Abstract
B cells and antibodies constitute an important element in different inflammatory diseases of the central nervous system (CNS). Autoantibodies can serve as a biomarker to identify disease subgroups and may in addition contribute to the pathogenic process. One candidate autoantigen for multiple sclerosis (MS) is myelin oligodendrocyte glycoprotein (MOG). MOG is localized at the outermost surface of myelin in the CNS and has been the focus of extensive research for more than 30 years. Its role as an important autoantigen for T cells and as a target of demyelinating autoantibodies has been established in several variants of experimental autoimmune encephalomyelitis (EAE), an animal model of MS. The literature regarding antibodies to MOG in MS patients is confusing and contradictory. Recent studies, however, have described high levels of antibodies to conformationally correct MOG in pediatric acquired demyelination, both acute disseminated encephalomyelitis (ADEM) and MS. In adult MS, such antibodies are rarely found and then only at low levels. In this review, we summarize key findings from animal models and patient studies, discuss challenges in detecting anti-MOG antibodies in patients and present recent approaches to identifying new autoantigens in MS.
Keywords: Myelin oligodendrocyte glycoprotein, detection methods of autoantibodies, multiple sclerosis, ADEM
Introduction
Inflammatory demyelinating diseases of the central nervous system (CNS) comprise a broad spectrum of mechanistically heterogeneous disorders of which multiple sclerosis (MS) is the most common [Meinl et al. 2010]. There is strong evidence based on histopathological investigations and clinical response to plasma exchange [Keegan et al. 2005] that antibody-dependent mechanisms contribute to the pathogenesis in at least a subset of patients with CNS inflammation [Meinl et al. 2006]. The recently discovered aquaporin (AQP)4-specific autoantibodies allow the differentiation of neuromyelitis optica (NMO) from MS [Weinshenker et al. 2006]. It is tempting to speculate that certain subsets of MS patients can be identified based on their autoantibody profile. Myelin oligodendrocyte glycoprotein (MOG) is one of the best studied candidates, and is the focus of this review.
Historically: why is MOG interesting as a target?
The interest in MOG is based on animal experiments showing that MOG is a target of demyelinating antibodies. Initially, it was described that CNS homogenate contains a target of antibodies mediating complement-dependent demyelination in guinea pigs [Lebar et al. 1976, 1986]. A monoclonal antibody (mAb), 8-18C5 against rat cerebellar glycoproteins was raised in mice [Linnington et al. 1984] and used to define a glycoprotein in CNS myelin that was later named MOG [Linington and Lassmann, 1987]. With the help of this mAb, rat MOG cDNA was isolated and subsequently sequenced [Gardinier et al. 1992]. The sera of experimental autoimmune encephalomyelitis (EAE) guinea pigs were shown to have a demyelinating activity which was correlated to anti-MOG antibody titers [Linington and Lassmann, 1987].
Lessons from animal models
MOG can be involved in CNS autoimmunity in principally two different ways. First, MOG-specific T cells evoke CNS inflammation. Second, anti-MOG antibodies lead to the induction of demyelination. Demyelination is a characteristic feature of MS lesions indicated by the presence of naked demyelinated axons.
The development of encephalitogenic T cells and demyelinating antibodies upon immunization with MOG depends largely on the strain of mice or rats. In Dark Agouti (DA) rats, immunization with MOG in complete Freund’s adjuvant (CFA) results in a prominent T-cell response with only slight demyelination [Storch et al. 1998]. Brown Norway (BN) rats, on the other hand, develop a strong antibody response upon active immunization with MOG and incomplete Freund’s adjuvant (IFA) [Storch et al. 1998]. Immunizing Lewis rats with murine MOG and CFA causes a rather mild form of EAE, characterized by occasional focal demyelination with only small numbers of T cells in the CNS, suggesting that MOG is a poor encephalitogen in the Lewis rat [Adelmann et al. 1995]. It has been proposed that this difference is due to higher expression of MOG in the CNS of BN rats compared with Lewis rats and that this increase in protein expression is caused by polymorphisms in the non-coding region of the MOG gene [Pagany et al. 2003].
SJL/J mice, but not C57Bl/6 mice, are able to mount a pathogenic anti-MOG antibody response upon active immunization with recombinant rat MOG and IFA [Bourquin et al. 2003]. The inability of C57BL/6 mice to produce antibodies against rat MOG that recognize MOG on the cell surface seems to be caused by the MHC class II haplotype H-2b [Bourquin et al. 2003]. This animal model has unfolded interesting differences between human and rodent MOG. Immunizing with human MOG, instead of rat MOG, leads to B-cell dependent EAE in these mice [Marta et al. 2005]. Although anti-MOG antibodies are generated in both cases, only antibodies generated against human MOG, but not those generated against rat MOG, are pathogenic and cause EAE upon transfer to recipient mice. B-cell-independent EAE, in contrast, cannot be generated with human MOG in these mice, because the encephalitogenic T-cell response is dependent on a serine at position 42, where human MOG comprises a proline [Oliver et al. 2003]. Substituting proline with serine in human MOG allows B-cell-independent EAE induction with the human MOG mutant in C57Bl/6 mice [Marta et al. 2005].
Using anti-MOG-antibodies, a two-hit model for the induction of demyelination was developed. Encephalitogenic T cells initiate an inflammation and compromise the integrity of the blood–brain barrier. Anti-MOG antibodies are then able to access the CNS and mediate demyelination [Linington et al. 1988; Schluesener et al. 1987]. The two-hit model is also supported by experiments with mice carrying a MOG-specific transgenic B-cell receptor or T-cell receptor (TCR): C57Bl/6 mice with a knock-in of the H chain of the pathogenic MOG-specific mAb 8-18C5 were generated [Litzenburger et al. 1998]. These mice have high titers of functional autoreactive MOG-specific B cells and serum antibodies but the mice do not develop spontaneous EAE. Upon a slight challenge with encephalitogenic T cells, a fulminant demyelination develops [Litzenburger et al. 1998]. A spontaneous relapsing–remitting mouse has been developed [Pollinger et al. 2009]. These transgenic SJL/J mice carry a TCR specific for the MOG peptide 92-106 and develop EAE with a high frequency. The mice produce MOG-specific antibodies; depletion of B cells with an anti-CD20 mAb suppresses spontaneous EAE development. MOG-deficient TCR transgenic mice, in contrast, do not produce anti-MOG antibodies and do not develop relapsing–remitting EAE [Pollinger et al. 2009].
Anti-(AQP)-4 antibodies from NMO-patients have been shown to induce lesions in the CNS of mice and rats after opening of the blood–brain barrier by encephalitogenic T cells [Bennett et al. 2009; Bradl et al. 2009; Kinoshita et al. 2009]. When injected directly into the brain together with complement, rather than peripherally, these anti-(AQP)-4 antibodies are even able to induce lesions in mice lacking T cells [Saadoun et al. 2011].
Also in primates, such as common marmosets (Callithrix jacchus), MOG immunization induces demyelinating antibodies and encephalitogenic T cells [‘t Hart et al. 2004]. Immunization of C. jacchus with myelin basic protein (MBP) results in clinically mild EAE without demyelination. Subsequent passive transfer of IgG against whole white matter causes demyelination and clinical aggravation, this effect is also achieved by passive transfer of either polyclonal antirat MOG antibodies or the anti-MOG mAb 8-18C5 [Genain and Hauser, 1996]. Immunizing marmosets with either the extracellular domain of rat MOG or synthetic 20-mer peptides revealed that only antibodies generated against the protein, but not against the peptides, cause demyelination and severe EAE in marmosets [von Budingen et al. 2002].
Structure, abundance and localization of MOG
MOG is a quantitatively minor component of CNS myelin (less than 0.05% of all CNS myelin proteins) but its localization on the outermost surface of myelin [Brunner et al. 1989] makes it accessible for antibodies (Figure 1). Other more abundant myelin components, such as myelin basic protein, are inaccessible for antibodies. Rat MOG together with the Fab fragment of the mAb 8-18C5 [Breithaupt et al. 2003] and mouse MOG [Clements et al. 2003] have been crystallized. The demyelinating mAb 8-18C5 recognizes two residues (His103 and Ser 104) at the membrane-distal surface of MOG [Breithaup et al. 2008]. MOG features an IgV-like fold with a single glycosylation site (Asn-31). Similarities in the amino acid sequence and in the three-dimensional structure of MOG and the milk protein butyrophilin (BTN) put forward a role for BTN in the sensitization of T and B cells against MOG [Breithaupt et al. 2008; Guggenmos et al. 2004].
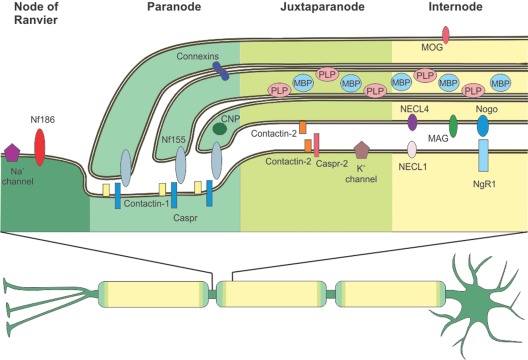
Figure 1.
Distribution of central nervous system (CNS) myelin proteins. A neuron with a myelinated axon is depicted. Myelin enwraps the axon at intervals called internodes omitting small openings termed nodes of Ranvier. Adjacent to the nodes of Ranvier are the paranode and the juxtaparanode. All four zones have a characteristic protein composition, as depicted in the upper part of the picture. At th nodes of Ranvier, Neurofascin 186 (NF186) supports the clustering of Na+ channels. To allow saltatory conduction, the nodal Na+ channels are separated from the juxtaparanodal K+ channels via the paranode, where the myelin protein Neurofascin 155 (NF155) binds tightly to the axonal complex of Contactin-1 and Contactin-associated protein (Caspr). Connexins form gap junctions between myelin layers at the paranode. 2’3’-cyclic-nucleotide 3’-phosphodiesterase (CNP) is an abundant cytoplasmic myelin protein, predominantly found at the paranode. At the juxtaparanode, clustered K+ channels are associated to Caspr-2 and Contactin-2 is found both at the innermost myelin-sheath and the axon, allowing it to bind to itself. Typical myelin proteins are found at the internode: Proteolipid protein (PLP) and Myelin basic protein (MBP) are the major myelin proteins, the quantitatively minor Myelin oligodendrocyte glycoprotein (MOG) is found at the outermost surface of the myelin sheath. Nectin-like (NECL) adhesion proteins and Myelin associated glycoprotein (MAG) are found at the periaxonal space. Nogo is also predominantly found at the adaxonal myelin membrane, its receptor Nogo-receptor 1 (NgR1) is found at the axonal membrane.
The exact function of MOG remains unclear but its structure and localization suggest a role as an adhesion molecule, possibly gluing CNS myelin fibers together [Clements et al. 2003]. MOG itself is also able to bind the complement component C1q and might therefore regulate the classical complement pathway [Johns and Bernard, 1997]. A recent study [Cong et al. 2011] showed that MOG might also function as a host cell receptor for the rubella virus. A MOG knockout mouse, however, showed no obvious phenotype [Delarasse et al. 2003]. Essential features of MOG are summarized in Table 1.
Table 1.
Important features of MOG.
| Size | 218 amino acids/27 kDa |
| Abundance and occurrence | Restricted to CNS, on the outermost surface of myelin sheaths and on oligodendrocytes |
| 0.05% of myelin proteins | |
| Membrane topology | Single span transmembrane protein: |
| – extracellular domain (amino acids 1–117) | |
| – one transmembrane domain | |
| – one C-terminal integral membrane domain [Della Gaspera et al. 1998] | |
| Function | Unknown. |
| No phenotype in knockout mouse [Delarasse et al. 2003]. Probably adhesion of myelin fibers [Clements et al. 2003] or regulator of classical complement pathway [Johns and Bernard, 1997]. Potential host cell receptor of the rubella virus [Cong et al. 2011]. | |
| Structure | IgV-like fold, single glycosylation site (Asn-31), one disulphide bond (Cys24–Cys98) [Breithaupt et al. 2003; Clements et al. 2003]. |
| Sequence conservation | More than 90% among mammals [Pham-Dinh et al. 1993]. |
| Possible pathogenic relevance | Target of encephalitogenic T cells. |
| Target of demyelinating Abs in many species including primates. |
MOG, myelin oligodendrocyte glycoprotein; CNS, central nervous system; IgV, immunoglobulin V; Abs, antibodies.
Anti-MOG antibodies in adult MS patients: different detection methods and contradictory results
Enzyme-linked immunosorbent assay
Table 2 gives an overview over important studies assaying anti-MOG antibodies in patients with CNS inflammation and controls with different techniques. The first studies of anti-MOG antibodies in MS patients employed enzyme-linked immunosorbent assay (ELISA) assays with MOG purified from human brain white matter to determine the anti-MOG antibody titer in patients’ sera and cerebrospinal fluid (CSF) [Xiao et al. 1991]. Using this approach, anti-MOG antibodies were detected in the CSF of a subset of MS patients, but also in the control groups. Similar results were obtained in a later study for which the extracellular domain of human MOG was expressed in Escherichia coli, without refolding of the antigen [Reindl et al. 1999]. Using native full-length mouse MOG from transfected mammalian cells, anti-MOG antibodies in both MS patients and healthy controls were detected and it was shown that the levels of IgM antibodies were higher in patients with a first demyelinating event, as compared with MS patients with a relapse or healthy donors [Gaertner et al. 2004]. The group also claimed to observe a correlation of anti-MOG IgG levels and disease activity, as those IgG antibodies were found to be higher in MS patients with a relapse or secondary progressive MS patients, as compared with healthy donors and MS patients in remission [Gaertner et al. 2004].
Table 2.
Methods and studies for anti-MOG antibody detection in patients with CNS inflammation and controls.
| Study | Technique | Antigen | Patient sample tested | Percentage of patients with demyelinating disorders declared anti-MOG positive | Percentage of controls declared anti-MOG positive |
|---|---|---|---|---|---|
| [Xiao et al. 1991] | ELISA | MOG from human white matter | Plasma and CSF from adult MS patients and controls | Plasma: 0% | Plasma: 0% |
| CSF: 23% | CSF: 3–7% | ||||
| [Lindert et al. 1999] | Western Blot | Human MOG expressed in E. coli | Plasma from adult MS patients and controls | 54% | 22% |
| [Reindl et al. 1999] | Western Blot, ELISA | Human MOG expressed in E. coli | Serum and CSF from adults MS patients and controls | Serum: 38% | Serum: 3–53% |
| CSF: 33% | CSF: 0–53% | ||||
| [Haase et al. 2001] | Cell-based assay (flow cytometry), peptide ELISA | Human MOG transfected LTK3- cells, Synthetic human MOG peptides | Purified anti-MOG antibodies from serum of adult MS patients and controls | ELISA: 100% | ELISA: 100% |
| Flow cytometry: 6% | Flow cytometry: 0% | ||||
| [Berger et al. 2003; Kuhle et al. 2007; Pelayo et al. 2007] | Western blot | Human MOG expressed in E. coli | Serum of adult CIS patients | 62% | No controls |
| [Gaertner et al. 2004] | ELISA | Mouse MOG transfected AG8 cells | Serum from adult MS patients and controls | N/S | N/S |
| [O’Connor et al. 2005] | ELISA (DELFIA), solution-phase RIA | Refolded human MOG, expressed in E. coli; in vitro translated human MOG | Purified IgG from MS lesions, serum and CSF from adult MS patients and controls | CNS tissue: 50% | CNS tissue: 0–25% |
| CSF: 5% | CSF: 0% | ||||
| Serum: 3% | Serum: 0% | ||||
| [Zhou et al. 2006] | Cell-based assay (flow cytometry, Immunocytochemistry) | Human MOG transfected LN18 cells | Serum of adult MS patients and controls | 22-41% | 4% |
| [Lalive et al. 2006] | Cell-based assay (flow cytometry), ELISA | Human MOG transfected CHO cells, human MOG expressed in E. coli | Serum of adult MS patients and controls | N/S | N/S |
| [O’Connor et al. 2007] | Tetramer radioimmunoassay, cell-based assay (flow cytometry), ELISA (DELFIA) | Tetramers of human MOG extracellular domain; Jurkat cells transfected with MOG-EGFP; human MOG expressed in E. coli, refolded | Serum and CSF of pediatric ADEM and MS patients and adult MS patients and controls | Serum and CSF: | Serum: 1% |
| ADEM: 19% | CSF: 6% | ||||
| MS: 0-8% | |||||
| CIS: 0% | |||||
| [Menge et al. 2007b] | ELISA, LiPhELIA | Human MOG, expressed in E. coli | Serum of adult MS patients and controls | LiPhELIA: 0% | LiPhELIA: 0% |
| [Wang et al. 2008] | ELISA | Human MOG, expressed in E. coli | Serum of MS patients before and after disease onset and controls | 52% | 43% |
| [McLaughlin et al. 2009] | Cell-based assay (Flow cytometry) | Human MOG-GFP transfected Jurkat cells | Serum and CSF pediatric MS and ADEM patients, serum of adult MS patients and controls | Pediatric: 21% | Pediatric: 0–6% |
| Adult: 4% | Adult: 0–5% | ||||
| [Brilot et al. 2009] | Cell-based assay (Flow cytometry) | Human MOG transfected LN18 cells | Serum and CSF of pediatric ADEM and CIS patients; Serum of adult MS patients and controls | ADEM and CIS children: 47% | Pediatric: 0–7% |
| Adult MS: 0% | |||||
| [Klawiter et al. 2010] | ELISA | Human MOG, expressed in insect cells | Serum and CSF of MS patients and controls | 37% (rMOG index >0.7) | 8% (rMOG index >0.7) |
| [Selter et al. 2010] | Cell-based assay (Flow cytometry) | Human MOG transfected LN18 cells | Serum of pediatric ADEM and CIS patients and controls | ADEM: 47% | 0% |
| CIS: 36% | |||||
| [Probstel et al. 2011] | Cell-based assay (Flow cytometry) | Human MOG, transfected TE671 cells | Serum of pediatric ADEM, CIS and MS patients, adult MS patients and controls | ADEM: 35% | 0% |
| CIS: 29% | |||||
| Pediatric MS: 15% | |||||
| Adult MS: 6% | |||||
| [Lalive et al. 2011] | Cell-based assay (flow cytometry), ELISA, LiPhELIA, | Human MOG transfected CHO cells, human MOG expressed in E. coli | Serum of pediatric ADEM, MS and viral encephalitis patients and controls | Flow cytometry: ADEM: 27% | 0% |
| MS: 5% | |||||
| Viral encephalitis: 0% | |||||
| ELISA: | |||||
| ADEM 55% | |||||
| MS: 14% | |||||
| LiPhELIA: 0% | |||||
| Viral encephalitis: 0% | |||||
| [Di Pauli et al. 2011] | Cell-based assay (Immunocytochemistry), ELISA | Human MOG-EmGFP transfected HEK293A cells, human MOG expressed by E. coli, refolded | Serum and CSF of pediatric and adult ADEM, CIS and MS patients and controls | Immunocytochem: | Immunocytochem: |
| ADEM: 44% | 0-2% | ||||
| CIS: 8% | ELISA: 4–11% | ||||
| MS: 2% | |||||
| ELISA: | |||||
| ADEM: 24% | |||||
| CIS:8% | |||||
| MS: 15% | |||||
| [Gori et al. 2011] | ELISA | Rat MOG expressed in E. coli, refolded | Serum and CSF from adult MS patients and controls | 0% | 0% |
MOG, myelin oligodendrocyte glycoprotein; CNS, central nervous system; CSF, cerebrospinal fluid; MS, multiple sclerosis; ELISA, enzyme-linked immunosorbent assay; CIS, clinically isolated syndrome; ADEM, acute disseminated encephalomyelitis; LiPhELIA, liquid phase enzyme-linked immuno assay, DELFIA, dissociation enhanced lanthanide fluoroimmunoassay; RIA, radioimmunoassay; CHO, Chinese hamster ovary.
Seemingly healthy young adults with sera containing anti-MOG IgG antibodies, as determined by ELISA using recombinant human MOG, were reported to have a slightly higher risk of developing MS, compared with matched controls [Wang et al. 2008]. This association between anti-MOG status and disease risk disappeared after normalizing for the anti-EBNA IgG titer [Wang et al. 2008]. Another group [Klawiter et al. 2010] detected antibodies against human MOG expressed in insect cells with a secondary antibody recognizing all antibody isotypes in sera and CSF of both MS patients and controls. The group calculated an rMOG index as a marker for intrathecal anti-MOG Ig production and reported that this index was slightly higher in MS patients than in controls [Klawiter et al. 2010].
Anti-MOG antibodies were isolated by affinity chromatography to MOG coupled agarose and the binding of these antibodies to synthetic MOG peptides was assessed in an ELISA [Haase et al. 2001]. Antibodies from both patients and controls bound to several peptides, without showing a specific MS-associated binding pattern. By comparing the binding of the same antibodies to cell-bound MOG in a flow-cytometry assay, one out of 17 MS samples and none of the nine healthy controls bound MOG in its native conformation [Haase et al. 2001].
In a recent study [Gori et al. 2011], rat MOG was expressed in E. coli and refolded. The correct folding of the antigen was demonstrated by far-UV circular dichroism. No antibodies in either MS patients or controls were detected.
Antibody binding to the extracellular domain of recombinant human MOG, expressed in E. coli, was not seen in solution, but in solid-phase, antibodies from both MS patients and healthy controls bound to the same MOG construct [Menge et al. 2007b]. It seems conceivable that the affinity of anti-MOG antibodies is not high enough to allow detection of antibody binding in solution.
Anti-MOG Ig in MS tissue
IgG from the CNS parenchyma of autopsy samples from MS patients were isolated and their binding to refolded E. coli MOG in solid phase and to in vitro translated human MOG in solution phase was assessed [O’Connor et al. 2005]. The group identified IgG binding to MOG in solid phase in a subgroup of MS patients, but not in the CSF or serum. One out of 37 MS patients and no controls showed binding to the solution-phase MOG protein [O’Connor et al. 2005]. Binding of immunogold-labeled MOG and MBP peptides to CNS tissue of MS patients and marmosets with MOG-induced EAE was described [Genain et al. 1999]. The group concluded that these MOG peptides bind to anti-MOG antibodies present on the tissue. This intriguing finding awaits further confirmation and elaboration.
Western blot
Western blot analysis detects antibodies to denatured proteins. Using this technique, antibodies to MOG were found in MS patients and in healthy controls [Lindert et al. 1999; Reindl et al. 1999]. It had been proposed that the anti-MOG and anti-MBP antibody status of clinically isolated syndrome (CIS) patients, as determined by Western blotting using MOG expressed in E. coli, could be predictive of the progression to MS [Berger et al. 2003]. In this study, patients with antibodies against both MBP and MOG had a significantly higher relapse risk, compared with antibody-seronegative CIS patients. These results were refuted by later studies [Kuhle et al. 2007; Pelayo et al. 2007] that used the same methods.
Cell-based assays
To detect antibodies to native MOG, cell lines were transfected to express MOG on the surface. Binding of antibodies to this surface expressed MOG can be detected and quantified by fluorescence-activated cell sorting (FACS) or immunocytochemistry [Brilot et al. 2009; Di Pauli et al. 2011; Haase et al. 2001; Lalive et al. 2006, 2011; McLaughlin et al. 2009; O’Connor et al. 2007; Probstel et al. 2011; Zhou et al. 2006]. The first study [Haase et al. 2001] that used MOG-transfected cells initially obtained MOG-binding IgG from 200 to 300 ml of serum using E. coli produced MOG for affinity purification. As mentioned above, antibody binding to MOG-transfected cells was seen in material obtained from 1/17 MS patients and in 0/9 preparations from controls.
A major advance in our understanding of antibodies to MOG was obtained in a study that analyzed acute disseminated encephalomyelitis (ADEM) patients along with adult MS patients [O’Connor et al. 2007]. ADEM is an acute disorder of the CNS affecting mostly children under the age of 10. This typically monophasic disease is characterized by an acute inflammation and demyelination of the CNS and usually succeeds a viral infection or vaccination [Tenembaum et al. 2002]. This study [O’Connor et al. 2007] used both cell-bound MOG and a tetramer-based assay (see below). It showed that a proportion of patients with ADEM have high antibodies to MOG, whereas in adult MS patients, such antibodies are barely detectable.
All subsequent studies that used a cell-based assay and included ADEM, pediatric and adult MS, basically confirmed this observation [Brilot et al. 2009; Di Pauli et al. 2011; Lalive et al. 2011; McLaughlin et al. 2009; Probstel et al. 2011].
A MOG tetramer radioimmunoassay
Further analysis showed that the proportion of anti-MOG positive children can be identified with a tetramer radioimmunoassay in which four human MOG extracellular domains are tetramerized and used to detect antibodies via immunoprecipitation [O’Connor et al. 2007]. This technique is more sensitive than conventional solution-phase assays, as the avidity of the antigen is higher and it detects antibodies to the correctly folded MOG protein more specifically than conventional ELISA, because the MOG tetramer does not bind antibodies to linear MOG epitopes. With this technique, the group was able to detect high levels of anti-MOG antibodies in ADEM patients, but hardly in MS patients and control groups. The tetramer radioimmunoassay and the cell-based assay essentially identified the same group of anti-MOG positive patients.
Anti-MOG antibodies in a proportion of pediatric MS and ADEM
Using a tetramer radioimmunoassay, antibodies to conformationally intact MOG were detected in a subset of ADEM patients and these antibodies are rather rare in adult-onset MS cases [O’Connor et al. 2007]. Anti-MOG antibodies were also found in the serum of a subset of pediatric MS cases using cells transfected with MOG-GFP [McLaughlin et al. 2009]. All studies analyzing anti-MOG reactivity in pediatric patients noted a higher reactivity in young children (age <10 years) [Brilot et al. 2009; McLaughlin et al. 2009; Probstel et al. 2011]. No linkage between anti-MOG and anti-Epstein–Barr virus (EBV) antibodies was seen [Selter et al. 2010]. Cells transfected with hMOG are significantly more prone to natural killer (NK)-mediated cytotoxicity after incubation with purified serum IgG from anti-MOG positive patients, as compared with IgG from anti-MOG negative patients [Brilot et al. 2009]. Another group [Di Pauli et al. 2011] used immunocytochemistry to assess anti-MOG staining in a longitudinal study. They found a correlation between a decline in antibody titers and full recovery in ADEM patients. In a 5-year follow up, all 16 ADEM patients studied showed a rapid decline of anti-MOG antibodies [Probstel et al. 2011]. Strikingly, anti-MOG antibodies detected in the same study in pediatric MS patients persist in six of eight patients with fluctuations and were even shown to increase. High titer anti-MOG-antibodies are also present in a proportion of children with optic neuritis (ON) and are more frequent in children with recurrent ON, as compared to children with monophasic ON [Rostasy et al. 2012].
Summarizing view on anti-MOG Ig in pediatric and adult MS and ADEM
Technical challenges
Taken together, a general view is emerging that cell-bound assays are the best suited tools for analyzing antibodies to conformationally correct MOG. With a cell-bound assay and with the tetramer assay a proportion of children with ADEM or MS can be identified and clearly distinguished from controls. ELISA and Western blotting detect antibodies to incorrectly folded or denatured MOG. Those antibodies are not pathogenic, because they do not necessarily recognize MOG as it is present in the CNS. The disadvantage of using cell-bound assays, on the other hand, is that the cell surface is a very complex, indefinable milieu, compared with an ELISA plate. This high complexity makes it difficult to compare the results of different groups working with different cell lines and different protocols. It would therefore be worthwhile to develop an ELISA assay in which the human MOG protein is provided in its correctly folded structure only.
Anti-MOG Ig in adult and childhood MS/ADEM
Antibodies against correctly folded MOG are preferably found in pediatric MS and ADEM patients. In adult MS patients, such anti-MOG antibodies are rarely detected. The reason for this phenomenon is yet uncertain, but the different pathogeneses of these two CNS disorders (ADEM and MS) might play a role. ADEM usually succeeds a viral infection or vaccination and it has been proposed that antibodies produced against a viral structure might attack the immune system [Menge et al. 2007a]. Another explanation for the absence of anti-MOG antibodies in adult MS patients might be that anti-MOG antibodies are present in a few patients at the time of initiation of CNS inflammation, but disappear in the following years. A recent longitudinal study noted the persistence of anti-MOG IgG with fluctuations for a period of up to 5 years in children with MS [Probstel et al. 2011]. The further long-term follow up of these and other anti-MOG positive patients will provide further insight.
Possible pathogenic relevance of anti-MOG Ig in childhood demyelination
The formal proof of pathogenicity of human anti-MOG IgG might require transfer experiments which have not yet been performed. However, the features of anti-MOG IgG in pediatric demyelination strongly suggest that these antibodies are pathogenic. This view is based on the comparison with prerequisites of pathogenicity of anti-MOG antibodies established in animal experiments. First, pathogenic anti-MOG antibodies recognize the conformationally correct MOG in a cell-bound assay [Brehm et al. 1999]. As discussed above, several groups have now identified anti-MOG antibodies in a proportion of pediatric demyelination with such an assay. Second, in animal models the complement fixing ability of anti-MOG was linked to their demyelinating activity [Piddlesden et al. 1993]. The pediatric anti-MOG antibodies are mostly of the complement-activating isotype IgG1 [McLaughlin et al. 2009; Probstel et al. 2011]; in vitro experiments examining their complement fixing activity in the context of myelin and oligodendrocytes are still missing. In addition to complement, antibody-dependent cellular cytotoxicity might also contribute to tissue destruction and antibody-dependent cellular cytotoxicity activity of human anti-MOG antibodies has been observed in vitro [Brilot et al. 2009]. Third, a breached blood–brain barrier, e.g. in the context of a T-cell-mediated encephalitis is crucial for the pathogenicity of anti-MOG antibodies, since anti-MOG antibodies are not pathogenic in the absence of CNS inflammation [Litzenburger et al. 1998]. Gadolinium-enhanced lesions are found in the majority of pediatric MS and ADEM patients [Poser and Brinar, 2007; Waubant et al. 2009]. These MRI data point out the disturbed blood–brain barrier in pediatric demyelination indicating that anti-MOG antibodies will have access to CNS myelin.
Consequences for the therapy of anti-MOG positive patients?
The number of drugs available for the therapy of inflammatory CNS diseases is increasing [Kieseier and Stuve, 2011]. This offers the possibility for further treatment optimization, provided biomarkers are available to identify patient subgroups. An example is the detection of anti-aquaporin-4 antibodies in NMO patients. The presence of these autoantibodies has therapeutic consequences, since many observations support the application of anti-CD20 treatment in NMO patients [Pellkofer et al. 2011]. In contrast, IFNβ might not be the ideal treatment for many NMO patients, since it may worsen the disease [Palace et al. 2010]. This might be due to the induction of the B-cell survival factor BAFF by IFNβ [Krumbholz et al. 2008]. Up to now experiences with IFNβ treatment in anti-MOG positive patients have not been reported. It seems plausible that patients with anti-MOG antibodies rather benefit from plasma exchange or a B-cell-directed therapy. A reduction of anti-MOG antibodies after B-cell-directed therapy would be expected, if short-lived plasma blasts rather than long-lived plasma cells are the source of these antibodies. The rapid decline of anti-MOG Ig in ADEM patients [Probstel et al. 2011] suggests that in these patients the antibodies are derived from short-lived plasma cells.
In search for other targets
The clinical observation that a proportion of MS patients benefit from plasma exchange [Keegan et al. 2005] and the absence of a decent MOG reactivity in the vast majority of adult MS patients suggests that targets of pathogenic antibodies in MS have yet to be identified. In a proteomic approach, glycoproteins were purified from human myelin, separated by two-dimensional gel electrophoresis and probed with patients’ sera for antigen recognition. The purification of myelin glycoproteins has two reasons. First, it enhances the sensitivity to detect antibodies to minor components of the myelin and second, glycoproteins (such as MOG) are frequently displayed on the cell surface and accessible for antibodies. Proteins recognized by patients’ Ig can be identified by mass spectrometry. With this approach, the two antigens neurofascin and contactin-2/TAG-1 have been identified [Derfuss et al. 2009; Mathey et al. 2007]. These two proteins are expressed by both oligodendrocytes and neurons and localized around the node of Ranvier [Derfuss et al. 2010]. A mAb to neurofascin mediated axonal injury in an animal model [Mathey et al. 2007], while T cells specific for contactin-2 directed encephalitis to the gray matter [Derfuss et al. 2009]. Another group also employed two-dimensional gel electrophoresis and mass spectrometry, without specific purification of glycoproteins, and found antibodies against transketolase and CNPaseI in the CSF of MS patients [Lovato et al. 2008]. Further work still has to identify the relevance of the reactivity to these new autoantigens for individual patients.
Patterns of autoantibody reactivities in the serum and CSF of MS patients have been described [Quintana et al. 2008]. Only binding to artificial peptides, but not to correctly folded proteins are observed in this study. The antigen recognition tested with these microarrays will therefore not necessarily mirror the reaction in the CNS of the patients and it remains open whether the detected antibodies, which may serve as a biomarker, are also pathogenic. The same might be true for lipid microarrays probing MS patients’ sera for binding to myelin sheath lipids [Kanter et al. 2006]. Hence, antigen microarrays can only give an idea of which antigens might play a role in MS.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant number SFB 571), the Verein zur Therapieforschung für Multiple-Sklerose-Kranke, the Bundesministerium für Bildung und Forschung (‘Krankheitsbezogenes Kompetenznetz Multiple Sklerose’) and the Gemeinnützige Hertie Stiftung.
The authors declare no conflicts of interest in preparing this article.
References
- Adelmann M., Wood J., Benzel I., Fiori P., Lassmann H., Matthieu J.M., et al. (1995) The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J Neuroimmunol 63: 17–27 [DOI] [PubMed] [Google Scholar]
- Bennett J.L., Lam C., Kalluri S.R., Saikali P., Bautista K., Dupree C., et al. (2009) Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 66: 617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T., Rubner P., Schautzer F., Egg R., Ulmer H., Mayringer I., et al. (2003) Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med 349: 139–145 [DOI] [PubMed] [Google Scholar]
- Bourquin C., Schubart A., Tobollik S., Mather I., Ogg S., Liblau R., et al. (2003) Selective unresponsiveness to conformational B cell epitopes of the myelin oligodendrocyte glycoprotein in H-2b mice. J Immunol 171: 455–461 [DOI] [PubMed] [Google Scholar]
- Bradl M., Misu T., Takahashi T., Watanabe M., Mader S., Reindl M., et al. (2009) Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol 66: 630–643 [DOI] [PubMed] [Google Scholar]
- Brehm U., Piddlesden S.J., Gardinier M.V., Linington C. (1999) Epitope specificity of demyelinating monoclonal autoantibodies directed against the human myelin oligodendrocyte glycoprotein (MOG). J Neuroimmunol 97: 9–15 [DOI] [PubMed] [Google Scholar]
- Breithaupt C., Schafer B., Pellkofer H., Huber R., Linington C., Jacob U. (2008) Demyelinating myelin oligodendrocyte glycoprotein-specific autoantibody response is focused on one dominant conformational epitope region in rodents. J Immunol 181: 1255–1263 [DOI] [PubMed] [Google Scholar]
- Breithaupt C., Schubart A., Zander H., Skerra A., Huber R., Linington C., et al. (2003) Structural insights into the antigenicity of myelin oligodendrocyte glycoprotein. Proc Natl Acad Sci U S A 100: 9446–9451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brilot F., Dale R.C., Selter R.C., Grummel V., Kalluri S.R., Aslam M., et al. (2009) Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol 66: 833–842 [DOI] [PubMed] [Google Scholar]
- Brunner C., Lassmann H., Waehneldt T.V., Matthieu J.M., Linington C. (1989) Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2’,3’-cyclic nucleotide 3’-phosphodiesterase in the CNS of adult rats. J Neurochem 52: 296–304 [DOI] [PubMed] [Google Scholar]
- Clements C.S., Reid H.H., Beddoe T., Tynan F.E., Perugini M.A., Johns T.G., et al. (2003) The crystal structure of myelin oligodendrocyte glycoprotein, a key autoantigen in multiple sclerosis. Proc Natl Acad Sci U S A 100: 11059–11064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong H., Jiang Y., Tien P. (2011) Identification of the myelin oligodendrocyte glycoprotein as a cellular receptor for rubella virus. J Virol, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarasse C., Daubas P., Mars L.T., Vizler C., Litzenburger T., Iglesias A., et al. (2003) Myelin/oligodendrocyte glycoprotein-deficient (MOG-deficient) mice reveal lack of immune tolerance to MOG in wild-type mice. J Clin Invest 112: 544–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Gaspera B., Pham-Dinh D., Roussel G., Nussbaum J.L., Dautigny A. (1998) Membrane topology of the myelin/oligodendrocyte glycoprotein. Eur J Biochem 258: 478–484 [DOI] [PubMed] [Google Scholar]
- Derfuss T., Linington C., Hohlfeld R., Meinl E. (2010) Axo-glial antigens as targets in multiple sclerosis: implications for axonal and grey matter injury. J Mol Med 88: 753–761 [DOI] [PubMed] [Google Scholar]
- Derfuss T., Parikh K., Velhin S., Braun M., Mathey E., Krumbholz M., et al. (2009) Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc Natl Acad Sci U S A 106: 8302–8307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pauli F., Mader S., Rostasy K., Schanda K., Bajer-Kornek B., Ehling R., et al. (2011) Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol 138: 247–254 [DOI] [PubMed] [Google Scholar]
- Gaertner S., de Graaf K.L., Greve B., Weissert R. (2004) Antibodies against glycosylated native MOG are elevated in patients with multiple sclerosis. Neurology 63: 2381–2383 [DOI] [PubMed] [Google Scholar]
- Gardinier M.V., Amiguet P., Linington C., Matthieu J.M. (1992) Myelin/oligodendrocyte glycoprotein is a unique member of the immunoglobulin superfamily. J Neurosci Res 33: 177–187 [DOI] [PubMed] [Google Scholar]
- Genain C.P., Cannella B., Hauser S.L., Raine C.S. (1999) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med 5: 170–175 [DOI] [PubMed] [Google Scholar]
- Genain C.P., Hauser S.L. (1996) Allergic encephalomyelitis in common marmosets: pathogenesis of a multiple sclerosis-like lesion. Methods 10: 420–434 [DOI] [PubMed] [Google Scholar]
- Gori F., Mulinacci B., Massai L., Avolio C., Caragnano M., Peroni E., et al. (2011) IgG and IgM antibodies to the refolded MOG(1-125) extracellular domain in humans. J Neuroimmunol 233: 216–220 [DOI] [PubMed] [Google Scholar]
- Guggenmos J., Schubart A.S., Ogg S., Andersson M., Olsson T., Mather I.H., et al. (2004) Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. J Immunol 172: 661–668 [DOI] [PubMed] [Google Scholar]
- Haase C.G., Guggenmos J., Brehm U., Andersson M., Olsson T., Reindl M., et al. (2001) The fine specificity of the myelin oligodendrocyte glycoprotein autoantibody response in patients with multiple sclerosis and normal healthy controls. J Neuroimmunol 114: 220–225 [DOI] [PubMed] [Google Scholar]
- Johns T.G., Bernard C.C. (1997) Binding of complement component Clq to myelin oligodendrocyte glycoprotein: a novel mechanism for regulating CNS inflammation. Mol Immunol 34: 33–38 [DOI] [PubMed] [Google Scholar]
- Kanter J.L., Narayana S., Ho P.P., Catz I., Warren K.G., Sobel R.A., et al. (2006) Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med 12: 138–143 [DOI] [PubMed] [Google Scholar]
- Keegan M., Konig F., McClelland R., Bruck W., Morales Y., Bitsch A., et al. (2005) Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet 366: 579–582 [DOI] [PubMed] [Google Scholar]
- Kieseier B.C., Stuve O. (2011) A critical appraisal of treatment decisions in multiple sclerosis—old versus new. Nat Rev Neurol 7: 255–262 [DOI] [PubMed] [Google Scholar]
- Kinoshita M., Nakatsuji Y., Kimura T., Moriya M., Takata K., Okuno T., et al. (2009) Neuromyelitis optica: Passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun 386: 623–627 [DOI] [PubMed] [Google Scholar]
- Klawiter E.C., Piccio L., Lyons J.A., Mikesell R., O’Connor K.C., Cross A.H. (2010) Elevated intrathecal myelin oligodendrocyte glycoprotein antibodies in multiple sclerosis. Arch Neurol 67: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbholz M., Faber H., Steinmeyer F., Hoffmann L.A., Kumpfel T., Pellkofer H., et al. (2008) Interferon-beta increases BAFF levels in multiple sclerosis: implications for B cell autoimmunity. Brain 131: 1455–1463 [DOI] [PubMed] [Google Scholar]
- Kuhle J., Pohl C., Mehling M., Edan G., Freedman M.S., Hartung H.P., et al. (2007) Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med 356: 371–378 [DOI] [PubMed] [Google Scholar]
- Lalive P.H., Hausler M.G., Maurey H., Mikaeloff Y., Tardieu M., Wiendl H., et al. (2011) Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult Scler 17: 297–302 [DOI] [PubMed] [Google Scholar]
- Lalive P.H., Menge T., Delarasse C., Della G.B., Pham-Dinh D., Villoslada P., et al. (2006) Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc Natl Acad Sci U S A 103: 2280–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebar R., Boutry J.M., Vincent C., Robineaux R., Voisin G.A. (1976) Studies on autoimmune encephalomyelitis in the guinea pig. II. An in vitro investigation on the nature, properties, and specificity of the serum-demyelinating factor. J Immunol 116: 1439–1446 [PubMed] [Google Scholar]
- Lebar R., Lubetzki C., Vincent C., Lombrail P., Boutry J.M. (1986) The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clin Exp Immunol 66: 423–434 [PMC free article] [PubMed] [Google Scholar]
- Lindert R.B., Haase C.G., Brehm U., Linington C., Wekerle H., Hohlfeld R. (1999) Multiple sclerosis: B- and T-cell responses to the extracellular domain of the myelin oligodendrocyte glycoprotein. Brain 122: 2089–2100 [DOI] [PubMed] [Google Scholar]
- Linington C., Bradl M., Lassmann H., Brunner C., Vass K. (1988) Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol 130: 443–454 [PMC free article] [PubMed] [Google Scholar]
- Linington C., Lassmann H. (1987) Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol 17: 61–69 [DOI] [PubMed] [Google Scholar]
- Linnington C., Webb M., Woodhams P.L. (1984) A novel myelin-associated glycoprotein defined by a mouse monoclonal antibody. J Neuroimmunol 6: 387–396 [DOI] [PubMed] [Google Scholar]
- Litzenburger T., Fassler R., Bauer J., Lassmann H., Linington C., Wekerle H., et al. (1998) B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med 188: 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovato L., Cianti R., Gini B., Marconi S., Bianchi L., Armini A., et al. (2008) Transketolase and 2’,3’-cyclic-nucleotide 3’-phosphodiesterase type I isoforms are specifically recognized by IgG autoantibodies in multiple sclerosis patients. Mol Cell Proteomics 7: 2337–2349 [DOI] [PubMed] [Google Scholar]
- Marta C.B., Oliver A.R., Sweet R.A., Pfeiffer S.E., Ruddle N.H. (2005) Pathogenic myelin oligodendrocyte glycoprotein antibodies recognize glycosylated epitopes and perturb oligodendrocyte physiology. Proc Natl Acad Sci U S A 102: 13992–13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey E.K., Derfuss T., Storch M.K., Williams K.R., Hales K., Woolley D.R., et al. (2007) Neurofascin as a novel target for autoantibody-mediated axonal injury. J Exp Med 204: 2363–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Chitnis T., Newcombe J., Franz B., Kennedy J., McArdel S., et al. (2009) Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J Immunol 183: 4067–4076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinl E., Derfuss T., Linington C. (2010) Identifying targets for autoantibodies in CNS inflammation: Strategies and achievements. Clin Exp Neuroimmunol 1: 47–60 [Google Scholar]
- Meinl E., Krumbholz M., Hohlfeld R. (2006) B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol 59: 880–892 [DOI] [PubMed] [Google Scholar]
- Menge T., Kieseier B.C., Nessler S., Hemmer B., Hartung H.P., Stuve O. (2007a) Acute disseminated encephalomyelitis: an acute hit against the brain. Curr Opin Neurol 20: 247–254 [DOI] [PubMed] [Google Scholar]
- Menge T., von Budingen H.C., Lalive P.H., Genain C.P. (2007b) Relevant antibody subsets against MOG recognize conformational epitopes exclusively exposed in solid-phase ELISA. Eur J Immunol 37: 3229–3239 [DOI] [PubMed] [Google Scholar]
- O’Connor K.C., Appel H., Bregoli L., Call M.E., Catz I., Chan J.A., et al. (2005) Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J Immunol 175: 1974–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor K.C., McLaughlin K.A., De Jager P.L., Chitnis T., Bettelli E., Xu C., et al. (2007) Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med 13: 211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver A.R., Lyon G.M., Ruddle N.H. (2003) Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol 171: 462–468 [DOI] [PubMed] [Google Scholar]
- Pagany M., Jagodic M., Bourquin C., Olsson T., Linington C. (2003) Genetic variation in myelin oligodendrocyte glycoprotein expression and susceptibility to experimental autoimmune encephalomyelitis. J Neuroimmunol 139: 1–8 [DOI] [PubMed] [Google Scholar]
- Palace J., Leite M.I., Nairne A., Vincent A. (2010) Interferon beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol 67: 1016–1017 [DOI] [PubMed] [Google Scholar]
- Pelayo R., Tintore M., Montalban X., Rovira A., Espejo C., Reindl M., et al. (2007) Antimyelin antibodies with no progression to multiple sclerosis. N Engl J Med 356: 426–428 [DOI] [PubMed] [Google Scholar]
- Pellkofer H.L., Krumbholz M., Berthele A., Hemmer B., Gerdes L.A., Havla J., et al. (2011) Long-term follow-up of patients with neuromyelitis optica after repeated therapy with rituximab. Neurology 76: 1310–1315 [DOI] [PubMed] [Google Scholar]
- Pham-Dinh D., Mattei M.G., Nussbaum J.L., Roussel G., Pontarotti P., Roeckel N., et al. (1993) Myelin/oligodendrocyte glycoprotein is a member of a subset of the immunoglobulin superfamily encoded within the major histocompatibility complex. Proc Natl Acad Sci U S A 90: 7990–7994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddlesden S.J., Lassmann H., Zimprich F., Morgan B.P., Linington C. (1993) The demyelinating potential of antibodies to myelin oligodendrocyte glycoprotein is related to their ability to fix complement. Am J Pathol 143: 555–564 [PMC free article] [PubMed] [Google Scholar]
- Pollinger B., Krishnamoorthy G., Berer K., Lassmann H., Bosl M.R., Dunn R., et al. (2009) Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med 206: 1303–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poser C.M., Brinar V.V. (2007) Disseminated encephalomyelitis and multiple sclerosis: two different diseases - a critical review. Acta Neurol Scand 116: 201–206 [DOI] [PubMed] [Google Scholar]
- Probstel A.K., Dornmair K., Bittner R., Sperl P., Jenne D., Magalhaes S., et al. (2011) Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology 77: 580–588 [DOI] [PubMed] [Google Scholar]
- Quintana F.J., Farez M.F., Viglietta V., Iglesias A.H., Merbl Y., Izquierdo G., et al. (2008) Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A 105: 18889–18894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reindl M., Linington C., Brehm U., Egg R., Dilitz E., Deisenhammer F., et al. (1999) Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 122: 2047–2056 [DOI] [PubMed] [Google Scholar]
- Rostasy K., Mader S., Schanda K., Huppke P., Gartner J., Kraus V., et al. (2012) Anti-MOG antibodies in children with optic neuritis. Arch Neurol, in press [DOI] [PubMed] [Google Scholar]
- Saadoun S., Waters P., Macdonald C., Bridges L.R., Bell B.A., Vincent A., et al. (2011) T cell deficiency does not reduce lesions in mice produced by intracerebral injection of NMO-IgG and complement. J Neuroimmunol 235: 27–32 [DOI] [PubMed] [Google Scholar]
- Schluesener H.J., Sobel R.A., Linington C., Weiner H.L. (1987) A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol 139: 4016–4021 [PubMed] [Google Scholar]
- Selter R.C., Brilot F., Grummel V., Kraus V., Cepok S., Dale R.C., et al. (2010) Antibody responses to EBV and native MOG in pediatric inflammatory demyelinating CNS diseases. Neurology 74: 1711–1715 [DOI] [PubMed] [Google Scholar]
- Storch M.K., Stefferl A., Brehm U., Weissert R., Wallstrom E., Kerschensteiner M., et al. (1998) Autoimmunity to myelin oligodendrocyte glycoprotein in rats mimics the spectrum of multiple sclerosis pathology. Brain Pathol 8: 681–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenembaum S., Chamoles N., Fejerman N. (2002) Acute disseminated encephalomyelitis: a long-term follow-up study of 84 pediatric patients. Neurology 59: 1224–1231 [DOI] [PubMed] [Google Scholar]
- ‘t Hart B.A., Laman J.D., Bauer J., Blezer E., van K.Y., Hintzen R.Q. (2004) Modelling of multiple sclerosis: lessons learned in a non-human primate. Lancet Neurol 3: 588–597 [DOI] [PubMed] [Google Scholar]
- von Budingen H.C., Hauser S.L., Fuhrmann A., Nabavi C.B., Lee J.I., Genain C.P. (2002) Molecular characterization of antibody specificities against myelin/oligodendrocyte glycoprotein in autoimmune demyelination. Proc Natl Acad Sci U S A 99: 8207–8212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Munger K.L., Reindl M., O’Reilly E.J., Levin L.I., Berger T., et al. (2008) Myelin oligodendrocyte glycoprotein antibodies and multiple sclerosis in healthy young adults. Neurology 71: 1142–1146 [DOI] [PubMed] [Google Scholar]
- Waubant E., Chabas D., Okuda D.T., Glenn O., Mowry E., Henry R.G., et al. (2009) Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol 66: 967–971 [DOI] [PubMed] [Google Scholar]
- Weinshenker B.G., Wingerchuk D.M., Pittock S.J., Lucchinetti C.F., Lennon V.A. (2006) NMO-IgG: a specific biomarker for neuromyelitis optica. Dis Markers 22: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B.G., Linington C., Link H. (1991) Antibodies to myelin-oligodendrocyte glycoprotein in cerebrospinal fluid from patients with multiple sclerosis and controls. J Neuroimmunol 31: 91–96 [DOI] [PubMed] [Google Scholar]
- Zhou D., Srivastava R., Nessler S., Grummel V., Sommer N., Bruck W., et al. (2006) Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc Natl Acad Sci U S A 103: 19057–19062 [DOI] [PMC free article] [PubMed] [Google Scholar]