A, Toshiyuki T, Akira S, Toshio H, et al. The clinical evaluation of mouth rinse for periodontal disease. Jpn J Conserv Dent. 2000;43:703–711. [Google Scholar]
- 20.Fischman SL. Clinical index systems used to assess the efficacy of mouthrinses on plaque and gingivitis. J Clin Periodontol. 1988;15:506–510. doi: 10.1111/j.1600-051x.1988.tb01022.x. [DOI] [PubMed] [Google Scholar]
- 21.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 22.Löe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38(Suppl):610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 23.Axelsson P, Lindhe J. Efficacy of mouthrinses in inhibiting dental plaque and gingivitis in man. J Clin Periodontol. 1987;14:205–212. doi: 10.1111/j.1600-051x.1987.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 24.Engelberger T, Hefti A, Kallenberger A, Rateitschak KH. Correlations among Papilla Bleeding Index, other clinical indices and histologically determined inflammation of gingival papilla. J Clin Periodontol. 1983;10:579–589. doi: 10.1111/j.1600-051x.1983.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 25.Zimmer S, Kolbe C, Kaiser G, Krage T, Ommerborn M, Barthel C. Clinical efficacy of flossing versus use of antimicrobial rinses. J Periodontol. 2006;77:1380–1385. doi: 10.1902/jop.2006.050362. [DOI] [PubMed] [Google Scholar]
- 26.Mankodi S, Bauroth K, Witt JJ, Bsoul S, He T, Gibb R, et al. A 6-month clinical trial to study the effects of a cetylpyridinium chloride mouthrinse on gingivitis and plaque. Am J Dent. 2005;18 Spec No:9A–14A. [PubMed] [Google Scholar]
- 27.Allen DR, Davies R, Bradshaw B, Ellwood R, Simone AJ, Robinson R, et al. Efficacy of a mouthrinse containing 0.05% cetylpyridinium chloride for the control of plaque and gingivitis: a 6-month clinical study in adults. Compend Contin Educ Dent. 1998;19(2 Suppl):20–26. [PubMed] [Google Scholar]
- 28.Nelson RF, Rodasti PC, Tichnor A, Lio YL. Comparative study of four over-the-counter mouthrinses claiming antiplaque and/or antigingivitis benefits. Clin Prev Dent. 1991;13:30–33. [PubMed] [Google Scholar]
- 29.Charles CH, Mostler KM, Bartels LL, Mankodi SM. Comparative antiplaque and antigingivitis effectiveness of a chlorhexidine and an essential oil mouthrinse: 6-month clinical trial. J Clin Periodontol. 2004;31:878–884. doi: 10.1111/j.1600-051X.2004.00578.x. [DOI] [PubMed] [Google Scholar]