Abstract
Satureja spicigera (Lamiaceae) grows wildly in Northwest of Iran. In this study, bioassay-guided isolation and identification of the main compounds has been reported using various chromatographic methods and comparison of their spectral data with those reported in the literature. Brine shrimp lethality and four cancerous cell lines HT29/219, Caco2, NIH-3T3, and T47D were used for cytotoxicity evaluations. From the aerial parts of S. spicigera, nine known compounds including two flavanones, 5,7,3′,5′-tetrahydroxy flavanone (8) and 5,4′-dihydroxy-3′-methoxyflavanone-7-(6′′-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (9), one dihydrochalcone, nubigenol (7), together with thymoquinone (1), thymol (2), carvacrol (3), β-sitosterol (4), ursolic acid (5) and oleanolic acid (6) were identified. Among the isolated chalcone and flavanones, compound 8 was effective against Artemia salina larva (LC50= 2 μg/mL) and only the compound 9 demonstrated IC50 value of 98.7 μg/mL on the T47D (human, breast, ductal carcinoma). Other compounds did not show significant inhibition of the cell growth.
1. Introduction
The genus Satureja (Lamiaceae) has 13 species in Iran and is called Marzeh. One of these species is S. spicigera that grows wildly in Northwest of Iran [1, 2]. Recent phytochemical studies indicated the presence of flavanones (naringenin, aromadendrin, eriodictyol, taxifolin, and flavanone trimethyl ether) and flavones (apigenin, luteolin, diosmetin, genkwanin, ladanein, thymosin, thymonin, cirsimaritin, and xanthomicrol) in some species of Satureja [3–5].
So far, antimicrobial [6], spasmoltic [7], anti-HIV [8], antiviral [9], antioxidant [10] and cytotoxic activities [11, 12] have been reported from several species of this genus. Volatile composition of some Iranian species of Satureja has been investigated [13]. Recently, we have reported the chemical composition of the volatile oil of S. spicigera [14]. The oil of S. spicigera was rich in monoterpenes (89.9%) with thymol (37.3%) as the major compound [14]. Comparison of some Satureja species by phylogenetic and chemotaxonomic analysis showed that the genetic distance between S. spicigera and S. mutica is close [15]. Antibacterial activity of the oil of S. spicigera has been reported against B. subtilis, S. aureus, E. coli, and K. pneumonia [16]. Also, we reported the trypanocidal activities of the several extracts of S. spicigera and other species of this genus [17–19]. A literature survey has shown that the phytochemical constituents of S. spicigera were not previously published. Therefore, we aim to report the isolation, structural elucidation, and cytotoxicity of the main constituents of S. spicigera for the first time.
2. Material and Methods
2.1. General Procedures
1H- and 13C-NMR spectra were measured on a Bruker Avance TM 500 DRX (500 MHz for 1H and 125 MHz for 13C) spectrometer with tetramethylsilane as an internal standard, and chemical shifts are given in δ (ppm). HR-ESITOFMS were obtained on a Bruker MicroTOFII mass spectrometer. The FT-IR spectra were recorded on a Nicolet 550 instrument. Silica gel 60F254 precoated plates (Merck TM) were used for TLC. The spots were detected by spraying anisaldehyde-H2SO4 reagent followed by heating (120°C for 5 minuets).
2.2. Plant Material
Aerial parts of Satureja spicigera (C. Koch) Boiss, at flowering stage, were collected from Astara in the Northwest of Iran, in September 2004. Voucher specimen (78410 TARI) was deposited at the Herbarium of the Institute of Forests and Rangelands Researches. Plant specimen was identified by Dr. Vali-allah Mozaffarian from the same institute.
2.3. Extraction, Isolation, and Structural Elucidation
The flowered aerial parts of S. spicigera (1 kg) was cut into small pieces and extracted with ethyl acetate and methanol, consequently, at room temperature. The ethyl acetate extract (23 g) was subjected to silica gel column chromatography (CC) with hexane : CHCl3 (8 : 2), CHCl3 : AcOEt (2 : 8), and AcOEt as eluent to give six fractions (A–F). The fraction C (7 g) was submitted to silica gel CC with hexane : AcOEt (19 : 1) to obtain five fractions C1–C5. The fraction C2 (97 mg) was chromatographed on sephadex LH20 with AcOEt : MeOH (3 : 1) to result compound 1 (14 mg). The fraction C3 (1 g) was subjected to CC with hexane : AcOEt (9 : 1) to give four fractions C31–C34. The compounds 2 (35 mg) and 3 (15 mg) were given from the fractions C32 and C33, respectively, using sephadex LH20 eluted with AcOEt : MeOH (3 : 1). The fraction C5 (400 mg) was submitted to silica gel CC with hexane : AcOEt (9 : 1) to obtain compound 4 (26 mg).
The MeOH extract (70 g) was successively subjected to silica gel CC with hexane : AcOEt (8 : 2), CHCl3 : AcOEt (8 : 2), AcOEt, and MeOH as eluent to give eight fractions (M1–M8). Fraction M3 (5.3 g) was fractionated on CC with CHCl3 : AcOEt (8 : 2) to yield compounds 5 (320 mg) and 6 (45 mg). The fraction M4 (690 mg) was chromatographed on sephadex LH20 with MeOH to result M41–M43. Fraction M43 (44 mg) was purified on sephadex LH20 with MeOH to yield compound 7 (15 mg). Compound 8 (26 mg) was yielded from fraction M5 (1.9 g) using twice sephadex LH20 with MeOH. The fraction M8 (9 g) was submitted to reverse- phase (C8) CC with aqueous MeOH (30%, 50%, and 100%) to give 7 fractions (M81–M87). Compound 9 (90 mg) was obtained from fraction M87 (3.3 g) using sephadex LH20 with MeOH.
2.4. Brine Shrimp Lethality Assay (BSA)
Gohari et al. reported the method which was adopted to study the cytotoxic activity of the compounds [11]. Water life brand brine shrimp (Artemia salina) eggs were purchased from the Shalat Center (Tehran). The eggs were hatched in a flask containing 300 mL artificial seawater made by dissolving distilled water. The flask was well aerated with the aid of an air pump and kept in a water bath at 29-30°C. A bright light was left on. The nauplii hatched within 48 h. The extracts and pure compounds were dissolved in normal saline. Different concentrations were obtained by serial dilution. Solution of each concentration (500 μL) was transferred into clean 24-well plates via a pipette, and aerated seawater having 10–20 nauplii (500 μL) was added. A check count was performed and the number alive noted after 24 h. The mortality end point of the bioassay was determined as the absence of controlled forward motion during 30 seconds of observation. The controls used were seawater and berberine hydrochloride (LC50 = 26 μg/mL). Lethality percentage was determined and LC50 calculated based on probit analysis with 95% of confidence interval [11].
2.5. Cell Cultures and Cytotoxicity Assay
Four cancerous cell lines HT29/219 (human, colon, epithelial-like, carcinoma), Caco2 (human, colon, adenocarcinoma), NIH-3T3 (Swiss NIH mouse, embryo fibroblast), and T47D (human, breast, ductal carcinoma) were purchased from the Pasteur Institute, Tehran, Iran. The cells were maintained in RPMI 1640, supplemented with 10% fetal bovine serum, 0.28 units/mL insulin, 100 μg/mL streptomycin, 100 units/mL penicillin, and 0.3 mg/mL glutamine. The cells were grown at 37°C in a humidified atmosphere of 5% CO2, in air.
The cytotoxicity of the compounds isolated from S. spicigera was assayed using the MTT cytotoxicity assay. The cells (3 × 10 4) were plated in 500 μL of medium/well in 48-well plates (NUNC Cell Culture Flasks, Denmark). After an overnight incubation at 37°C, in 5% CO2, and a humidified atmosphere, the samples were added to the cells to a final concentration of 500 μg/mL. “Methotrexate” (positive control) and pure compounds were examined at concentrations ranging from 5, 10, 20, 40, 80, and 100 μg/mL. The plates were incubated at 37°C, in 5% CO2, humidified atmosphere, for 48 hours. After 48 hours, 50 μL of 5 mg/mL MTT (dissolved in PBS) was added per well. After three hours of incubation, the MTT solution was removed and the cells were washed with 100 μL of PBS, twice. One hundred and fifty microlitres of DMSO was added per well, to solubilize the formazan crystals. The optical densities of the wells were then measured at 570 nm (690 nm reference wavelength). By referring to the control (medium with DMSO), the cell survival was assessed [20].
3. Results
Dried aerial parts of S. spicigera, collected during full flowering stage, were successively extracted with ethyl acetate and methanol. These extracts were concentrated and examined with brine shrimp lethality assay (BSA). Both extracts showed cytotoxic activity against Artemia salina larvae, so were used for further isolation on silica gel and sephadex column chromatography to obtain compounds 1–9 (Figure 1). Isolated compounds, 1–7, were identified as thymoquinone (1), thymol (2), carvacrol (3), β-sitosterol (4), ursolic acid (5), oleanolic acid (6), and nubigenol (7) by comparison of their spectral data with those reported in literature [11, 21–23]. The results have a good agreement with references. Full assignments of the compounds 8 and 9, performed by 2D-NMR and HR-ESITOFMS techniques, have been reported in this paper, because they have not been found in the literature review [24, 25].
Figure 1.
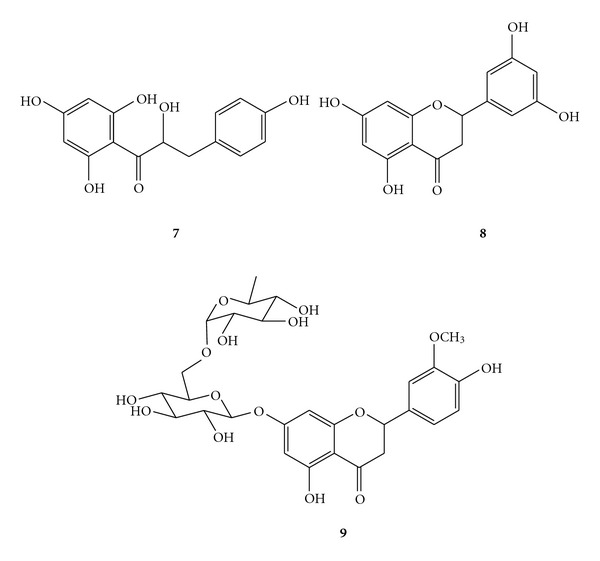
Chemical structures of the isolated dihydrochalcone and flavanones from Satureja spicigera.
The results obtained from BSA showed that the compound 8 (LC50 = 2 μg/mL) was effective against larvae of Artemia salina. This is a toxic compound compared to berberine hydrochloride (LC50 = 26 μg/mL) which was used as a positive control. Among the three various compounds of S. spicigera, the compound 9 demonstrated IC50 value of 98.7 μg/mL on the T47D (human, breast, ductal carcinoma) (P < 0.01). The other compounds did not show significant inhibition of the cell growth or proliferation, even compound 8 which showed toxicity against brine shrimp larvae (Table 3).
Table 3.
The effects of dihydrochalcone and flavanones isolated from S. spicigera on the viability of various cell lines using MTT assay.
| Sample | Cell linesa (MTT assay) | |||
|---|---|---|---|---|
| T47D | Caco-2 | HT-29 | NIH 3T3 | |
| 7 | 132.0 | 143.0 | >150 | 112.0 |
| 8 | >150 | >150 | >150 | 100.7 |
| 9 | 98.7 | >150 | >150 | 140.4 |
aResults are expressed as IC50 values (μg/mL), Key to cell Lines employed: HT-29 and Caco-2 (colon adenocarcinoma); T47D (breast carcinoma); NIH 3T3 (Swiss embryo fibroblast).
5,7,3′,5′-Tetrahydroxy Flavanone (8) —
Pale yellow crystal; melting point 264–268°C; IR (CHCl3) ν max 3353 (O–H), 2974, 2924, 2855 (C–H), 1627, 1598 (C=O), 1435, 1114, 695 cm−1; HR-ESITOFMS m/z 311.0526 [M + Na]+ (calcd for C15H12O6Na, 311.0532). NMR spectra are shown in Table 1.
Table 1.
NMR spectra of the compound 8 in DMSO-d 6.
| No. | 13C-NMR | 1H-NMR | HMBC |
|---|---|---|---|
| 2 | 78.4 | 5.37 (dd, J = 12.4, 2.9 Hz, 1H) | H-3b, H-2′ or H-6′ |
| 3 | 42.1 | 2.69 (dd, J = 17.0, 2.9 Hz, 1H) | |
| 3.18 (dd, J = 17.0, 12.5 Hz, 1H) | |||
| 4 | 196.2 | H-3a, H3b | |
| 5 | 162.9 | H-6 | |
| 6 | 95.8 | 5.88 (d, J = 1.2 Hz, 1H) | 5-OH |
| 7 | 166.7 | H-6 or H-8 | |
| 8 | 95.0 | 5.88 (d, J = 1.2 Hz, 1H) | |
| 9 | 163.4 | H-8, 5-OH | |
| 10 | 101.7 | H-6 or H-8, 5-OH | |
| 1′ | 129.4 | H-3b, H-2′ or H-6′ | |
| 2′ | 117.9 | 6.75 (brs, 1H) | H-4′ |
| 3′ | 145.7 | H-4′ | |
| 4′ | 114.3 | 6.88 (brs, 1H) | H-2′ or H-6′ |
| 5′ | 145.2 | H-4′, H-2′ or H-6′ | |
| 6′ | 115.3 | 6.75 (brs, 1H) | |
| 5-OH | 12.14 (s, 1H) | ||
| 7-OH | 10.76 (s, 1H) | ||
| 3′-OH | 9.00 (s, 1H) | ||
| 5′-OH | 9.05 (s, 1H) |
5,4′-Dihydroxy-3′-methoxyflavanone-7-(6′′-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside (9) —
White amorphous crystal; melting point 256°C; HR-ESITOFMS m/z 633.1790 [M + Na]+ (calcd for C28H34O15Na, 633.1795). NMR data is shown in Table 2.
Table 2.
NMR spectra of the compound 9 in DMSO-d 6.
| No. | 13C-NMR | 1H-NMR | HMBC |
|---|---|---|---|
| 2 | 78.4 | 5.50 (dd, J = 12.5, 3.0 Hz, 1H) | H-3b, H-2′ |
| 3 | 42.1 | 2.77 (dd, J = 17.5, 3.2 Hz, 1H) | |
| 3.14 (m, 1H) | |||
| 4 | 197.1 | H-3a, H-3b | |
| 5 | 162.5 | H-6 | |
| 6 | 96.4 | 6.13 (d, J = 2.5 Hz, 1H) | H-8 |
| 7 | 165.2 | H-6, H-8 | |
| 8 | 95.6 | 6.14 (d, J = 2.5 Hz, 1H) | |
| 9 | 163.1 | H-8 | |
| 10 | 103.4 | H-6 | |
| 1′ | 131.9 | H-2, H-6′ | |
| 2′ | 114.2 | 6.94 (m, 1H) | |
| 3′ | 146.5 | H-5′, OCH3 | |
| 4′ | 148.0 | ||
| 5′ | 112.1 | 6.94 (m, 1H) | |
| 6′ | 118.0 | 6.89 (dd, J = 8.0, 2.0 Hz, 1H) | H-2, H-2′ |
| –OCH3 | 55.7 | 3.79 (s, 3H) | |
| 5-OH | 12.08 | ||
| Glc-1′′ | 99.5 | 4.97 (d, J = 7.5 Hz, 1H) | |
| 2′′ | 73.0 | 3.21 (m, 1H) | |
| 3′′ | 76.3 | 3.26 (m, 1H) | |
| 4′′ | 69.6 | 3.15 (m, 1H) | |
| 5′′ | 75.5 | 3.54 (m, 1H) | |
| 6′′ | 66.1 | 3.37 (m, 1H) | H-1′′′ |
| 3.80 (m, 1H) | |||
| Rha-1′′′ | 100.6 | 4.51 (d, J = 1.0 Hz, 1H) | |
| 2′′′ | 70.7 | 3.62 (m, 1H) | H-1′′′ |
| 3′′′ | 70.3 | 3.42 (m, 1H) | |
| 4′′′ | 72.1 | 3.17 (m, 1H) | |
| 5′′′ | 68.4 | 3.42 (m, 1H) | |
| 6′′′ | 17.9 | 1.08 (d, J = 6.0 Hz, 3H) | H-1′′′ |
References
- 1.Rechinger KH. Flora Iranica, Labiatae. Vol. 150. Graz, Austria: AcademischeDruck-U-Verganstalt; 1986. [Google Scholar]
- 2.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran, Iran: Farhang Moaser; 1996. [Google Scholar]
- 3.Skoula M, Grayer RJ, Kite GC. Surface flavonoids in Satureja thymbra and Satureja spinosa (Lamiaceae) Biochemical Systematics and Ecology. 2005;33(5):541–547. [Google Scholar]
- 4.de Rojas VRS, Somoza B, Ortega T, Villar AM. Isolation of vasodilatory active flavonoids from the traditional remedy Satureja obovata . Planta Medica. 1996;62(3):272–274. doi: 10.1055/s-2006-957876. [DOI] [PubMed] [Google Scholar]
- 5.Saeidnia S, Nourbakhsh MS, Gohari AR, Davood A. Isolation and identification of the main compounds of Satureja sahendica Bornm. Australian Journal of Basic and Applied Sciences. 2011;5:1450–1453. [Google Scholar]
- 6.Feresin GE, Tapia AA, Bustos DA. Antibacterial activity of some medicinal plants from San Juan, Argentina. Fitoterapia. 2000;71(4):429–432. doi: 10.1016/s0367-326x(00)00128-3. [DOI] [PubMed] [Google Scholar]
- 7.Hajhashemi V, Sadraei H, Ghannadi AR, Mohseni M. Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. Journal of Ethnopharmacology. 2000;71(1-2):187–192. doi: 10.1016/s0378-8741(99)00209-3. [DOI] [PubMed] [Google Scholar]
- 8.Bedoya LM, Sanchez-Palomino S, Abad MJ, Bermejo P, Alcami J. Anti-HIV activity of medicinal plant extracts. Journal of Ethnopharmacology. 2001;77(1):113–116. doi: 10.1016/s0378-8741(01)00265-3. [DOI] [PubMed] [Google Scholar]
- 9.Abad MJ, Bermejo P, Gonzales E, Iglesias I, Irurzun A, Carrasco L. Antiviral activity of Bolivian plant extracts. General Pharmacology. 1999;32(4):499–503. doi: 10.1016/s0306-3623(98)00214-6. [DOI] [PubMed] [Google Scholar]
- 10.Radonic A, Milos M. Chemical composition and in vitro evaluation of antioxidant effect of free volatile compounds from Satureja montana L. Free Radical Research. 2003;37(6):673–679. doi: 10.1080/1071576031000105643. [DOI] [PubMed] [Google Scholar]
- 11.Gohari AR, Hadjiakhoondi A, Sadat-Ebrahimi SE, Saeidnia S, Shafiee A. Cytotoxic terpenoids from Satureja macrantha C. A. Mey. Daru. 2005;13(4):177–181. [Google Scholar]
- 12.Gohari AR, Saeidnia S, Gohari MR, Moradi-Afrapoli F, Malmir M, Hadjiakhoondi A. Bioactive flavonoids from Satureja atropatana Bonge. Natural Product Research. 2009;23(17):1609–1614. doi: 10.1080/14786410902800707. [DOI] [PubMed] [Google Scholar]
- 13.Gohari AR, Hadjiakhoondi A, Shafiee A, Ebrahimi ES, Mozaffarian VA. Chemical composition of the essential oils of Satureja atropatana and Satureja mutica growing wild in Iran. Journal of Essential Oil Research. 2005;17(1):17–18. [Google Scholar]
- 14.Gohari AR, Hadjiakhoondi A, Sadat-Ebrahimi E, Saeidnia S, Shafiee A. Composition of the volatile oils of Satureja spicigera and Satureja macrantha from Iran. Flavour and Fragrance Journal. 2005;21(2):348–350. [Google Scholar]
- 15.Sepehrizadeh Z, Saeidnia S, Gohari AR, Shapourabadi MB, Tabatabaei-Yazdi M, Hadjiakhoondi A. Comparison of some Satureja species by phylogenetic and chemotaxonomic analysis. International Journal of Biology and Biotechnology. 2006;3:677–680. [Google Scholar]
- 16.Eftekhar F, Raei F, Yousefzadi M, Ebrahimi SN, Hadian J. Antibacterial activity and essential oil composition of Satureja spicigera from Iran. Zeitschrift fur Naturforschung. C. 2009;64(1-2):20–24. doi: 10.1515/znc-2009-1-204. [DOI] [PubMed] [Google Scholar]
- 17.Gohari AR, Saeidnia S, Hadjiakhoondi A. Trypanocidal activity of the essential oil of Satureja macrantha and its main volatile components. International Journal of Essential Oil Therapeutics. 2007;1(4):184–186. [Google Scholar]
- 18.Gohari AR, Saeidnia S, Hadjiakhoondi A, Naghinejad A, Yagura T. Trypanocidal activity of some medicinal plants against the epimastigotes of Trypanosoma cruzi . Journal of Medicinal Plants. 2008;7(4):22–26. [Google Scholar]
- 19.Saeidnia S, Gohari AR, Kiuchi F, Honda G. In vitro anti-epimastigote activity of some Iranian medicinal plants. Iranian Journal of Pharmaceutical Research. 2005;2:101–103. [Google Scholar]
- 20.Momtaz S, Lall N, Hussein A, Ostad S, Abdollahi M. Investigation of the possible biological activities of a poisonous South African plant; Hyaenanche globosa (Euphorbiaceae) Pharmacognosy Magazine. 2010;6(21):34–41. doi: 10.4103/0973-1296.59964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Exarchou V, Godejohann M, van Beek TA, Gerothanassis IP, Vervoort J. LC-UV-Solid-Phase extraction-NMR-MS combined with a cryogenic flow probe and its application to the identification of compounds present in Greek Oregano. Analytical Chemistry. 2003;75(22):6288–6294. doi: 10.1021/ac0347819. [DOI] [PubMed] [Google Scholar]
- 22.Matlin SA, Prazeres MA, Bittner M, Silva M. Norditerpene dilactones from Podocarpus saligna. Phytochemistry. 1984;23(12):2863–2866. [Google Scholar]
- 23.Saeidnia S, Gohari AR, Uchiyama N, Ito M, Honda G, Kiuchi F. Two new monoterpene glycosides and trypanocidal terpenoids from Dracocephalum kotschyi . Chemical and Pharmaceutical Bulletin. 2004;52(10):1249–1250. doi: 10.1248/cpb.52.1249. [DOI] [PubMed] [Google Scholar]
- 24.Nessa F, Ismail Z, Mohamed N, Haris MRHM. Free radical-scavenging activity of organic extracts and of pure flavonoids of Blumea balsamifera DC leaves. Food Chemistry. 2004;88(2):243–252. [Google Scholar]
- 25.Chen Y, Liu J, Davidson RS, Howarth OW. Isolation and structure of clematine, a new flavanone glycoside from Clematis armandii Franch. Tetrahedron. 1993;49(23):5169–5176. [Google Scholar]


