Abstract
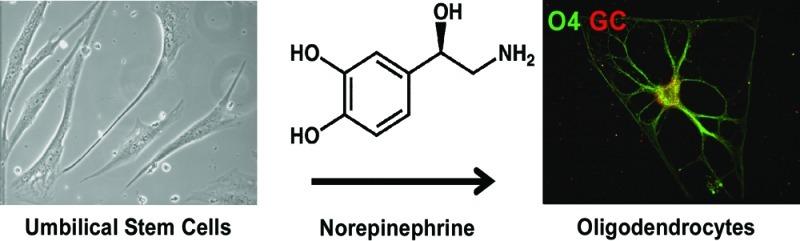
Umbilical cord stem cells would be a favorable alternative to embryonic stem cells for therapeutic applications. In this study, human multipotent progenitor cells (MLPCs) from umbilical cord were differentiated into oligodendrocytes by being exposed to a range of microenvironmental chemical and physical cues. Chemical cues were represented by a novel defined differentiation medium containing the neurotransmitter norepinephrine (NE). Under traditional two-dimensional conditions, the MLPCs differentiated into oligodendrocyte precursors but did not progress further. However, in a three-dimensional environment, the MLPCs differentiated into committed oligodendrocytes that expressed myelin basic protein. The apparent method of interaction of NE in stimulating the differentiation process was shown to occur through the adenergic pathway, while all prior differentiation methods have used other routes. This novel method of obtaining functional human oligodendrocytes from MLPCs would eliminate many of the difficulties associated with their differentiation from embryonic stem cells.
Keywords: Immunochemistry, in vitro test, mesenchymal stem cell, neural cell, surface modification, three-dimensional
There is a crucial need to establish alternatives to human embryonic stem cells (hESC) as a source of stem cells for clinical applications. This is especially true for applications to disease and injury in the central nervous system (CNS), as recent clinical trials have been delayed by the Federal Drug Administration for hESC but have been relatively straightforward for adult stem cells and stem cells from other sources. To that end, this study represents the first example of small molecule induction of oligodendrocytes from stem cells by activation of noradrenergic signaling. The multipotent progenitor cells (MLPCs) from a human umbilical cord were differentiated into oligodendrocytes in the presence of norepinephrine (NE) in a three-dimensional (3D) environment. Differentiation of these cells in a two-dimensional (2D) environment was not sufficient to allow complete maturation.
Oligodendrocytes, the cells that are responsible for myelination of axons in the CNS, originate from neuroepithelium during development1−6 and, like most other cells in the CNS, arise from Sox-1 positive neuroepithelial cells of the neural tube.7−9 The progression of the oligodendroglial lineage is characterized by morphological changes and acquisition of specific surface antigens.10−14 The oligodendrocyte progenitors can be detected with the A2B5 antibody followed by the expression of the O4 sulfatide, which persists in ramified but immature oligodendrocytes. Committed oligodendrocytes lose A2B5 reactivity after they begin to express O1 galactocerebroside. Differentiated oligodendrocytes, which are postmitotic and richly multipolar cells, express myelin basic protein (MBP) upon maturation and initiate the myelination of neurons in the CNS. In this study, oligodendrocytes were generated from Sox-1 positive MLPCs from human umbilical cord. It is possible that MLPCs, like cells of the CNS and early waves of multipotent MSCs, originate from neuroepithelium.15,16 MLPCs from umbilical cord are collected at birth and have the potential to give rise to all three embryonic layers.17
Differentiation of oligodendrocytes in the local tissue environment depends on gradients of soluble factors and physical cues. One of the soluble factors is norepinephrine (NE), a small molecule neurotransmitter released from noradrenergic neurons. The effect of NE on oligodendrocyte differentiation is not yet well understood. However, it has been reported that noradrenergic fibers contact oligodendrocytes at sites that resemble symmetrical synapses, suggesting that oligodendrocytes could be a primary target of NE.18 It has been determined that NE binds to and activates α- and β-adrenergic receptors (ARs), and oligodendrocytes express both α-1 and β-ARs.19−22 It had also been demonstrated that activation of α-1 adrenergic signaling influenced the formation of processes and the production of myelin and that activation of β-adrenergic signaling by NE inhibited proliferation and accelerated lineage progression.19−21,23,24 It was suggested that β-AR-mediated signaling may be restricted to the proliferative phases of oligodendrocyte development and dismantled after the arrest of proliferation.
The physical cues that modify differentiation are defined by mechanical forces and local architecture.25,26 These conserved and evolutionarily ancient features, although largely unknown, may be the source of many developmental cues. During development, signals are transduced to a stem or progenitor cells’ nuclei through changes in the cytoskeleton. For example, it has been shown that the fate of oligodendrocyte precursors is controlled by both spatial and geometric characteristics of an axonal niche.27 We have previously demonstrated how physical as well as chemical cues control the function of endothelial and neuronal cells in a defined system.28−32
In this study, a 3D environment that guided differentiation of MLPCs into mature oligodendrocytes was constructed. A nonbiological growth promoting substrate trimethoxy-silylpropyl-diethylenetriamine (DETA) was utilized as a physical cue as it had been demonstrated in the past to promote CNS cell growth and differentiation.28,33,34 The triamine moiety of DETA resembles the structure of spermidine, a well-known growth factor35,36 that we believe is responsible for its cytophilic properties. The chemical cues were represented by a number of soluble factors of which NE had the key function. The soluble factors alone under the 2D conditions were able to induce differentiation of MLPCs along the initial stages of the oligodendrocyte lineage; however, mature oligodendrocytes were obtained only under 3D conditions.
To date, human oligodendrocytes have been produced mostly from embryonic or fetal stem cells, raising ethical considerations.37−40 MLPCs, unlike embryonic stem cells, do not spontaneously differentiate in vitro yet are capable of extensive differentiation and expansion17 and could generate unlimited numbers of human oligodendrocytes. This will be especially important as stem cell-derived therapeutics are developed for demyelinating diseases such as multiple sclerosis (MS). From a technological standpoint, this system would also be advantageous as the time to differentiation is much shorter for the MLPCs than for hESC, and the MLPSs also can be induced using small molecules, without genetic manipulation, in a defined, serum-free system.
Results and Discussion
MLPCs and Oligodendrocytes May Share a Neuroepithelial Origin
Oligodendrocytes arise from the Sox1 positive neuroepithelium during development. Induction of oligodendrocyte fate is characterized by expression of A2B5 and PDGFR-α.41,42 To explore whether untreated MLPCs could have some neuroepithelial or oligodendrocyte progenitor characteristics, we performed immunocytochemical analysis for expression of Sox1, A2B5, and PDGFR-α. The results indicated that untreated MLPCs were Sox1 positive. This suggests that MLPCs, like oligodendrocytes, originate from the neuroepithelium. Untreated cells were PDGFR-α positive and A2B5 negative but expressed PDGFR-β (Figure 1). The negative expression of A2B5 and positive staining for PDGFR-β (Figure 1) distinguished the untreated MLPCs from oligodendrocyte progenitor cells. These results are interesting when compared to recently published findings demonstrating that Sox1+ neuroepithelium also gives rise to the first wave of multipotent mesenchymal stem cells, generated during development.15,16
Figure 1.
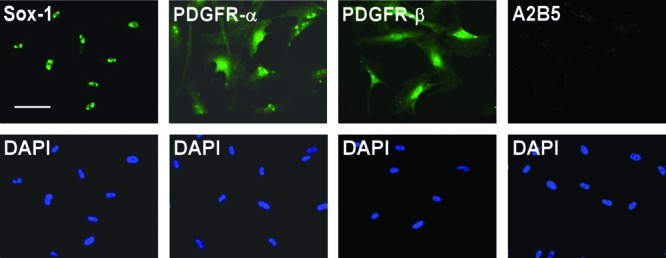
Immunocytochemical analysis of untreated MLPC suggests a neuroepithelial origin. Untreated MLPCs expressed the neuroepithelial marker Sox-1 and stained positively for PDGFR-α and PDGFR-β and negatively for A2B5. The scale bar is 100 μm (20× magnification).
Differentiation of MLPCs along an Oligodendrocyte Lineage in a 2D Environment
To investigate the effect of noradrenergic signaling on the oligodendrocyte development, MLPCs were differentiated in a defined, serum-free culture system. Prior to differentiation, the MLPCs were plated on DETA-coated coverslips. DETA is an aminosilane that forms a covalently bound, uniform, self-assembled monolayer on the glass surface. DETA contains a triamine moiety that resembles spermidine, a known growth factor, in its terminal group. At physiologic pH, these amines carry partial positive charges, providing a hydrophilic surface promoting cellular attachment. DETA is nondigestible by matrix metalloproteases secreted by the cells, and therefore, it promotes long-term cell survival. Prior to experiments, DETA surfaces were characterized by XPS analysis to ensure that the complete monolayer was formed during the self-assembly process and the hydrophilic property of surfaces was confirmed by static contact angle measurements of 45.6 ± 2°. Once characterized, DETA-coated coverslips were used for plating and differentiation of MLPCs.
Plated MLPCs were allowed to evenly spread and expanded either for 3 days or to 60% confluence. Then the culture medium was replaced with the preinduction medium supplemented with bFGF, EGF, and PDGF-AA. After 24 h, cells were transferred into the differentiation medium, containing growth factors bFGF, EGF, and PDGF-AA along with K252a, heparin, forskolin, and NE. The essential factors were NE, forskolin, and K252a, as the desired morphology was not observed in the absence of any of these factors (Figure 2A). Both forskolin and K252a are frequently used during stem cell differentiation; however, norepinephrine emerged as the novel stem cell differentiation factor. The absence of the growth factors increased the differentiation rate but resulted in a decreased level of survival and less elaborate process formation. After the transfer into the differentiation medium, the MLPCs exhibited cell shape changes, from that of a fibroblast morphology to refractile cell bodies.
Figure 2.
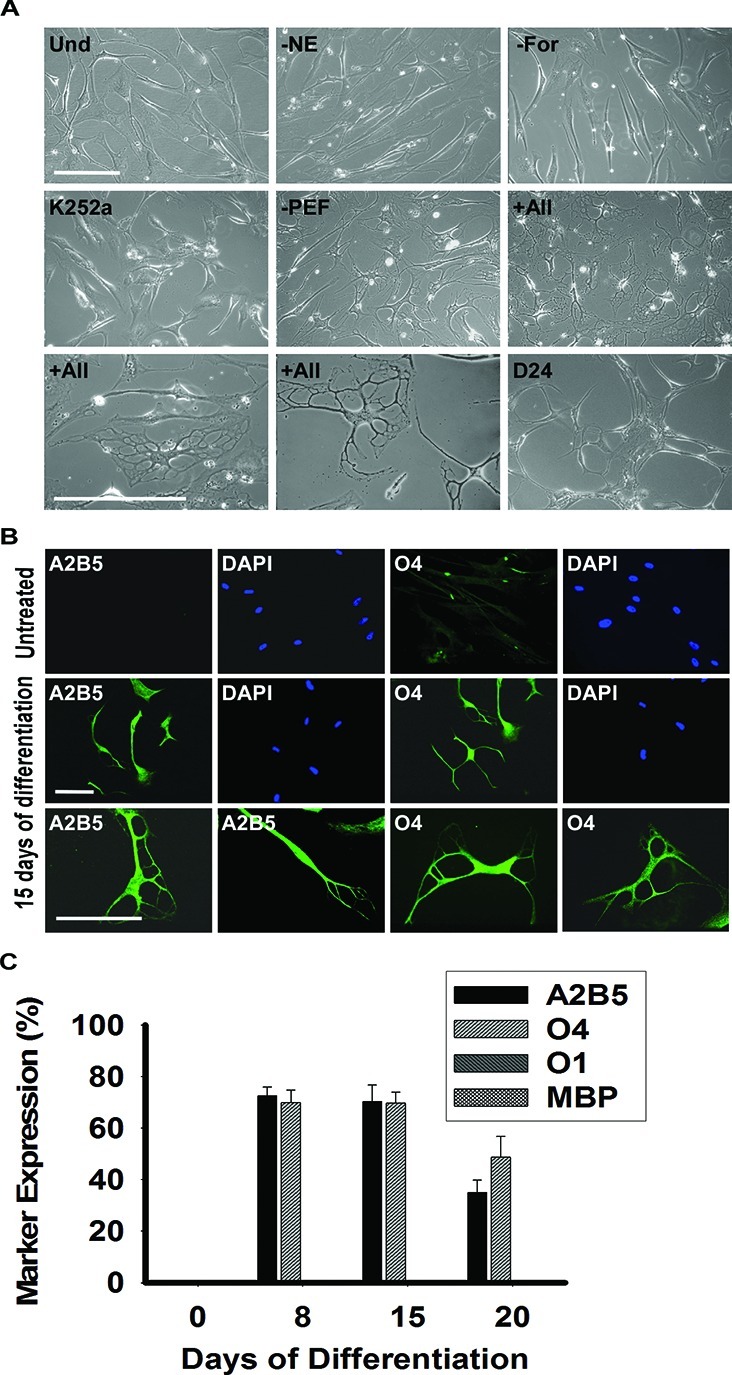
Phase contrast images and immunocytochemical analysis of differentiating MLPCs in a 2D environment. (A) Undifferentiated MLPCs (Und) exhibited fibroblast morphology. Cells after 15 days in differentiation medium without NE, forskolin (For), or K252a retained their fibroblast morphology. Process formation was visible in the medium without growth factors PDGF-AA, EGF, and bFGF (PEF). Refractile cell bodies and an increased level of process formation were observed in the presence of all factors indicated above for days 15–20. Cells lost their multipolar morphology and became bipolar or spindle-shaped after approximately 20–24 days. The scale bars are 100 μm (20× and 40× magnification). (B) Immunocytochemical analysis of differentiating MLPCs in a 2D environment. The untreated MLPCs showed negative staining for A2B5 and faint staining for O4. After differentiating for 15 days, cells exhibited positive staining for A2B5 and O4, characteristics of immature oligodendrocyte precursor cells. The scale bars are 100 μm (rows 1 and 2, 20× magnification; row 3, 40× magnification). (C) MLPCs do not differentiate into committed oligodendrocytes in a 2D environment. After differentiation for 8 days, 72.4% of cells were positive for A2B5 and 69.9% for O4, and after 15 days, 70.3% of MLPCs exhibited positive staining for A2B5 and 69.7% for O4. After 20 days, 35.0% of cells remained A2B5 positive and 49.7% O4 positive. Expression of O1 galactocerebroside and MBP was absent in both untreated and differentiating cells. Error bars represent the SD.
Within 8 days, approximately 70% of cells developed multiple processes, and Figure 2A reflects the morphology development at day 15. Immunocytochemical analysis was performed using the antibodies for specific stages of oligodendrocyte differentiation (Figure 2B). The untreated MLPCs showed negative staining for A2B5 and faint staining for O4. Cells were also negative for the more mature oligodendrocyte markers O1 galactocerebroside and MBP. However, after differentiation for 8 days, 72.4 ± 3.4% of cells exhibited positive staining for A2B5 and 69.9 ± 4.9% for O4, but expression of O1 galactocerebroside and MBP was absent in the 2D environment.
The results indicate that in response to the treatment, the majority of MLPCs acquired cellular characteristics of immature oligodendrocyte precursors. The expression of A2B5 and O4, accompanied by a multiprocess morphology, persisted to day 15, but the precursors did not achieve a fully differentiated phenotype. After 15 days, cells began to lose their multiprocess morphology and became mostly bipolar and spindle-shaped (Figure 2A). At day 20, 35.0 ± 4.8% of cells remained A2B5 positive and 49.7 ± 7.9% O4 positive and O1 and MBP negative (Figure 2C). In addition, limited cell survival was observed after 20 days.
Differentiation of MLPCs in a 3D Environment
Because it has been shown to be an important feature in cellular development, we examined the effect of a simple 3D environment on oligodendrocyte lineage progression. To construct this 3D environment, we differentiated cells between two coverslips. Initially, undifferentiated cells were plated onto DETA-coated coverslips at the bottom of 12-well plates. At 60% confluence, the culture medium was replaced with the preinduction medium and then an unmodified glass coverslip was placed atop the cultured cells.
Cell flattening and spreading were observed in the 3D environment within 24 h (Figure 3B). After 24 h, the preinduction medium was replaced with differentiation medium and there was a further increase in the level of cell flattening (Figure 3C), but within 10 days, cells began to form processes and the cell bodies slowly contracted. Process development and branching continued for 3 weeks. After 30 days, approximately 85% of the cells had elaborated an extensive network of processes (Figure 3D–F). The presence of PDGF was required for process formation, as in its absence cells progressed through initial differentiation stages but lost their multiprocess morphology after 2 weeks. The presence of bFGF and EGF was not essential but resulted in an increased level of branching and the development of highly elaborated processes (Figure 3G–I). The influence of bFGF and EGF on oligodendrocyte process regeneration has been previously reported,43,44 and our results suggest a role for both growth factors in process formation in vitro, as well.
Figure 3.
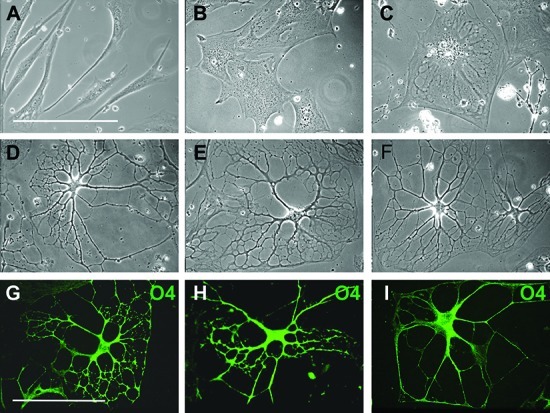
3D environment promotes further differentiation of MLPCs. (A–F) Phase contrast images of differentiating MLPCs. (A) The untreated cells displayed typical fibroblast morphology. (B) Cells exhibited flattening and spreading at 24 h in the 3D environment. (C) Cells after 8 days in the differentiation medium displayed an increased level of flattening. (D–F) After differentiation for 30 days, approximately 80% of cells revealed extensive processes. (G–I) Growth factors influenced the development of processes. (G and H) Immunostained cells displaying increased levels of branching and development of processes in the presence of bFGF and EGF. (I) Simple processes were observed in the absence of bFGF and EGF. Scale bars are 100 μm (40× magnification).
Immunocytochemical analysis revealed that after differentiation for 20 days 81.8 ± 6.6% of cells expressed the oligodendroglial marker A2B5 and 80.6 ± 2.9% O4 (Figure 4C). After 30 days, 57.7 ± 3.6% of the cells stained positively for A2B5, 79.6 ± 2.9% for O4, 42.1 ± 2.7% for the committed oligodendrocyte marker O1 galactocerebroside, and 15.2 ± 0.5% for MBP (Figure 4A–C). The 3D environment appeared to play a key role during differentiation, oligodendrocyte commitment, and lineage progression. Even after the removal of NE from the differentiation medium after 20 days, the cells retained their differentiated morphology after an additional 10 days in culture.
Figure 4.
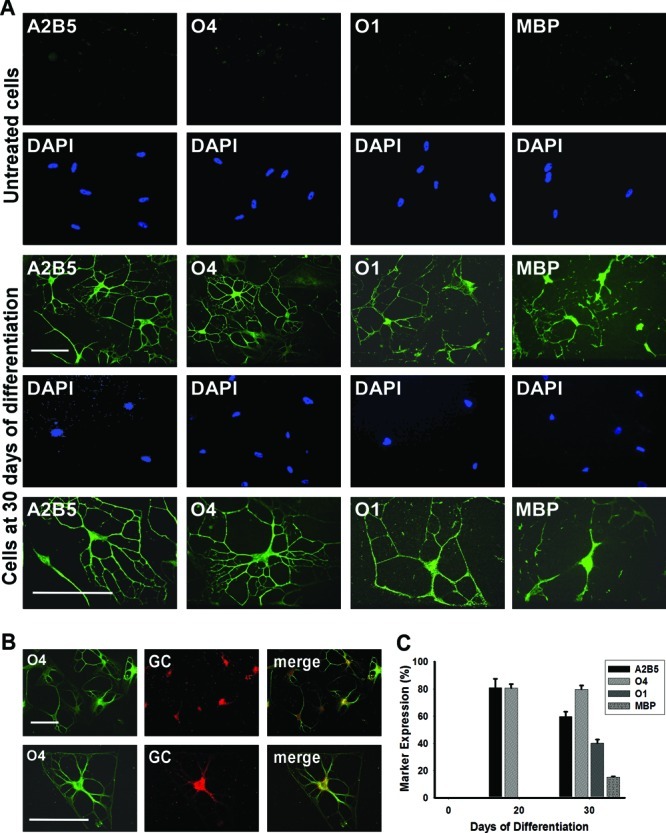
MLPCs differentiate into committed oligodendrocytes in a 3D environment. (A) Immunocytochemical analysis of differentiating MLPCs in a 3D environment. The untreated MLPCs indicated negative staining for A2B5, faint staining for O4, and negative staining for O1 galactocerebroside and MBP. After differentiation for 30 days, cells exhibited intensely positive staining for A2B5 and O4. Cells also expressed O1 galactocerebroside and MBP, characteristic of committed oligodendrocytes. Scale bars are 100 μm (20× and 40× magnification). (B) Coexpression of O4 and galactocerebroside (GC) in the differentiated cells. After differentiation for 30 days, GC was expressed in O4 positive cells. Scale bars are 100 μm (row 1, 20× magnification; row 2, 40× magnification). (C) Progression of differentiation in a 3D environment. After differentiation for 20 days, 81.8% of cells expressed oligodendroglial marker A2B5 and 80.6% O4 and were negative for O1 and MBP. After differentiation for 30 days, 57.7% of cells stained positively for A2B5, 79.6% for O4, 42.1% for committed oligodendrocyte marker O1, and 15.2% for MBP. Error bars represent the SD.
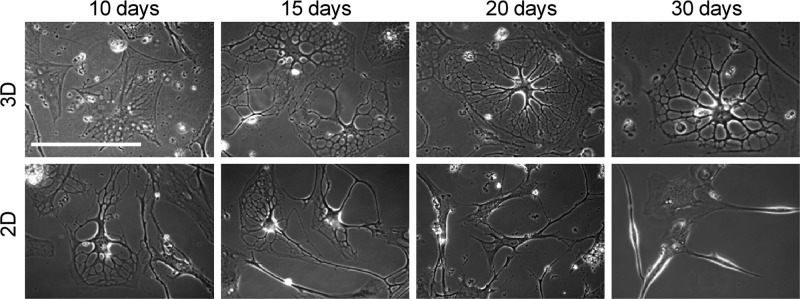
This is in contrast to the cells in the 2D environment that appeared to dedifferentiate after 20 days in culture and never expressed oligodendrocyte markers O1 and MBP. A direct comparison of the morphological differences over a 30 day period is shown in Figure 5.
Figure 5.
Morphological comparison of differentiating cells in 3D and 2D environments. Differentiating MLPCs exhibit different morphologies in 3D and 2D environments over time. Cells displayed the formation of extensive processes at day 15 in both 2D and 3D environments. However, process development and branching were visible at day 30 in only the 3D environment. Differentiating cells lost their multipolar morphology and became bipolar or spindle-shaped with decreased viability by day 30 in the 2D environment.
The contribution of the surface chemistry of the top coverslip was also investigated as it has been shown to have a dramatic effect on cellular response and differentiation.30,31,45,46 To determine the most appropriate 3D conditions for differentiation, the top glass coverslips were modified with various surface chemistries that promote or repel cell adhesion (Table 1).
Table 1. Percentages of Cells Developing Processes in Response to Top Coverslip Modification and Contact Angles for Each Surfacea.
| top coverslip modification |
||||
|---|---|---|---|---|
| development of processes (%) | DETA | PEG | 13F | unmodified glass |
| day 20 | 66.9 ± 2.8 | 53.2 ± 1.3 | 38.1 ± 3.1 | 77.6 ± 2.2 |
| day 30 | 69.2 ± 10.4 | 55.5 ± 8.2 | b | 85.0 ± 2.1 |
| contact angle | 46 ± 2° | 37 ± 2° | 88 ± 2° | <5° |
Data are means ± SD for three coverslips after differentiation for 20 and 30 days.
No surviving cells were observed on day 30.
Unmodified glass coverslips were used as a control. To promote cell adhesion, the top coverslip was coated with a DETA monolayer. This environment, in which cells were attached to both top and bottom coverslips, produced initially good differentiation because DETA is a cell adhesion- and cell survival-promoting molecule. However, strong cell adhesion to both the top and bottom coverslips eventually caused an increased level of cell death, probably because of damage from cell movement during feeding and evaluation.
For the inverse situation, the top coverslip was coated with polyethylene glycol (PEG) or with a nonadhesive fluorinated silane (13F) monolayer. The PEG coating is hydrophilic and had a measured contact angle of 37 ± 2° but is a protein cell resistant surface, while 13F is a hydrophobic surface and had a measured contact angle of 88 ± 2°. XPS analysis confirmed the presence of a monolayer for both surface modifications. The PEG-coated top coverslips resulted in less differentiation, and 13F-coated coverslips triggered significant cell death. Thus, it was determined that the glass coverslip controls were the most suitable top surfaces, as the cells did not adhere well to the glass and remained on the bottom DETA-coated coverslips after the top had been removed for analysis.
MLPCs Express Functional ARs in the 3D System
To investigate the role of adrenergic signaling mechanisms in differentiation of oligodendrocyte from the MLPCs, immunocytochemical analysis was performed for expression of α- and β-ARs. The findings indicated that MLPCs already expressed β1-ARs on the cell surface before differentiation (Figure 6A). No expression of β2-ARs before or during differentiation was detected. Expression of α1-ARs was first observed at the nucleus, and the intensity of staining significantly increased after treatment with preinduction medium supplemented with bFGF, EGF, and PDGF-AA (Figure 6B). Further analysis revealed that bFGF alone was able to increase the level of nuclear expression of α1-ARs (Figure 6C) in a time- and dose-dependent manner (results not shown). On day 15, the α1-ARs began to relocate to the cell surface. On day 30, differentiated cells expressed α1-ARs at the surface of cell bodies and to a lesser degree in the processes (Figure 6B). This surface expression was observed only in cells with a multiprocess morphology, whereas in cells exhibiting a flat morphology, or undifferentiated cells, the α1-ARs remained at the nucleus. These results are consistent with recent findings demonstrating nuclear localization of α1-ARs, in which a signal is transduced from the nucleus to the plasma membrane, providing a new model for α1-AR signaling.47,48 In this previously published model, activation of nuclear α1-ARs resulted in localization of activated ERK at the plasma membrane. In our studies, the same initial expression patterns were noted in both the 2D and 3D systems, but the cells began to lose viability past day 20 under the 2D conditions.
Figure 6.
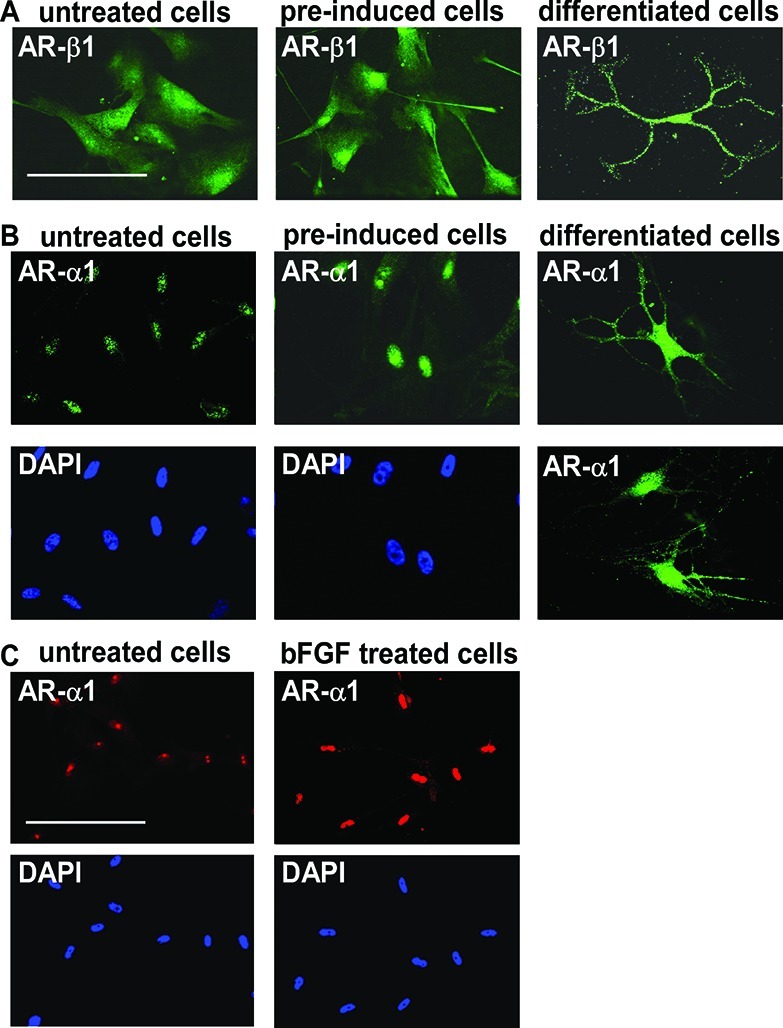
Expression of α1- and β1-ARs. (A) Positive staining for β1-ARs in undifferentiated cells, cells preinduced for 24 h with bFGF, EGF, and PDGF-AA, and cells after differentiation for 30 days. The scale bar is 100 μm. (B) Nuclear expression of α1-ARs in undifferentiated cells, intensive nuclear staining in cells preinduced for 24 h with bFGF, EGF, and PDGF-AA, and surface expression after differentiation for 30 days. (C) Nuclear expression of α1-ARs in undifferentiated cells and evidence of a significant increase in staining intensity after treatment with bFGF alone for 24 h. Scale bars are 100 μm (40× magnification).
Activation of Both α- and β-AR Is Essential for Differentiation in the 3D System
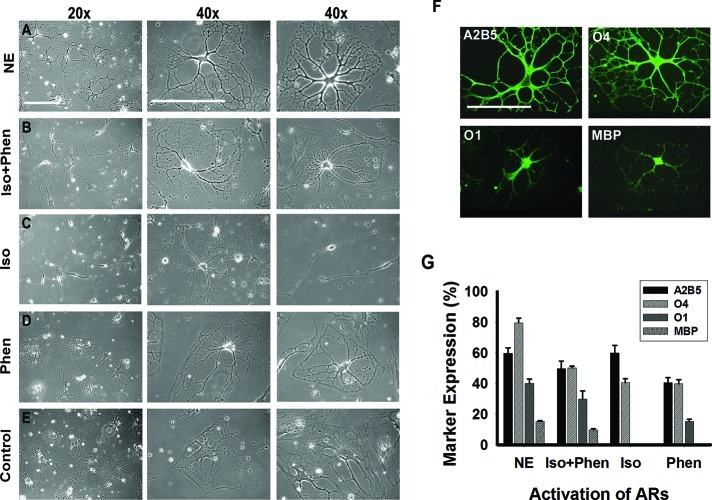
To assess the role of each adrenergic receptor in the differentiation process, NE was substituted in the differentiation medium with equimolar concentrations of the β-AR agonist isoproterenol, the α1-AR agonist phenylephrine, or both agonists. Daily treatment with isoproterenol induced morphological changes, cell body contraction, and formation of processes within the first 15 days. However, approximately 60% of the cells displayed a bipolar morphology resembling that of immature oligodendrocyte progenitors. Cells did not change their bipolar morphology within 30 days of differentiation (Figure 7C) and exhibited enhanced cell death. To characterize these cells, immunocytochemical analysis on day 30 was done. We observed that 57.9 ± 4.9% of the cells expressed A2B5 and 42.5 ± 2.7% O4; however, the cells were O1 and MBP negative (Figure 7G). These results demonstrated that stimulation of β-AR with isoproterenol induced the initial stages of differentiation. However, β-AR treatment alone was not sufficient to direct the MLPCs into a more mature stage of differentiation.
Figure 7.
Influence of ARs on differentiation of MLPC into oligodendrocytes. (A) Phase contrast images of cells after differentiation for 30 days, in the presence of NE, that exhibited a complex multipolar morphology. (B) Cells differentiated for 30 days in the medium in which NE was substituted with the β-AR agonist isoproterenol and the α1-AR agonist phenylephrine. Cells were morphologically comparable to cells treated with NE. (C) Cells differentiated in the presence of the β-AR agonist isoproterenol frequently displayed a bipolar morphology, resembling immature oligodendrocyte progenitors. (D) Substitution of NE with the α1-AR agonist phenylephrine resulted in a mature morphology in 10% of cells. The remainder of the cells showed only partial process development or remained flat. (E) Cells differentiated for 30 days without activation of ARs by NE or AR agonists continued to exhibit a mostly flat morphology. (F) Stimulation of both α1- and β1-ARs is required for optimal differentiation. Immunocytochemical analysis of cells differentiated for 30 days in the presence of the β-AR agonist isoproterenol and the α1-AR agonist phenylephrine. Cells stained positively for A2B5, O4, O1, and MBP. Scale bars are 100 μm. (G) Comparison of differentiation levels achieved by activation of both ARs by NE, by both AR agonists (Iso+Phen), by β-AR by isoproterenol (Iso), and by α1-AR agonist phenylephrine (Phen). Error bars represent the SD.
Daily treatment of the MLPCs with the α1-AR agonist phenylephrine initially resulted in only modest effects. Good survival was observed, but the majority of cells maintained a flat morphology. Within 15 days of differentiation, approximately 40% of the cells began to develop processes; however, on day 30, only 15% of the cells exhibited a more mature morphology with developed processes. The majority of cells showed only partial process development and branching or maintained a flat morphology (Figure 7D). Immunocytochemical analysis on day 30 revealed that 40.5 ± 3.4% of cells stained positively for A2B5, 39.8 ± 2.8% for O4, and 15.2 ± 1.5% for O1 (Figure 7G). The results suggested that activation of α1-AR could play a role in more advanced stages of differentiation in which cells start to lose expression of A2B5 and begin to express O1.
Daily treatments of cells with both isoproterenol and phenylephrine resulted in formation of multiple processes, and the treated cells became morphologically similar to those treated with NE (Figure 7A,B). On day 30, 48.7 ± 4.9% of the cells stained positively for A2B5, 50.2 ± 1.1% for O4, 28.9 ± 5.2% for O1, and 9.7 ± 0.7% for MBP (Figure 7F,G). The results indicate that stimulation of both the α1- and β-ARs is required for optimal differentiation. This suggests a close interplay between both ARs, ultimately resulting in the expression of genes essential for oligodendrocyte development.
Human umbilical cord stem cells are a highly promising source of cells for tissue regeneration.49 Compared to adult stem cells obtained from bone marrow, these cells are immature and elicit a low incidence of graft rejection, graft-versus-host disease, and low post-transplant infections.49 Besides this biological advantage, these stem cells are abundantly available and routinely harvested without risk to the donor.50 Compared to embryonic stem cells obtained from human embryos, human umbilical cord stem cells not only are more easily obtained but also may be more suitable for cellular therapeutics. Embryonic stem cells have been associated with reports of the uncontrollable development of teratomas in a syngeneic transplantation model, imprinting-related developmental abnormalities, and ethical issues. Human umbilical cord stem cells, readily available and differentiated by environmental signals, offer some potential therapeutic advantages.
This study has indicated that MLPCs display extreme sensitivity to their environment. Their fate depends not only on soluble factors but also on the surrounding physical cues. The combination of these external signals altered the cell morphology and fate decision to differentiate along the oligodendrocyte lineage. Electron microscopy studies have provided evidence of a noradrenergic control of the oligodendroglial and astroglial cells throughout the cortex.18 Oligodendrocytes were the major target of the noradrenergic fibers, exhibiting a light thickening at the sites of contact. It was reported that oligodendrocytes expressed α1 and β-ARs and their activation by NE-accelerated differentiation of the oligodendrocyte precursors.19,20,22 In spite of this, there are no known studies using NE as a key factor to induce differentiation of stem cells into oligodendrocytes. To explore this possibility, MLPCs were analyzed for expression of ARs and found that the undifferentiated cells expressed both α1-ARs and β1-ARs. The β1-ARs were localized on the surface before and during differentiation. Surprisingly, typical surface expression of the α1-ARs was not observed; instead, the α1-ARs were localized at the nucleus. The intensity of nuclear staining significantly increased after treatment with bFGF. However, as the cells exhibited a more differentiated phenotype after differentiation for 15 days, relocation of α1-ARs to the surface was observed. Nuclear localization of α1-ARs is consistent with a recently proposed model for α1-AR signaling in cardiac myocytes. In this new model, activation of α1-AR signaling was initiated at the nuclear membrane and resulted in localization of activated ERK in calveolae at the plasma membrane.47,48
Differentiation was initiated by the transfer of MLPCs into the differentiation medium in a 2D environment. The differentiation medium contained NE along with forskolin, K252a, heparin, PDGF-AA, bFGF, and EGF. Within 8 days, process formation was observed and immunocytochemical analysis indicated a positive reactivity to A2B5 and O4 primary antibodies. In spite of this, cells did not progress further along the oligodendrocyte lineage. After 2 weeks, cells exhibited a bipolar and spindlelike morphology and remained A2B5 and O4 positive but O1 negative, and a prolonged differentiation time significantly increased the level of cell death.
A 3D microenvironment was constructed to combine chemical and physical cues and further influence a lineage commitment. The MLPCs responded to the 3D environment initially by cell flattening and later, within 2 weeks, formation of processes. At 30 days, 42.1 ± 2.7% of cells expressed the O1 antigen, indicating terminally differentiated oligodendrocytes, and 15.2 ± 0.5% of the cells expressed MBP with an increased level of cell survival. The differentiated cells survived for more than 40 days in culture. It has been demonstrated that stimulation of β-ARs induces differentiation through an increase in the intracellular level of cAMP and through the activation of proteins known to be involved in cell cycle arrest.19 There are also studies revealing roles of p38MAPK and Erk1/2 in differentiation of oligodendrocyte progenitors.51 Both ARs can activate p38MAPK, while Erk1/2 is a downstream target of α1-AR. It was shown in these studies that p38MAPK activity was required for the progression of bipolar early progenitors (A2B5 positive and O4 negative) to multipolar late progenitors (O4 positive and O1 negative), and Erk1/2 activity was essential for the progression of late progenitors to oligodendrocytes.52
Our results indicated that the majority of cells treated with the β-AR agonist isoproterenol remained bipolar even after differentiation for 30 days. Immunocytochemical analysis indicated differentiation arrest at the stage where A2B5 positive cells begin to express O4, perhaps because of insufficient activation of Erk1/2 signaling. In contrast, cells treated with the α1-AR agonist phenylephrine showed a higher level of survival, and after differentiation for 30 days, 15.2 ± 1.5% of cells had progressed to the O1 positive stage, possibly because of an increased level of stimulation of the Erk1/2 signaling pathway. However, simultaneous activation of both receptors by NE, or by both agonists, was the best strategy, possibly by supplying the optimal cAMP levels and stimulating essential signaling pathways engaged in the close interplay during differentiation.
This study demonstrates the significance of the cellular microenvironment as a driving aspect in human stem cell differentiation. A 3D environment was constructed, and novel signaling pathways were utilized to induce differentiation of MLPCs; neither condition alone produced functional differentiation. The mechanical cues in combination with soluble factors influenced the progression of MLPCs along the oligodendrocyte lineage. Future studies will improve our understanding of the role of the microenvironment and noradrenergic signaling in oligodendrocyte differentiation and will determine the applicability of cells in studying and treating conditions such as MS, neuropathies, and traumatic brain injury. However, it is reasonable to assume that this methodology would be directly applicable to SOX1 positive cells of neuroepithelial origin and possibly other stem cells.
Methods
Modification of Surfaces
Glass coverslips were cleaned using an HCl/methanol mixture (1:1), soaked in concentrated H2SO4 for 30 min, and then rinsed in doubly deionized H2O. Coverslips were then boiled in deionized water, rinsed with acetone, and dried in an oven. The trimethoxysilylpropyldiethylenetriamine (DETA, United Chemical Technologies), tridecafluoro-1,1,2,2-tetrahydroctyl-1-trichlorosilane (13F, Gelest), and polyethylene glycol (PEG, Sigma Chemical Co., St. Louis, MO) monolayers were formed by the reaction of the cleaned surfaces with a 0.1% (v/v) mixture of the organosilane in toluene (Fisher T2904). The DETA coverslips were heated to just below the boiling point of toluene for 30 min, rinsed with toluene, reheated to just below the boiling temperature, and then dried in an oven. Surfaces were characterized by contact angle and X-ray photoelectron spectroscopy methods as described previously.46
Cell Culture
MLPCs, human umbilical cord blood-derived clonal cell lines, passage 5, were purchased from BioE Inc. (St. Paul, MN). The company describes the cells as CD34, CD45, SSEA-3, SSEA-4, STRO-1, SCF, CD9, and CD133 positive early in culture. They also indicate that they are a unique phenotype because they are CD34 and CD45 positive and differ morphologically from mesenchymal and hematopoietic stem cells. They have a normal and stable karyotype; they are capable of differentiating into all three embryonic layers, and they have been cloned from a single cell. The cells were cultured in tissue culture-treated T-75 flasks and maintained in high-glucose Dulbecco’s modified Eagle's medium (DMEM, Gibco BRL, Rockville, MD) with the addition of 15% fetal bovine serum (Stem Cell Technologies, Vancouver, BC), and 1% Antibiotic Antimycotic (Gibco BRL) at 37 °C in a humidified atmosphere containing 5% CO2. The medium was changed every 3–4 days. Cells were passaged by trypsinization (0.05% trypsin/EDTA solution; Gibco BRL) upon reaching 60% confluence and replated at a 1:3 ratio. Passage 8 was used for the experiments unless otherwise indicated.
Induction of Oligodendrocyte Differentiation
The MLPCs were seeded on DETA-coated glass coverslips (18 mm) at a density of 4 × 103 cells/cm2 in growth medium. After 3 days, at 60% confluency, cells were incubated for 24 h in preinduction medium, which consisted of DMEM, 15% FBS, FGF-2, EGF (10 ng/mL each; R&D Systems, Minneapolis, MN), and PDGF-AA (10 ng/mL; Chemicon International, Temecula, CA). To initiate differentiation, the preinduction medium was removed, and the cells were washed twice with Hanks’ balanced salt solution (Gibco BRL) and transferred to serum-free differentiation medium. Differentiation medium consisted of DMEM, N2 supplement (1%; Gibco BRL), 10 μM forskolin, 5 units/mL heparin (Sigma Chemical Co.), 5 nM K252a, FGF-2, EGF, PDGF-AA (10 ng/mL each), and 20 μM NE (Sigma Chemical Co.). The differentiation medium was changed every other day, while NE was added daily. For agonist experiments, norepinephrine was substituted with either the α1-adrenoceptor agonist isoproterenol or the β-receptor agonist isoproterenol (20 μM each; Sigma Chemical Co.).
Construction of the 3D Environment
MLPCs were plated on DETA-coated glass coverslips (18 mm) in growth medium. At 60% confluence, growth medium was replaced with preinduction medium. After the medium had been replaced, another set of coverslips was placed atop the cells. Prior to the placement, top coverslips were sterilized with ethanol and washed with preinduction medium. After 24 h, preinduction medium was removed, and the cells were washed twice with Hanks’ balanced saltssolution and transferred to serum-free differentiation medium. The differentiation medium was changed every other day, and NE was added daily.
Morphological Analysis
Phase contrast images were taken with a commercial Nikon Coolpix 990 camera using the Zeiss Axiovert S100 microscope. Pictures were analyzed using Scion Image (Scion Corp., Frederick, MD).
Immunocytochemistry
To characterize cells by immunocytochemistry, the top coverslips, if present, were first carefully removed. Cells were then briefly washed with Hanks’ balanced salt solution and fixed in 4% paraformaldehyde for 18 min. Fixed cells were stored in PBS, permaebilized with 0.5% Triton X-100 in PBS for 7 min, blocked with 5% donkey serum for 1 h, and then incubated with the primary antibody overnight at 4 °C. Primary antibodies were mouse monoclonal A2B5 (MAB312, 1:250), O4 (MAB345, 1:100), O1 (MAB344, 1:200), MBP (1:25), and rabbit polyclonal anti-galactocerebroside (AB142, 1:200) (all from Chemicon International); mouse monoclonal β2-AR (sc-81577, 1:200) and rabbit polyclonal β1-AR (sc-567, 1:250) (from Santa Cruz Biotechnology); and α1-AR (ab3462, 1:1000; Abcam Inc., Cambridge, MA). After being washed with PBS, cells were incubated with an Alexa Fluor 488-conjugated anti-mouse IgG or Alexa Fluor 488-conjugated anti-rabbit IgG for 2 h at room temperature. After being washed with PBS, the coverslips were mounted with Vectashield mounting medium (H1000, Vector Laboratories, Burlingame, CA) onto slides. For visualization of cellular nuclei, the specimens were counterstained with DAPI. Immunoreactivity was observed and analyzed by using an Ultra VIEWLCI confocal imaging system (Perkin-Elmer).
Quantification
Morphological and immunocytochemical quantification was performed on undifferentiated stem cells or cells during various differentiation stages. For each coverslip, at least 10 pictures were taken from randomly chosen views under 20× magnification. All the marker positive cells were counted, as well as the total number of cells in these views. At least three coverslips in each group were quantified, and data were expressed as the average ± standard deviation (SD). Statistical differences between different experimental groups were analyzed via the one-way ANOVA (analysis of variance) test and an independent Student’s t test.
Glossary
Abbreviations
- ARs
adrenergic receptors
- bFGF
basis fibroblast growth factor
- cAMP
cyclic adenosine monophosphate
- CNS
central nervous system
- DETA
trimethoxy-silylpropyl-diethylenetriamine
- EGF
epidermal growth factor
- Erk
extracellular signal-regulated kinase
- hESC
human embryonic stem cells
- MBP
myelin basic protein
- MLPCs
multipotent progenitor cells
- MS
multiple sclerosis
- NE
norepinephrine
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- PEG
polyethylene glycol
- p38MAPK
p38 mitogen-activated protein kinase
- SD
standard deviation
- SOX1
SRY-related HMG box1
- 2D
two-dimensional
- 3D
three-dimensional
- 13F
fluorinated silane.
Funding for this research was provided by National Institutes of Health Grant R01-NS050452.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Bunge M. B.; Bunge R. P.; Pappas G. D. (1962) Electron microscopic demonstration of connections between glia and myelin sheaths in the developing mammalian central nervous system. J. Cell Biol. 12, 448–453. [DOI] [PubMed] [Google Scholar]
- Bunge R. P. (1968) Glial cells and the central myelin sheath. Physiol. Rev. 48, 197–251. [DOI] [PubMed] [Google Scholar]
- Hirano A. (1968) A confirmation of the oligodendroglial origin of myelin in the adult rat. J. Cell Biol. 38, 637–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N.; Pringle N.; Richardson W. D. (2008) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos. Trans. R. Soc. London, Ser. B 363, 71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentas D. M.; Miller R. H. (1998) Regulation of oligodendrocyte development. Mol. Neurobiol. 18, 247–259. [DOI] [PubMed] [Google Scholar]
- Peters A. (1964) Observations on the Connexions between Myelin Sheaths and Glial Cells in the Optic Nerves of Young Rats. J. Anat. 98, 125–134. [PMC free article] [PubMed] [Google Scholar]
- LeVine S. M.; Goldman J. E. (1988) Embryonic divergence of oligodendrocyte and astrocyte lineages in developing rat cerebrum. J. Neurosci. 8, 3992–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll E.; Miller R. H. (1993) Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development 118, 563–573. [DOI] [PubMed] [Google Scholar]
- Warf B. C.; Fok-Seang J.; Miller R. H. (1991) Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J. Neurosci. 11, 2477–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R.; Pfeiffer S. E. (1992) Novel stage in the oligodendrocyte lineage defined by reactivity of progenitors with R-mAb prior to O1 anti-galactocerebroside. J. Neurosci. Res. 32, 309–316. [DOI] [PubMed] [Google Scholar]
- Curtis R.; Cohen J.; Fok-Seang J.; Hanley M. R.; Gregson N. A.; Reynolds R.; Wilkin G. P. (1988) Development of macroglial cells in rat cerebellum. I. Use of antibodies to follow early in vivo development and migration of oligodendrocytes. J. Neurocytol. 17, 43–54. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S. E.; Warrington A. E.; Bansal R. (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol. 3, 191–197. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H.; del Cerro C.; Kies M. W.; Herndon R. M. (1985) Immunocytochemistry of myelin basic proteins in adult rat oligodendroglia. J. Neuroimmunol. 7, 355–363. [PubMed] [Google Scholar]
- Volpe J. J. (2008) Neurology of the Newborn, 5th ed., Elsevier Health Sciences, Amsterdam. [Google Scholar]
- Miller F. D. (2007) Riding the waves: Neural and nonneural origins for mesenchymal stem cells. Cell Stem Cell 1, 129–130. [DOI] [PubMed] [Google Scholar]
- Takashima Y.; Era T.; Nakao K.; Kondo S.; Kasuga M.; Smith A. G.; Nishikawa S. (2007) Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 129, 1377–1388. [DOI] [PubMed] [Google Scholar]
- van de Ven C.; Collins D.; Bradley M. B.; Morris E.; Cairo M. S. (2007) The potential of umbilical cord blood multipotent stem cells for nonhematopoietic tissue and cell regeneration. Exp. Hematol. 35, 1753–1765. [DOI] [PubMed] [Google Scholar]
- Paspalas C. D.; Papadopoulos G. C. (1996) Ultrastructural relationships between noradrenergic nerve fibers and non-neuronal elements in the rat cerebral cortex. Glia 17, 133–146. [DOI] [PubMed] [Google Scholar]
- Ghiani C. A.; Eisen A. M.; Yuan X.; DePinho R. A.; McBain C. J.; Gallo V. (1999) Neurotransmitter receptor activation triggers p27(Kip1)and p21(CIP1) accumulation and G1 cell cycle arrest in oligodendrocyte progenitors. Development 126, 1077–1090. [DOI] [PubMed] [Google Scholar]
- Khorchid A.; Cui Q.; Molina-Holgado E.; Almazan G. (2002) Developmental regulation of α1A-adrenoceptor function in rat brain oligodendrocyte cultures. Neuropharmacology 42, 685–696. [DOI] [PubMed] [Google Scholar]
- Khorchid A.; Larocca J. N.; Almazan G. (1999) Characterization of the signal transduction pathways mediating noradrenaline-stimulated MAPK activation and c-fos expression in oligodendrocyte progenitors. J. Neurosci. Res. 58, 765–778. [PubMed] [Google Scholar]
- Ventimiglia R.; Greene M. I.; Geller H. M. (1987) Localization of β-adrenergic receptors on differentiated cells of the central nervous system in culture. Proc. Natl. Acad. Sci. U.S.A. 84, 5073–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asotra K.; Macklin W. B. (1993) Protein kinase C activity modulates myelin gene expression in enriched oligodendrocytes. J. Neurosci. Res. 34, 571–588. [DOI] [PubMed] [Google Scholar]
- Cohen R. I.; Almazan G. (1993) Norepinephrine-stimulated PI hydrolysis in oligodendrocytes is mediated by α1A-adrenoceptors. NeuroReport 4, 1115–1118. [PubMed] [Google Scholar]
- Burdick J. A.; Vunjak-Novakovic G. (2009) Review: Engineered Microenvironments for Controlled Stem Cell Differentiation. Tissue Eng., Part A 15, 205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V.; Sheetz M. (2006) Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. S.; Kelland E. E.; Tokar E.; De la Torre A. R.; Chan J. R. (2008) The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc. Natl. Acad. Sci. U.S.A. 105, 14662–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M.; Bhargava N.; Gregory C.; Riedel L.; Molnar P.; Hickman J. J. (2005) Adult rat spinal cord culture on an organosilane surface in a novel serum-free medium. In Vitro Cell. Dev. Biol.: Anim. 41, 343–348. [DOI] [PubMed] [Google Scholar]
- Schaffner A. E.; Barker J. L.; Stenger D. A.; Hickman J. J. (1995) Investigation of the factors necessary for growth of hippocampal neurons in a defined system. J. Neurosci. Methods 62, 111–119. [DOI] [PubMed] [Google Scholar]
- Spargo B. J.; Testoff M. A.; Nielsen T. B.; Stenger D. A.; Hickman J. J.; Rudolph A. S. (1994) Spatially controlled adhesion, spreading, and differentiation of endothelial cells on self-assembled molecular monolayers. Proc. Natl. Acad. Sci. U.S.A. 91, 11070–11074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger D. A.; Pike C. J.; Hickman J. J.; Cotman C. W. (1993) Surface determinants of neuronal survival and growth on self-assembled monolayers in culture. Brain Res. 630, 136–147. [DOI] [PubMed] [Google Scholar]
- Varghese K.; Das M.; Bhargava N.; Stancescu M.; Molnar P.; Kindy M. S.; Hickman J. J. (2009) Regeneration and characterization of adult mouse hippocampal neurons in a defined in vitro system. J. Neurosci. Methods 177, 51–59. [DOI] [PubMed] [Google Scholar]
- Das M.; Molnar P.; Devaraj H.; Poeta M.; Hickman J. (2003) Electrophysiological and morphological characterization of rat embryonic motoneurons in a defined system. Biotechnol. Prog. 19, 1756–1761. [DOI] [PubMed] [Google Scholar]
- Ravenscroft M. S.; Bateman K. E.; Shaffer K. M.; Schessler H. M.; Jung D. R.; Schneider T. W.; Montgomery C. B.; Custer T. L.; Schaffner A. E.; Liu Q. Y.; Li Y. X.; Barker J. L.; Hickman J. J. (1998) Developmental neurobiology implications from fabrication and analysis of hippocampal neuronal networks on patterned silane-modified surfaces. J. Am. Chem. Soc. 120, 12169–12177. [Google Scholar]
- Eisenberg T.; Knauer H.; Schauer A.; Büttner S.; Ruckenstuhl C.; Carmona-Gutierrez D.; Ring J.; Schroeder S.; Magnes C.; Antonacci L.; Fussi H.; Deszcz L.; Hartl R.; Schraml E.; Criollo A.; Megalou E.; Weiskopf D.; Laun P.; Heeren G.; Breitenbach M.; Grubeck-Loebenstein B.; Herker E.; Fahrenkrog B.; Fröhlich K.-U.; Sinner F.; Tavernarakis N.; Minois N.; Kroemer G.; Madeo F. (2009) Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11, 1305–1314. [DOI] [PubMed] [Google Scholar]
- Kaeberein M. (2009) Spermidine surprise for a long life. Nat. Cell Biol. 11, 1277–1278. [DOI] [PubMed] [Google Scholar]
- Hu B. Y.; Du Z. W.; Li X. J.; Ayala M.; Zhang S. C. (2009) Human oligodendrocytes from embryonic stem cells: Conserved SHH signaling networks and divergent FGF effects. Development 136, 1443–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Qu Y.; Stewart T. J.; Howard M. J.; Chakrabortty S.; Holekamp T. F.; McDonald J. W. (2000) Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc. Natl. Acad. Sci. U.S.A. 97, 6126–6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor G. I.; Totoiu M. O.; Haque N.; Carpenter M. K.; Keirstead H. S. (2005) Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia 49, 385–396. [DOI] [PubMed] [Google Scholar]
- Rogister B.; Ben-Hur T.; Dubois-Dalcq M. (1999) From neural stem cells to myelinating oligodendrocytes. Mol. Cell. Neurosci. 14, 287–300. [DOI] [PubMed] [Google Scholar]
- Behar T. N. (2001) Analysis of fractal dimension of O2A glial cells differentiating in vitro. Methods 24, 331–339. [DOI] [PubMed] [Google Scholar]
- Pringle N. P.; Yu W. P.; Guthrie S.; Roelink H.; Lumsden A.; Peterson A. C.; Richardson W. D. (1996) Determination of neuroepithelial cell fate: Induction of the oligodendrocyte lineage by ventral midline cells and sonic hedgehog. Dev. Biol. 177, 30–42. [DOI] [PubMed] [Google Scholar]
- Gogate N.; Verma L.; Zhou J. M.; Milward E.; Rusten R.; O’Connor M.; Kufta C.; Kim J.; Hudson L.; Dubois-Dalcq M. (1994) Plasticity in the adult human oligodendrocyte lineage. J. Neurosci. 14, 4571–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp P. E.; Adams M. H. (2004) Epidermal growth factor promotes oligodendrocyte process formation and regrowth after injury. Exp. Cell Res. 296, 135–144. [DOI] [PubMed] [Google Scholar]
- Ravenscroft-Chang M. S.; Stohlman J. M.; Molnar P.; Natarajan A.; Canavan H. E.; Teliska M.; Stancescu M.; Krauthamer V.; Hickman J. J. (2010) Altered calcium dynamics in cardiac cells grown on silane-modified surfaces. Biomaterials 31, 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman J. J.; Bhatia S. K.; Shoen P.; Pike C.; Cotman C. W.; Stenger D. A. (1994) Rational Pattern Design for In Vitro Cellular Networks. J. Vac. Sci. Technol., A 12, 607–616. [Google Scholar]
- Huang Y.; Wright C. D.; Merkwan C. L.; Baye N. L.; Liang Q.; Simpson P. C.; O’Connell T. D. (2007) An α1A-adrenergic-extracellular signal-regulated kinase survival signaling pathway in cardiac myocytes. Circulation 115, 763–772. [DOI] [PubMed] [Google Scholar]
- Wright C. D.; Chen Q.; Baye N. L.; Huang Y.; Healy C. L.; Kasinathan S.; O’Connell T. D. (2008) Nuclear α1-adrenergic receptors signal activated ERK localization to caveolae in adult cardiac myocytes. Circ. Res. 103, 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Badri N. S.; Hakki A.; Saporta S.; Liang X.; Madhusodanan S.; Willing A. E.; Sanberg C. D.; Sanberg P. R. (2006) Cord blood mesenchymal stem cells: Potential use in neurological disorders. Stem Cells Dev. 15, 497–506. [DOI] [PubMed] [Google Scholar]
- Kögler G.; Sensken S.; Airey J. A.; Trapp T.; Müschen M.; Feldhahn N.; Liedtke S.; Sorg R. V.; Fischer J.; Rosenbaum C.; Greschat S.; Knipper A.; Bender J.; Degistirici O.; Gao J.; Caplan A. I.; Colletti E. J.; Almeida-Porada G.; Müller H. W.; Zanjani E.; Wernet P. (2004) A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 200, 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat N. R.; Zhang P.; Mohanty S. B. (2007) p38 MAP kinase regulation of oligodendrocyte differentiation with CREB as a potential target. Neurochem. Res. 32, 293–302. [DOI] [PubMed] [Google Scholar]
- Baron W.; Metz B.; Bansal R.; Hoekstra D.; de Vries H. (2000) PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: Regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol. Cell. Neurosci. 15, 314–329. [DOI] [PubMed] [Google Scholar]