Abstract
Objectives:
To evaluate the comparative cost efficiency across the European Union G5 countries of the erythropoiesis-stimulating agents (ESAs) epoetin α (originator [Eprex®] and biosimilar [Binocrit®]; once weekly), epoetin β (NeoRecormon®; once weekly), and darbepoetin α (Aranesp®; once weekly or once every 3 weeks) under different scenarios of fixed and weight-based dosing in the management of chemotherapy-induced anemia.
Methods:
Direct costs of ESA treatment were calculated for one patient with cancer undergoing chemotherapy (six cycles at 3-week intervals) with ESA initiated at week 4 and continued for 15 weeks. Five scenarios were developed under fixed and weight-based dosing: continuous standard dose for 15 weeks; sustained dose escalation to 1.5× or double the standard dose at week 7, continued for 12 weeks; and discontinued dose escalation to 1.5× or double the standard dose at week 7 for a 3-week period, then 9 weeks of standard dose.
Results:
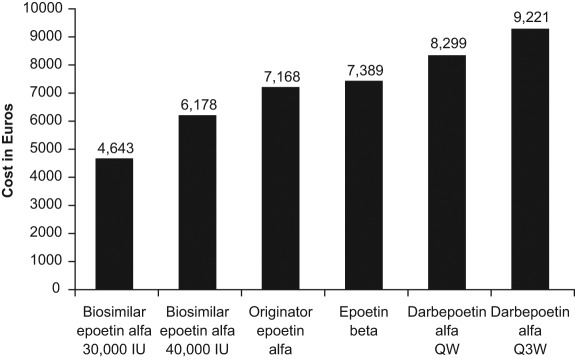
Under fixed dosing, the average cost of biosimilar epoetin α treatment across scenarios was €4643 (30,000 IU) or €6178 (40,000 IU). Corresponding estimates were €7168 for originator epoetin α, €7389 for epoetin β, €8299 for darbepoetin α once weekly, and €9221 for darbepoetin α once every 3 weeks. Under weight-based dosing, the average cost of biosimilar epoetin α treatment across scenarios was €4726. Corresponding estimates were €5484 for originator epoetin α, €5652 for epoetin β, and €8465 for both darbepoetin α once weekly and once every three weeks.
Conclusion:
Managing chemotherapy-induced anemia with biosimilar epoetin α is consistently cost efficient over treatment with originator epoetin α, epoetin β, and darbepoetin α under both fixed and weight-based dosing scenarios.
Keywords: anemia, biosimilars, cost efficiency, cost savings, erythropoiesis-stimulating agents, erythropoietin
Introduction
The European Cancer Anaemia Survey (ECAS) highlighted the high prevalence and incidence of anemia among patients with cancer in Europe [Ludwig et al. 2004]. Among more than 15,000 patients evaluated, the prevalence of anemia (hemoglobin [Hb] <12 g/dl) at enrolment was 39%; 67% of patients had a Hb level less than 12 g/dl recorded at least once during the survey. The incidence of anemia was 54% overall and 63% among patients who received chemotherapy [Ludwig et al. 2004].
Treatment options for anemia include blood transfusions, iron supplementation (for patients with absolute or functional iron deficiency), and erythropoiesis-stimulating agents (ESAs) [Aapro and Link, 2008]. Blood transfusions are often recommended for use only in patients with a Hb level less than 9 g/dl [Aapro and Link, 2008; Bokemeyer et al. 2007]. In addition, there are a number of issues around blood transfusions [Aapro and Link, 2008; Upile et al. 2008]. These include concerns about continued availability of blood due to falling donor numbers, the risk of transfusion-transmitted diseases, the increasingly rigorous screening of donors and blood to reduce the risk of disease transmission, the risk of tumor growth promotion and worsening of prognosis, and the real cost of the product and its administration. Despite these concerns, transfusions are still frequently used in many countries while in others there has been an increased use of ESAs [Ludwig et al. 2009].
Use of ESAs provides more sustained anemia correction compared with transfusions, and is more convenient for patients [Bokemeyer et al. 2007]. Use of ESAs in congruence with European Organisation for Research and Treatment of Cancer (EORTC) guidelines leads to improved results in terms of Hb outcomes [Aapro et al. 2009]. Several ESA products are available in Europe for the treatment of chemotherapy-induced anemia in patients with cancer. These include epoetin α (Eprex®, Janssen-Cilag, Neuss, Germany), epoetin β (NeoRecormon®, F. Hoffman-La Roche, Basel, Switzerland), epoetin θ (Eporatio®, Teva, Petach Tikva, Israel), and darbepoetin α (Aranesp®, Amgen Europe, Breda, The Netherlands). Clinical practice guidelines consider all ESAs to be equivalent in relation to effectiveness and safety [Schrijvers et al. 2010]. A biosimilar epoetin α was approved by the European Medicines Agency (EMA) in 2007, and is marketed as Binocrit® (Sandoz, Holzkirchen, Germany), Epoetin α Hexal® (Hexal, Holzkirchen, Germany) and Abseamed® (Medicie Arzneimittel, Iserlohn, Germany) at a lower price than the originator products. Another biosimilar ESA has also been approved by the EMA (epoetin ζ), and is marketed as Retacrit® (Hospira, Royal Leamington Spa, UK) and Silapo® (STADA, Bad Vilbel, Germany).
The recommended initial fixed doses are 40,000 IU/week for originator epoetin α and biosimilar epoetin α, 30,000 IU/week for epoetin β, and 150 µg for darbepoetin α once weekly or 500 µg if given once every 3 weeks. Weight-based dosing typically assumes 450 IU/kg/week for originator epoetin α, biosimilar epoetin α and epoetin β, 2.25 µg/kg for darbepoetin α once weekly, and 6.75 µg/kg for darbepoetin α every three weeks. If Hb has not increased by at least 1 g/dl after 4 weeks, according to the product information the dose of originator epoetin α, biosimilar epoetin α and epoetin β may be escalated up to 200% of the standard dose. Treatment should be discontinued if, after 8 weeks, Hb has not increased by at least 1 g/dl. For darbepoetin α, if the clinical response of the patient (fatigue, Hb response) is inadequate after 9 weeks, further therapy may not be effective.
Biosimilar, or similar biological medicinal product, is a regulatory term used to define medicines that are similar to a biological medicine that has already been approved and whose patent has expired [Roger, 2010]. To obtain EMA approval, a biosimilar has to demonstrate comparability to the reference product in terms of quality, safety, and efficacy. Biosimilar epoetin α (Binocrit®) has comparable efficacy and safety to its reference product (Eprex®) [Weigang-Köhler et al. 2009; Haag-Weber et al. 2009], and has also been shown to be effective in real-life clinical practice. Initial treatment with biosimilar epoetin α 30,000 IU or 40,000 IU per week has been shown to produce a similar Hb response [Kerkhofs et al. 2012], and the overall response rate for biosimilar epoetin α in this study was similar to that observed for originator ESAs in an earlier study [Ludwig et al. 2009].
Despite concerns about increased risk of thrombovascular events, and regulatory warnings about possible ESA-induced tumor progression and mortality, available data suggest that ESAs are effective when used for labeled indications in patients with cancer, with a favorable risk–benefit profile [Aapro and Spivak, 2009]. Meta-analyses also indicate that ESAs have a neutral effect on overall survival when used in accordance with approved indications (i.e. patients with cancer who are receiving chemotherapy) [Glaspy et al. 2010; Tonelli et al. 2009].
Comparative cost efficiency (or budget impact) studies assess the actual cost of treatment that buyers or payers would incur and are considered independent of outcomes (unlike cost-effectiveness studies, which evaluate cost relative to differential outcomes). For instance, biosimilar filgrastim (Zarzio®, Sandoz) has been shown to be the most cost-efficient approach to prevent or treat chemotherapy-induced febrile neutropenia under various treatment scenarios from 1 to 14 days relative to the originator filgrastim (Neupogen®, Amgen) and its pegylated formulation (Neulasta®, Amgen) [Aapro et al. 2011]. Given the equivalence of originator and biosimilar ESAs in terms of outcomes, actual cost is the purchase differentiator.
There is a real choice available to physicians and pharmacists when selecting different ESA products. As previously indicated, there appears to be no significant difference in the effectiveness and safety of the different agents in managing chemotherapy-induced anemia [Schrijvers et al. 2010]. Each agent can also be used in fixed and weight-based dosing scenarios. To enable a clinically relevant comparison, this study compares the cost of each ESA in a range of different fixed and weight-based dosing schedules. Physicians and pharmacists can then select the appropriate comparisons to match their own clinical practice to estimate the comparative cost efficiency (or budget impact) of switching between different originator and biosimilar ESAs or dosing regimens. The study planned to evaluate the cost efficiency of different ESA preparations. We modeled ESA use in the five largest countries by population (Germany, France, UK, Italy, Spain), which together make up more than half the EU population (316 million out of 501 million) [Eurostat European Statistics, 2011]. Specifically, the study compared the population-weighted direct costs of managing anemia with originator epoetin α, epoetin β, darbepoetin α, and biosimilar epoetin α in one patient with cancer undergoing a chemotherapy regimen of six cycles at 3-week intervals (18 weeks) and with ESA initiation at week 4 and continued for a total of 15 weeks. Five scenarios were developed under both fixed and weight-based dosing: continuous standard dose of each ESA for 15 weeks; sustained dose escalation of each ESA to 1.5 times or double the standard dose at week 7 and continued for 12 weeks; and discontinued dose escalation to 1.5 times or double the standard dose of each ESA at week 7 for a 3-week period, then 9 weeks of standard dose. The second objective was to determine the relative cost savings of treatment with biosimilar epoetin α over the originator ESAs.
Methods
Cost model
Cost was defined as the direct costs incurred by a buyer or payer for buying or reimbursing any of the four agents to treat anemia in one patient with cancer undergoing a chemotherapy regimen of six cycles at 3-week intervals (18 weeks). Consistent with the cost-efficiency analysis for granulocyte colony-stimulating factor [Aapro et al. 2011], indirect costs were not considered as the focus was on the actual cost and associated budget impact of a purchasing or reimbursement decision: the incurred cost of delivering goods as subtracted from total revenue in the calculation of gross margin prior to consideration of administrative and selling costs. The public pack price (in euros) was used for all but the UK, for which the negotiated NHS price was used (in British pounds converted to euros at exchange rate of 1.13499).
Model assumptions
First, treatment was defined as a chemotherapy regimen of six cycles at 3-week intervals, for a total 18 weeks. A cycle was defined as the 3-week period starting with the week in which chemotherapy was administered plus the ensuing 2 weeks. Second, weekly ESA treatment was assumed to have been initiated at the start of the second chemotherapy cycle (week 4) and continued over each subsequent cycle, including the 2 weeks following the last chemotherapy cycle. Thus ESA treatment was assumed to last for a total of 15 weeks. Third, to fully capture physician prescribing behaviors, both fixed and weight-based dosing was considered. Fixed ESA dosing was set at 40,000 IU per week for originator epoetin α, 30,000 and 40,000 IU per week for biosimilar epoetin α, 30,000 IU per week for epoetin β, and either 150 μg once weekly or 500 μg once every 3 weeks for darbepoetin α; these were selected on the basis of recommended initial doses for the various agents, and taking account of commonly used and effective doses as reported in the literature [Kerkhofs et al. 2011; Ludwig et al. 2009]. For weight-based dosing, we used the average weight of 68 kg reported for patients in the Anaemia Cancer Treatment (ACT) study, all of whom had anemia and were treated with ESAs [Ludwig et al. 2009]. For originator epoetin α, epoetin β, and biosimilar epoetin α, a posology of 450 IU/kg/week was used for a weekly dose of 30,600 IU. Posologies for darbepoetin α were 2.25 μg/kg/week for the once weekly and 6.75 μg/kg/week for the once every 3 week formulations, for doses of 153 μg and 459 μg for the two regimens, respectively. Fourth, five scenarios were applied to both the fixed and weight-based dosing schemes. The continuous standard dose (CSD) scenario assumed that the standard dose of each ESA was administered for 15 weeks. Two sustained dose escalation (SDE) scenarios specified a dose escalation to 1.5 times or double the standard dose at week 7 of the chemotherapy regimen (start of cycle 3) and continued for the remaining 12 weeks. Under the discontinued dose escalation (DDE) scenario, ESA treatment was escalated to 1.5 times or double the standard dose at week 7 for a 3-week period, followed by a return to standard dose ESA treatment for the remaining 9 weeks. Fifth, we assumed all ESA agents to be equally effective in managing chemotherapy-induced anemia. Lastly, population estimates were as per 1 January 2011; that is, capturing the population at the close of 2010. Hence, 2010 prices were used in the analysis.
Analyses
Table 1 summarizes the calculations to derive the cost estimates for the different agents. The following steps were taken:
Table 1.
Calculations used to derive the cost estimates for the different erythropoiesis-stimulating agents.
| Public pack price |
NHS price |
|||||
|---|---|---|---|---|---|---|
| Germany | France | Italy | Spain | UK | ||
| Price parity conversion (€/1000 IU) | ||||||
| Originator epoetin α 40,000 IU | 9.39 | 7.90 | 11.39 | 10.01 | 7.53 | |
| Epoetin β 30,000 IU | 9.42 | 8.19 | 12.42 | 9.55 | 7.96 | |
| Darbepoetin α 150 µg | 13.56 | 8.04 | 12.66 | 9.60 | 8.33 | |
| Biosimilar epoetin α 40,000 IU | 9.27 | 6.82 | 9.68 | 7.67 | 5.78 | |
| Germany | France | Italy | Spain | UK | G5 median | |
| Weekly price (€/1000 IU equivalent dose) | ||||||
| Originator epoetin α | 375.60 | 316.00 | 455.60 | 400.40 | 301.20 | 375.60 |
| Epoetin β | 376.80 | 327.60 | 496.80 | 382.00 | 318.40 | 376.80 |
| Darbepoetin α | 542.40 | 321.60 | 506.40 | 384.00 | 333.20 | 384.00 |
| Biosimilar epoetin α | 370.80 | 272.80 | 387.20 | 306.80 | 231.20 | 306.80 |
| Germany | France | Italy | Spain | UK | Weighted G5 | |
| Weighted weekly price* (€/1000 IU equivalent dose) | ||||||
| Originator epoetin α | 97.16 | 65.07 | 87.40 | 58.47 | 59.50 | 367.60 |
| Epoetin β | 97.47 | 67.46 | 95.30 | 55.79 | 62.90 | 378.91 |
| Darbepoetin α | 140.30 | 66.22 | 97.14 | 56.08 | 65.83 | 425.57 |
| Biosimilar epoetin α | 95.92 | 56.17 | 74.28 | 44.80 | 45.67 | 316.84 |
Weighted price per country is calculated by multiplying price per 1000 IU equivalent dose and proportional population weight.
Price parity conversion
Not all the five largest EU countries share a common currency. To permit a comparison that allows for fluctuation between the euro and the British pound, we first converted all prices for the four products in each of the G5 countries to the base of ‘price per 1000 IU epoetin α’ for product and country. This conversion put the cost-of-treatment estimates for each agent on a price parity basis expressed in 1000 IU of epoetin α. Specifically, we used the public pack price (or NHS price for the UK) per 1000 IU epoetin α as the benchmark, assuming a standard regimen of 40,000 IU/week. Taking the public pack or NHS price for epoetin β and darbepoetin α and, under consideration of the recommended regimens for these agents to achieve therapeutic equivalence with epoetin α, we converted the purchasing price of these agents to the epoetin α base; that is, the cost of epoetin β 30,000 IU/week and darbepoetin α 150 μg expressed in epoetin α price per 1000 IU. This brought all agents considered onto the pricing platform in commercial use.
Price per week of treatment per 1000 IU equivalent dose in each G5 country
Using the parity prices, we calculated the weekly cost of treatment for each product in each of the five countries.
Population-weighted price per week of treatment per 1000 IU equivalent dose
To determine the weighted G5 price for 1 week of treatment with each product, we multiplied a product’s price in a given country by the proportional population weight for that country. Thus, the weighted G5 cost for 1 week of treatment with originator epoetin α was €367.60, €378.91 for epoetin β, €425.57 for darbepoetin α, and €316.84 for biosimilar epoetin α.
Results
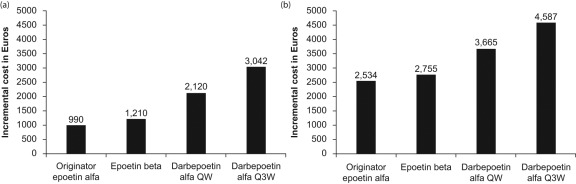
Table 2 presents the cost of 15 weeks of treatment and the percentage savings possible with use of 30,000 IU and 40,000 IU biosimilar epoetin α for each of the five scenarios under a fixed-dosing scheme. The average cost across scenarios for 15 weeks of treatment with biosimilar epoetin α was €4643 (30,000 IU) or €6178 (40,000 IU), compared with €7168 for originator epoetin α, €7389 for epoetin β, €8299 for darbepoetin α once weekly, and €9221 for darbepoetin α once every 3 weeks (Figure 1). The average incremental costs (across the five scenarios) over biosimilar epoetin α of fixed-dose treatment with the originator products are shown in Figure 2. The percentage savings achieved by treating patients with biosimilar epoetin α 40,000 IU were 13.8% over originator epoetin α, 16.4% over epoetin β, 25.5% over darbepoetin α once weekly, and 33.0% over darbepoetin α once every 3 weeks (Table 2). For biosimilar epoetin α 30,000 IU, the average savings were 35.4% over originator epoetin α, 37.3% over epoetin β, 44.2% over darbepoetin α once weekly, and 49.7% over darbepoetin α once every three weeks.
Table 2.
Cost of erythropoiesis-stimulating agent (ESA) treatment and percentage savings associated with use of biosimilar epoetin α: fixed-dosing scenarios.
| ESA cost (€) |
Relative savings with use of biosimilar epoetin α 40,000 IU (%) |
Relative savings with use of biosimilar epoetin α 30,000 IU (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | Originator epoetin α | Epoetin β | Darbepoetin α QW | Darbepoetin α Q3W | Biosimilar epoetin α 40,000 IU | Biosimilar epoetin α 30,000 IU | Originator epoetin α | Epoetin β | Darbepoetin α QW | Darbepoetin α Q3W | Originator epoetin α | Epoetin β | Darbepoetin α QW | Darbepoetin α Q3W |
| Continuous standard dose | 5514 | 5684 | 6384 | 7093 | 4753 | 3564 | 13.8 | 16.4 | 25.5 | 33.0 | 35.4 | 37.3 | 44.2 | 49.7 |
| Sustained dose escalation (1.5×) | 7720 | 7957 | 8937 | 9930 | 6654 | 4990 | 13.8 | 16.4 | 25.5 | 33.0 | 35.4 | 37.3 | 44.2 | 49.7 |
| Discontinued dose escalation (1.5×) | 6065 | 6252 | 7022 | 7802 | 5228 | 3921 | 13.8 | 16.4 | 25.5 | 33.0 | 35.4 | 37.3 | 44.2 | 49.7 |
| Sustained dose escalation (2.0×) | 9925 | 10,231 | 11,490 | 12,767 | 8555 | 6416 | 13.8 | 16.4 | 25.5 | 33.0 | 35.4 | 37.3 | 44.2 | 49.7 |
| Discontinued dose escalation (2.0×) | 6617 | 6820 | 7660 | 8511 | 5703 | 4277 | 13.8 | 16.4 | 25.5 | 33.0 | 35.4 | 37.3 | 44.2 | 49.7 |
| Average | 7168 | 7389 | 8299 | 9221 | 6178 | 4643 | 13.8 | 16.4 | 25.5 | 33.0 | 35.4 | 37.3 | 44.2 | 49.7 |
QW, once weekly; Q3W, once every 3 weeks.
Figure 1.
Average total cost of 15 weeks’ treatment with the different erythropoiesis-stimulating agents (ESAs) across five fixed-dosing scenarios. Costs are based on population-weighted price per week in the EU G5 countries. QW, once weekly; Q3W, once every 3 weeks.
Figure 2.
Average incremental cost of 15 weeks’ treatment with the different erythropoiesis-stimulating agents (ESAs) across five fixed-dosing scenarios. Costs are based on population-weighted price per week in the EU G5 countries: (A) relative to biosimilar epoetin α 40,000 IU; (B) relative to biosimilar epoetin α 30,000 IU. QW, once weekly; Q3W, once every 3 weeks.
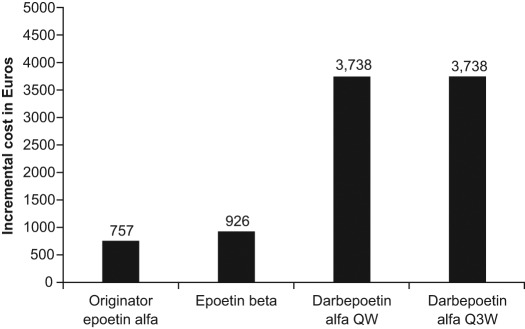
Table 3 details the results for the five scenarios when weight-based dosing was applied, in this case for a patient weighing 68 kg. The average cost for 15 weeks of treatment across the scenarios was €5484 for originator epoetin α, €5652 for epoetin β, and €8465 for darbepoetin α once weekly or once every 3 weeks compared with €4726 for biosimilar epoetin α. The average incremental costs (across the five scenarios) over biosimilar epoetin α of weight-based treatment with the originator products are shown in Figure 3. Using biosimilar epoetin α yielded savings of 13.8% over originator epoetin α, 16.4% over epoetin β, and 44.2% over darbepoetin α once weekly or once every 3 weeks.
Table 3.
Cost of erythropoiesis-stimulating agent (ESA) treatment and percentage savings associated with use of biosimilar epoetin α: weight-based-dosing scenarios.
| ESA cost (€) |
Relative savings with use of biosimilar epoetin α (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Scenario | Originator epoetin α | Epoetin β | Darbepoetin α QW | Darbepoetin α Q3W | Biosimilar epoetin α | Originator epoetin α | Epoetin β | Darbepoetin α QW | Darbepoetin α Q3W |
| Continuous standard dose | 4218 | 4348 | 6511 | 6511 | 3636 | 13.8 | 16.4 | 44.2 | 44.2 |
| Sustained dose escalation (1.5×) | 5905 | 6087 | 9116 | 9116 | 5090 | 13.8 | 16.4 | 44.2 | 44.2 |
| Discontinued dose escalation (1.5×) | 4640 | 4783 | 7162 | 7162 | 3999 | 13.8 | 16.4 | 44.2 | 44.2 |
| Sustained dose escalation (2.0×) | 7593 | 7826 | 11720 | 11720 | 6544 | 13.8 | 16.4 | 44.2 | 44.2 |
| Discontinued dose escalation (2.0×) | 5062 | 5218 | 7813 | 7813 | 4363 | 13.8 | 16.4 | 44.2 | 44.2 |
| Average | 5484 | 5652 | 8465 | 8465 | 4726 | 13.8 | 16.4 | 44.2 | 44.2 |
QW, once weekly; Q3W, once every 3 weeks.
Figure 3.
Average incremental cost (relative to biosimilar epoetin α) of 15 weeks’ treatment with the different erythropoiesis-stimulating agents (ESAs) across five weight-based dosing scenarios. Costs are based on population-weighted price per week in the EU G5 countries. QW, once weekly; Q3W, once every 3 weeks.
Discussion
The main conclusion from this comparative cost-efficiency analysis comparing biosimilar epoetin α with originator ESAs is that, for the EU G5, the cost of treating anemia in one patient with cancer during one line of chemotherapy (whether using fixed or weight-based dosing) is lowest for the biosimilar ESA, followed by originator epoetin α, epoetin β, darbepoetin α once weekly and finally darbepoetin α once every 3 weeks. With the efficacy and effectiveness of all four agents considered equivalent, biosimilar epoetin α provides favorable value (defined as quality over cost) in the management of chemotherapy-induced anemia. For fixed-dosing schedules, the average cost savings associated with use of biosimilar epoetin α 40,000 IU ranged from €990 (13.8%) to €3042 (33.0%), while for biosimilar epoetin α 30,000 IU the average savings ranged from €2534 (35.4%) to €4587 (49.7%). For weight-based dosing, the cost savings with the use of biosimilar epoetin α ranged from €757 (13.8%) to €3738 (44.2%). It is interesting to note that European prescribing information for darbepoetin α does not contain explicit advice on dose escalation, while the US prescribing information does. In the present analysis, biosimilar epoetin α remains cost efficient relative to darbepoetin α for both fixed and weight-based dosing even if only continuous standard dosing is considered.
Our model assumes that all the ESAs have similar efficacy and safety, which is justified on the basis of available data and recommendations in clinical practice guidelines [Schrijvers et al. 2010]. Dose requirements for biosimilar epoetin α are also similar to those for short- and long-acting originator ESAs, as indicated by a large study in patients (n = 1695) with anemia secondary to chronic kidney disease [Ode et al. 2011].
ESAs have two potentially useful actions in the treatment of chemotherapy-induced anemia: correcting anemia to improve quality of life and avoiding the need for transfusions [Aapro and Link, 2008]. The relative cost effectiveness of each management strategy for chemotherapy-induced anemia differs with each aim along with the different target Hb levels for treatment. The data presented in this paper help inform the choice between different types of ESA products. In choosing between a policy of transfusion or ESAs for managing chemotherapy-induced anemia, more complex analyses are needed [Littlewood et al. 2006]. One could argue that the cost of red blood cell transfusions should be entered into the cost-efficiency equations for managing chemotherapy-induced anemia. Using estimates from six studies covering five European countries, the population-weighted inflation-adjusted cost of two units of red blood cell transfusions in 2010 was estimated at €878 [Abraham and Sun, 2012]. If three transfusions of two units each were needed to correct anemia and maintain adequate Hb levels during a six-cycle chemotherapy regimen, matching the transfusion-related cost of €2634 would require significant discounting by ESA manufacturers. However, substitution of ESAs by transfusions is not the issue for the following reasons. First, red blood cell transfusion does transiently increase Hb levels, but it does not provide the short- to medium-term quality-of-life benefits that come with the erythropoietic stimulation achieved with ESAs [Jones et al. 2004; Kimel et al. 2008]. Second, transfusions are recommended to correct very low (<9 g/dl) Hb levels, but not for Hb titers above that level [Aapro and Link, 2008; Bokemeyer et al. 2007]. Third, transfusions come with their own safety, convenience and quality-of-life issues [Aapro and Link, 2008; Upile et al. 2008]. Fourth, the increase in Hb from transfusions is only transient. Hence it is not a matter of weighing the cost of transfusions to the cost of ESA treatment. Instead, analyses should focus on the cost of transfusions plus the cost of managing transfusion-related adverse events versus the cost of ESA treatment plus the cost of managing ESA-related adverse events. To our knowledge, such cost-effectiveness analysis has not yet been done. There are, however, data to indicate that for a comparable increase in quality of life, ESA treatment costs five to seven times less than transfusion [Cornes et al. 2007]. Finally, due to falling donor numbers and enhanced screening for pathogens, blood is a scarce resource [Aapro and Link, 2008; Stramer et al. 2009]; indeed, the EU has issued a number of directives on strategies to conserve blood supplies. Moreover, older blood (blood stored for >28 days) has reduced red cell function and viability following transfusion, and subsequently reduced O2-carrying potential [D’Almeida et al. 2000; Koch et al. 2008]. In summary, there are various clinical, pharmacological, efficacy, safety, as well as policy and economic elements to consider in the decisions of if and when to prescribe red blood cell transfusions.
As indicated in the ACT study, many physicians do not intervene to correct anemia until Hb levels have fallen below 9 g/dl [Ludwig et al. 2009]. Possible explanations for this include late diagnosis, concerns about the impact of ESAs on survival leading to a more conservative approach to anemia management in this population, or cost of ESA use. Biosimilar ESAs such as biosimilar epoetin α have been developed specifically to lower costs of treatment with difficult-to-make, expensive biological agents. Use of biosimilar ESAs can provide savings in hospital budgets, expand access to ESA treatment, and provide physicians with the latitude to treat anemia earlier (in accordance with current labels and guidelines). Consequently, more patients would benefit from improved quality of life and scarce blood resources would be conserved.
Supplementing ESA therapy with intravenous iron has been shown to increase hemoglobin response rates compared with ESA alone [Auerbach et al. 2004, 2010; Bastit et al. 2008], to levels similar to those observed for transfusions. This has also been recently demonstrated for biosimilar epoetin α [Kerkhofs et al. 2012]. In this audit of patients with solid tumors and chemotherapy-induced anemia, the hemoglobin response rate was 77% among patients treated with biosimilar epoetin α alone, and 93% among those who received supplemental intravenous iron. Other data indicate that addition of intravenous iron to ESA therapy increases Hb response and decreases ESA dose requirement in patients with cancer and anemia [Hedenus et al. 2007]. While the full impact of adding intravenous iron to ESA therapy is outside the scope of the current cost model, it is likely that a greater response rate could be achieved with a lower ESA dose, further reducing the cost of ESA treatment across the therapeutic class [Szucs et al. 2011].
Immunogenicity is a particular safety concern with all biopharmaceuticals, including biosimilars. As of December 2011, the estimated exposure to biosimilar epoetin α was 122,000 patient-years (across both oncology and nephrology indications) [Sandoz, data on file], and no cases of antibodies or other unexpected safety concerns have been reported with the commercially available product. Similarly, no cases of immunogenicity have been reported in patients with cancer receiving any ESA for chemotherapy-induced anemia [Macdougall et al. 2012].
The relative cost efficiency of biosimilar epoetin α is important in itself at the microeconomic level of purchasing and reimbursement decisions for a defined patient panel. The macroeconomic benefits should be considered as well, especially if the savings achieved with biosimilar epoetin α treatment can be reallocated to provide other patients within a panel with treatments for which otherwise no funds would be available. Consider, for instance, how savings from supportive cancer care with biosimilar epoetin α at a fixed dose of 40,000 IU/week might translate into greater patient access to primary cancer care with such life-saving agents as rituximab (Mabthera®, Roche) and trastuzumab (Herceptin®, Roche). The weighted EU G5 median cost of treatment for diffuse large B-cell non-Hodgkins lymphoma with rituximab is €11,320. Treating human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer with trastuzumab is estimated to cost €29,784. If the estimated 94,587 patients with cancer in the G5 countries treated with an ESA other than biosimilar epoetin α were converted to the latter, savings between €110,605,428 and 204,688,976 could be achieved. Using the lower conservative estimate, an additional 9771 rituximab treatments would become available in the EU G5, meeting the needs of 65.1% of patients with the above type of lymphoma. Similarly, an additional 3714 trastuzumab treatments would meet the need of 40.3% of the EU G5 population of patients with HER2-positive metastasized breast cancer (data not reported, but available from the corresponding author). The savings created in supportive cancer care would indeed facilitate substantially improved patient access to primary cancer treatment.
A potential limitation of our analysis is the use of weighted average unit costs based on public pack prices (and the NHS negotiated price for the UK) rather than actual product costs (which can vary within and between countries, and will change over time in response to national and international market forces). Use of weighted average unit costs has the benefit of smoothing any cost differentials between the countries and provides a generalized estimate of ESA costs for the five countries included in the study [Aapro et al. 2011]. Cost-efficiency analyses for purchasing and reimbursement decisions in individual markets should use the unit dose costs for that market. In addition, our model does not take into account discounts that often apply in hospital- or tender-driven markets; often in these settings the price differential between a biosimilar and originator is greater than indicated by the list price, and the cost savings possible through the use of biosimilar ESA are likely to be higher than our analysis indicates.
Conclusion
This analysis shows that, based on weighted EU G5 cost estimates, the budget impact of managing chemotherapy-induced anemia with biosimilar epoetin α is consistently cost efficient over treatment with originator epoetin α, epoetin β, and darbepoetin α under both fixed and weight-based dosing and across various scenarios of standard and escalated dose regimens. Our analysis demonstrates that, relative to originator ESAs, biosimilar epoetin α is the most cost-efficient ESA to correct anemia and maintain adequate hemoglobin levels in patients with cancer undergoing chemotherapy.
Acknowledgments
Medical writing support was provided by Tony Reardon of Spirit Medical Communications Ltd and funded by the sponsor. The services provided by I. Abraham and D. Sun were part of the contract for scientific services made by the sponsor to Matrix45.
Footnotes
This study was sponsored by an unrestricted grant from Sandoz Biopharmaceuticals (Sandoz International GmbH).
M. Aapro and P. Cornes have served as consultants to the sponsor. I. Abraham and D. Sun are employees of Matrix45. By company policy, employees are prohibited from owning equity in client organizations (except through mutual funds or other independently administered collective investment instruments) or contracting independently with client organizations. Matrix45 provides similar services to those described in this article to other biopharmaceutical companies on a non-exclusivity basis.
References
- Aapro M., Cornes P., Abraham I. (2011) Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegylated filgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract 24 May [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Aapro M.S., Link H. (2008) September 2007 update on EORTC guidelines and anaemia management with erythropoiesis-stimulating agents. Oncologist 13(Suppl. 3): 33–36 [DOI] [PubMed] [Google Scholar]
- Aapro M., Spivak J.L. (2009) Update on erythropoiesis-stimulating agents and clinical trials in oncology. Oncologist 14(Suppl. 1): 6–15 [DOI] [PubMed] [Google Scholar]
- Aapro M., Van Erps J., MacDonald K., Soubeyran P., Muenzberg M., Turner M., et al. (2009) Managing cancer-related anaemia in congruence with the EORTC guidelines is an independent predictor of haemoglobin outcome: initial evidence from the RESPOND study. Eur J Cancer 45: 8–11 [DOI] [PubMed] [Google Scholar]
- Abraham I., Sun D. (2012) The cost of blood transfusion in Europe an estimate from six studies. Transfusion. DOI: 10.1111/j.1537-2995.2011.03532.x 10.1111/j.1537-2995.2011.03532.x [DOI] [PubMed] [Google Scholar]
- Auerbach M., Ballard H., Trout J.R., McIlwain M., Ackerman A., Bahrain H., et al. (2004) Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol 22: 1301–1307 [DOI] [PubMed] [Google Scholar]
- Auerbach M., Silberstein P.T., Webb R.T., Averyanove A., Ciuleanu T.E., Shao J., et al. (2010) Darbepoetin alfa 300 or 500 µg once every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. Am J Hematol 85: 655–663 [DOI] [PubMed] [Google Scholar]
- Bastit L., Vandebroek A., Altintas S., Gaede B., Pintér T., Suto T.S., et al. (2008) Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J Clin Oncol 26: 1611–1618 [DOI] [PubMed] [Google Scholar]
- Bokemeyer C., Aapro M.S., Courdi A., Foubert J., Link H., Osterborg A., et al. (2007) EORTC guidelines for the use of erythropoietic proteins in anaemic patients with cancer: 2006 update. Eur J Cancer 43: 258–270 [DOI] [PubMed] [Google Scholar]
- Cornes P., Coiffier B., Zambrowski J.J. (2007) Erythropoietic therapy for the treatment of anemia in patients with cancer: a valuable clinical and economic option. Curr Med Res Opin 23: 357–368 [DOI] [PubMed] [Google Scholar]
- D’Almeida M.S., Jagger J., Duggan M., White M., Ellis C., Chin-Yee I.H. (2000) A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med 10: 291–303 [DOI] [PubMed] [Google Scholar]
- Eurostat European Statistics (2011) Available at: http://epp.eurostat.ec.europa.eu/tgm/table.do?tab=table&language=en&pcode=tps00001&tableSelection=1&footnotes=yes&labeling=labels&plugin=1 (accessed 20 November 2011).
- Glaspy J., Crawford J., Vansteenkiste J., Henry D., Rao S., Bowers P., et al. (2010) Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes. Br J Cancer 102: 301–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag-Weber M., Vetter A., Thyroff-Friesinger U. (2009) Therapeutic equivalence, long-term efficacy and safety of HX575 in the treatment of anemia in chronic renal failure patients receiving hemodialysis. Clin Nephrol 72: 380–390 [PubMed] [Google Scholar]
- Hedenus M., Birgegård G., Näsman P., Ahlberg L., Karlsson T., Lauri B., et al. (2007) Addition of intravenous iron to epoetin β increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 21: 627–632 [DOI] [PubMed] [Google Scholar]
- Jones M., Schenkel B., Just J., Fallowfield L. (2004) Epoetin alfa improves quality of life in patients with cancer: results of meta-analysis. Cancer 101: 1720–1732 [DOI] [PubMed] [Google Scholar]
- Kerkhofs L., Boschetti G., Lugini A., Stanculeanu D., Garcia Palomo A. (2012) Use of biosimilar epoetin to increase hemoglobin levels in patients with chemotherapy-induced anemia: real-life clinical practice. Future Oncol. DOI: 10.2217/FON.12.39 10.2217/FON.12.39 [DOI] [PubMed] [Google Scholar]
- Kimel M., Leidy N.K., Mannix S., Dixon J. (2008) Does epoetin alfa improve health-related quality of life in chronically ill patients with anemia? Summary of trials of cancer, HIV/AIDS, and chronic kidney disease. Value Health 11: 57–75 [DOI] [PubMed] [Google Scholar]
- Koch C.G., Li L., Sessler D.I., Figueroa P., Hoeltge G.A., Mihaljevic T., Blackstone E.H. (2008) Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 358: 1229–1239 [DOI] [PubMed] [Google Scholar]
- Littlewood T., Zambrowski J-J., Cornes P. (2006) Treating anaemia: deconstructing healthcare costs. Curr Med Res Opin 22(Suppl. 4): S23–S33 [Google Scholar]
- Ludwig H., Aapro M., Bokemeyer C., Macdonald K., Soubeyran P., Turner M., et al. (2009) Treatment patterns and outcomes in the management of anaemia in cancer patients in Europe: findings from the anaemia Cancer Treatment (ACT) study. Eur J Cancer 45: 1603–1615 [DOI] [PubMed] [Google Scholar]
- Ludwig H., Van Belle S., Barrett-Lee P., Birgegård G., Bokemeyer C., Gascón P., et al. (2004) The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur J Cancer 40: 2293–2306 [DOI] [PubMed] [Google Scholar]
- Macdougall I.C., Roger S.D., De Francisco A., Goldsmith D.J, Schellekens H., Ebbers H., et al. (2012) Antibody-mediated pure red cell aplasia in chronic kidney disease patients receiving erythropoiesis-stimulating agents: new insights. Kidney Int 81: 727–732 [DOI] [PubMed] [Google Scholar]
- Ode M., Roth K., Locatelli F., Hörl W. (2011) Switch from a broad range of erythropoiesis-stimulating agents to HX575 (biosimilar epoetin alfa): a 6-month prospective multicenter study. Poster presentation at the XLVIII ERA-EDTA Congress, Prague, Czech Republic, June 2011 [Google Scholar]
- Roger S.D. (2010) Biosimilars: current status and future directions. Expert Opin Biol Ther 10: 1011–1018 [DOI] [PubMed] [Google Scholar]
- Schrijvers D., De Samblanx H., Roila F; ESMO Guidelines Working Group (2010) Erythropoiesis-stimulating agents in the treatment of anaemia in cancer patients: ESMO Clinical Practice Guidelines for use. Ann Oncol 21(Suppl. 5): v244–v247 [DOI] [PubMed] [Google Scholar]
- Stramer S.L., Hollinger F.B., Katz L.M., Kleinman S., Metzel P.S., Gregory K.R., et al. (2009) Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion 49(Suppl. 2): 1S–29S [DOI] [PubMed] [Google Scholar]
- Szucs T.D., Blank P.R., Schwenkglenks M., Aapro M. (2011) Potential health economic impact of intravenous iron supplementation to erythropoiesis-stimulating agent treatment in patients with cancer- or chemotherapy-induced anaemia. Oncology 81: 45–49 [DOI] [PubMed] [Google Scholar]
- Tonelli M., Hemmelgarn B., Reiman T., Manns B., Reaume M.N., Lloyd A., et al. (2009) Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ 180: E62–E71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upile T., Jerjes W., Sandison A., Singh S, Rhys-Evans P., Sudhoff H., et al. (2008) The direct effects of stored blood products may worsen prognosis of cancer patients; shall we transfuse or not? An explanation of the adverse oncological consequences of blood product transfusion with a testable hypothesis driven experimental research protocol. Med Hypotheses 71: 498–492 [DOI] [PubMed] [Google Scholar]
- Weigang-Köhler K., Vetter A., Thyroff-Friesinger U. (2009) HX575, recombinant human epoetin alfa, for the treatment of chemotherapy-associated symptomatic anaemia in patients with solid tumours. Onkologie 32: 168–174 [DOI] [PubMed] [Google Scholar]