Abstract
Background:
In the BIG 1-98 trial objective cognitive function improved in postmenopausal women 1 year after cessation of adjuvant endocrine therapy for breast cancer. This report evaluates changes in subjective cognitive function (SCF).
Methods:
One hundred postmenopausal women, randomised to receive 5 years of adjuvant tamoxifen, letrozole, or a sequence of the two, completed self-reported measures on SCF, psychological distress, fatigue, and quality of life during the fifth year of trial treatment (year 5) and 1 year after treatment completion (year 6). Changes between years 5 and 6 were evaluated using the Wilcoxon signed-rank test. Subjective cognitive function and its correlates were explored.
Results:
Subjective cognitive function and the other patient-reported outcomes did not change significantly after cessation of endocrine therapy with the exception of improvement for hot flushes (P=0.0005). No difference in changes was found between women taking tamoxifen or letrozole. Subjective cognitive function was the only psychosocial outcome with a substantial correlation between year 5 and 6 (Spearman's R=0.80). Correlations between SCF and the other patient-reported outcomes were generally low.
Conclusion:
Improved objective cognitive function but not SCF occur following cessation of adjuvant endocrine therapy in the BIG 1-98 trial. The substantial correlation of SCF scores over time may represent a stable attribute.
Keywords: subjective cognitive function, quality of life, breast cancer, aromatase inhibitor, tamoxifen, letrozole
Cognitive deficits following adjuvant breast cancer therapy have been increasingly investigated over the past decade. Earlier studies focused mainly on chemotherapy (Vardy et al, 2007; Vardy, 2009), but more recent research has examined the potential aetiological role of adjuvant endocrine therapies (Jenkins et al, 2004; Bender et al, 2007; Hermelink et al, 2008; Jenkins et al, 2008; Collins et al, 2009; Debess et al, 2010; Phillips et al, 2010; Schilder et al, 2010; Phillips et al, 2011a, 2011b). In these previous studies, objective cognitive function was usually measured with standardised neuropsychological tests (Vardy, 2009; Phillips et al, 2011a, 2011b). If subjective cognitive complaints were assessed, a range of validated self-report questionnaires, ‘self-developed’ non-validated questionnaires and/or semi-structured interviews were used (Pullens et al, 2010). The prevalence of subjective cognitive dysfunction in women with breast cancer ranged from 21 to 90%, and there was no association between objective cognitive function and SCF (Pullens et al, 2010). Most studies on cognitive function also included measures of psychological distress (mainly depression and anxiety), quality of life (QOL), and fatigue as potential covariates.
Irrespective of therapy received (i.e., chemotherapy or endocrine therapy or both), or of study design (cross-sectional vs longitudinal), studies in women with breast cancer have consistently reported that objective cognitive function is not related to psychological distress (Jenkins et al, 2004; Shilling et al, 2005; Bender et al, 2006; Hermelink et al, 2007), fatigue (Fan et al, 2005; Bender et al, 2006; Jenkins et al, 2006; Mehnert et al, 2007; Collins et al, 2009), or QOL (Fan et al, 2005; Shilling et al, 2005; Jenkins et al, 2006; Mehnert et al, 2007). Only one study found that better scores in cognitive function were weakly related to less fatigue 2 years after chemotherapy completion (Schilder et al, 2008). In contrast, several studies found SCF to be associated with psychological distress (Castellon et al, 2004; Jenkins et al, 2004; Hermelink et al, 2007; Jenkins et al, 2008; Weis et al, 2009). There are few longitudinal studies that have investigated subjective cognitive and psychosocial changes in women with breast cancer during adjuvant endocrine therapy (Jenkins et al, 2006; Jenkins et al, 2008; Collins et al, 2009; Legault et al, 2009), and none of them specifically studied changes in these factors after ceasing therapy. One study examined the relationship between cognitive limitations and tamoxifen or aromatase inhibitors in employed breast cancer survivors on average 3 years after primary treatment (Breckenridge et al, 2012). Exposure to endocrine therapy was not related to scores on the objective measures, but moderately related to perceived attentional problems at work and perceived cognitive functioning in everyday life.
We have previously reported results from the BIG 1-98 study on objective cognitive function (Phillips et al, 2010; Phillips et al, 2011a, 2011b). There was a statistically significant improvement in objective cognitive function after cessation of adjuvant endocrine therapy, with an effect size large enough to be considered clinically meaningful (Phillips et al, 2011a, 2011b). In this report, we present the findings on SCF, psychological distress, fatigue, and QOL. Thus, objectives of the study reported here were (1) to evaluate changes in SCF, psychological distress, fatigue, and QOL 1 year after the cessation of adjuvant endocrine therapy in postmenopausal women with early breast cancer, who were randomised within a double blind controlled trial (BIG1-98) (Thurlimann et al, 2005; Mouridsen et al, 2009) to receive adjuvant tamoxifen or letrozole alone or in sequence; (2) to evaluate whether there are differences in changes in SCF and other psychosocial outcomes 1 year after the cessation of adjuvant endocrine therapy between women taking tamoxifen or letrozole for the last 3 of 5 years of trial treatment; and (3) to evaluate the relationship between cognitive function (objective and subjective), psychological distress, fatigue, and QOL while still on treatment and 1 year after cessation. In addition, subjective complaints of cognitive dysfunction and their correlates were explored.
Materials and methods
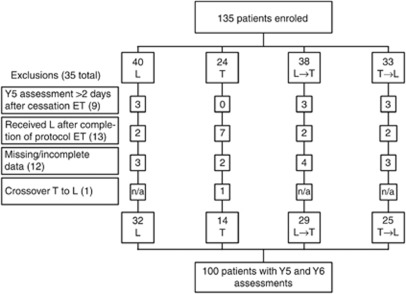
The BIG 1-98 trial (March 1998–May 2003) randomised 8010 postmenopausal women with hormone receptor-positive tumours to receive one of four adjuvant endocrine therapy options after stratification by institution and chemotherapy (Figure 1). A sub-study assessed cognitive function and psychosocial factors at year 5 (during the fifth year on endocrine therapy (Phillips et al, 2010) and at year 6 (∼1 year after ceasing therapy (Phillips et al, 2011a, 2011b). The sub-study protocol was approved by the local and International Breast Cancer Study Group (IBCSG) ethics committees and the required health authorities of each participating centre. All women gave informed consent to participate in the sub-study and parent study.
Figure 1.
CONSORT diagram of the BIG 1-98 Cognitive Function Substudy. T=tamoxifen for 5 years; L=letrozole for 5 years; ET=endocrine therapy; T→L=tamoxifen for 2 years followed by letrozole for 3 years; L→T=letrozole for 2 years followed by tamoxifen for 3 years; Y5=cognitive function assessment taken at the end of 5 years of ET; Y6=cognitive function assessment taken ∼1 year after the completion of ET.
Cognitive function and patient-reported outcomes
Objective cognitive function was assessed using a brief computerised test battery (CogState Ltd; http://www.cogstate.com), which is free from practice effects (Collie et al, 2001; Falleti et al, 2003; Snyder et al, 2005; Vardy et al, 2006). Testing consisted of five non-verbal tasks, measuring the speed of psychomotor function, visual attention, attention and working memory, and visual learning and memory. In addition, two verbal learning and memory tasks required subjects to learn a 12-word shopping list, and then to recall this list after 20 min. Details of the test battery have been described elsewhere (Phillips et al, 2010). For the seven tasks, a composite score, representing the age-adjusted average standardised score of each task for each individual, was calculated (Phillips et al, 2010).
After cognitive testing, women completed several questionnaires. Subjective cognitive function was assessed with the Cognitive Failures Questionnaire (CFQ (Broadbent et al, 1982)), a 25-item self-report measure that assesses a person's failures in memory, perception, attention, and motor function over the past 6 months. The response categories range from 0 (‘never’) to 4 (‘very often’). Studies exploring potential CFQ subscales vary considerably with regard to the number of subscales identified, so in this study only the global summary score was used (Broadbent et al, 1982). To measure psychological distress, a 12-item version of the General Health Questionnaire (GHQ-12 (Goldberg, 1992; Politi et al, 1994; Schmitz et al, 1999; Donath, 2001) was used. In addition, the Brief Fatigue Inventory (BFI (Mendoza et al, 1999), a 9-item instrument to assess severity of fatigue and its inference with daily living in a 24-h period, was administered. Various global and symptom-specific QOL domains were measured by linear analogue self-assessment indicators (Bernhard et al, 1997). Validated language versions were used where available, otherwise a standard translation procedure was performed (forward-backward).
Statistical considerations
Changes in SCF and each of the patient-reported outcome measures from year 5 to year 6 were evaluated by subtracting year 5 from year 6 scores. Higher scores reflect a poorer condition; thus, a negative change in the score indicates an improvement. Owing to the skewness in the distribution of responses for the BFI, GHQ, and QOL measures at the year 5 assessment, analyses were performed using nonparametric methods. The stratified Wilcoxon signed-rank test was used to evaluate changes 1 year after cessation of adjuvant endocrine therapy (regardless of the type of endocrine therapy received). The Van Elteren Wilcoxon rank-sum test, stratified on language, was used to test the difference in the changes from year 5 to year 6 between women taking tamoxifen and women taking letrozole at year 5. Spearman's correlation coefficients (R) were obtained for pairwise associations among the CogState Battery composite score and the self-reported measures.
Where available, cut-offs were applied to identify women with mental disorders or severe fatigue. A widely accepted convention to define a case of mental disorder using the GHQ-12 is a score ⩾3 (Goldberg et al, 1997). A score ⩾31 is considered as cut-off for screening of mental disorder for the QOL mood scale (Singer et al, 2008). A BFI score ⩾8 represents severe fatigue (Chang et al, 2007). In the absence of an established cut-off for the CFQ total score, we used a distribution-based measure and focused on women who reported the highest 33% of CFQ total scores at year 5.
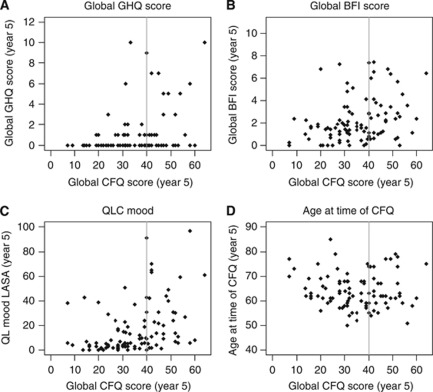
In order to characterise women who reported the highest 33% of CFQ total scores at year 5 (still on treatment), relationships between CFQ scores and their year-5 BFI, GHQ and mood scores, age at this time, and year 6-CFQ scores were explored graphically (scatter plots). These variables were chosen a priori because of research showing strong associations between SCF and fatigue, depression, anxiety and mood, and because of age being considered an important covariate of cognitive function. Women who reported the highest 33% of CFQ total scores at year 5 were further characterised with regard to specific complaints (on CFQ individual item level) by exploring the relationship between individual complaints at year 5 and year 6.
Results
Of 135 women recruited to this study, 35 were ineligible for this analysis (see Figure 1 for reasons), leaving 100 women. The year-6 assessment was undertaken a median of 365.5 days (range 191–699 days) after ceasing protocol endocrine therapy.
Most patient and disease characteristics (i.e., age, history of head injury, neurological disorder, anxiety or depression, currently treated for anxiety, depression or mental disorder, alcohol consumption, ECOG status, chemotherapy received, hormone replacement therapy, ER/PgR status, tumour size, and local therapy) were balanced between women who had both assessments vs those who had only year-5 assessment (drop-outs N=23; see Figure 1), except that women who had four or more positive nodes were more likely to drop out by the year-6 assessment (P=0.01). No statistically significant differences were found for patient and disease characteristics between the women taking tamoxifen vs letrozole at year 5.
Differences between treatments groups at year 5
At year 5, no differences were found for SCF (P=0.79), fatigue (P=0.58), psychological distress (P=0.16), or the QOL indicators between women taking tamoxifen or letrozole.
Changes from on- to off-treatment
Overall, no significant change was found from on-treatment to 1-year off-treatment for SCF or any of the patient-reported outcomes (Table 1), except for a significant decrease in hot flushes (mean change (s.d.)=−10.3 (30.87); median change (range)=−2 (−99, 100), P=0.0005). Likewise, none of the changes in SCF and other patient-reported outcomes differed significantly between women taking tamoxifen compared with letrozole (data not shown).
Table 1. Descriptive statistics of patient-reported outcomes by time point and change in score.
|
Year 5(during the fifth year on ET)
|
Year 6 (∼1 year after ceasing ET)
|
Change (year 6–year 5)
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Med | Min | Max | N | Med | Min | Max | Mean | Med | s.d. | Min | Max | Pa | |
| CFQ | 99 | 35.00 | 7.00 | 64.00 | 100 | 35.0 | 3.00 | 65.00 | −1.10 | −1.00 | 7.33 | −27.00 | 14.00 | 0.25 |
| BFI | 98 | 1.61 | 0.00 | 7.44 | 98 | 2.11 | 0.00 | 8.44 | 0.17 | 0.28 | 1.77 | −6.78 | 4.44 | 0.12 |
| GHQ | 100 | 0.00 | 0.00 | 10.00 | 99 | 0.00 | 0.00 | 10.00 | 0.13 | 0.00 | 2.38 | −10.00 | 10.00 | 0.28 |
| QOL indicators | ||||||||||||||
| Physical well-being | 100 | 9.5 | 0 | 94 | 98 | 13.0 | 0 | 100 | 1.09 | 0.5 | 22.64 | −79 | 99 | 0.45 |
| Mood | 100 | 8.0 | 0 | 97 | 98 | 12.0 | 0 | 100 | 1.45 | 0.0 | 20.69 | −61 | 94 | 0.56 |
| Tiredness | 100 | 22.0 | 0 | 96 | 98 | 30.0 | 0 | 100 | 4.05 | 4.5 | 28.49 | −80 | 76 | 0.09 |
| Appetite | 100 | 5.5 | 0 | 84 | 98 | 6.5 | 0 | 100 | 0.16 | 0.0 | 22.65 | −72 | 99 | 0.70 |
| Hot flushes | 100 | 20.0 | 0 | 100 | 98 | 6.0 | 0 | 100 | −10.3 | −2.0 | 30.87 | −99 | 100 | 0.0005 |
| Feeling sick | 100 | 2.0 | 0 | 77 | 98 | 1.0 | 0 | 100 | −0.66 | 0.0 | 18.18 | −76 | 100 | 0.32 |
| Effort to cope | 100 | 6.0 | 0 | 88 | 99 | 4.0 | 0 | 100 | −2.44 | −1.0 | 20.59 | −88 | 100 | 0.12 |
| Arm restriction | 100 | 3.0 | 0 | 95 | 99 | 4.0 | 0 | 100 | 1.73 | 0.0 | 23.26 | −76 | 100 | 0.38 |
| Subjective health | 100 | 10.0 | 0 | 86 | 98 | 11.0 | 0 | 100 | 0.14 | 0.0 | 20.53 | −85 | 100 | 0.85 |
Abbreviations: ET=endocrine therapy; Med=median; Min=minimum; Max=maximum; CFQ=Cognitive Failure Questionnaire; BFI=Brief Fatigue Inventory; GHQ=General Health Questionnaire. For all outcomes, higher scores reflect a poorer condition, and a negative change indicates an improvement.
P-value calculated using the Wilcoxon signed-rank test.
Prevalence of severe fatigue and psychiatric or mental disorder
Regarding fatigue, no woman had a BFI score considered as representing severe fatigue at year 5 and only one experienced severe fatigue at year 6. About the same proportion of women had GHQ scores defined as a case of mental disorder at year 5 (14%) and year 6 (18%). Of those, 12 women changed from ‘no case’ at year 5 to ‘case’ at year 6; however, 8 women improved, changing from ‘case’ to ‘no case’. The proportion of women with mood scores indicative for a mental disorder remained stable with 20% of women at year 5 and 18% at year 6. Of those, 9 women worsened, changing from not impaired at year 5 to impaired at year 6; 11 women improved, going from impaired to not impaired.
Relationship between cognitive function and patient-reported outcomes
There were no notable correlations between objective cognitive function (composite score) and the various patient-reported outcomes at year 5 or 6 (correlation coefficients ranged from R=−0.15 to R=0.18). In addition, no notable correlations were found between individual Cogstate tasks and SCF, with correlation coefficients ranging from R=−0.09 to R=0.14 at year 5 and from R=−0.02 to R=0.19 at year 6. Subjective cognitive function showed a low correlation with fatigue (BFI, R=0.33), psychological distress (GHQ, R=0.31), mood (R=0.45), tiredness (R=0.41), and physical well-being (R=0.37) and no correlations with the other QOL indicators at year 5. Similarly, at year 6 correlations between SCF and fatigue (BFI, R=0.37), psychological distress (GHQ, R=0.42), and tiredness (R=0.41) were low, and no correlations with the other QOL indicators were found.
Subjective cognitive complaints and their correlates
For women with CFQ scores in the highest third, the lowest score was 40 at the year-5 assessment. Thirty-eight women (38%) at year 5 and 36 women (36%) at year 6 had a CFQ score of ⩾40.
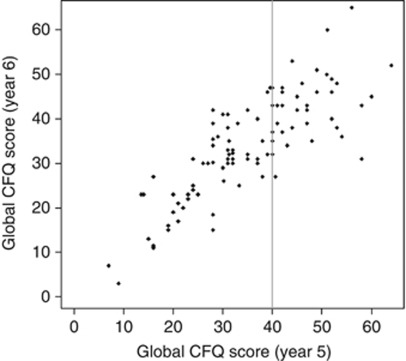
Figure 2A–D shows plots of CFQ scores by GHQ, BFI, mood scores, and age at year 5 for all patients. Whereas there seems to be some tendency for women who reported the highest 33% of SCF scores (vertical line represents the cut-off) to have worse BFI and mood scores, this is not seen with the GHQ score or age. Women with more cognitive complaints at year 5 (i.e., still on treatment) also reported more complaints at year 6 (i.e., after ceasing endocrine treatment; R=0.80), as shown in Figure 3. In contrast, correlations between on- and off-treatment scores were lower for BFI (R=0.53), GHQ (R=0.39) or the various QOL indicators (ranging from R=0.32 to 0.62).
Figure 2.
Plots of SCF on psychological distress (GHQ, A), fatigue (BFI, B), mood (C) and age (D) at year 5. The vertical line represents the cut-off for women who reported the highest 33% of SCF (CFQ) scores.
Figure 3.
Plot of SCF (CFQ) scores at year 5 vs score at year 6 (Spearman's R=0.80) for the entire sample.
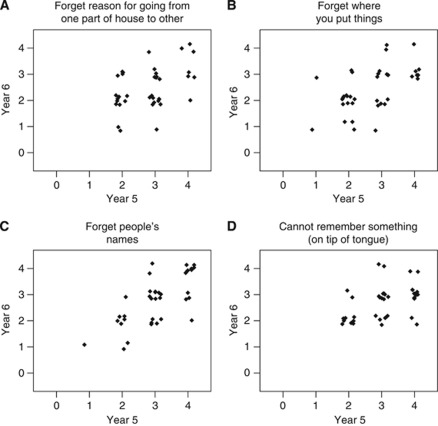
There were moderate-to-high correlations between responses at year 5 and those at year 6 for individual CFQ items (ranging from R=0.24 to 0.77). When the individual complaints of women with the highest 33% of CFQ total scores at year 5 were explored (Figure 4), four items pertaining to memory lapses (i.e., ‘forgetting reason for going from one part of house to the other’, ‘forget where you put things’, ‘forget people's names’, ‘cannot remember something (on tip of tongue)’), had high responses for both years 5 and 6, suggesting that these were the most bothersome specific complaints in this particular subgroup of women, and that they remained a problem during treatment (year 5) and 1 year after treatment cessation (year 6).
Figure 4.
Plots of four selected CFQ items (A–D) at year 5 vs score at year 6 for women who reported the highest 33% of SCF (CFQ) scores.
In summary, although objective cognitive function improved 1 year after treatment completion in the BIG 1-98 Trial (Phillips et al, 2011a, 2011b), hot flushes reduced, and SCF and the other psychosocial measures remained stable with no difference between women taking tamoxifen and letrozole.
Discussion
In this sub-study, SCF, fatigue, psychological distress, and QOL did not change between the fifth year on adjuvant endocrine therapy and 1 year after treatment cessation in postmenopausal women with early-stage breast cancer. The only exception was a significant decrease in hot flushes, which is expected on withdrawal of endocrine therapy. Similarly, the proportion of women who were at risk for a mental disorder was stable, with rates <20% at year 5 and 6. Other studies on cognitive function in women with breast cancer using also the GHQ-12 to control for mental disorder have reported similar rates of risks for mental disorder in women receiving radiotherapy and/or endocrine therapy (Jenkins et al, 2006), but higher rates in women receiving chemotherapy (Shilling et al, 2005).
To our knowledge, this is the first study reporting on subjective cognitive changes in women after cessation of endocrine therapy for early-stage breast cancer within a randomised controlled trial setting. Most studies reporting on changes in SCF have focused on subjective cognitive changes after chemotherapy (Hurria et al, 2006; Jenkins et al, 2006; Shilling and Jenkins, 2007; Jansen et al, 2008; Quesnel et al, 2009). In the study by Jenkins (Jenkins et al, 2006), one group of patients received radiotherapy and/or endocrine therapy only. In this group no subjective cognitive changes were found over time, but patients were still taking endocrine treatment at the time of their follow-up assessment. In a randomised double-blind chemoprevention trial (IBIS II trial (Jenkins et al, 2008), postmenopausal women at high risk for breast cancer did not report changes in SCF (assessed with the CFQ) during the first two years on the aromatase inhibitor anastrozole.
The fact that in our study SCF remained stable suggests that it may represent a stable attribute. The argument, made by some authors, that subjective cognitive complaints in women treated for breast cancer may solely represent psychological distress or fatigue (Morse et al, 2003; Pullens et al, 2010) is undermined by our finding of only weak-to-moderate associations among SCF and these measures. Similarly, the correlation between on- and off-treatment scores was low for psychosocial distress, moderate for fatigue but substantial for SCF. An alternative hypothesis is therefore that self-report instruments purporting to measure cognitive function, may in fact be measuring a stable, psychological factor. In one study of patients receiving chemotherapy for breast cancer, the personality trait ‘negative affectivity’ was one of the determinants of cognitive self-reports (Hermelink et al, 2010).
Our exploratory analysis showed that among women in the highest third of SCF scores at the end of treatment, there were few specific cognitive problems persisting over time, in particular four specific lapses concerning memory. Shilling and Jenkins (2007) interviewed women with breast cancer receiving adjuvant therapy, who reported memory problems regarding the kind of problems they encountered. Few were able to mention more than one to two specific cognitive problems. A notable problem was remembering what they are doing, or were supposed to have done that is similar to the problem of ‘forgetting reason for going from one part of house to the other’ in our study. Other frequently reported problems (e.g., forgetting the names of people, forgetting appointments, forgetting where things are, and remembering a word they wished to use) were also consistent with the kind of problems reported as most bothersome by the women who reported the highest 33% of SCF scores. There was no indication that these women differed substantially from the rest of the sample with respect to age, psychological distress or fatigue. This is in contrast to studies reporting evidence for a moderate-to-strong relationship between SCF and psychological distress, fatigue or QOL during and after adjuvant treatment for breast cancer (Jenkins et al, 2006; Shilling and Jenkins, 2007; Weis et al, 2009; Breckenridge et al, 2012).
In line with the existing evidence, we found no association between objective cognitive function and SCF. It has been argued that the reason for this dissociation may be that standardised neuropsychological tests are insufficiently sensitive to detect mild impairments in cancer patients (Schagen et al, 2009). Furthermore, objective cognitive tests are usually conducted at specific time points in a clearly defined test situation, and therefore may fail to assess cognitive function in relevant everyday settings (Sbordone, 2001) or to cover a broader time period (Tannock et al, 2004).
There are some limitations to consider. In the absence of an established cut-off or normative data for the CFQ, we had to rely on an arbitrary, distribution-based criterion. This frame of reference allowed an exploration of the clinical observation, that there is a subgroup of patients with complaints of cognitive impairment persisting after completing adjuvant therapy. The lack of a definition for subjective cognitive impairment is a general problem; only 7 out of 27 studies conducted in women with breast cancer (Pullens et al, 2010) described the definition of subjective cognitive dysfunction, using either theoretical definitions or cut-offs. At the time the study was designed, subjective data from women with breast cancer indicated that they were concerned most about their ability to concentrate and remember, and none of the available studies had highlighted executive function as an area of specific concern. In a recent review, subjective cognitive deficits consisted of problems with memory, concentration, language, and self-reported retardation in mental processes or lower effectiveness (Pullens et al, 2010). Yet, it is possible that our study missed patients with executive function deficits, which may be correlated with changes in SCF. We had no assessment of cognitive function before the start of endocrine therapy and were not able to control for the potential preexistence of cognitive impairment.
In conclusion, it is reassuring that objective cognitive function improves significantly after ceasing endocrine therapy (Phillips et al, 2011a, 2011b). However, given the results reported here, there is a subgroup of women who may not feel that their everyday cognitive abilities, in particular those related to memory, are better after ceasing therapy. The quite clear disconnect between objective cognitive function and SCF, whatever the underlying reason, is a crucial issue that must be addressed in subsequent research, in order for the research community to respond effectively to the concerns of women about their cognition during and after breast cancer treatment.
Acknowledgments
We thank the women who participated in this trial, collaborators, and funding sources. Acknowledgement for involved individuals is given in the Appendix. This work was supported by Novartis and coordinated by IBCSG. Support for the IBCSG: Swedish Cancer Society; The Cancer Council Australia; Australian New Zealand Breast Cancer Trials Group; Frontier Science and Technology Research Foundation; Swiss Group for Clinical Cancer Research (SAKK); National Cancer Institute at the National Institutes of Health (Grant number CA-75362); Cancer Research Switzerland/Oncosuisse; and the Foundation for Clinical Cancer Research of Eastern Switzerland (OSKK); The Cancer Council Victoria, Dr John Colebatch Clinical Research Fellowship (to KAP). The substudy was funded by Novartis.
APPENDIX
BIG 1-98 Collaborative Group Participants in cognitive function substudy
Steering Committee: B Thürlimann (Chair), S Aebi, L Blacher, H Bonnefoi, A S Coates, T Cufer, B Ejlertsen, J F Forbes, R D Gelber, A Giobbie-Hurder, A Goldhirsch, A Hiltbrunner, S B Holmberg, R Maibach, A Martoni, L Mauriac, G MacGrogan, H T Mouridsen, R Paridaens, D Phuong, K N Price, M Rabaglio, B B Rasmussen, M M Regan, A Santoro, I E Smith, A Wardley, and G Viale. Novartis: H A Chaudri-Ross, and S Segal.
IBCSG Foundation Council (members from 1998 to 2010): S Aebi, A S Coates, M Colleoni, J P Collins, H Cortés Funes, R D Gelber, A Goldhirsch, M Green, A Hiltbrunner, S B Holmberg, P Karlsson, I Kössler, I Láng, J Lindtner, F Paganetti, M de Stoppani, C-M Rudenstam, H-J Senn, R Stahel, B Thürlimann, and A Veronesi.
Coordinating Center (Berne, Switzerland): M Castiglione (Chief Executive Officer 1998–2007), A Hiltbrunner (Director), M Rabaglio, G Egli, H Hawle, B Cliffe, S Ribeli-Hofmann, F Munarini, R Kammler, R Studer, B Ruepp, R Maibach, and N Munarini.
Quality of Life Office (Bern, Switzerland): J Bernhard, and K Ribi.
Statistical Center (Dana-Farber Cancer Institute, Boston, MA, USA): R D Gelber (Director), M M Regan (Group Statistician), K N Price (Director of Scientific Administration), A Giobbie-Hurder (Trial Statistician), A Keshaviah, H Litman, B F Cole, Z Sun, P K Gray, H Huang, L J Somos, B Timmers, and L Nickerson.
Data Management Center (Frontier Science and Technology Research Foundation, Amherst, NY, USA): L Blacher (Director of Data Management), T Heckman Scolese (Coordinating Data Manager), M Belisle, M Caporale, J Celano, L Dalfonso, L Dooley, S Fischer, K Galloway, J Gould, R Hinkle, M Holody, G Jones, R Krall, S Lippert, J Meshulam, L Mundy, A Pavlov-Shapiro, K Scott, M Scott, S Shepard, J Swick, L Uhteg, D Weinbaum, C Westby, and T Zielinski.
Breast International Group (BIG)
International Breast Cancer Study Group
Australian New Zealand Breast Cancer Trials Group (ANZ BCTG): R D Snyder, J F Forbes, and F Boyle; ANZ BCTG Operations Office (Newcastle, Australia): D Lindsay, D Preece, J Cowell, D Talbot, and A Whipp.
Australia: The Cancer Council Victoria, Melbourne: K Wysman, S Vickery, N Ranieri, B Gleeson, B Scher, F Abell, R Basser, R Bell, B Brady, D Blakey, P Briggs, I Burns, P Campbell, M Chao, J Chirgwin, B Chua, K Clarke, J Collins, R De Boer, J C Din, R Doig, A Dowling, R Drummond, N Efe, S T Fan, M Francis, P Francis, V Ganju, P Gibbs, G Goss, M Green, P Gregory, J Griffiths, I Haines, M Henderson, R Holmes, P James, J Kiffler, M Lehman, M Leyden, L Lim, G Lindeman, R Lynch, B Mann, J McKendrick, S McLachlan, R McLennan, G Mitchell, S Mitra, C Murphy, I Parker, K Phillips, I Porter, G Richardson, J Scarlet, S Sewak, J Shapiro, R Snyder, R Stanley, C Steer, D Stoney, A Strickland, G Toner, C Underhill, K White, M White, A Wirth, and S Wong; W P Holman Clinic, Prince of Wales Hospital, Sydney: C Lewis, A Zaat, B Brigham, D Goldstein, and M Friedlander.
New Zealand: Auckland Hospital: V J Harvey, J Proctor, J Millet, B Joppa, B Evans, W Jones, M McCrystal, D Porter, P Thompson, and M Vaughan.
Italy: Istituto Europeo di Oncologia, Milano: M Colleoni, G Viale, P Veronesi, G Peruzzotti, L Corsetto, R Ghisini, G Renne, A Luini, L Orlando, R Torrisi, A Rocca, T De Pas, E Munzone, V Galimberti, S Zurrida, M Intra, F Nolé, R Orecchia, G Martinelli, F de Braud, and A Goldhirsch.
Switzerland: Swiss Group for Clinical Cancer Research (SAKK): University Hospital Basel, Basel: C Rochlitz, E Müller, R Herrmann, D Oertli, E Wight, and H Moch; Institute of Oncology of Southern Switzerland: Ospedale San Giovanni, Bellinzona: J Bernier, L Bronz, F Cavalli, E Gallerani, A Richetti, and A Franzetti; Ospedale Regionale di Lugano (Civico and Italiano), Lugano: M Conti-Beltraminelli, M Ghielmini, T Gyr, S Mauri, and P C Saletti; Ospedale Regionale Beata Vergine, Mendrisio: A Goldhirsch, O Pagani, R Graffeo, M Locatelli, S Longhi, P C Rey, and M Ruggeri; Ospedale Regionale La Carità, Locarno: E Zucca and D Wyss; Istituto Cantonale di Patologia, Locarno: L Mazzucchelli, E Pedrinis, and T Rusca; Inselspital, Berne: S Aebi, M F Fey, M Castiglione, and M Rabaglio; Kantonsspital Olten, Olten: S Aebi, M F Fey, M Zuber, and G Beck; Kantonsspital St. Gallen, St. Gallen: B Thürlimann, D Köberle, F Weisser, S Mattmann, A Müller, T Cerny, B Späti, M Höfliger, G Fürstenberger, B Bolliger, C Öhlschlegel, U Lorenz, M Bamert, J Kehl-Blank, and E Vogel.
Belgium: Institut Jules Bordet, Bruxelles: J M Nogaret, V Robberecht, V Garreau, and F Cardoso.
United Kingdom: University of Dundee, Dundee: A M Thompson, B Massie, and J A Dewar.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Novartis contracted with the International Breast Cancer Study Group (IBCSG) for provision of services related to the conduct and management of the trial. Dr Thürlimann owns stock in Novartis; Dr Cardoso has received consulting and/or lecture fees from Novartis, Dr Thompson and Dr Goldhirsch have received honoraria from Novartis. The remaining authors have no conflicts to report.
References
- Bender CM, Sereika SM, Berga SL, Vogel VG, Brufsky AM, Paraska KK, Ryan CM (2006) Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology 15: 422–430 [DOI] [PubMed] [Google Scholar]
- Bender CM, Sereika SM, Brufsky AM, Ryan CM, Vogel VG, Rastogi P, Cohen SM, Casillo FE, Berga SL (2007) Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause 14: 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard J, Hurny C, Coates AS, Peterson HF, Castiglione-Gertsch M, Gelber RD, Goldhirsch A, Senn HJ, Rudenstam CM (1997) Quality of life assessment in patients receiving adjuvant therapy for breast cancer: the IBCSG approach. The International Breast Cancer Study Group. Ann Oncol 8: 825–835 [DOI] [PubMed] [Google Scholar]
- Breckenridge LM, Bruns GL, Todd BL, Feuerstein M (2012) Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psychooncology 21: 43–45 [DOI] [PubMed] [Google Scholar]
- Broadbent DE, Cooper PF, FitzGerald P, Parkes KR (1982) The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol 21(Pt 1): 1–16 [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA (2004) Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol 26: 955–969 [DOI] [PubMed] [Google Scholar]
- Chang YJ, Lee JS, Lee CG, Lee WS, Lee KS, Bang SM, Wang XS, Mendoza TR, Cleeland CS, Yun YH (2007) Assessment of clinical relevant fatigue level in cancer. Support Care Cancer 15: 891–896 [DOI] [PubMed] [Google Scholar]
- Collie A, Darby D, Maruff P (2001) Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med 35: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S (2009) Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psychooncology 18: 811–821 [DOI] [PubMed] [Google Scholar]
- Debess J, Riis JO, Engebjerg MC, Ewertz M (2010) Cognitive function after adjuvant treatment for early breast cancer: a population-based longitudinal study. Breast Cancer Res Treat 121: 91–100 [DOI] [PubMed] [Google Scholar]
- Donath S (2001) The validity of the 12-item General Health Questionnaire in Australia: a comparison between three scoring methods. Aust N Z J Psychiatry 35: 231–235 [DOI] [PubMed] [Google Scholar]
- Falleti MG, Maruff P, Collie A, Darby DG, McStephen M (2003) Qualitative similarities in cognitive impairment associated with 24 h of sustained wakefulness and a blood alcohol concentration of 0.05%. J Sleep Res 12: 265–274 [DOI] [PubMed] [Google Scholar]
- Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, Tannock IF (2005) Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 23: 8025–8032 [DOI] [PubMed] [Google Scholar]
- Goldberg D (1992) General health questionnaire (GHQ-12) edn. NFER-Nelson: Windsor, UK [Google Scholar]
- Goldberg DP, Gater R, Sartorius N, Ustun TB, Piccinelli M, Gureje O, Rutter C (1997) The validity of two versions of the GHQ in the WHO study of mental illness in general health care. Psychol Med 27: 191–197 [DOI] [PubMed] [Google Scholar]
- Hermelink K, Henschel V, Untch M, Bauerfeind I, Lux MP, Munzel K (2008) Short-term effects of treatment-induced hormonal changes on cognitive function in breast cancer patients: results of a multicenter, prospective, longitudinal study. Cancer 113: 2431–2439 [DOI] [PubMed] [Google Scholar]
- Hermelink K, Küchenhoff H, Untch M, Bauerfeind I, Lux MP, Bühner M, Manitz J, Fensterer V, Münzel K (2010) Two different sides of 'chemobrain': determinants and nondeterminants of self-perceived cognitive dysfunction in a prospective, randomized, multicenter study. Psychooncology 19: 1321–1328 [DOI] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Munzel K (2007) Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer 109: 1905–1913 [DOI] [PubMed] [Google Scholar]
- Hurria A, Goldfarb S, Rosen C, Holland J, Zuckerman E, Lachs MS, Witmer M, van Gorp WG, Fornier M, D'Andrea G, Moasser M, Dang C, Van Poznak C, Robson M, Currie VE, Theodoulou M, Norton L, Hudis C (2006) Effect of adjuvant breast cancer chemotherapy on cognitive function from the older patient's perspective. Breast Cancer Res Treat 98: 343–348 [DOI] [PubMed] [Google Scholar]
- Jansen CE, Dodd MJ, Miaskowski CA, Dowling GA, Kramer J (2008) Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology 17: 1189–1195 [DOI] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, Shah E, Stein R, Whitehead S, Winstanley J (2006) A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94: 828–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S (2004) Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology 13: 61–66 [DOI] [PubMed] [Google Scholar]
- Jenkins VA, Ambroisine LM, Atkins L, Cuzick J, Howell A, Fallowfield LJ (2008) Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II). Lancet Oncol 9: 953–961 [DOI] [PubMed] [Google Scholar]
- Legault C, Maki PM, Resnick SM, Coker L, Hogan P, Bevers TB, Shumaker SA (2009) Effects of tamoxifen and raloxifene on memory and other cognitive abilities: cognition in the study of tamoxifen and raloxifene. J Clin Oncol 27: 5144–5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert A, Scherwath A, Schirmer L, Schleimer B, Petersen C, Schulz-Kindermann F, Zander AR, Koch U (2007) The association between neuropsychological impairment, self-perceived cognitive deficits, fatigue and health related quality of life in breast cancer survivors following standard adjuvant versus high-dose chemotherapy. Patient Educ Couns 66: 108–118 [DOI] [PubMed] [Google Scholar]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL (1999) The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 85: 1186–1196 [DOI] [PubMed] [Google Scholar]
- Morse R, Rodgers J, Verrill M, Kendell K (2003) Neuropsychological functioning following systemic treatment in women treated for breast cancer: a review. Eur J Cancer 39: 2288–2297 [DOI] [PubMed] [Google Scholar]
- Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, Smith I, Mauriac L, Forbes JF, Price KN, Regan MM, Gelber RD, Coates AS (2009) Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Eng J Med 361: 766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Aldridge J, Ribi K, Sun Z, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J (2011a) Cognitive function in postmenopausal breast cancer patients one year after completing adjuvant endocrine therapy with letrozole and/or tamoxifen in the BIG 1-98 trial. Breast Cancer Res Treat 126: 221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Ribi K, Fisher R (2011b) Do aromatase inhibitors have adverse effects on cognitive function? Breast Cancer Res 13: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA, Ribi K, Sun Z, Stephens A, Thompson A, Harvey V, Thurlimann B, Cardoso F, Pagani O, Coates AS, Goldhirsch A, Price KN, Gelber RD, Bernhard J (2010) Cognitive function in postmenopausal women receiving adjuvant letrozole or tamoxifen for breast cancer in the BIG 1-98 randomized trial. Breast 19: 388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi PL, Piccinelli M, Wilkinson G (1994) Reliability, validity and factor structure of the 12-item General Health Questionnaire among young males in Italy. Acta Psychiatr Scand 90: 432–437 [DOI] [PubMed] [Google Scholar]
- Pullens MJ, De Vries J, Roukema JA (2010) Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psychooncology 19: 1127–1138 [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H (2009) Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat 116: 113–123 [DOI] [PubMed] [Google Scholar]
- Sbordone RJ (2001) Limitations of neuropsychological testing to predict the cognitive and behavioral functioning of persons with brain injury in real-world settings. NeuroRehabilitation 16: 199–201 [PubMed] [Google Scholar]
- Schagen SB, Das E, van Dam FS (2009) The influence of priming and pre-existing knowledge of chemotherapy-associated cognitive complaints on the reporting of such complaints in breast cancer patients. Psychooncology 18: 674–678 [DOI] [PubMed] [Google Scholar]
- Schilder CM, Eggens PC, Seynaeve C, Linn SC, Boogerd W, Gundy CM, Beex LV, Van Dam FS, Schagen SB (2008) Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol 48: 76–85 [DOI] [PubMed] [Google Scholar]
- Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Huizenga HM, Nortier JW, van de Velde CJ, van Dam FS, Schagen SB (2010) Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the Neuropsychological Side Study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol 28: 1294–1300 [DOI] [PubMed] [Google Scholar]
- Schmitz N, Kruse J, Tress W (1999) Psychometric properties of the General Health Questionnaire (GHQ-12) in a German primary care sample. Acta Psychiatr Scand 100: 462–468 [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V (2007) Self-reported cognitive problems in women receiving adjuvant therapy for breast cancer. Eur J Oncol Nurs 11: 6–15 [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Morris R, Deutsch G, Bloomfield D (2005) The effects of adjuvant chemotherapy on cognition in women with breast cancer--preliminary results of an observational longitudinal study. Breast 14: 142–150 [DOI] [PubMed] [Google Scholar]
- Singer S, Danker H, Dietz A, Hornemann B, Koscielny S, Oeken J, Matthaus C, Vogel HJ, Krauss O (2008) Screening for mental disorders in laryngeal cancer patients: a comparison of 6 methods. Psychooncology 17: 280–286 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Werth J, Giordani B, Caveney AF, Feltner D, Maruff P (2005) A method for determining the magnitude of change across different cognitive functions in clinical trials: the effects of acute administration of two different doses alprazolam. Hum Psychopharmacol 20: 263–273 [DOI] [PubMed] [Google Scholar]
- Tannock IF, Ahles TA, Ganz PA, Van Dam FS (2004) Cognitive impairment associated with chemotherapy for cancer: report of a workshop. J Clin Oncol 22: 2233–2239 [DOI] [PubMed] [Google Scholar]
- Thurlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Rabaglio M, Smith I, Wardley A, Price KN, Goldhirsch A (2005) A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Eng J Med 353: 2747–2757 [DOI] [PubMed] [Google Scholar]
- Vardy J (2009) Cognitive function in breast cancer survivors. Cancer Treat Res 151: 387–419 [DOI] [PubMed] [Google Scholar]
- Vardy J, Rourke S, Tannock IF (2007) Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol 25: 2455–2463 [DOI] [PubMed] [Google Scholar]
- Vardy J, Wong K, Yi QL, Park A, Maruff P, Wagner L, Tannock IF (2006) Assessing cognitive function in cancer patients. Support Care Cancer 14: 1111–1118 [DOI] [PubMed] [Google Scholar]
- Weis J, Poppelreuter M, Bartsch HH (2009) Cognitive deficits as long-term side-effects of adjuvant therapy in breast cancer patients: 'subjective' complaints and 'objective' neuropsychological test results. Psychooncology 18: 775–782 [DOI] [PubMed] [Google Scholar]