Abstract
Summary
The ability to taste the bitter compound phenylthiocarbamide (PTC) and related chemicals is bimodal, and all human populations tested to date contain some people who can and some people who cannot taste PTC. Why this trait has been maintained in the population is uncertain but this polymorphism may influence food selection, nutritional status or thyroid metabolism. The gene product that gives rise to this phenotype is unknown, and its characterization would provide insight into the mechanism of bitter taste perception. Although this trait is often considered a simple Mendelian trait, i.e. one gene-two alleles, a recent linkage study found a major locus on chromosome 5p15 and evidence for an additional locus on chromosome 7. The development of methods to identify these genes will provide a good stepping-stone between single-gene disorders and polygenic traits.
1. Introduction
About 66 years ago, A. L. Fox, a Du Pont chemist, reported a startling accidental discovery (Anonymous 1931, Fox 1932). Boyd (1950) describes the event:
Dr A. L. Fox had occasion to prepare a quantity of phenyl-thio-carbamide… As he was placing this compound in a bottle some of it was dispersed into the air as dust. Thereupon another occupant of the laboratory complained of the bitter taste of the dust. This surprised Fox, who being much closer to the scene of operations had of course inhaled more of the dust, but had perceived no taste. He was so positive that the stuff was tasteless that he went so far as to taste some of the crystals directly, finding them as tasteless as chalk. Nevertheless the other chemist was convinced the substance was bitter and was confirmed in this impression when he in turn tasted the crystals and found them to be intensely bitter. Naturally a lively argument arose. In an attempt to settle it, the two chemists called in various other laboratory workers, friends and other people with whom they could establish contact. Some people declared the substance was tasteless and some again found it bitter.
The threshold at which people can taste phenylthiocarbamide (PTC) is bimodal, and some people are tasters and others are nontasters (Hartmann 1939, Riddell and Wybar 1944, Kalmus 1952). Family and twin studies suggest this trait is inherited as a Mendelian recessive, with two alleles typically represented as T and t, with T representing the ‘tasting’ allele and t the ‘non-tasting’ allele (Blakeslee 1931, Snyder 1931, Blakeslee 1932, Levit and Soboleva 1935, Lee 1937, Rife 1938, Hogben 1946, Matsunaga and Tsuji 1957, Merton 1958, Pons 1960, Kaplan and Fischer 1965, Martin 1975, Rao and Morton 1977, Forrai and Bankovi 1984, Whissell-Buechy 1990b). The evidence for a genetic component underlying the PTC tasting ability is so strong that it was once used in paternity tests before DNA markers were available (Cardullo and Holt 1951). The ability to taste PTC is listed as a genetic trait (McKusick 1995), (MIM No. 171200) and has been referred to as an ‘honorary blood group’.
Opening any genetics or anthropology journal published after 1930, one can hardly find an issue without a paper on the genetics of PTC. Indeed, the taste-blindness of PTC is perhaps the most studied trait in human genetics, second only to the ABO blood group system. However, almost 70 years after Fox’s discovery, the genetic study of PTC ability has not advanced at the same rate as the genetics of other inherited phenotypes. The gene has not been characterized.
PTC tasting ability is not just one of many seemingly innocuous human traits (such as tongue-rolling or arm-folding) that are interesting but not worth pursuing the underlying genetic variability. PTC blindness is reportedly associated with food preferences and several diseases, especially disorders of thyroid metabolism. Characterization of the PTC gene would provide a powerful tool to further examine and delineate each of these associations. The exact mechanism of taste transduction is still poorly understood and has lagged behind the biology of other sensory modalities such as auditory, olfactory, mechanioreception and photoreception. The characterization of the PTC gene would provide an opportunity to investigate gustatory function, an interface where ‘physiology and psychology meet’ (Adrian 1963). In this paper, we will review genetic studies of PTC blindness and the evidence for the pleiotropic effects of this gene, strategies for gene identification and recent linkage results.
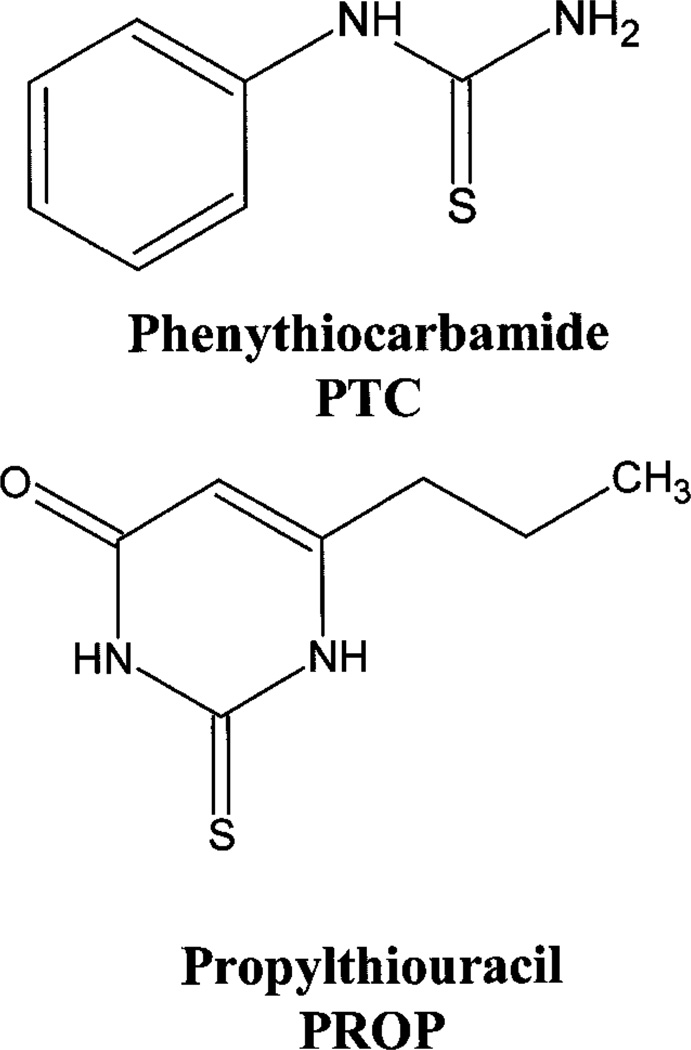
The ability to taste PTC and PROP are correlated and reflect the same polymorphism. 6-n-Propylthiouracil (PROP) and PTC are chemically related compounds (figure 1) and the taste responses to both are correlated in humans (Barnicot, Harris and Kalmus 1951, Lawless 1980, Hooper and Bartoshuk 1983). Electrophysiological evidence in primates suggested PTC and PROP produce nearly identical responses (Scott et al. 1998). Earlier studies used PTC, but most investigators have switched to PROP because it lacks the sulphurous odour of PTC and, because PROP is used as a medication to treat Graves’ Disease, safety limits can be set for its use (Fischer and Griffin 1964, Wheatcroft and Thornburn 1972, Lawless 1980). To preserve the history of this locus, however, we will refer the major gene that confers the bitter taste polymorphism as the PTC locus, and assume that PROP is a surrogate for PTC.
Figure 1.
Chemical structure of PTC and propylthiouracil.
Is individual variability in PTC sensitivity due to the presence or absence of a receptor contained in the taste cells? Fox (1932) first suggested that the taste of PTC and other related compounds was closely connected with the presence of the C=S group. Further investigations suggested the N—C=S group might be more critical (Hopkins 1942, Harris and Kalmus 1949–1951) (see figure 1). Nontasters may lack a receptor for PTC on the tongue that recognizes this N—C=S group (Fischer and Griffin 1964).
A family was identified that appeared to have an interesting mutation of the PTC locus, and the data collected from that family is consistent with the PTC gene being a taste receptor (Skude 1959, Skude 1960, Skude 1963). The people in the family perceived the taste of PTC to be sweet rather than bitter. This observation is interesting because PTC is structurally similar to a compound called Dulcin, which is very sweet (Cook 1933, Cohen and Ogdon 1949a). These families may harbour a mutation of the PTC receptor, such that they respond to PTC as sweet rather than bitter.
Although appealing, a simple receptor hypothesis may be incorrect. The perception of bitter compounds not containing the N—C=S group and other non-bitter oral stimuli are influenced by PTC genotype (Falconer 1946–47, Kalmus 1958, Fischer and Griffin 1964, Dawson and West 1967, Hall et al. 1975, Gent and Bartoshuk 1983, Leach and Noble 1986, Mela 1989, Karrer and Bartoshuk 1991, Looy, Callaghan and Weingarten et al. 1992, Looy and Weingarten 1992, Bartoshuk 1993, Bartoshuk, Duffy and Miller 1994, Lucchina, Bartoshuk, Duffey et al. 1995, Drewnowski, Henderson and Shore 1997a, Tepper and Nurse 1997, Bartoshuk, Duffey, Lucchina et al. 1998, Drewnowski, Henderson, Shore et al. 1998b) but see Boughter and Whitney (1993). Furthermore, taste-bud distribution is anatomically different between people who experience the taste of PROP as intensely bitter compared with those who do not (Miller and Reedy 1990, Bartoshuk et al. 1994). Taken together, these observations are difficult to reconcile with the hypothesis that nontasters lack a membrane-bound receptor in the taste cell sensitive specifically to PTC and structurally similar compounds.
The origins of the PTC polymorphism might not be the lack of a taste cell receptor, but rather the lack of a compound in saliva that allows people to taste PTC. The clearest demonstration of the validity of this hypothesis would be if nontasters could taste PTC, mixed in taster saliva. The results of these types of experiments are negative, however (Hartmann 1939, Cohen and Ogdon 1949b). Furthermore, when saliva is removed from the tongue, tasters remain tasters (Salmon and Blakeslee 1935). There is one report of a taster who could not taste a PTC related compound when his own saliva was removed and replaced with the saliva of a nontaster (Fischer and Griffin 1964), but nonspecific effects of the testing condition could have led to this result. It is therefore unlikely that PTC nontasters lack a salivary protein that permits PTC to be tasted.
1.1. Modifiers of the genotype–phenotype relationship
Although the PTC polymorphism has been regarded as a single locus trait, most investigators have pointed out its complex features, and have proposed that certain subject characteristics and environmental factors may alter the phenotype. The most robust modifier of the genotype± phenotype relationship is sex. Women are more likely to be tasters and can taste PTC at lower concentrations than can men (Fernberger 1932, Boyd and Boyd 1936, Hartmann 1939, Falconer 1946–47, Barnicot 1950, Beach 1953, Matsunaga, Suzuki, Itoh et al. 1954, Buchi 1955, Buchi and Roy 1955, Kumar 1955, Pons 1955, Simmons, Graydon, Semple et al. 1956, Kalmus 1958, Vyas, Bhatia, Banker et al. 1958, Leguebe 1960, Kumar and Sastry 1961, Sheba 1962, Bhattacharya 1964, Das and Mukherjee 1964, Kaplan and Fischer 1965, Khullar 1965, Romanus 1965, Say, Kiran, Altay et al. 1966, Boobphanirojana, Chetanasilpin, Saengudom et al. 1970, Patel 1971, Scott-Emuakpor, Uviovo and Warren 1975, Mitchell, Cook and Sunderland 1977, Ranganayaki and Injeti 1979, Babu, Jaikishan and Veerraju 1984, Parveen, Goni and Shah 1990, Sengupta and Dutta 1991, Balakrishna, Ramesh and Veerraju 1992, Sudhakar, Babu and Padma 1992, Bartoshuk et al. 1994, Devi, Lakshmi and Veerraju 1995, Sato, Okada, Miyamoto et al. 1997). Not all investigators measuring male and female subjects found statistically reliable differences. Of these, however, it is striking that almost all studies show this tendency for women to be sensitive tasters compared with men (Rikimaru 1936b, Boyd and Boyd 1937, Kalmus 1952, Das and Ghosh 1954, Bhattacharjee 1956, Saldanha 1958, Soltan and Bracken 1958, Akesson 1959a, Saldanha and Becak 1959, Freire-Maia, Freire-Maia and Quelce-Salgado 1960, Beiguelman 1962, Saldanha 1962, Sharma 1962, Vyas, Bhatia, Sukumaran et al. 1962, Das, Mukherjee and Bhattacharjee 1963, Pullin and Sunderland 1963, Kalmus, De Garay, Rodart et al. 1964, Tiwari 1966, Dawson and West 1967, Sharma 1967, Srivastava and Tyagi 1967, Tiwari and Bhasin 1967, Parmar 1968, Eriksson, Fellman, Forsius et al. 1970, Alsbirk and Alsbirk 1972, Akcasu, Kameswaran, Sanyal et al. 1974, Than-Sint and Mya-Tu 1974, Rastogi and Tyagi 1975, Akcasu and Ozalp 1977, Ibraimov and Mirrakhimov 1979, Mathur, Mather and Bahadur 1983, Reddy 1983, Bokesoy and Togan 1987, Ramana and Naidu 1992, Kranzler, Moore and Hesselbrock 1996, DiCarlo and Powers 1998). Only in a handful of cases, have investigators found marginal evidence that males are more sensitive than females (Thambipillai 1955–56, Kumar 1957, Sheba 1962, Saldanha and Nacrur 1963, Jenkins 1965, Agrawal 1966, Cartwright and Sunderland 1967, Kalmus 1967, Hashem and Khalifa 1968, Chattopadhyay 1971, Chandraiah and Bahadur 1975, Shah and Sattar 1981, Panayotou, Kritsikis and Bartsocas 1983, Babu et al. 1984, Yanagida 1988, Odeigah 1994, Rao and Jaikishan 1995), and in many of these studies, the subjects were children or adolescents. This observation, taken together with evidence that PTC sensitivity fluxes over the menstrual cycle (Kaplan and Fischer 1965, Bhatia, Sharma and Mehta 1981) suggest sex hormones may influence PTC sensitivity.
Although women can taste PTC at lower concentrations and are more likely to be tasters than are men, the mode of transmission for PTC taster status does not follow an X-linked inheritance pattern. Furthermore, with the exception of one study (McDonald 1979), the ability to taste PTC does not cosegregate with X-linked forms of colour blindness (Buchi and Roy 1955, Bhattacharjee 1956, Fernandes, Junqueira, Kalmus et al. 1957, Junqueira et al. 1957, Kalmus 1957, Freire-Maia et al. 1960, Pullin and Sunderland 1963, Kalmus, Kalmus, Wishart et al. 1964, Agrawal 1968, Sunderland and Ryman 1968, Lightman, Carr-Locke and Pickles 1970, Bonne, Ashbel, Berlin et al. 1972, Set 1973, Seth and Seth 1973, Barnicot and Woodburn 1975, Sirajuddin 1977, Bhattacharjee, Chowdhuri and Chatterjee 1978, Goud and Rao 1979a, b, Ranganayaki and Injeti 1979, Bhalla, Bhatia, Sood et al. 1980, Ramesh, Kumar and Murty 1981, Sharma and Bhalla 1981, Chowdhury 1988, Bagga and Seth 1992, Balakrishna et al. 1992, Ramana and Naidu 1992, Sudhakar et al. 1992, Singh and Bagga 1994, Devi et al. 1995). Modifier loci that increase PTC taste sensitivity, however, may lie on the X chromosome or may be autosomal genes regulated by sex hormones.
In addition to gender, smoking and ageing have been suggested as other modifiers of the PTC phenotype± genotype relationship. Either the chronic or acute effects of smoking might alter the perception of PTC (Hall and Blakeslee 1945, Srivastava 1959, Kaplan and Glanville 1964, Kaplan, Glanville and Fischer 1965) but not all studies are consistent with this hypothesis (Salmon and Blakeslee 1935, Falconer 1946–47, Pons 1955, Freire-Maia 1960, Leguebe 1960, Sharma 1962). Smoking may somehow interfere with or desensitize the function of some taste receptors. Likewise, the ability to taste PTC has been suggested to decline with age (Kalmus and Trotter 1962, Kaplan and Fischer 1965, Dass 1976, Whissell-Buechy 1990a, Schiffman, Gatlin, Frey, et al. 1994, Reed, Bartoshuk, Duffey et al. 1995), but some investigators do not observe such effects (Leguebe 1960, Sharma 1967, Koertvelyessy, Crawford and Hutchinson 1982, Koertvelyessy and Crawford 1990). The majority of these studies suggest that PTC acuity does decline with age, but the effects are modest.
Occasionally, identical twins are discordant for tasting ability and therefore environmental influences, such as illness, may change taster status (Ardashnikov, Lichtenstein, Martynova et al. 1936, Rife 1938). For instance, head injury and otitis media (ear infection) may influence the taster phenotype (Bartoshuk, Duffey, Reed et al. 1996). Overall, subjects with otitis media find the bitter taste of PTC-related compounds more intense compared with subjects without a history of otitis media; the reverse is true for subjects with and without a history of head trauma. Both of these events (ear infections and head trauma) may influence taste by damaging gustatory nerves, and the specific alterations in taste depend on the location and extent of the damage.
2. Population genetics
The population genetics of PTC has received extensive investigation. The ability to taste PTC has been tested extensively in various populations around the world (table 1) and has been partially reviewed (Cohen and Ogdon 1949a, Boyd 1950, Das 1966, Mourant, Kopec and Domaniewska-Sobczak 1976, Tills, Kopec and Hills 1983, Nasidze 1995, Mattes and Beauchamp 2000). The populations tested have varied from outbred groups such as University students in large cities (e.g. Fernberger 1932) to small, genetically isolated groups such as the Samaritans (e.g. Bonne 1966). Geographic proximity is a poor predictor of allele frequency because groups that live nearby but do not intermarry can have large differences in the proportion of tasters and nontasters (e.g. Babu et al. 1996). Several trends are apparent, however. The nontaster frequency is much higher than the prevalence of typical Mendelian genetic disease. With the exception of one small group of Brazilian Indians (Kalmus 1957), nontasters exist in all populations studied. In some subgroups, the frequency of nontasters is higher than the frequency of tasters (e.g. some tribes and castes in India, table 1). These observations, taken in conjunction with the presence of polymorphism in other mammalian species such as primates (Fisher 1939, Chiarelli 1963, Eaton and Gavan 1965, Smith, Lorey and Small 1981), cats (Bolekhan, Semenov, Gerasimova et al. 1997) and perhaps pigs (Braude 1949), suggests the creation of this polymorphism occurred before the dispersion of humans throughout the continents.
Table 1.
Worldwide population variation in nontaster frequency.
| Country or continent |
Group | n | % Nontaster |
Reference |
|---|---|---|---|---|
| Africa | Africans | 74 | 2.7 | Barnicot 1950 |
| Africa | Amhara | 123 | 12.2 | Bat-Miriam et al. 1962 |
| Africa | Arabs from Syria | 400 | 36.5 | Parr 1934 |
| Africa | Armenians from Syria | 294 | 32.0 | Parr 1934 |
| Africa | Assiut | 480 | 24.0 | Boyd 1950 |
| Africa | Bari | 70 | 7.1 | Rife 1953 |
| Africa | Billen, Ethiopian | 104 | 3.9 | Bat-Miriam et al. 1962 |
| Africa | Boarij Syria | 119 | 32.0 | Boyd 1950 |
| Africa | Bush | 85 | 7.1 | Jenkins 1965 |
| Africa | Cyrenaicans | 65 | 20.0 | Jenkins 1965 |
| Africa | Dinka | 132 | 34.8 | Rife 1953 |
| Africa | Egyptians | 208 | 24.1 | Parr 1934 |
| Africa | Egyptians | 569 | 22.0 | Boyd 1950 |
| Africa | Egyptians | 1078 | 8.2 | Hashem and Khalifa 1968 |
| Africa | Egyptians, Bagdad Christians | 60 | 27.0 | Boyd 1950 |
| Africa | Egyptians, Bagdad Jewish | 168 | 17.0 | Boyd 1950 |
| Africa | Egyptians, Bagdad Moslems | 322 | 29.0 | Boyd 1950 |
| Africa | Fezzanites | 52 | 17.3 | Jenkins 1965 |
| Africa | Galla | 110 | 15.5 | Bat-Miriam et al. 1962 |
| Africa | Giriama Bantu | 208 | 3.8 | Allison 1951 |
| Africa | Guraghe | 108 | 13.9 | Bat-Miriam et al. 1962 |
| Africa | Hadza in North Tanzania | 118 | 23.7 | Barnicot and Woodburn 1975 |
| Africa | Ibadan, Western Nigeria | 191 | 13.7 | Kalmus 1967 |
| Africa | Kenyan | 375 | 4.8 | Sunderland and Rosa 1975 |
| Africa | Kgalagadi | 38 | 5.2 | Jenkins 1965 |
| Africa | Libyans | 167 | 19.8 | Sunderland and Rosa 1975 |
| Africa | Melinde Arabs | 63 | 25.4 | Allison 1951 |
| Africa | Meshghara Syria; Christians | 96 | 30.0 | Boyd 1950 |
| Africa | Meshghara Syria; Moslems | 171 | 18.0 | Boyd 1950 |
| Africa | Mixed Sudanese | 51 | 17.6 | Rife 1953 |
| Africa | N Falasha | 24 | 8.3 | Bat-Miriam et al. 1962 |
| Africa | Nigeria | 970 | 12.6 | Odeigah 1994 |
| Africa | Nigeria | 2013 | 12.5 | Scott-Emuakpor et al. 1975 |
| Africa | Northern Sudanese | 100 | 4.0 | Rife 1953 |
| Africa | Nuer | 110 | 18.2 | Rife 1953 |
| Africa | S Falasja | 109 | 8.3 | Bat-Miriam et al. 1962 |
| Africa | Shilluk | 105 | 20.0 | Rife 1953 |
| Africa | Tigré | 119 | 5.0 | Bat-Miriam et al. 1962 |
| Africa | Tripolitanians | 50 | 21.5 | Jenkins 1965 |
| Africa | Urban Bantu | 86 | 2.3 | Jenkins 1965 |
| Australia | Aboriginal | 50 | NG | Lugg 1968 |
| Australia | Aborigines | NG | 50.0 | Simmons et al. 1957 |
| Australia | Aborigines | 152 | 49.3 | Simmons et al. 1954a |
| Central America | Miskito in Nicaragua | 96 | 20.8 | Stefano and Molieri 1976 |
| Central America | Rama in Nicaragua | 79 | 1.3 | Stefano and Molieri 1976 |
| Central America | Mexican | 1689 | 10.4 | Kalmus et al. 1964 |
| Central America | Sumo in Nicaragua | 85 | 8.2 | Stefano and Molieri 1976 |
| China | Chinese Immigrants | 66 | 10.6 | Barnicot 1950 |
| China | Chinese Immigrants | 167 | 6.0 | Parr 1934 |
| China | Han Lan Zhou City | 538 | 11.0 | Zhang et al. 1988 |
| China | Dong Xiang Gansu Province | 831 | 18.4 | Zhang et al. 1988 |
| China | Kazak Gansu Province, Akscu | 161 | 19.3 | Zhang et al. 1988 |
| China | YuGru Gansu Province, Sunan County | 486 | 23.0 | Zhang et al. 1988 |
| China | Hui Gansu Province, Linxia County | 1323 | 17.6 | Zhang et al. 1988 |
| China | Tu Qinghai Province, Huzhy County | 801 | 18.4 | Zhang et al. 1988 |
| China | SaLa Qinghai Province, Xuhua Country | 1077 | 8.5 | Zhang et al. 1988 |
| China | Tibetan Gansu Province | 914 | 13.6 | Zhang et al. 1988 |
| China | Mongolian Gansu, Suhua County | 332 | 16.0 | Zhang et al. 1988 |
| China | Bao’an Gansu, Jishishan Country | 545 | 5.1 | Zhang et al. 1988 |
| China | Han Chinese in Shanghai | 106 | 10.0 | Guo et al. 1998 |
| Europe | Cumbria | 854 | 19.7 | Mitchell et al. 1977 |
| Europe | Danes | 314 | 31.8 | Mohr 1951 |
| Europe | England, Derbyshire | 653 | 36.8 | Cartwright and Sunderland 1967 |
| Europe | England, Lancaster | 835 | 27.5 | Cartwright and Sunderland 1967 |
| Europe | England, Northeast | 777 | 28.9 | Sunderland 1966 |
| Europe | English | 581 | 28.0 | Akcasu et al. 1974 |
| Europe | Finns | 202 | 29.2 | Allison and Nevanlinna 1952 |
| Europe | Finns | 761 | 22.1 | Eriksson et al. 1970 |
| Europe | Irish from Dublin | 618 | 27.0 | Boyd 1950 |
| Europe | Isle of Man | 699 | 27.8 | Mitchell et al. 1977 |
| Europe | Lapps | 140 | 6.9 | Allison and Nevanlinna 1952 |
| Europe | Nellim Fisher Lapps | 76 | 10.5 | Eriksson et al. 1970 |
| Europe | Nellim Skolt Lapps | 138 | 29.7 | Eriksson et al. 1970 |
| Europe | Norwegian Lapps | 255 | 17.6 | Monn 1969 |
| Europe | Salamis Island | 183 | 32.2 | Panayotou et al. 1983 |
| Europe | Sardinians | 541 | 27.1 | Maxia et al. 1975 |
| Europe | Sevettijärvi Skolt Lapps | 251 | 28.3 | Eriksson et al. 1970 |
| Europe | Spaniards | 306 | 24.8 | Pons 1955 |
| Europe | Spaniards, San Sebastian, Spain | 172 | 27.0 | Boyd 1950 |
| Europe | Swedish | 1051 | 12.8 | Romanus 1965 |
| Europe | Swedish, Southern | 200 | 32.0 | Akesson 1959a |
| Europe | Ǻland, Main Island | 522 | 26.2 | Eriksson and Forsius 1964 |
| Europe | Sottunga | 38 | 18.4 | Eriksson and Forsius 1964 |
| Europe | Kökar | 121 | 34.7 | Eriksson and Forsius 1964 |
| Europe | Welsh | 252 | NG | Beach 1953 |
| Europe | Welsh | 398 | 28.0 | Boyd 1950 |
| Europe | Welsh, Carmarthenshire | 271 | 35.2 | Partridge 1962 |
| Europe | Welsh, Pembrokeshire | 1005 | 27.6 | Pullin and Sunderland 1963 |
| Europe | Yugoslavian | 459 | 30.9 | Grünwald and Herman 1963 |
| Greenland | Eskimos from Umanaq | 129 | 53.5 | Alsbirk and Alsbirk 1972 |
| India | Ahom | 123 | 21.1 | Sengupta and Dutta 1991 |
| India | Angami Nagas | 150 | 6.3 | Seth and Seth 1973 |
| India | Anglo-Indians | 160 | 28.1 | Bhattacharya 1964 |
| India | Audich Brahman | 200 | 35.0 | Parikh et al. 1969a |
| India | Audichya Brahmans | 200 | 37.0 | Vyas et al. 1962 |
| India | Bado Gadaba | 409 | 53.6 | Das et al. 1963 |
| India | Bagatha | 483 | 37.9 | Babu et al. 1996 |
| India | Baghdadi Jews | 200 | 20.5 | Sirsat 1956 |
| India | Bareng Paroja | 439 | 49.9 | Das et al. 1963 |
| India | Bene-Israel Jews | 200 | 20.0 | Sirsat 1956 |
| India | Bengali Brahims of Lucknow | 300 | 27.7 | Deb and Shukla 1981 |
| India | Bhangi Harijans | 200 | 43.0 | Vyas et al. 1962 |
| India | Bhil | 188 | 45.2 | Vyas et al. 1962 |
| India | Bod Mali | 70 | 20.0 | Babu et al. 1996 |
| India | Bodh of Lahaul | 110 | 11.8 | Chowdhury 1988 |
| India | Bohras | 130 | 45.4 | Hakim et al. 1973 |
| India | Brahim of Orissa | 56 | 33.9 | Tripathy 1969 |
| India | Brahims | 165 | 20.0 | Chandraiah and Bahadur 1975 |
| India | Brahims of Narendra Nagar | 170 | 29.4 | Rani and Seth 1981 |
| India | Brahmin | 132 | 26.5 | Srivastava and Tyagi 1967 |
| India | Brahmin, Maharashtrian | 58 | 27.5 | Singh and Bagga 1994 |
| India | Brahmins | 242 | 23.6 | Tiwari and Bhasin 1967 |
| India | Brahmins of Bhimtal | 217 | 17.5 | Singh 1975 |
| India | Chandraseniya Kayasth Prabhu | 200 | 46.5 | Sanghvi and Khanolkar 1949–50 |
| India | Chenchu | 132 | 37.9 | Simmons et al. 1953a |
| India | Chenchu | 227 | 55.1 | Sirajuddin 1977 |
| India | Chettibalija | 155 | 40.0 | Devi et al. 1995 |
| India | Chitpavan | 200 | 34.5 | Sanghvi and Khanolkar 1949–50 |
| India | Cutchi Lohana | 200 | 39.0 | Vyas et al. 1962 |
| India | Danguria | 126 | 15.1 | Srivastava 1961 |
| India | Delhi and Madras | 301 | 28.0 | Akcasu et al. 1974 |
| India | Delhi students | 102 | 29.0 | Bhatia et al. 1979 |
| India | Desasth | 200 | 42.5 | Sirsat 1956 |
| India | Desasth Rigvedi Brahman | 100 | 36.0 | Sanghvi and Khanolkar 1949–50 |
| India | Dhanka | 211 | 56.3 | Vyas et al. 1962 |
| India | Dhobis | 165 | 4.8 | Ranganayaki and Injeti 1979 |
| India | Dhobis | 205 | 12.7 | Balakrishna et al. 1992 |
| India | Dhodia | 83 | 42.2 | Vyas et al. 1962 |
| India | Dogra Brahmins | 111 | 39.7 | Sharma and Bhalla 1981 |
| India | Dogra Rajputs | 79 | 38.0 | Sharma and Bhalla 1981 |
| India | Dubla | 207 | 45.4 | Vyas et al. 1962 |
| India | Gadaba | 193 | 15.0 | Babu et al. 1996 |
| India | Gamit | 200 | 53.5 | Vyas et al. 1962 |
| India | Gorkhas | 202 | 14.4 | Parmar 1968 |
| India | Harijans | 77 | 31.2 | Chandraiah and Bahadur 1975 |
| India | Hill Kolams | 224 | 50.9 | Ramesh et al. 1981 |
| India | Hill Rajputs, Cis-Himalayan | 159 | 24.5 | Bhalla et al. 1980 |
| India | Hindu Gujjars | 200 | 56.5 | Balgir 1992 |
| India | Hindus | 334 | 59.0 | Dhesi et al. 1972 |
| India | Immigrant Burmese | 208 | 19.7 | Agrawal 1966 |
| India | Iranis | 200 | 25.0 | Sirsat 1956 |
| India | Jalaris | 215 | 15.8 | Rao and Jaikishan 1995 |
| India | Jalary | 103 | 47.6 | Reddy 1983, Reddy 1988 |
| India | Jatapu | 158 | 25.3 | Babu et al. 1996 |
| India | Jats | 564 | 38.0 | Chattopadhyay 1971 |
| India | Jat-Sikhs | 536 | 59.3 | Dhesi et al. 1972 |
| India | Juang of Orissa | 75 | 38.7 | Das et al. 1978 |
| India | Kapol Vania | 200 | 51.5 | Vyas et al. 1962 |
| India | Karana of Orissa | 41 | 36.6 | Tripathy 1969 |
| India | Karnataka | 353 | 45.6 | Srivastava 1980 |
| India | Kashmiri | 800 | 11.8 | Parveen et al. 1990 |
| India | Kayastha | 114 | 29.0 | Srivastava and Tyagi 1967 |
| India | Kayasthas of Lucknow | 300 | 30.0 | Deb and Shukla 1981 |
| India | Keet | 223 | 36.8 | Das 1971 |
| India | Keet of Assam | 223 | 36.8 | Das and Buragohain 1969 |
| India | Khandayat of Orissa | 49 | 18.4 | Tripathy 1969 |
| India | Khasis | 317 | 21.8 | Miki et al. 1960 |
| India | Khattri | 75 | 29.3 | Srivastava and Tyagi 1967 |
| India | Khojas | 222 | 41.4 | Hakim et al. 1973 |
| India | Kodava of Kodagu | 233 | 32.8 | Saheb et al. 1979 |
| India | Koli | 128 | 38.3 | Vyas et al. 1962 |
| India | Kolis, Cis-Himalayan | 142 | 27.5 | Bhalla et al. 1980 |
| India | Konda | 234 | 53.4 | Das and Mukherjee 1964 |
| India | Konda Dora | 350 | 35.1 | Babu et al. 1996 |
| India | Konda Kammaras | 413 | 36.1 | Babu et al. 1984 |
| India | Kondhs or Orissa | 51 | 64.7 | Tripathy 1966 |
| India | Kota of Nilgiri Hills | 534 | 40.8 | Ghosh 1973 |
| India | Koya Dora | 359 | 12.8 | Babu et al. 1996 |
| India | Koya Dora | 569 | 51.0 | Goud and Rao 1979a |
| India | Koya Dora | 505 | 28.5 | Parmar 1968 |
| India | Kumaonis | 194 | 12.4 | Seth 1962 |
| India | Kurmi Mahato | 111 | 49.5 | Basu et al. 1966 |
| India | Lad Vania | 200 | 33.5 | Parikh et al. 1969a |
| India | Ladakhis | 53 | 5.7 | Bhalla 1972 |
| India | Lahaulis | 274 | 12.7 | Sharma 1967 |
| India | Lampadi | 142 | 45.1 | Goud and Rao 1979a |
| India | Lepchas | 154 | 7.2 | Miki et al. 1960 |
| India | Leva Patidars | 200 | 32.5 | Vyas et al. 1962 |
| India | Lower Caste | 130 | 9.2 | Srivastava and Tyagi 1967 |
| India | Mahars | 200 | 43.0 | Parikh et al. 1969b |
| India | Manne Dora | 380 | 34.5 | Ramana and Naidu 1992 |
| India | Manne Kolams | 343 | 55.7 | Ramesh et al. 1981 |
| India | Manzai Mali | 317 | 27.4 | Babu et al. 1996 |
| India | Maratha | 200 | 42.5 | Sanghvi and Khanolkar 1949–50 |
| India | Maratha, Maharashtrian | 989 | 31.4 | Singh and Bagga 1994 |
| India | Mathur Kayasths | 422 | 57.8 | Mathur et al. 1983 |
| India | Mikir of Assam | 114 | 11.4 | Dass 1976 |
| India | Misgars | 153 | 45.1 | Hakim et al. 1973 |
| India | Moplahs | 186 | 48.4 | Hakim et al. 1973 |
| India | Muslim | 39 | 20.5 | Srivastava and Tyagi 1967 |
| India | Muslim | 106 | 38.7 | Bhattacharjee 1956 |
| India | Muslim Gujjars | 200 | 42.5 | Balgir 1992 |
| India | Muslims | 105 | 20.0 | Chandraiah and Bahadur 1975 |
| India | Muslims | 250 | 42.4 | Hakim et al. 1973 |
| India | Muslims of Lucknow | 300 | 28.0 | Srivastava 1976 |
| India | Naika | 78 | 46.2 | Vyas et al. 1962 |
| India | Naikpod | 154 | 64.3 | Goud and Rao 1979a |
| India | Nicobarese | 83 | 18.6 | Agrawal 1968 |
| India | Nokte Naga | 271 | 13.7 | Kumar 1955 |
| India | Non-Jat Sikhs | 285 | 56.3 | Dhesi et al. 1972 |
| India | Northern Pahira | 206 | 41.6 | Basu et al. 1966 |
| India | Ollaro | 227 | 52.6 | Das and Mukherjee 1964 |
| India | Other castes of Orissa | 49 | 28.6 | Tripathy 1969 |
| India | Pachhimaha | 87 | 14.9 | Srivastava 1964 |
| India | Pallar | 110 | 43.6 | Buchi 1955 |
| India | Paniyan | 204 | 12.7 | Das and Ghosh 1954 |
| India | Panjabis | 322 | 32.0 | Sharma 1962 |
| India | Paraja of Orissa | 85 | 41.3 | Das et al. 1978 |
| India | Pardhans | 140 | 62.9 | Goud and Rao 1979a |
| India | Pardhans | 202 | 51.5 | Ramesh et al. 1981 |
| India | Pareng | 232 | 52.9 | Das and Mukherjee 1964 |
| India | Parsis | 200 | 21.5 | Sirsat 1956 |
| India | Pathan | 150 | 26.0 | Srivastava 1974 |
| India | Raj Gonds | 163 | 54.6 | Goud and Rao 1979a |
| India | Raj Gonds | 239 | 47.3 | Ramesh et al. 1981 |
| India | Rajbanshi | 580 | 40.7 | Das et al. 1967 |
| India | Rajputs | 45 | 8.9 | Srivastava and Tyagi 1967 |
| India | Rajputs | 229 | 25.3 | Tiwari and Bhasin 1967 |
| India | Rajputs of Palampur | 87 | 29.9 | Bagga and Seth 1992 |
| India | Rana | 155 | 18.1 | Srivastava 1964 |
| India | Rana of Orissa | 29 | 31.0 | Das et al. 1978 |
| India | Rarhi Brahmin | 143 | 35.1 | Bhattacharjee 1956 |
| India | Rastogis of Lucknow | 300 | 17.0 | Rastogi and Tyagi 1975 |
| India | Reddys | 183 | 21.3 | Chandraiah and Bahadur 1975 |
| India | Relli | 175 | 1.7 | Ranganayaki and Injeti 1979 |
| India | Riang | 401 | 16.2 | Kumar and Sastry 1961 |
| India | Samantha | 250 | 9.2 | Babu et al. 1996 |
| India | Savara | 200 | 26.5 | Babu et al. 1996 |
| India | Sayyad | 150 | 26.7 | Srivastava 1974 |
| India | Scheduled, Maharashtrian | 1073 | 35.4 | Singh and Bagga 1994 |
| India | Shompens of Nicobar | 54 | 33.3 | Agrawal 1969 |
| India | Sikkimese Lepchas | 107 | 13.1 | Bhattacharjee et al. 1978 |
| India | Sindhi | 480 | 29.2 | Khullar 1965 |
| India | Southern Pahira | 671 | 65.7 | Basu et al. 1966 |
| India | Spitians | 110 | 12.0 | Sharma 1967 |
| India | Suddha | 200 | 26.5 | Sanghvi and Khanolkar 1949–50 |
| India | Sukla | 100 | 33.0 | Sanghvi and Khanolkar 1949–50 |
| India | Swangla of Lahaul | 100 | 14.0 | Chowdhury 1988 |
| India | Talavia Dubla | 212 | 42.9 | Vyas et al. 1962 |
| India | Tibetan refugees | 230 | 17.4 | Patel 1971 |
| India | Tibetans | 216 | 10.7 | Sharma 1967 |
| India | Tibetans | 400 | 14.7 | Tiwari 1966 |
| India | Tibetans | 106 | 12.2 | Bhalla 1972 |
| India | Uttar Pradesh | 344 | 34.9 | Srivastava 1959 |
| India | Vadabalija of Penticotta | 266 | 36.5 | Reddy 1983, Reddy 1988 |
| India | Vadabalija of Vadapeta | 213 | 39.9 | Reddy 1983, Reddy 1988 |
| India | Vadabaljas | 200 | 12.5 | Ranganayaki and Injeti 1979 |
| India | Vadnagara | 108 | 25.0 | Sirsat 1956 |
| India | Vaisha | 67 | 34.3 | Srivastava and Tyagi 1967 |
| India | Vaishya | 351 | 35.3 | Das 1971 |
| India | Vaishya of Assam | 351 | 35.3 | Das and Buragohain 1969 |
| India | Visa Oswal Jain | 200 | 30.0 | Parikh et al. 1969a |
| India | Vyshyas | 131 | 17.6 | Chandraiah and Bahadur 1975 |
| India | Wad Balgel | 114 | 66.7 | Agrawal 1964 |
| India | Weavers | 112 | 24.1 | Chandraiah and Bahadur 1975 |
| Jamacia | Jamaicans | 682 | 9.4 | Terry 1950 |
| Japan | Ainu | 328 | 6.4 | Simmons et al. 1953b |
| Japan | Ainu-Japanese | 275 | 5.1 | Simmons et al. 1953b |
| Japan | Formosans | 3172 | 6.8 | Rikimaru 1936a |
| Japan | Formosans | 5933 | 7.1 | Rikimaru 1936b |
| Japan | Japanese | 295 | 7.1 | Saldanha 1958 |
| Japan | Japanese | 314 | 33.1 | Yanagida 1988 |
| Japan | Japanese | 656 | 8.0 | Tsuji 1957 |
| Japan | Japanese | 915 | 9.4 | Sato et al. 1997 |
| Japan | Japanese | 916 | 23.0 | Suzuki 1949 |
| Japan | Japanese | 921 | 9.1 | Fukuoka 1936 |
| Japan | Japanese | 1600 | 12.0 | Matsunaga et al. 1954 |
| Japan | Japanese | 5871 | 13.1 | Rikimaru 1936a |
| Japan | Japanese | 8824 | 14.3 | Rikimaru 1936b |
| Japan | Natives | 1756 | 1.8 | Rikimaru 1936b |
| Korea | Koreans | 771 | 15.1 | Kang et al. 1967 |
| Middle East | Armenians from Beyrouth and Ghazir | 311 | 25.0 | Boyd 1950 |
| Middle East | Aschkenasim | 245 | 31.5 | Parr 1934 |
| Middle East | Balkan immigrants in Israel | 101 | 21.8 | Sheba 1962 |
| Middle East | Berber in Israel | 464 | 33.6 | Guttman et al. 1967 |
| Middle East | Cochin immigrants in Israel | 41 | 31.7 | Sheba 1962 |
| Middle East | Cochin in Israel | 402 | 42.0 | Guttman et al. 1967 |
| Middle East | Djerva immigrants in Israel | 383 | 42.0 | Guttman et al. 1967 |
| Middle East | European Ashkenazim immigrants | 440 | 20.7 | Sheba 1962 |
| Middle East | Gerba immigrants in Israel | 41 | 41.5 | Sheba 1962 |
| Middle East | Habbanite | 506 | 19.8 | Bonne et al. 1972 |
| Middle East | Iraqi and Persian immigrants in Israel | 336 | 16.1 | Sheba 1962 |
| Middle East | Kurdistan immigrants in Israel | 129 | 14.0 | Sheba 1962 |
| Middle East | Kurdistan immigrants in Israel | 455 | 26.8 | Guttman et al. 1967 |
| Middle East | Libya immigrants in Israel | 501 | 22.4 | Guttman et al. 1967 |
| Middle East | North-African immigrants in Israel | 340 | 15.0 | Sheba 1962 |
| Middle East | Samaritans in Israel | 125 | 6.4 | Bonne 1966 |
| Middle East | Semenites | 59 | 32.3 | Parr 1934 |
| Middle East | Sephardim | 175 | 28.0 | Parr 1934 |
| Middle East | Yemen immigrants in Israel | 261 | 18.0 | Sheba 1962 |
| Middle East | Yemen immigrants in Israel | 498 | 26.3 | Guttman et al. 1967 |
| New Guinea | Pygmies in Netherland New Guinea | 178 | 36.0 | Graydon et al. 1958 |
| New Guinea | West Nakanai, New Britain | 352 | 34.4 | Simmons et al. 1956 |
| North America | Alaskan Eskimos | 68 | 26.0 | Allison and Blumberg 1959 |
| North America | American Indians admixed | 110 | 12.8 | Parr 1934 |
| North America | American Indians, Kansas | 183 | 6.1 | Parr 1934 |
| North America | American students, Gentile | 232 | 20.3 | Rife and Schonfeld 1944 |
| North America | American students, Jewish | 82 | 14.7 | Rife and Schonfeld 1944 |
| North America | Americans of African ancestry | 107 | 7.5 | Setterfield et al. 1936 |
| North America | Americans of African ancestry | 533 | 23.5 | Parr 1934 |
| North America | Americans of European ancestry | 210 | 28.0 | Taylor 1961 |
| North America | Americans of European ancestry | 291 | 35.4 | Matson 1938 |
| North America | Americans of European ancestry | 477 | 17.8 | Setterfield et al. 1936 |
| North America | Blackfeet Indians | 129 | 8.5 | Matson 1938 |
| North America | Caucasian Americans, USA | 439 | 30.9 | Parr 1934 |
| North America | Caucasian Americans, USA | 3643 | 29.8 | Parr 1934 |
| North America | Flathead Indians | 442 | 17.4 | Matson 1938 |
| North America | Mennonite | 1157 | 25.0 | Koertvelyessy et al. 1982 |
| North America | Micmac Indians | 496 | 29.1 | Chiasson 1963 |
| North America | Papago Indians Arizona | 70 | 1.4 | MacRoberts 1964 |
| North America | Ramah, New Mexico, USA | 269 | 2.0 | Boyd 1950 |
| Polynesia | Cook Island | 215 | 16.3 | Simmons et al. 1955 |
| Polynesia | Eastern and Central Islands | 126 | 7.9 | Simmons and Graydon 1957 |
| Philippine | Negritos | 73 | 13.7 | Pascasio et al. 1974 |
| Philippine | Philippine, non-Negrito | 200 | 2.0 | Pascasio et al. 1974 |
| Puerto Rico | Puerto Ricans | 1693 | 10.3 | Thieme 1952 |
| Russia | Kharkov | 486 | 37.0 | Boyd 1950 |
| Russia | Kirghiz children | 734 | 29.1 | Ibraimov and Mirrakhimov 1979 |
| Russia | Kirghiz students | 640 | 19.6 | Ibraimov and Mirrakhimov 1979 |
| Russia | Russian students | 245 | 31.9 | Ibraimov and Mirrakhimov 1979 |
| Russia | Siberians | 137 | 5.8 | Rychkov and Borodina 1969 |
| Russia | Tiflis | 455 | 23.0 | Boyd 1950 |
| Russia | Zagorsk | 237 | 41.0 | Boyd 1950 |
| South America | Amoreeiras | 70 | 8.6 | Kalmus 1957 |
| South America | Ashkenazic Jews in Brazil | 244 | 27.9 | Saldanha and Becak 1959 |
| South America | Brazilian patients and staff | 162 | 35.1 | Kalmus, 1957 |
| South America | Brazilian students | 148 | 33.8 | Kalmus 1957 |
| South America | Brazilians of African ancestry | 90 | 12.1 | Kalmus 1957 |
| South America | Brazilians of African ancestry | 123 | 10.6 | Saldanha 1962 |
| South America | Buzios, Victoria | 73 | 13.7 | Kalmus 1957 |
| South America | Caboclos | 74 | 24.3 | Kalmus 1957 |
| South America | Caboclos, admixed | 18 | 11.1 | Kalmus 1957 |
| South America | Carajas, Brazil | 86 | 0.0* | Kalmus 1957 |
| South America | Carajas, Brazil | 86 | 0.0* | Junqueira et al. 1957 |
| South America | Chileans | 316 | 17.4 | Saldanha and Nacrur 1963 |
| South America | Curitiba | 92 | 26.0 | Freire-Maia and Quelce-Salgado 1960 |
| South America | Highland Quechua, Peru | 319 | 3.1 | Frisancho et al. 1977 |
| South America | Ilhabella | 77 | 20.8 | Kalmus 1957 |
| South America | Ilhabella, admixed | 43 | 16.3 | Kalmus 1957 |
| South America | Japanese immigrants | 89 | 9.0 | Kalmus 1957 |
| South America | Japanese immigrants in Brazil | 300 | 12.7 | Beiguelman 1962 |
| South America | Jivaro Indians, Ecuador | 327 | 2.1 | Sunderland and Ryman 1968 |
| South America | Kaingangs | 77 | 2.6* | Kalmus 1957 |
| South America | Kaingangs | 77 | 2.6* | Fernandes et al. 1957 |
| South America | Lowland Mestizo, Peru | 805 | 7.3 | Frisancho et al. 1977 |
| South America | Lowland Quechua, Peru | 672 | 7.1 | Frisancho et al. 1977 |
| South America | Manaus Amazonia | 90 | 15.6 | Kalmus 1957 |
| South America | Piracicaba, admixed | 41 | 9.8 | Kalmus 1957 |
| South America | Presidente Prudente | 73 | 8.0 | Freire-Maia and Quelce-Salgado 1960 |
| South America | Rio de Jan, admixed | 203 | 11.9 | Kalmus 1957 |
| South America | Russian immigrants in Brazil | 60 | 43.3 | Freire-Maia et al. 1960 |
| South America | Salvador, Ba | 34 | 38.0 | Freire-Maia and Quelce-Salgado 1960 |
| South America | Southern Peruvian Quechua | 522 | 2.9 | Garruto et al. 1975 |
| South America | Tucano Indians, Brazil | 128 | 6.3 | Montenegro 1964 |
| South-East Asia | Burmese medical students | 300 | 12.0 | Than-Sint and Mya-Tu 1974 |
| South-East Asia | Chinese immigrants in Singapore | 50 | 2.0 | Lugg 1955 |
| South-East Asia | European immigrants in Singapore | 50 | 19.6 | Lugg 1955 |
| South-East Asia | Malay | 237 | 16.0 | Thambipillai 1955–56 |
| South-East Asia | Malay immigrants | 50 | 15.6 | Lugg 1955 |
| South-East Asia | Malay, Negrito | 50 | 18.0 | Lugg 1956–57 |
| South-East Asia | Malay, Senoi | 50 | 4.0 | Lugg 1956–57 |
| South-East Asia | Tamil Indians in Singapore | 50 | 26.8 | Lugg 1955 |
| South-East Asia | Thailand | 56 | 5.4 | Simmons et al. 1954b |
| South-East Asia | Thailand | 460 | 9.7 | Boobphanirojana et al. 1970 |
| South-West Asia | Iraqi | 110 | 21.8 | Shah and Sattar 1981 |
| South-West Asia | Kurdish in Iran | 346 | 27.5 | Lightman et al. 1970 |
| South-West Asia | Turkey | 315 | 5.0 | Akcasu et al. 1974 |
| South-West Asia | Turkey | 366 | 11.2 | Bokesoy and Togan 1987 |
| South-West Asia | Turkey | 684 | 4.1 | Akcasu and Ozalp 1977 |
| South-West Asia | Turkey | 2000 | 20.0 | Say et al. 1966 |
Studies varied in the methods to classify nontasters.
May represent the same dataset published twice.
Refers to the subcontinent of India.
NG = not given.
2.1. PTC heterozygotes: selectively advantageous?
Based on the ubiquitous variation in PTC-tasting ability in various populations and chimpanzees, Fisher, Ford and Huxley (1939) speculated that heterozygotes have a selective advantage over homozygotes. Otherwise, in the time elapsed since the creation of these alleles, selection, or genetic drift, or both, would have eliminated one of the alleles from the population (Fisher 1939). The nature of the proposed selective advantage is unknown. One hypothesis, however, is that genotype at the PTC locus may influence food selection through its effects on bitter taste sensitivity. Given that bitterness is associated with toxic compounds, PTC tasters may be more likely to avoid such toxic compounds while PTC nontasters might be willing to eat a broader variety of foods (Drewnowski and Rock 1995, Tepper 1998). These differences in food selection of bitter tasting foods may, in turn, influence metabolism and physiology (Greene 1974, Davis 1978). There is no single food category or food type, however, that is always preferred or avoided by PTC tasters (Fischer and Griffin 1961, Fischer, Griffin, England et al. 1961, Fischer, Griffin, and Kaplan 1963, Glanville and Kaplan 1965, Forrai and Bankovi 1984, Niewind, Krondl and Shrott 1988, Mattes and Labov 1989, Jerzsa-Latta, Krondl and Coleman 1990, Anliker, Bartoshuk, Ferris et al. 1991, Frank and van der Klaauw 1994, Noble 1994, Akella 1997, Drewnowski, Henderson and Shore 1997b, Drewnowski et al. 1998a, Drewnowski et al. 1998b), reviewed in (Reed 1999, Mattes and Beauchamp 2000) and therefore further research is needed to understand how PTC genotype influences food selection.
2.2. PTC taster status and alcoholism
Alcoholism or drinking behaviour is associated with PTC insensitivity in some (Peeples 1962, Pelchat and Danowski 1992, DiCarlo and Powers 1998, Intranuovo and Powers 1998) but not all studies (Sharma 1962, Kang, Cho and Yurn 1967, Reid, Brunt and Bias 1968, Smith 1972, Swinson 1973, Swinson 1983, Kranzler et al. 1996). Interestingly, PROP retards alcohol-induced liver disease (Orrego, Blake, Blendis et al. 1994). Perhaps PTC taster status may be related to the individual differences in the consequences of chronic alcohol consumption.
2.3. PTC taster status and diseases
There have been reports of associations or lack of associations between PTC taste status and diseases and traits not directly related to taste. These include diabetes (Terry and Segall 1947, Terry 1950, Akesson 1959b, Bayani-Sioson 1964, Schelling, Tetreault, Lasagna et al. 1965, Rao and Sisodia 1970, Ali, Azad Khan, Mahtab et al. 1994), dental caries (Chung, Witkop and Henry 1962), eye disease (Becker and Morton 1964, Suzuki, Takeuchi and Kitazawa 1966, Alsbirk and Alsbirk 1972, Kalmus and Lewkonia 1973), thyroid disorders (Harris, Kallmus and Trotter 1949, Kitchin, Howel-Evans, Clarke et al. 1959, Shepard and Gartler 1960, Fraser 1961, Shepard 1961, Brand 1963, Hollingsworth 1963, Bayani-Sioson 1964, Azevedo, Krieger, Mi et al. 1965a, Covarrubias, Barzelatto, Stevenson et al. 1965, De Luca and Cramarossa 1965, Paolucci, Ferro-Luzzi, Modiano et al. 1971, Mendez de Araujo, Salzano and Wolff 1972, Persson, Kolendorf and Kolendorf 1972, Facchini, Abbati and Campagnoni 1990, Haque 1990, Koertvelyessy and Crawford 1990, Facchini, Pettener, Rimondi et al. 1997), schizophrenia (Freire-Maia, Karam, Mehl et al. 1968, Schlosberg and Baruch 1992), gastrointestinal ulcers (Kaplan, Fischer, Glanville et al. 1964, Stanchev, Tsonev and Minchev 1985, Li, McIntosh, Byth et al. 1990), depression (Whittemore 1986, 1990), personality characteristics (Very and Iacono 1968, Mascie-Taylor, McManus, MacLarnon et al. 1983, Kimmel and Lester 1987), mental function (Azevedo, Snyder and Krieger 1965b, Karam and Freire-Maia 1967, Greene 1974), growth variation (Johnson, Hertzog and Malina 1966, Whissell-Buechy and Wills 1989), malignant tumours (Milunicova, Jandova and Skoda 1969, Ahnja, Reddy and Reddy 1977) and susceptibility to infectious disease (Saldanha 1956, Akesson 1959b, Bayani-Sioson 1964, Beiguelman 1964, Brand 1964, Rao 1972, Ghei and Vaidya 1977). The reports of association between diseases and taster status may be due to chance associations; there are several instances of an initial report and one or more subsequent failures to replicate. In these cases, differences between studies in the characteristics of subjects or low statistical power may explain discordant results. Also, genetic association studies are prone to false positive results due to population stratification, which may be present in some but not all study populations. Finally, the associations may be genuine, and could occur either because the taster locus is in linkage disequilibrium with other loci that predispose to a disease, or because the PTC locus has pleiotropic effects, or because the disease process changes PTC taster status.
2.4. Mode of inheritance, segregation analysis and heritability estimations
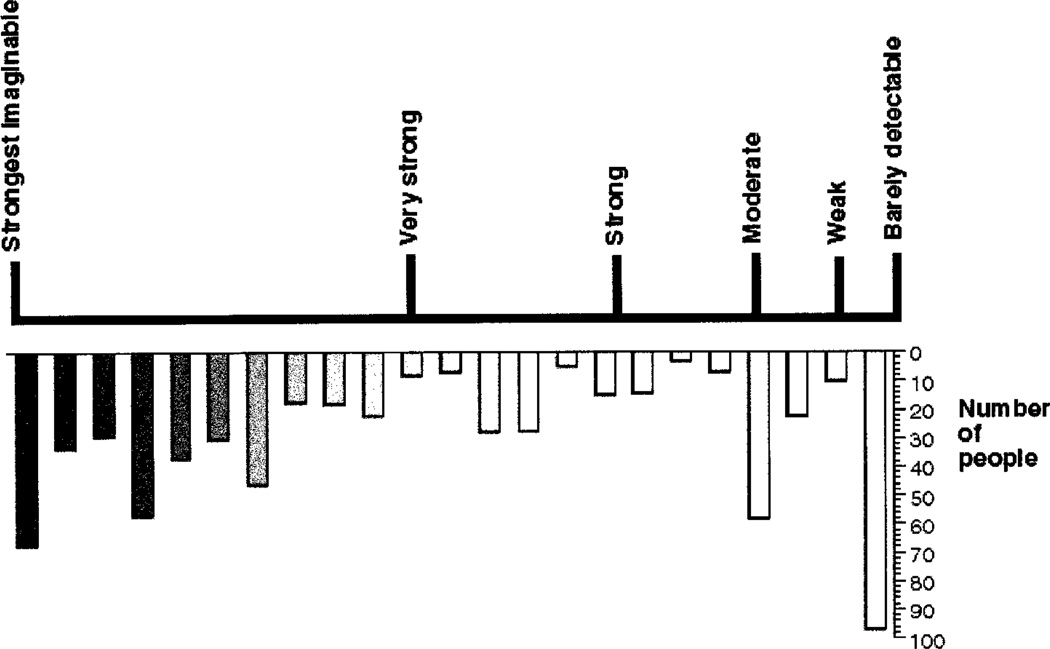
Although the inability to taste PTC, or PTC taste blindness, has often been cited as a classical textbook example of a Mendelian trait, there are controversies surrounding its exact mode of inheritance. Incomplete dominance (Falconer 1946–47, Martin 1975, Jones and McLachlan 1991, Bartoshuk et al. 1994, 1996, Reed et al. 1995), multiple alleles (Rychkov and Borodina 1969, Rychkov and Borodina 1973, Ibraimov and Mirrakhimov 1979) and multigenic inheritance have all been suggested (Boyd and Boyd 1937, Boyd 1950, Babu et al. 1984, Olson, Boehnke, Neiswanger et al. 1989). Distributions of the rating of concentrated PROP solutions demonstrate bimodallity, but there is a broad range of intensity ratings within the taster group (figure 2).
Figure 2.
Distribution of the ratings for a suprathreshold concentration of propylthiouracil, adjusted for sex and age effects.
Reddy and Rao (1989) reexamined the genetics of PTC taste thresholds by studying 100 nuclear families (Reddy and Rao 1989). They concluded that variability in thresholds is controlled by a major locus with incomplete dominance as well as by a multifactorial component. Olson et al. (1989) studied 120 families and concluded that the data fitted best a two-locus model in which one locus controls PTC tasting and the other locus controls general taste ability (Olson et al. 1989). Results of studies that identify two types of nontasters, those with a specific inability to taste PTC and those with more generalized deficits in gustatory abilities, appear to be consistent with this hypothesis (Frank and Korchmar 1985). However, it is important to bear in mind that possible measurement error, misclassifications, or hidden non-paternity might reduce support for a single-locus hypothesis, making multilocus hypothesis more acceptable. Additional segregation analysis of genotyped individuals (to exclude non-paternity) would make analysis and conclusions about heritability and mode of inheritance more robust.
When measured as a quantitative trait, it is evident that not all of the phenotypic variance in PTC taste perception is heritable. Morton, Cantor, Corey et al. (1981) reported a broad heritability of 55% for taste threshold for PTC (Morton et al.1981). The modifiers of the genotype–phenotype relationship such as gender, age, smoking, history of otitis media and head trauma may partially account for the nonheritable fraction of the phenotypic variance.
In the last decade, genetic analysis has advanced to such a degree that mapping and characterizing rare disease genes is straightforward, and significant progress has been made toward mapping multigenic traits and diseases. The PTC polymorphism, because it combines both bimodal and continuous variation, is an appealing model to develop methods and strategies for complex traits. Also, since the phenotyping is relatively stable, inexpensive, can be measured accurately, and the gene frequency is high, it is an ideal complex trait to test out current gene mapping methodology, given that the detection of genes for other complex traits has been difficult. Issues pertinent to the eventual characterization of this trait are discussed below.
Rodent models are undesirable to map the PTC locus. Because the mouse’s genome is well characterized and shares many phenotypic characteristics with humans, it has gained widespread use as an experimental model in genetics. However, it is generally accepted that the mouse is not a good model system for PTC genetics. Genetic variation does account for individual and strain differences in rodents’ perception of PTC (Richter and Clisby 1941, Hoshishima, Yokoyama and Seto 1962, Klein and DeFries 1970a, b, Tobach, Bellin and Das 1974, Lush 1986). However, none of the loci that influence the perception of propylthiouracil in mice are likely to be the human PTC locus (Whitney and Harder 1986, Harder, Boughter and Whitney 1996, Boughter and Whitney 1998, Harder and Whitney 1998). Moreover, the chorda tympani and glossopharyngel taste nerves are not more variable in response to PTC than to other bitter compounds (Dahl, Erikson and Simon 1997), suggesting that mice lack the extreme bimodality of the response compared with humans. Therefore, the species differences between mice and humans in PTC taste perception make the mouse of limited use for genetic investigation.
Other models might be suitable to map the PTC trait. A mutant strain of fruit flies (Drosophila melanogaster) displays bimodal sensitivity to PTC (Ogita 1958, Davring and Sunner 1971). Primates display the polymorphism (Fisher 1939, Chiarelli 1963, Eaton and Gavan 1965, Smith et al. 1981) as do cats (Bolekhan et al. 1997) and perhaps pigs (Braude 1949). The two best-characterized mammalian genomes are the human and the mouse and, because a well-characterized genome is essential for gene mapping and characterization, humans are the experimental species of choice.
2.5. Phenotypic considerations in gene mapping experiments
To conduct a mapping study for any trait, an accurate measure of the phenotype is essential. Two methods have been used to phenotype subjects for PTC status. One method is to determine the lowest concentration of PTC or related compound that a subject can identify or detect (threshold methods). Harris and Kalmus devised a popular threshold method widely used by other investigators (Harris and Kalmus 1949). In this test, subjects sip increasing concentrations of PTC until they detect a taste. Then four cups of PTC at that concentration, as well as four cups of water are offered to the subject, and the subject is asked to sort the cups into two groups, based upon the taste. There are other less popular variations of threshold methods, but the results obtained from all such methods are in reasonably good agreement (Lawless 1980). Refinements, such as the choice of solvent, may improve the discrimination between tasters and nontasters (Masuoka, Lee, Hatjopoulos et al. 1995, Lee and O’Mahony 1998). Several investigators attempted to further subdivide subjects by their PTC ability and by other taste abilities, such as sensitivity to quinine or perception of sodium benzoate (Hoover 1956, Fischer and Griffin 1964). Testing subjects for their ability to detect other types of chemicals at low concentrations is helpful to find nontasters that have nonspecific taste loss.
The other method is for subjects to rate the intensity of a concentration of PTC well above that which a taster can taste (suprathreshold methods). Suprathreshold methods have been recently refined (Bartoshuk et al. 1996). Using a scale that reduces ceiling effects increases the sensitivity of suprathreshold intensity measures (Lucchina, Curtis, Putnam et al. 1998). It is unclear whether the threshold or suprathreshold phenotype is the most direct reflection of the genotype, and therefore both methods of phenotyping will be useful in gene mapping studies.
2.6. Statistical methods and strategies for gene identification
Traditional parametric linkage methods may have low power to detect linkage for the PTC gene because of its high allele frequency. A significant fraction of the variation of PTC tasting in humans appears to be polygenic, and therefore parametric methods are a less desirable choice to map this trait. Affected sibling pair methods may be better suited to mapping the PTC locus. These methods do not rely on models that specify mode of inheritance, can detect multiple loci, and are capable of analysing quantitative phenotypes such as threshold or suprathreshold measures of PTC status. Strictly speaking, however, these methods are unproven, because no gene has been cloned based solely on these methods alone.
Because this taste polymorphism is found in primates (Fisher 1939, Chiarelli 1963, Eaton and Gavan 1965), PTC tasting ability may be an ancient polymorphism, not a relatively new human mutation. Consequently, linkage methods such as homozygosity mapping, which is very powerful for mapping rare recessive diseases (Guo 1997), will have little power to map this trait.
2.7. Fine-scale mapping based on linkage disequilibrium and association studies
Once the gene is mapped by linkage methods, fine mapping will facilitate gene identification. Recently developed methods for fine-scale mapping based on linkage disequilibrium may be useful, provided linkage disequilibrium is present in the region containing the PTC locus (Xiong and Guo 1997a, b).
An association study compares DNA samples from populations of individuals with and without a particular trait, in our case PTC blindness, to determine which alleles are associated with the trait. An allele is said to be associated with this trait when carriers of this allele are more frequent among PTC nontasters than tasters. Association studies can be more powerful than traditional linkage studies to detect genes responsible for a given trait (Risch and Merikangas 1996, see also Xiong and Guo 1998). This approach may be a promising way to identify candidate genes responsible for PTC blindness.
2.8. Results of gene mapping experiments
Early studies investigated linkage between the PTC locus and blood groups or other genetic traits (Hogben and Pollack 1935, Burks and Wyandt 1941, Kloepfer 1946, Sanger and Race 1951, Holt, Thompson, Sanger et al. 1952, Umansky, Reid, Corcoran et al. 1966, Gedde-Dahl and Monn 1967, Fu, Azevedo and Moreton 1968, Gedde-Dahl and Monn 1968, Blondheim and Reznik 1971, Chautard-Freire-Maia 1974, Conneally, Nance and Huntzinger 1974, Crandall and Spence 1974, Conneally, Dumont-Driscoll, Huntzinger et al. 1976, Keats, Morton and Rao 1978, Spence, Falk, Neiswanger et al. 1984, O’Hanlon, Weissbecker, Cortessis et al. 1988, Bhatkar, Nallulwar and Katti 1989). While some investigators detected linkage between the PTC and a polymorphism in a blood group antigen (KELL) on chromosome 7 (Umansky et al. 1966, Chautard-Freire-Maia 1974, Conneally et al. 1974, 1976, Keats et al. 1978), other investigators did not (Holt et al. 1952, Crandall and Spence 1974, Spence et al. 1984).
A recent linkage study using sibling pair methods has indicated that the primary PTC locus maps to chromosome 5p15, with at least one additional locus on chromosome 7 (Reed, Nanthakumar , North et al. 1999). The initial linkages to chromosome 7 and the subsequent non-replication described in the preceding paragraph appears to have occurred because the chromosome 7 linkage accounts for less variance than the locus on chromosome 5 and may be more important in threshold rather than suprathreshold sensitivity. Polymorphic markers near the PTC locus identified within 5p15 are differentially transmitted to taster children by heterozygous parents, suggested linkage disequilibrium (Reed et al. 1999). These preliminary results suggested fine-scale mapping based on linkage disequilibrium methods are likely to be useful in narrowing the interval containing the PTC locus.
2.9. Possible identification of a family of bitter taste receptors and the PTC gene
Two groups of investigators have identified genes that may be bitter taste receptors (Alder et al. 2000, Matsunami et al. 2000). The bitter genes identified are G-protein coupled receptors, which is the expected structure of taste receptors, and at least one of these genes has been shown to be functional (Chandrashekar et al. 2000). Of the putative bitter receptors sequenced to date, five are located in areas of linkage to PTC (5p15, T2R1; 7q31, T2R3, T2R4, T2R5) and therefore are candidates for the PTC receptor.
The most definitive demonstration that a candidate gene is responsible for the PTC phenotype is the discovery of an allele that co-segregates with the taste phenotype. Sequencing efforts are underway to identify allelic variability within these genes, and to examine the relationship between genotype and phenotype.
3.0. Identification of the PTC loci will further our understanding of sensory biology and the genetics of complex traits
One of the investigators to first describe the heritability of the PTC polymorphism demonstrated differences in chemosensory experience by small experiments, interjected between dinner courses at a meeting of American Association for the Advancement of Science in 1934 (Blakeslee 1935). These experiments highlighted the individual differences in our sensory world. After almost 70 years, the origins of that variability, apparently due in large part to genetic factors, remains a conundrum. Through the characterization of PTC genetics, we will be in a better position to understand the origins of these individual sensory experiences. This achievement would assist in elucidating the mechanism of taste transduction. In addition, the mapping of the PTC genes will provide a powerful tool to examine the genetic basis for food preferences and the relationship between taste status and health outcomes. It also provides a testing ground for current gene-mapping methodology, which have not yet been successful for complex traits.
Acknowledgements
This work was supported by NIH R01GM56515 and R29GM52205 to SWG, NIH R03DC03509 to DRR, and NIH grants R01DK44073 and R01DK48095 to R. Arlen Price. We thank Michael G. Tordoff, R. Arlen Price, and Gary K. Beauchamp for their comments on an earlier draft of this manuscript. The authors are grateful for the ongoing advice and encouragement of Linda M. Bartoshuk. SWG also wishes to thank Chang-Jiang Zheng for his stimulating discussions of the topic. We thank Rachel Hertz and Weidong Li for translation of research articles. The assistance of Duc Bach, Karynn Henry, Nicole Baker, Cassandra George and Richard Joseph in obtaining reference materials is gratefully acknowledged.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors (see comments) Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Adrian E. Opening address. In: Zotterman Y, editor. Olfaction and Taste. New York: Macmillan; 1963. pp. 1–4. [Google Scholar]
- Agrawal HN. A short note on a study of A.B.O. blood groups, P.T.C test sensitivity, middle phalangeal hairs and sickle cell traits among the Wad Balgei of Andhra Pradesh. Bulletin of Anthropological Survey of India. 1964;13:111–113. [Google Scholar]
- Agrawal HN. A study of ABO blood groups, P.T.C. taste sensitivity, sickle cell trait and middle phalangeal hairs among the Burmese immigrants of Andaman Islands. The Eastern Anthropologist. 1966;19:107–117. [Google Scholar]
- Agrawal HN. ABO blood groups, P.T.C. taste sensitivity, sickle cell trait, middle phalangeal hairs, and colour blindness in the costal Nicobarese of Great Nicobar. Acta Genetica et Statistica Medica. 1968;18:147–154. doi: 10.1159/000152131. [DOI] [PubMed] [Google Scholar]
- Agrawal HN. An anthropological study of the Shompens of Great Nicobar. Human Heredity. 1969;19:312–315. doi: 10.1159/000152234. [DOI] [PubMed] [Google Scholar]
- Ahnja YR, Reddy OS, Reddy SS. PTC taste-sensitivity among women with carcinoma of the cervix. Anthropologist—Delhi. 1977;24:40–42. [Google Scholar]
- Akcasu A, Ozalp E. Distribution of taste thresholds for phenylthiocarbamide among different age groups in Turkey. Pahlavi Medical Journal. 1977;8:294–304. [PubMed] [Google Scholar]
- Akcasu A, Kameswaran L, Sanyal RK, West GB. Taste thresholds of phenylthiocarbamate. Behaviour Genetic. 1974;4:305–315. doi: 10.1007/BF01066152. [DOI] [PubMed] [Google Scholar]
- Akella GD, Henderson SA, Drewnowski A. Sensory acceptance of Japanese green tea and soy products is linked to genetic sensitivity to 6-n-propylthiouracil. Nutrition and Cancer. 1997;29:146–151. doi: 10.1080/01635589709514616. [DOI] [PubMed] [Google Scholar]
- Akesson HO. Taste deficiency for phenyl-thio-urea in Southern Sweden. Acta Genetica Medicae et Gemellogoiae. 1959a;8:431–433. [PubMed] [Google Scholar]
- Akesson HO. Taste sensitivity to phenyl-thio-urea in tuberculosis and diabetes mellitus. Annals of Human Genetics. 1959b;23:262–265. doi: 10.1111/j.1469-1809.1959.tb01469.x. [DOI] [PubMed] [Google Scholar]
- Ali SG, Azad Khan AK, Mahtab H, Khan AR, Muhibullah M. Association of phenylthiocarbamide taste sensitivity with diabetes mellitus in Bangladesh. Human Hereditary. 1994;44:14–17. doi: 10.1159/000154183. [DOI] [PubMed] [Google Scholar]
- Allison AC. A note on taste-blindness in Kenya Africans and Arabs. Man. 1951;204:119–120. [Google Scholar]
- Allison AC, Blumberg BS. Ability to taste phenylthiocarbamide among Alaskan Eskimos and other populations. Human Biology. 1959;31:352–359. [Google Scholar]
- Allison AC, Nevanlinna HR. Taste-deficiency in Lappish and Finnish populations. Annals of Eugentics London. 1952;17:113–114. doi: 10.1111/j.1469-1809.1953.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Alsbirk KE, Alsbirk PH. PTC taste sensitivity in Greenland Eskimos from Umanaq. Distribution and correlation to ocular anterior chamber depth. Human Hereditary. 1972;22:445–452. doi: 10.1159/000152522. [DOI] [PubMed] [Google Scholar]
- Anliker JA, Bartoshuk L, Ferris AM, Hooks LD. Children’s food preferences and genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) American Journal of Clinical Nutrition. 1991;54:316–320. doi: 10.1093/ajcn/54.2.316. [DOI] [PubMed] [Google Scholar]
- Anonymous. Six in ten ‘tasteblind’ to bitter chemical. Science News Letter. 1931;9:249. [Google Scholar]
- Ardashnikov SN, Lichtenstein EA, Martynova RP, Soboleva GV, Postnikova EN. The diagnosis of zygosity in twins. Three instances of differences in taste acuity in identical twins. Journal of Heredity. 1936;27:465–468. [Google Scholar]
- Azevedo E, Krieger H, Mi MP, Morton NE. PTC taste sensitivity and endemic goiter in Brazil. American Journal of Human Genetics. 1965a;17:87–90. [PMC free article] [PubMed] [Google Scholar]
- Azevedo E, Snyder LH, Krieger H. Phenylthiocarbamide-tasting in the mentally immature. The Lancet. 1965b;i:1223–1224. [PubMed] [Google Scholar]
- Babu BV, Kusuma YS, Naidu JM. Genetic diversity of PTC taste sensitivity among tribal and caste populations of Andhra Pradesh, India. Zeitschrift für Morphologie und Anthropologie. 1996;81:217–221. [PubMed] [Google Scholar]
- Babu MS, Jaikishan G, Veerraju P. Variation in taste perception to P.T.C. in two tribal populations of Andhra Pradesh. Indian Anthropologist. 1984;14:63–68. [Google Scholar]
- Bagga A, Seth PK. Incidence of PTC tasters and defective colour vision among Rajputs of Palampur. Man in India. 1992;72:69–72. [Google Scholar]
- Balakrishna A, Ramesh M, Veerraju P. PTC taste sensitivity and red green colour-blindness among Dhobis. Man in India. 1992;72:65–68. [Google Scholar]
- Balgir R. Anthropogenetic variations among the two breeding isolates of Gujjars of Northwest India. South Asian Anthropologist. 1992;13:23–29. [Google Scholar]
- Barnicot NA. Taste deficiency for phenylthiourea in African Negroes and Chinese. Annals of Eugentics London. 1950;15:248–254. doi: 10.1111/j.1469-1809.1949.tb02440.x. [DOI] [PubMed] [Google Scholar]
- Barnicot N, Woodburn J. Colour-blindness and sensitivity to PTC in Hadza. Annals of Human Biology. 1975;2:61–68. doi: 10.1080/03014467500000571. [DOI] [PubMed] [Google Scholar]
- Barnicot NA, Harris H, Kalmus H. Taste thresholds of further eighteen compounds and their correlation with P.T.C. thresholds. Annals of Eugentics. 1951;16:119–127. doi: 10.1111/j.1469-1809.1951.tb02464.x. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Genetic and pathological taste variation: what can we learn from animal models and human disease? Ciba Foundation Symposium. 1993;179:251–262. doi: 10.1002/9780470514511.ch16. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Lucchina LA, Prutkin J, Fast K. PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Annals of New York Academy of Science. 1998;855:793–796. doi: 10.1111/j.1749-6632.1998.tb10660.x. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ. PTC/PROP tasting: anatomy, psychophysics, and sex effects. [published erratum appears in Physiology of Behaviour, 1995, 58, 203] Physiology of Behaviour. 1994;56:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Reed D, Williams A. Supertasting, earaches and head injury: genetics and pathology alter our taste worlds. Neuroscience Biobehaviour Review. 1996;20:79–87. doi: 10.1016/0149-7634(95)00042-d. [DOI] [PubMed] [Google Scholar]
- Basu A, Sarkar J, Roy B, Dasgupta P, Sarkar SS. Anthropo-genetic investigations in the Dalma and the Ajodhya Hills and their neighborhood. Science and Culture. 1966;32:273–275. [Google Scholar]
- Bat-Miriam M, Adam A, Hananel Z. A survey of some gentical characters in Ethiopian tribes VI. Taste thresholds for phenylthiourea. American Journal of Physical Anthropology. 1962;20:190–193. doi: 10.1002/ajpa.1330200224. [DOI] [PubMed] [Google Scholar]
- Bayani-Sioson P. Is natural selection maintaining the ‘PTC polymorphism system’ in the population through susceptibility to disease conditions? Acta Medica Philippines. 1964;1:47–50. [PubMed] [Google Scholar]
- Beach SA. The frequency of taste-blindness in Welsh populations. Heredity. 1953;7:401–407. [Google Scholar]
- Becker B, Morton WR. Phenylthiourea taste testing and glaucoma. Archives of Ophthalmology. 1964;72:323–327. doi: 10.1001/archopht.1964.00970020323006. [DOI] [PubMed] [Google Scholar]
- Beiguelman B. Taste sensitivity to PTC among Japanese immigrants in Brasil. Review Brasil Biology. 1962;22:93–97. [PubMed] [Google Scholar]
- Beiguelman B. Taste sensitivity to phenylthiourea among patients affected with both tuberculosis and leprosy. Acta Genetica Medicae et Gemellogoiae. 1964;13:190–192. doi: 10.1017/s112096230001581x. [DOI] [PubMed] [Google Scholar]
- Bhalla V. Variations in taste threshold for PTC in populations of Tibet and Ladakh. Human Hereditary. 1972;22:453–458. doi: 10.1159/000152523. [DOI] [PubMed] [Google Scholar]
- Bhalla V, Bhatia K, Sood S, Aggarwal M. Genetic polymorphisms in Cis-Himalayan populations II. A1A2BO blood groups, ABH secretion, PTC tasting ability and colour blindness in Hill Rajputs and Kolis of Tirthan Valley (H. P.) Indian Anthropologist. 1980;10:55–59. [Google Scholar]
- Bhatia S, Sharma KN, Tandon OP, Singh S. Relation of PTC responses and secretor status to blood groups. Indian Journal of Physiology and Pharmacology. 1979;23:269–276. [PubMed] [Google Scholar]
- Bhatia S, Sharma KN, Mehta V. Taste responsiveness to phenyl-thio-carbamide and glucose during menstrual cycle. Current Science. 1981;50:980–983. [Google Scholar]
- Bhatkar RS, Nallulwar SC, Katti VA. The study of tasters and non-tasters of phenyl-thio-carbamide (PTC) and its relation to blood groups. Indian Journal of Physiology and Pharmacology. 1989;33:168–170. [PubMed] [Google Scholar]
- Bhattacharjee PN. A genetic survey in the Rarhi Brahmin and the Muslim of West Bengal: A1-A2-B-0, M-N, RH blood groups, ABH secretion, sickle-cell, P.T.C. taste, middle phalangeal hair and color blindness. Bulletin of the Department of Anthropology, Government of India. 1956;5:18–28. [Google Scholar]
- Bhattacharjee P, Chowdhuri D, Chatterjee S. Genetic variation among the Sikkimese Lepchas (A1 A2 BO, MN, Rh blood groups, PTC taste and colour blindness) and their affinity. Bulletin of the Anthropological Survey of India—Calcutta. 1978;27:24–31. [Google Scholar]
- Bhattacharya DK. Tasting of P.T.C. among the Anglo-Indians of India. Acta Genetica Medicae et Gemellologiae. 1964;13:159–166. doi: 10.1017/s1120962300015766. [DOI] [PubMed] [Google Scholar]
- Blakeslee AF. Genetics of sensory thresholds: taste for phenyl thio carbamide. Science. 1931;74:607. doi: 10.1073/pnas.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee AF. Genetics of sensory thresholds: taste for phenyl thio carbamide. Proceedings of the National Academy of Science. 1932;18:120–130. doi: 10.1073/pnas.18.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee AF. A dinner demonstration of threshold differences in taste and smell. Science. 1935;81:504–507. doi: 10.1126/science.81.2108.504. [DOI] [PubMed] [Google Scholar]
- Blondheim SH, Reznik L. The threshold for the smell of acetone and its relationship to the ability to taste phenythiocarbamate. Experientia. 1971;27:1282–1283. doi: 10.1007/BF02136687. [DOI] [PubMed] [Google Scholar]
- Bokesoy I, Togan I. Taste sensitivity to phenylthiocarbamide in Turkey. Gene Geography. 1987;1:99–101. [PubMed] [Google Scholar]
- Bolekhan EA, Semenov DG, Gerasimova IA, Samoilov MO. Use of phenylthiocarbamide for assessing cAMP-dependent resistance to anoxia in animals. Neuroscience, Behaviour and Physiology. 1997;27:268–271. doi: 10.1007/BF02462892. [DOI] [PubMed] [Google Scholar]
- Bonne B. Genes and phenotypes in the Samaritan isolate. American Journal of Physical Anthropology. 1966;24:1–20. doi: 10.1002/ajpa.1330240102. [DOI] [PubMed] [Google Scholar]
- Bonne B, Ashbel S, Berlin G, Sela B. The Habbanite isolate. III. Anthropometrics, taste sensitivity, and color vision. Human Heredity. 1972;22:430–444. doi: 10.1159/000152521. [DOI] [PubMed] [Google Scholar]
- Boobphanirojana P, Chetanasilpin M, Saengudom C, Flatz G. Phenylthiocarbamide taste thresholds in the population of Thailand. Humangenetik. 1970;10:329–334. doi: 10.1007/BF00278769. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Whitney G. Human taste thresholds for sucrose octaacetate. Chemical Senses. 1993;18:445–448. [Google Scholar]
- Boughter JD, Whitney G. Behavioral specificity of the bitter taste gene Soa. Physiology & Behavior. 1998;63:101–108. doi: 10.1016/s0031-9384(97)00398-3. [DOI] [PubMed] [Google Scholar]
- Boyd WC. Genetics and the Races of Man. An Introduction to Modern Physical Anthropology. Boston: Little, Brown and Company; 1950. [Google Scholar]
- Boyd WC, Boyd LG. New racial blood group studies in Europe and Egypt. Science. 1936;84:328–329. doi: 10.1126/science.84.2180.328. [DOI] [PubMed] [Google Scholar]
- Boyd WC, Boyd LG. Sexual and racial variations in ability to taste phenyl-thio-carbamide, with some data on the inheritance. Annals of Eugenics London. 1937;8:46–51. [Google Scholar]
- Brand N. Taste sensitivity and endemic goitre in Israel. Annals of Human Genetics, London. 1963;26:321–324. doi: 10.1111/j.1469-1809.1963.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Brand N. Taste response and poliomyelitis. Annals of Human Genetics, London. 1964;27:233–239. doi: 10.1111/j.1469-1809.1963.tb00789.x. [DOI] [PubMed] [Google Scholar]
- Braude R. Some observations on the behavior of pigs in an experimental piggery. Bulletin of Animal Behaviour. 1949;6:17–25. [Google Scholar]
- Buchi EC. Blood, secretion and taste among the Pallar, a South Indian community. The Anthropologist, University of Delhi. 1955;1:1–8. [Google Scholar]
- Buchi EC, Roy S. Taste, middle-phalangeal hair and colour vision of the Onge from Little Andaman. Bulletin Department Government, India. 1955;4:7–10. [Google Scholar]
- Burks BS, Wyandt H. Oval blood cells in human subjects tested for linkage with taste for PTC mid-digital hair, hair color, A-B agglutinogens, and sex. Genetics. 1941;26:223–233. doi: 10.1093/genetics/26.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardullo H, Holt LJ. Ability of infants to taste PTC: its application in cases of doubtful paternity. Proceedings of the Society of Experimental Biology and Medicine. 1951;76:589–592. doi: 10.3181/00379727-76-18569. [DOI] [PubMed] [Google Scholar]
- Cartwright RA, Sunderland E. Phenylthiocarbamide (PTC) tasting ability in populations in the north of England: with a note on Endemic Goitre. Acta Genetica et Statistica Medica. 1967:17. doi: 10.1159/000152186. [DOI] [PubMed] [Google Scholar]
- Chandraiah M, Bahadur B. P.T.C. tasting genes among six endogamous groups of Gadwal (A.P.) Journal of Physical Anthropology and Human Genetics. 1975;5:179–182. [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay PK. Taste sensitivity to phenylthiocarbamide among the Jats. Anatomischer Anzeiger. 1971;33:52–60. [PubMed] [Google Scholar]
- Chautard-Freire-Maia EA. Linkage relationships between 22 autosomal markers. Annals of Human Genetic. 1974;38:191–198. doi: 10.1111/j.1469-1809.1974.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Chiarelli B. Sensitivity to P.T.C. (phenyl-thio-carbamide) in primates. Folia Primatologica. 1963;1:88–94. [Google Scholar]
- Chiasson LP. Gene frequencies of the Micmac Indians. Journal of Heredity. 1963;54:229–236. doi: 10.1093/oxfordjournals.jhered.a107255. [DOI] [PubMed] [Google Scholar]
- Chowdhury AK. PTC taste sensitivity and colour-blindness among the Bodh and Swangla of Lahaul. Man in India. 1988;68:448–453. [Google Scholar]
- Chung CS, Witkop CJ, Henry JL. A genetic study of dental caries with special reference to PTC taste sensitivity. American Journal of Human Genetics. 1962;16:231–245. [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Ogdon DP. Taste blindness to phenyl-thio-carbamide and related compounds. Psychological Bulletin. 1949a;46:490–498. doi: 10.1037/h0060685. [DOI] [PubMed] [Google Scholar]
- Cohen J, Ogdon DP. Taste blindness to phenyl-thio-carbamide as a function of saliva. Science. 1949b;110:532–533. doi: 10.1126/science.110.2864.532. [DOI] [PubMed] [Google Scholar]
- Conneally PM, Nance WE, Huntzinger RS. Linkage analysis of Kell-Sutter and PTC loci. American Journal of Human Genetics. 1974;26:22a. [Google Scholar]
- Conneally PM, Dumont-Driscoll M, Huntzinger RS, Nance WE, Jackson CE. Linkage relations of the loci for Kell and phenylthiocarbamide taste sensitivity. Human Hereditary. 1976;26:267–271. doi: 10.1159/000152813. [DOI] [PubMed] [Google Scholar]
- Cook RC. Inherited variations in the sense of taste. American Mercury. 1933;28:67–69. [Google Scholar]
- Covarrubias E, Barzelatto J, Stevenson C, Bobadilla E, Pardo A. Taste sensitivity to phenyl-thio-carbamide and endemic goitre among Pewenche Indians. Nature. 1965;205:1036. [Google Scholar]
- Crandall BF, Spence MA. Linkage relations of the phenylthiocarbamide locus (PTC) Human Hereditary. 1974;24:247–252. doi: 10.1159/000152657. [DOI] [PubMed] [Google Scholar]
- Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Research. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- Das PB. Taste sensitivity to phenylthiourea in two caste groups of Assam. Man in India. 1971;51:252–256. [Google Scholar]
- Das PB, Buragohain SN. Taste sensitivity to phenylthiourea in two caste groups of Assam. Man in India. 1969;51:252–256. [Google Scholar]
- Das PK, Pattanaik G, Satpathy KC. PTC taste ability among Juang Paraja and Rana of Orissa. Society and Culture. 1978;9:55–60. [Google Scholar]
- Das SR. Application of phenylthiocarbamide taste character in the study of racial variation. Journal of Indian Anthropology Social Calcutta. 1966;1:63–80. [Google Scholar]
- Das SR, Ghosh L. A genetical survey among the Paniyan—a South Indian Aboriginal Tribe: (ABO, MN blood groups, secretor factor and taste ability) Bulletin of the Department of Anthropology Government of India. 1954;3:65–72. [Google Scholar]
- Das SR, Mukherjee DP. Phenylthiocarbamide taste sensitivity survey among the Pareng Gadaba, the Ollaro Gadaba, and the Konda Paroja of Koraput District, Orissa. Acta Genetica et Statistica Medica. 1964;14:168–176. doi: 10.1159/000151842. [DOI] [PubMed] [Google Scholar]
- Das SR, Mukherjee DP, Bhattacharjee PN. P.T.C. taste threshold distribution in the Bado Gadaba and the Bareng Paroja of Koraput district in Orissa. Acta Genetica et Statistica Medica. 1963;13:369–377. doi: 10.1159/000151821. [DOI] [PubMed] [Google Scholar]
- Das SR, Mukherjee DP, Bhattacharjee PN. Survey of the blood groups and PTC taste among the Rajbanshi caste of West Bengal (ABO, MNS, Rh, Duffy and Diego) Acta Genetica et Statistica Medica. 1967;17:433–445. doi: 10.1159/000152094. [DOI] [PubMed] [Google Scholar]
- Dass U. P.T.C. taste sensitivity among the Mikir of Assam. Bulletin of the Department of Anthropology Dibrugarh University. 1976;5:71–73. [Google Scholar]
- Davis RG. Increased bitter taste detection in Yucatan inhabitants related to coffee as a dietary source of niacin. Chemical Senses. 1978;3:423–429. [Google Scholar]
- Davring L, Sunner M. Cytogenetic effects of 2,4,5-trichlorophenoxyacetic acid on oogenesis and early embryogenesis in Drosophila melanogaster. Hereditas. 1971;68:115–122. doi: 10.1111/j.1601-5223.1971.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Dawson W, West GB. Taste polymorphism to anetholtrithione and phenylthiocarbamate. Annals of Human Genetics, London. 1967;30:273–276. doi: 10.1111/j.1469-1809.1967.tb00027.x. [DOI] [PubMed] [Google Scholar]
- DeLuca F, Cramarossa L. Phenylthiourea and endemic goitre. Lancet. 1965;1:1399–1400. [Google Scholar]
- Deb M, Shukla B. Taste sensitivity to PTC among the Bengali Brahmins and Kayasthas of Lucknow. Indian Journal of Physical Anthropology & Human Genetics. 1981;7:77–86. [Google Scholar]
- Devi KS, Lakshmi N, Veerraju P. Taste sensitivity to phenylthiocarbamide and colour blindness in Chettibalijas of Andhra Pradesh, South India. Journal of Human Ecology. 1995;6:57–58. [Google Scholar]
- Dhesi JS, Gupta AK, Saini RG. Frequency of PTC taste blindness among Punjabis. Current Science. 1972;41:119–120. [Google Scholar]
- DiCarlo ST, Powers AS. Propylthiouracil tasting as a possible genetic association marker for two types of alcoholism [In Process Citation] Physiology Behaviour. 1998;64:147–152. doi: 10.1016/s0031-9384(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Rock CL. The influence of genetic taste markers on food acceptance. American Journal of Clinical Nutrition. 1995;62:506–511. doi: 10.1093/ajcn/62.3.506. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Henderson SA, Shore AB. Genetic sensitvity to 6-n-propylthiouracil (PROP) and hedonic responses to bitter and sweet tastes. Chemical Senses. 1997a;22:27–37. doi: 10.1093/chemse/22.1.27. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Henderson SA, Shore AB. Taste responses to naringin, a flavonoid, and the acceptance of grapefruit juice are related to genetic sensitivity to 6-n-propylthiouracil. American Journal of Clinical Nutrition. 1997b;66:391–397. doi: 10.1093/ajcn/66.2.391. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Henderson SA, Barratt-Fornell A. Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiology Behaviour. 1998a;63:771–777. doi: 10.1016/s0031-9384(97)00540-4. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Henderson SA, Shore AB, Barratt-Fornell A. Sensory responses to 6-n-propylthiouracil (PROP) or sucrose solutions and food preferences in young women. Annals of New York Academy of Sciences. 1998b;855:797–801. doi: 10.1111/j.1749-6632.1998.tb10661.x. [DOI] [PubMed] [Google Scholar]
- Eaton JW, Gavan JA. Sensitivity to P-T-C among primates. American Journal of Physical Anthropology. 1965;23:381–388. doi: 10.1002/ajpa.1330230411. [DOI] [PubMed] [Google Scholar]
- Eriksson AW, Forsius H. Studies on human population genetics and anthropology in isolates on the Åland Islands. Journal of Genetics of Humans. 1964;13:60–75. [PubMed] [Google Scholar]
- Eriksson AW, Fellman J, Forsius H, Lehmann W. Phenylthiocarbamide tasting ability among Lapps and Finns. Human Hereditary. 1970;20:623–630. doi: 10.1159/000152368. [DOI] [PubMed] [Google Scholar]
- Facchini F, Abbati A, Campagnoni S. Possible relations between sensitivity to phenylthiocarbamide and goiter. Human Biology. 1990;62:545–552. [PubMed] [Google Scholar]
- Facchini F, Pettener D, Rimondi A, Sichimbaeva K, Riva P, Salvi P, Pretolani E, Fiori G. Taste sensitivity to PTC and thyroid function (FT4 and TSH) in high-and low-altitude Kirghiz populations in the Pamir. Human Biology. 1997;69:97–106. [PubMed] [Google Scholar]
- Falconer DS. Sensory thresholds for solutions of phenyl-thio-carbamide. Results of tests on a large sample, made by R.A. Fisher. Annals of Eugenics. 1946–47;13:211–222. [PubMed] [Google Scholar]
- Fernandes JL, Junqueira PC, Kalmus H, Ottensooser F, Pasqualin R, Wishart P. P.T.C. thresholds, colour vision and blood factors of Brazilian Indians I. Kaingangs. American Journal of Human Genetics. 1957;22:16–21. doi: 10.1111/j.1469-1809.1957.tb01294.x. [DOI] [PubMed] [Google Scholar]
- Fernberger SW. A preliminary study of taste deficiency. American Journal of Psychology. 1932;44:322–326. [Google Scholar]
- Fischer R, Griffin F. ‘Taste-blindness’ and variations in taste-threshold in relation to thyroid metabolism. Journal of Neuropsychiatry. 1961;3:98–104. [PubMed] [Google Scholar]
- Fischer R, Griffin F. Pharmacogenetic aspects of gustation. Drug Research (Arzneimittel-Forschung) 1964;14:673–686. [PubMed] [Google Scholar]
- Fischer R, Griffin F, England S, Garn SM. Taste thresholds and food dislikes. Nature. 1961;191:1328. doi: 10.1038/1911328a0. [DOI] [PubMed] [Google Scholar]
- Fischer R, Griffin F, Kaplan AR. Taste thresholds, cigarette smoking, and food dislikes. Medicina Experimentalis. 1963;9:151–167. doi: 10.1159/000135346. [DOI] [PubMed] [Google Scholar]
- Fisher R. Taste-testing the Anthropoid apes. Nature. 1939;144:750. [Google Scholar]
- Forrai G, Bankovi G. Taste perception for phenythiocarbamide and food choice—a Hungarian twin study. Acta Physiologic a Hungarica. 1984;64:33–40. [PubMed] [Google Scholar]
- Fox AL. The relationship between chemical constitution and taste. Proceedings of the National Academy of Sciences. 1932;18:115–120. doi: 10.1073/pnas.18.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RA, Korchmar DL. Gustatory processing differences in PTC tasters and non-tasters: a reaction time analysis. Physiology Behaviour. 1985;35:239–242. doi: 10.1016/0031-9384(85)90343-9. [DOI] [PubMed] [Google Scholar]
- Frank RA, van der Klaauw NJ. The contribution of chemosensory factors to individual differences in reported food preferences. Appetite. 1994;22:101–123. doi: 10.1006/appe.1994.1011. [DOI] [PubMed] [Google Scholar]
- Fraser GR. Cretinism and taste sensitivity to phenylthiocarbamide. The Lancet. 1961;1:964–965. doi: 10.1016/s0140-6736(61)91881-5. [DOI] [PubMed] [Google Scholar]
- Freire-Maia A. Smoking and P.T.C. sensitivity. Annals of Human Genetics, London. 1960;24:333–341. doi: 10.1111/j.1469-1809.1960.tb01746.x. [DOI] [PubMed] [Google Scholar]
- Freire-Maia A, Quelce-Salgado A. Taste sensitivity to P.T.C. in samples from three Brazilian populations. Annals of Human Genetics, London. 1960;24:97–102. doi: 10.1111/j.1469-1809.1959.tb01720.x. [DOI] [PubMed] [Google Scholar]
- Freire-Maia A, Freire-Maia N, Quelce-Salgado A. Genetic analysis in Russian immigrants. PTC sensitivity, finger prints, color vision, hand clasping, and arm folding. Amercan Journal of Physical Anthropology. 1960;18:235–240. doi: 10.1002/ajpa.1330180311. [DOI] [PubMed] [Google Scholar]
- Freire-Maia N, Karam E, Jr, Mehl H. P.T.C. sensitivity among psychiatric patients. Acta Genetica et Statistica Medica. 1968;18:31–37. doi: 10.1159/000152117. [DOI] [PubMed] [Google Scholar]
- Frisancho AR, Klayman JE, Schessler T, Way AB. Taste sensitivity to phenylthiourea (PTC), tongue rolling, and hand clasping among Peruvian and other Native American populations. Human Biology. 1977;49:155–163. [PubMed] [Google Scholar]
- Fu L, Azevedo E, Morton N. Evidence against the reported linkage to phosphoglucomutase (PGM1) and phenylthiocarbamide-tasting (PTC) Acta Genetica et Statistica Medica. 1968;18:416–419. doi: 10.1159/000152162. [DOI] [PubMed] [Google Scholar]
- Fukuoka G. Frequency of taste-blindness among the Japanese and related races. Eugentics News. 1936;21:52–54. [Google Scholar]
- Garruto R, Hoff C, Baker P, Jacob H. Phenotypic variation in ABO and Rh blood groups, PTC tasting ability, and lingual rotation among Southern Peruvian Quechua Indians. Human Biology. 1975;47:193–199. [PubMed] [Google Scholar]
- Gedde-Dahl T, Monn E. Linkage relations of the phosphoglucomutase PGM1 locus in man. Probable linkage to phenylthiocarbamid [sic] (PTC) taster locus. Acta Genetica et Statistica Medica. 1967;17:482–494. doi: 10.1159/000152102. [DOI] [PubMed] [Google Scholar]
- Gedde-Dahl TJ, Monn E. A note on the PGM1-PTC linkage relation. Acta Genetica et Statistica Medica. 1968;18:420. doi: 10.1159/000152163. [DOI] [PubMed] [Google Scholar]
- Gent JF, Bartoshuk LM. Sweetness of sucrose, neohesperidin, dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chemical Senses. 1983;7:265–272. [Google Scholar]
- Ghei SK, Vaidya MC. Taste deficiency to phenyl-thio-carbamide (P.T.C.) in leprosy. Journal of Anatomical Society of India. 1977;26:118–123. [Google Scholar]
- Ghosh AK. ABO blood groups and PTC taste sensitivity among the Kota of Nilgiri Hills. Human Heredity. 1973;23:78–82. doi: 10.1159/000152557. [DOI] [PubMed] [Google Scholar]
- Glanville EV, Kaplan AR. Food preference and sensitivity of taste for bitter compounds. Nature. 1965;4974:851–853. [Google Scholar]
- Goud JD, Rao PR. Colour-blindness and taste sensitivity to phenylthiocarbamide in tribal populations of Andhra Pradesh. Journal of Indian Anthropology Society. 1979a;14:269–274. [Google Scholar]
- Goud JD, Rao PR. Genetic distances among the five tribal populations of Andhra, Pradesh, South India. Anatomischer Anzeiger. 1979b;37:1–9. [PubMed] [Google Scholar]
- Graydon JJ, Semple NM, Simmons RT, Franken S. Blood groups in Pygmies of the Wissellakes in Netherlands New Guinea. American Journal of Physical Anthropology. 1958;16:149–171. [Google Scholar]
- Greene LS. Physical growth and development, neurological maturation, and behavioral functioning in two Ecuadorian Andean communities in which goiter is endemic. II. PTC taste sensitivity and neurological maturation. American Journal of Physical Anthropology. 1974;41:139–151. doi: 10.1002/ajpa.1330410118. [DOI] [PubMed] [Google Scholar]
- Grünwald P, Herman C. Study of several gene frequencies in Yugoslav population. Nature. 1963;199:830–831. doi: 10.1038/199830a0. [DOI] [PubMed] [Google Scholar]
- Guo S, Shen F, Wang Y, Zheng C. Threshold distribution of phenylthiocarbamide (PTC) in the Chinese population. Annals of New York Academy of Sciences. 1998;855:810–812. doi: 10.1111/j.1749-6632.1998.tb10664.x. [DOI] [PubMed] [Google Scholar]
- Guo SW. Linkage disequilibrium measures for fine-scale mapping: a comparison. Human Hereditary. 1997;47:301–314. doi: 10.1159/000154430. [DOI] [PubMed] [Google Scholar]
- Guttman R, Guttman L, Rosenzeig KA. Cross-ethnic variation in dental, sensory and perceptual traits: a nonmetric multivariate derivation of distance for ethnic groups and traits. American Journal of Physical Anthropology. 1967;27:259–276. doi: 10.1002/ajpa.1330270302. [DOI] [PubMed] [Google Scholar]
- Hakim SMA, Baxi AJ, Balakrishnan V, Kulkarni KV, Rao SS, Jhala HI. Blood groups, secretor status and ability to taste phenylthiocarbamide in some Muslim groups. Human Heredity. 1973;23:72–77. doi: 10.1159/000152556. [DOI] [PubMed] [Google Scholar]
- Hall AR, Blakeslee AF. Effect of smoking on taste threshold for phenyl-thio-carbamide (PTC) Proceedings National Academy of Science. 1945;31:390–396. doi: 10.1073/pnas.31.12.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MJ, Bartoshuk LM, Cain WS, Stevens JC. PTC taste blindness and the taste of caffeine. Nature. 1975;253:442–443. doi: 10.1038/253442a0. [DOI] [PubMed] [Google Scholar]
- Haque M. Association between PTC taster status and goitre among the Oraon of Shankargarh, Surguja. Journal of Anthropology Survey India. 1990;39:204–207. [Google Scholar]
- Harder DB, Whitney G. A common polygenic basis for quinine and PROP avoidance in mice. Chemical Senses. 1998;23:327–332. doi: 10.1093/chemse/23.3.327. [DOI] [PubMed] [Google Scholar]
- Harder DB, Boughter JD, Whitney G. PTC-avoidance polymorphism and other bitter-avoidance differences among mice in long-term preference tests. Chemical Senses. 1996;21:612. [Google Scholar]
- Harris H, Kalmus H. The measurement of taste sensitivity to phenylthiourea (P.T.C.) Annals of Eugentics, London. 1949;15:24–31. doi: 10.1111/j.1469-1809.1949.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Harris H, Kalmus H. Chemical specificity in genetical differences of taste sensitivity. Annals of Eugenics. 1949–1951;15:32–45. doi: 10.1111/j.1469-1809.1949.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Harris H, Kalmus H, Trotter WR. Taste sensitivity to phenylthiourea in goitre and diabetes. Lancet. 1949;2:1038–1039. doi: 10.1016/s0140-6736(49)91605-0. [DOI] [PubMed] [Google Scholar]
- Hartmann G. Application of individual taste difference towards phenyl-thio-carbamide in genetic investigations. Annals of Eugenics. 1939;9:123–135. [Google Scholar]
- Hashem N, Khalifa AS. Taste threshold for phenylthiocarbamide among Egyptians. Journal of Egyptian Medical Association. 1968;51:1022–1028. [PubMed] [Google Scholar]
- Hogben L. An Introduction to Mathematical Genetics. New York: Norton; 1946. [Google Scholar]
- Hogben L, Pollack R. A contribution to the relation of the gene loci involved in the isoagglutinin reaction, taste blindness, Friedreich’s ataxia and major brachydactyly of man. Journal of Genetics. 1935;31:353–361. [Google Scholar]
- Hollingsworth DR. Phenylthiourea taste testing in Hiroshima subjects with thyroid disease. Journal of Clinical Endocrinology. 1963;23:961–963. doi: 10.1210/jcem-23-9-961. [DOI] [PubMed] [Google Scholar]
- Holt HA, Thompson JS, Sanger R, Race RR. Linkage relations of the blood group genes of man. Heredity. 1952;6:213–216. [Google Scholar]
- Hooper JE, Bartoshuk LM. PTC taste blindness and caffeine: further considerations (Sarasota: AChemS) 1983 [Google Scholar]
- Hoover EF. Reliability of phenylthiocarbamide-sodium benzoate method of determining taste classification. Journal of Agricultural and Food Chemistry. 1956;4:345–348. [Google Scholar]
- Hopkins CY. Taste differences in compounds having the NCS linkage. Canadian Journal of Research B. 1942;20:268–273. [Google Scholar]
- Hoshishima K, Yokoyama S, Seto K. Taste sensitivity in various strains of mice. American Journal of Physiology. 1962;202:1200–1205. doi: 10.1152/ajplegacy.1962.202.6.1200. [DOI] [PubMed] [Google Scholar]
- Ibraimov A, Mirrakhimov MM. PTC-tasting ability in populations living in Kirghizia with special reference to hypersensitivity: its relation to age and sex. Human Genetics. 1979;46:97–105. doi: 10.1007/BF00278907. [DOI] [PubMed] [Google Scholar]
- Intranuovo LR, Powers AS. The perceived bitterness of beer and 6-n-propylthiouracil (PROP) taste sensitivity. Annals of New York Academy of Sciences. 1998;855:813–815. doi: 10.1111/j.1749-6632.1998.tb10665.x. [DOI] [PubMed] [Google Scholar]
- Jenkins T. Ability to taste phenylthiocarbamide among Kalahari Bushmen and Southern Bantu. Human Biology. 1965;37:371–374. [PubMed] [Google Scholar]
- Jerzsa-Latta M, Krondl M, Coleman P. Use and perceived attributes of cruciferous vegetables in terms of genetically-mediated taste sensitivity. Appetite. 1990;15:127–134. doi: 10.1016/0195-6663(90)90045-a. [DOI] [PubMed] [Google Scholar]
- Johnson FE, Hertzog KP, Malina RM. Phenylthiocarbamide taste sensitivity and its relationship to growth variation. American Journal of Physical Anthropology. 1966;24:253–256. doi: 10.1002/ajpa.1330240214. [DOI] [PubMed] [Google Scholar]
- Jones PN, McLachlan GJ. Fitting mixture distributions to phenylthiocarbamide (PTC) sensitivity. American Journal of Human Genetics. 1991;48:117–120. [PMC free article] [PubMed] [Google Scholar]
- Junqueira PC, Kalmus H, Wishart P. PTC. thresholds, color vision and blood factors of Brazilian Indians II. Carajas. Annals of Human Genetics. 1957;22:22–25. doi: 10.1111/j.1469-1809.1957.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Kalmus H. Inherited sense defects. Scientific American. 1952;186:64–70. [Google Scholar]
- Kalmus H. Defective colour vision PTC. tasting and drepanocytosis in samples from fifteen Brazilian populations. Annals of Human Genetics, London. 1957;21:313–317. doi: 10.1111/j.1469-1809.1972.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Kalmus H. Improvements in the classification of the taster genotypes. Annals of Human Genetics, London. 1958;22:222–230. doi: 10.1111/j.1469-1809.1958.tb01416.x. [DOI] [PubMed] [Google Scholar]
- Kalmus H. The frequency of PTC non-tasters in school children at Ibadan, western Nigeria. Human Biology. 1967;39:32–34. [PubMed] [Google Scholar]
- Kalmus H, Lewkonia I. Relation between some forms of glaucoma and phenylthiocarbamide tasting. British Journal of Ophthalmology. 1973;57:503–506. doi: 10.1136/bjo.57.7.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmus H, Trotter WR. Direct assessment of the effect of age on PTC. sensitivity. Annals of Human Genetics, London. 1962;26:145–149. doi: 10.1111/j.1469-1809.1962.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Kalmus H, De Garay AL, Rodarte U, Cobo L. The frequency of PTC tasting, hard ear wax colour blindness and other genetical characters in urban and rural Mexican populations. Human Biology. 1964;36:134–145. [PubMed] [Google Scholar]
- Kang YS, Cho WK, Yurn KS. Taste sensitivity to phenylthiocarbamide of Korean population. Eugenics Quarterly. 1967;14:1–6. [Google Scholar]
- Kaplan AR, Fischer R. Taste sensitivity for bitterness: some biological and clinical implications. In. In: Wortis J, editor. Recent Advances in Biological Psychiatry. New York: Plenum; 1965. pp. 183–196. [Google Scholar]
- Kaplan AR, Glanville EV. Taste thresholds for bitterness and cigarette smoking. Nature. 1964;202:1366. doi: 10.1038/2021366a0. [DOI] [PubMed] [Google Scholar]
- Kaplan AR, Fischer R, Glanville E, Powell W, Kamionkowski M, Fleshler B. Differential taste sensitivities in duodenal and gastric ulcer patients. Gastroenterology. 1964;47:604–609. [PubMed] [Google Scholar]
- Kaplan AR, Glanville EV, Fischer R. Cumulative effect of age and smoking on taste sensitivity in males and females. Journal of Gerontology. 1965;20:334–337. [Google Scholar]
- Karam E, Jr, Freire-Maia N. Phenylthiocarbamide and mental immaturity. Lancet. 1967;1:622. doi: 10.1016/s0140-6736(67)90474-6. [DOI] [PubMed] [Google Scholar]
- Karrer T, Bartoshuk L. Capsaicin desensitization and recovery on the human tongue. Physiology Behaviour. 1991;49:757–764. doi: 10.1016/0031-9384(91)90315-f. [DOI] [PubMed] [Google Scholar]
- Keats BJ, Morton NE, Rao DC. Possible linkages (lod score over 1.5) and a tentative map of the Jk-Km linkage group. Cytogenetics Cell Genetics. 1978;22:304–308. doi: 10.1159/000130960. [DOI] [PubMed] [Google Scholar]
- Khullar P. Taste sensitivity to phenylthiocarbamide among Sindhi children of Delhi. Anthropology, University of Delhi. 1965;12:17–24. [Google Scholar]
- Kimmel HL, Lester D. Personalities of those who can taste phenylthiocarbamide. Psychological Reports. 1987;61:586. doi: 10.2466/pr0.1987.61.2.586. [DOI] [PubMed] [Google Scholar]
- Kitchin FD, Howel-Evans W, Clarke CA, McConnell RB, Sheppard PM. PTC. taste response and thyroid disease. British Medical Journal. 1959;1:1069–1074. doi: 10.1136/bmj.1.5129.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T, DeFries J. Similar polymorphism of taste and sensitivity to PTC in mice and men. Nature. 1970a;225:555–557. doi: 10.1038/225555a0. [DOI] [PubMed] [Google Scholar]
- Klein T, DeFries J. Taste sensitvity in infrahuman species: use of a genetic model to test the validity of alternative measures. Behaviour Research Methods and Instruments. 1970b;2:106–107. [Google Scholar]
- Kloepfer HW. An investigation of 171 possible linkage relationships in man. Annals of Eugenics. 1946;13:35–72. doi: 10.1111/j.1469-1809.1946.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Koertvelyessy T, Crawford MH. Ptc taste response, T4 level, and age in non-smoking Mennonites. Collegium Antropologicum. 1990;14:317–323. [Google Scholar]
- Koertvelyessy TA, Crawford MH, Hutchinson J. PTC taste threshold distributions and age in Mennonite populations. Human Biology. 1982;54:635–646. [PubMed] [Google Scholar]
- Kranzler HR, Moore PJ, Hesselbrock VM. No association of PROP taster status and paternal history of alcohol dependence. Alcohol Clinical Experimental Research. 1996;20:1496–1500. doi: 10.1111/j.1530-0277.1996.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Kumar N. Taste, middle-phalangeal hair and occipital hair whorls among Nokte Naga. Bulletin of the Department of Anthropology , India. 1955;4:61–67. [Google Scholar]
- Kumar N. A genetic survey among the Tentulia Bagdi and the Duley of Hooghly district in West Bengal. Bulletin of the Department of Anthropology , Government, India. 1957;6:81–88. [Google Scholar]
- Kumar N, Sastry DB. A genetic survey among the Riang: a Mongoloid tribe of Tripura. Z Morph Anthropology. 1961;51:346–355. [Google Scholar]
- Lawless H. A comparison of different methods used to assess sensitivity to the taste of phenylthiocarbamide (PTC) Chemical Senses. 1980;5:247–256. [Google Scholar]
- Leach EJ, Noble AC. Comparison of bitterness of caffeine and quinine by a time-intensity procedure. Chemical Senses. 1986;11:339–345. [Google Scholar]
- Lee BF. A genetic analysis of taste deficiency in the American Negro. Ohio Journal of Science. 1937;34:337–342. [Google Scholar]
- Lee HD, O’Mahony M. PTC and PROP behave differently in tests of discrimination from their solvents. Chemical Senses. 1998;23:403–410. doi: 10.1093/chemse/23.4.403. [DOI] [PubMed] [Google Scholar]
- Leguebe A. Genetique et anthropologie de la sensibilite a la phenylthiocarbamide I Frequence du gene dans la population belge. Institut royal des Sciences naturelles de Belgique. 1960;36:1–27. [Google Scholar]
- Levit SG, Soboleva GV. Comparative intrapair correlations of fraternal twins and siblings. Journal of Genetics. 1935;30:389–396. [Google Scholar]
- Li ZL, McIntosh JH, Byth K, Stuckey B, Stiel D, Piper DW. Phenylthiocarbamide taste sensitivity in chronic peptic ulcer. Gastroenterology. 1990;99:66–70. doi: 10.1016/0016-5085(90)91230-4. [DOI] [PubMed] [Google Scholar]
- Lightman SL, Carr-Locke DL, Pickles HG. The frequency of PTC tasters and males defective in colour vision in a Kurdish population in Iran. Human Biology. 1970;42:665–669. [PubMed] [Google Scholar]
- Looy H, Weingarten HP. Facial expressions and genetic sensitivity to 6-n-propylthiouracil predict hedonic response to sweet. Physiology Behaviour. 1992;52:75–82. doi: 10.1016/0031-9384(92)90435-5. [DOI] [PubMed] [Google Scholar]
- Looy H, Callaghan S, Weingarten HP. Hedonic response of sucrose likers and dislikers to other gustatory stimuli. Physiology Behaviour. 1992;52:219–225. doi: 10.1016/0031-9384(92)90261-y. [DOI] [PubMed] [Google Scholar]
- Lucchina L, Bartoshuk L, Duffy V, Marks L, Ferris A. 6-N-Propylthiouracil perception affects nutritional status of independent living older females. Chemical Senses. 1995;20:735. [Google Scholar]
- Lucchina LA, Curtis OFT, Putnam P, Drewnowski A, Prutkin JM, Bartoshuk LM. Psychophysical measurement of 6-n-propylthiouracil (PROP) taste perception. Annals of New York Academy of Sciences. 1998;855:816–819. doi: 10.1111/j.1749-6632.1998.tb10666.x. [DOI] [PubMed] [Google Scholar]
- Lugg JWH. Thresholds of taste for phenylthiocarbamide in various ethnic groups. Journal of Physiology. 1955;129:47P. doi: 10.1113/jphysiol.1955.sp005387. [DOI] [PubMed] [Google Scholar]
- Lugg JWH. Taste-thresholds for phenylthiocarbamide of some population groups II. The thresholds of two uncivilized ethnic groups living in Malaya. Annals of Human Genetics, London. 1956–57;21:244–253. doi: 10.1111/j.1469-1809.1972.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Lugg JWH. Taste thresholds for phenylthiocarbamide of some population groups. IV. The thresholds of some Australian aboriginal and South Korean subjects. Annals of Human Genetics, London. 1968;32:43–51. [Google Scholar]
- Lush IE. Differences between mouse strains in their consumption of phenylthiourea (PTC) Heredity (Edinburgh) 1986;57:319–323. doi: 10.1038/hdy.1986.129. [DOI] [PubMed] [Google Scholar]
- MacRoberts MH. Taste sensitivity to phenylthiocarbamide (P.T.C.) among the Papago Indians of Arizona. Human Biology. 1964;36:28–31. [PubMed] [Google Scholar]
- Martin NG. Phenylthiocarbamide tasting in a sample of twins. Annals of Human Genetics, London. 1975;38:321–326. doi: 10.1111/j.1469-1809.1975.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Mascie-Taylor CG, McManus IC, MacLarnon AM, Lanigan PM. The association between phenylthiocarbamide (PTC) tasting ability and psychometric variables. Behaviour Genetics. 1983;13:191–196. doi: 10.1007/BF01065667. [DOI] [PubMed] [Google Scholar]
- Masuoka S, Lee HD, Hatjopoulos D, O’Mahony M. Taste discrimination using alternative solvents for PTC and NaCl. Chemical Senses. 1995;20:299–304. doi: 10.1093/chemse/20.3.299. [DOI] [PubMed] [Google Scholar]
- Mathur VD, Mathur S, Bahadur B. Taste deficiency for phenylthiocarbamide in Mathur Kayasths community of Hyderabad, A.P. Indian Journal of Physiology and Pharmacology. 1983;27:92–100. [PubMed] [Google Scholar]
- Matson GA. Blood groups and ageusia in Indians of Montana and Alberta. American Journal of Physical Anthropology. 1938;24:81–89. [Google Scholar]
- Matsunaga E, Tsuji T. Individual differences in taste-ability for some chemical compounds. Japan Journal of Human Genetics. 1957;2:79–80. [Google Scholar]
- Matsunaga E, Suzuki T, Itoh S, Sugimota R. Individual difference of taste-ability for phenyl-thio-carbamide. Sapporo Medical Journal. 1954;6:245–249. [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse (see comments) Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Mattes R, Labov J. Bitter taste responses to phenylthiocarbamide are not related to dietary goitrogen intake in human beings. Journal of the American Dietetic Association. 1989;89:692–694. [PubMed] [Google Scholar]
- Mattes RD, Beauchamp GK. Individual differences in bitter taste: dietary implications. In: Kunzendorf R, Wallace B, editors. Individual Differences in Conscious Experience. Amsterdam: John Benjamin; 2000. [Google Scholar]
- Maxia C, Cosseddu GG, Vona G, Floris G. The sensitivity to PTC. in 541 Sardinians. Journal of Human Evolution. 1975;4:281–286. [Google Scholar]
- McDonald HJ. Color deficiency and inability to taste phenylthiourea. New England Journal of Medicine. 1979;300:1224. doi: 10.1056/nejm197905243002124. [DOI] [PubMed] [Google Scholar]
- McKusick V. Mendelian Inheritance in Man. 10th edn. Baltimore: Johns Hopkins University Press; 1995. [Google Scholar]
- Mela DJ. Bitter taste intensity: the effect of tastant and thiourea taster status. Chemical Senses. 1989;14:131–135. [Google Scholar]
- Mendez de Araujo H, Salzano FM, Wolff H. New data on the association between PTC and thyroid diseases. Humangenetik. 1972;15:136–144. doi: 10.1007/BF00295740. [DOI] [PubMed] [Google Scholar]
- Merton BB. Taste sensitivity to PTC in 60 Norwegian families with 176 children. Confirmation of the hypothesis of single gene inheritance. Acta Genetica et Statistica Medica. 1958;8:114–128. doi: 10.1159/000151060. [DOI] [PubMed] [Google Scholar]
- Miki T, Tanaka T, Furuhata T. On the distribution of the ABO blood groups and the taste ability for phenyl-thio-carbamide (P.T.C.) of the Lepchas and the Khasis. Proceedings of the Japanese Academy. 1960;36:78–80. [Google Scholar]
- Miller IJ, Jr, Reedy FE., Jr Variations in human taste bud density and taste intensity perception. Physiology Behaviour. 1990;47:1213–1219. doi: 10.1016/0031-9384(90)90374-d. [DOI] [PubMed] [Google Scholar]
- Milunicova A, Jandova A, Skoda V. Phenylthiocarbamide tasting ability and malignant tumours. Human Heredity. 1969;19:398–401. doi: 10.1159/000152244. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Cook RM, Sunderland E. Phenylthiocarbamide (PTC) taste sensitivity in selected populations of the Isle of Man and Cumbria. Annals of Human Biology. 1977;4:431–438. doi: 10.1080/03014467700002411. [DOI] [PubMed] [Google Scholar]
- Mohr J. Taste sensitivity to phenylthiourea in Denmark. Annals of Eugenics. 1951;16:282–286. doi: 10.1111/j.1469-1809.1951.tb02480.x. [DOI] [PubMed] [Google Scholar]
- Monn E. Further data on the genetics of the ABO-, MN-and PTC-systems of the Norwegian Lapps. Human Heredity. 1969;19:678–683. doi: 10.1159/000152284. [DOI] [PubMed] [Google Scholar]
- Montenegro L. PTC. tasting among Tucano Indians. Annals of Human Genetics London. 1964;28:185–187. doi: 10.1111/j.1469-1809.1964.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Morton CC, Cantor RM, Corey LA, Nance WE. A genetic analysis of taste threshold for phenylthiocarbamide. Acta Genetica Medicae et Gemellogoiae. 1981;30:51–57. doi: 10.1017/s0001566000006619. [DOI] [PubMed] [Google Scholar]
- Mourant AE, Kopec AC, Domaniewska-Sobczak K. The Distribution of Human Blood Groups and other Polymorphisms. 2nd edn. London: Oxford University Press; 1976. [Google Scholar]
- Nasidze IS. Genetic polymorphisms of the Caucasus ethnic groups: distribution of some blood group genetic markers (Part II) Gene Geography. 1995;9:117–167. [PubMed] [Google Scholar]
- Niewind A, Krondl M, Shrott M. Genetic influences on the selection of brassica vegetables by elderly individuals. Nutrition Research. 1988;8:13–20. [Google Scholar]
- Noble AC. Bitterness in wine. Physiology Behaviour. 1994;56:1251–1255. doi: 10.1016/0031-9384(94)90373-5. [DOI] [PubMed] [Google Scholar]
- O’Hanlon K, Weissbecker K, Cortessis V, Spence MA, Azen EA. Genes for salivary proline-rich proteins and taste for phenylthiourea are not closely linked in humans. Cytogenetics Cell Genetics. 1988;49:315–317. doi: 10.1159/000132687. [DOI] [PubMed] [Google Scholar]
- Odeigah PG. Smell acuity for acetone and its relationship to taste ability to phenylthiocarbamide in a Nigerian population. East African Medical Journal. 1994;71:462–466. [PubMed] [Google Scholar]
- Ogita Z. The genetical relation between resistance to insecticides in general and that to phenylthiourea (PTU) and phenylurea (PU) in. Drosophila melanogaster Botyu-Kagaku. 1958;23:188–205. [Google Scholar]
- Olson JM, Boehnke M, Neiswanger K, Roche AF, Siervogel RM. Alternative genetic models for the inheritance of the phenylthiocarbamide taste deficiency. Genetics Epidemiology. 1989;6:423–434. doi: 10.1002/gepi.1370060305. [DOI] [PubMed] [Google Scholar]
- Orrego H, Blake JE, Blendis LM, Compton KV, Volpe R, Israel Y. Long-term treatment of alcoholic liver disease with propylthiouracil. Part 2, Influence of drop-out rates and of continued alcohol consumption in a clinical trial. Journal of Hepatology. 1994;20:343–349. doi: 10.1016/s0168-8278(94)80005-7. [DOI] [PubMed] [Google Scholar]
- Panayotou T, Kritsikis S, Bartsocas CS. Taste sensitivity to phenylthiocarbamide in the Salamis Island Population (Greece) Human Hereditary. 1983;33:179–180. doi: 10.1159/000153371. [DOI] [PubMed] [Google Scholar]
- Paolucci AM, Ferro-Luzzi A, Modiano G, Morpurgo G, Kanashiro VK. Taste sensitivity to phenylthiocarbamide (PTC) and endemic goiter in the Indian natives of Peruvian highlands. American Journal of Physical Anthropology. 1971;34:427–430. doi: 10.1002/ajpa.1330340312. [DOI] [PubMed] [Google Scholar]
- Parikh NP, Baxi AJ, Jhala HI. Blood groups, abnormal haemoglobins and other genetical characters in three Gujarati-speaking groups. Human Heredity. 1969a;19:486–489. doi: 10.1159/000152257. [DOI] [PubMed] [Google Scholar]
- Parikh NP, Baxi AJ, Jhala HI, Kulkarni KV. Blood groups and other genetical characters in Mahars—a socially low caste from Maharashtra. Indian Journal of Medical Research. 1969b;57:1467–1474. [PubMed] [Google Scholar]
- Parmar PK. Taste sensitivity to phenyl thiocarbamide (P.T.C.) in Gorkhas of Dhauladhar Range (Himachal Pradesh) The Eastern Anthropologist. 1968;21:267–277. [Google Scholar]
- Parr LW. Taste blindness and race. Journal Heredity. 1934;25:187–200. [Google Scholar]
- Partridge L. Taste blindness to PTC. in the Black Mountain Area of Carmarthenshire, Wales. Man. 1962;50:38–39. [Google Scholar]
- Parveen K, Goni M, Shah GM. Incidence of PTC. taste sensitivity and threshold in Kashmiri population. Indian Journal of Physiology Pharmacology. 1990;34:48–50. [PubMed] [Google Scholar]
- Pascasio FM, Bias WB, Manipol V, Campos P. Genetic marker systems in Philippine negritos. Birth Defects: Original Article Series. 1974;10:220–225. [PubMed] [Google Scholar]
- Patel S. ABO blood groups, PTC tasting and dermatoglyphics of Tibetans at Chandragiri, Orissa. American Journal of Physical Anthropology. 1971;35:149–150. doi: 10.1002/ajpa.1330350119. [DOI] [PubMed] [Google Scholar]
- Peeples EE. Taste Sensitivity to Phenylthiocarbamide in Alcoholics. Deland: Stetson University; 1962. p. 31. [Google Scholar]
- Pelchat ML, Danowski S. A possible genetic association between PROP-tasting and alcoholism. Physiology Behaviour. 1992;51:1261–1266. doi: 10.1016/0031-9384(92)90318-v. [DOI] [PubMed] [Google Scholar]
- Persson I, Kolendorf L, Kolendorf K. PTC taste sensitivity in toxic diffuse goitre. Human Heredity. 1972;22:459–465. doi: 10.1159/000152524. [DOI] [PubMed] [Google Scholar]
- Pons J. Taste sensitivity to phenylthiourea in Spaniards. Human Biology. 1955;27:153–160. [PubMed] [Google Scholar]
- Pons J. A contribution to the heredity of the P.T.C. taste character. Annals of Human Genetics, London. 1960;24:71–76. doi: 10.1111/j.1469-1809.1959.tb01716.x. [DOI] [PubMed] [Google Scholar]
- Pullin EW, Sunderland E. A survey of phenylthiovarbamide (P.T.C.)-tasting and colour-blindness in Pembrokeshire, Wales. Man. 1963;63:52–55. [Google Scholar]
- Ramana GV, Naidu JM. PTC taste sensitivity and colour blindness among Manne Dora. Man in India. 1992;72:455–458. [Google Scholar]
- Ramesh A, Kumar MV, Murty JS. Incidence of PTC taste sensitivity and red-green colour blindness in some tribal goups of Andhra Pradesh. Spectra of Anthropological Progress. 1981;3:35–39. [Google Scholar]
- Ranganayaki L, Njeti MS. Incidence of P.T.C. colourblindness among three endogamous castes of Vishakhapatnam (Andhra Pradesh) Indian Journal of Physical Anthropology and Human Genetics. 1979;5:169–170. [Google Scholar]
- Rani S, Seth PK. PTC taste sensitivity among the Brahmnis of Narendra Nagar (Uttar Pradesh, India) Indian Journal of Physical Anthropology & Human Genetic. 1981;7:161–166. [Google Scholar]
- Rao BB. Taste sensitivity to pheynl[sic]-thio-carbamide in leprosy and filariasis. Indian Anthropologist. 1972;2:124–129. [Google Scholar]
- Rao DC, Morton NE. Residual family resemblance for PTC taste sensitivity. Human Genetics. 1977;36:317–320. doi: 10.1007/BF00446282. [DOI] [PubMed] [Google Scholar]
- Rao GN, Sisodia P. Diabetes and phenylthiocarbamide (PTC) tasting ability. Journal of Association of Physicians India. 1970;18:577–581. [PubMed] [Google Scholar]
- Rao VLN, Jaikishan G. Variation in taste sensitivity among Jalaris of Andhra Pradesh. Journal of Human Ecology. 1995;6:149–150. [Google Scholar]
- Rastogi S, Tyagi D. Phenylthiocarbamide taste threshold distribution among the Rastogis of India. Acta Genetica Medicae et Gemellogoiae. 1975;24:167–168. doi: 10.1017/s112096230002206x. [DOI] [PubMed] [Google Scholar]
- Reddy BM. Variation in taste sensitivity to phenylthiocarbamide (PTC) among the marine fisherfolk of Puri. Man in India. 1983;63:417–421. [Google Scholar]
- Reddy BM. PTC taste sensitivity: a study on the relative usefulness of taste-nontaster proportions and mean threshold values in population comparisons. Journal of Indian Anthropologocal Society. 1988;23:260–265. [Google Scholar]
- Reddy BM, Rao DC. Phenylthiocarbamide taste sensitivity revisited: complete sorting test supports residual family resemblance. Genetics Epidemiology. 1989;6:413–421. doi: 10.1002/gepi.1370060304. [DOI] [PubMed] [Google Scholar]
- Reed DR. Behavioral modulation of the path from genotype to obesity phenotype. In: Ailhaud G, Guy-Grand B, editors. Progress in Obesity Research. Vol. 8. London: John Libbey; 1999. pp. 199–208. [Google Scholar]
- Reed DR, Nanthakumar E, North M, Bell C, Bartoshuk LM, Price RA. Localization of a gene for bitter taste perception to human chromosome 5p15. American Journal of Human Genetics. 1999;64:1478–1480. doi: 10.1086/302367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Bartoshuk LM, Duffy V, Marino S, Price RA. Propylthiouracil tasting: determination of underlying threshold distributions using maximum likelihood. Chemical Senses. 1995;20:529–533. doi: 10.1093/chemse/20.5.529. [DOI] [PubMed] [Google Scholar]
- Reid NCR, Brunt PW, Bias WB, Maddrey WC, Alonso BA, Iber FL. Genetic characteristics and cirrhosis: a controlled study of 200 patients. British Medical Journal. 1968;2:463–465. doi: 10.1136/bmj.2.5603.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CP, Clisby KH. Phenylthiocarbamide taste thresholds of rats and human beings. American Journal of Physiology. 1941;134:157–164. [Google Scholar]
- Riddell WJ, Wybar KC. Taste of thiouracil and phenylthiocarbamide. Nature. 1944;3917:669. [Google Scholar]
- Rife DC. Contributions of the 1937 National Twin’s Convention to research. Journal of Heredity. 1938;29:83–90. [Google Scholar]
- Rife DC. An investigation of genetic variability among Sudanese. American Journal of Physical Anthropology. 1953;11:189–202. doi: 10.1002/ajpa.1330110222. [DOI] [PubMed] [Google Scholar]
- Rife DC, Schonfeld MD. A comparison of the frequencies of certain genetic traits among Gentile and Jewish students. Human Biology. 1944;16:172–180. [Google Scholar]
- Rikimaru J-Y. A study of ‘taste-blindness’: I. The incidence among Japanese and Formosans, and divergences in race, age and sex. Japanese Journal of Psychology. 1936a;9:11–12. [Google Scholar]
- Rikimaru J-Y. Taste deficiency of Japanese and other races in Formosa. American Journal of Psychology. 1936b;48:649–653. [Google Scholar]
- Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science. 1996;273:1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Romanus T. The ability to taste PTC among Swedish men and women (nulliparae and others) Acta Genetica Medicae et Gemellogoiae. 1965;14:417–420. doi: 10.1017/s1120962300015110. [DOI] [PubMed] [Google Scholar]
- Rychkov YG, Borodina SR. Hypersensitivity to phenylthiocarbamide in one of the isolated populations of Siberia. Possible hypothesis of inheritance. Genetika. 1969;5:116–123. [Google Scholar]
- Rychkov YG, Borodina SR. Further investigations of the genetics of hypersensitivity to phenylthiocarbamide in man (experimental, population, and familial data) Genetika. 1973;9:141–152. [Google Scholar]
- Saheb SY, Gulati RK, Sastry DB, Raju CM, Sirajuddin SM. PTC taste sensitivity in Kodava population of Kodagu. Bulletin of the Anthropological Survey of India—Calcutta. 1979;28:66–75. [Google Scholar]
- Saldanha PH. Apparent pleiotropic effect of genes determining taste thresholds for phenylthiourea. Lancet. 1956;271:74. doi: 10.1016/s0140-6736(56)90429-9. [DOI] [PubMed] [Google Scholar]
- Saldanha PH. Taste thresholds for phenylthiorurea among Japanese. Annals of Human Genetics. 1958;22:380–384. doi: 10.1111/j.1469-1809.1958.tb01433.x. [DOI] [PubMed] [Google Scholar]
- Saldanha PH. Taste sensitivity to phenylthiourea among Brazilian negroes and its bearing on the problem of white-negro intermixture in Brazil. Human Biology. 1962;34:179–186. [PubMed] [Google Scholar]
- Saldanha PH, Becak W. Taste thresholds for phenylthiourea among Ashkenazic Jews. Science. 1959;129:150–151. doi: 10.1126/science.129.3342.150. [DOI] [PubMed] [Google Scholar]
- Saldanha PH, Nacrur J. Taste thresholds for phenylthiourea among Chileans. American Journal of Physical Anthropology. 1963;21:113–119. doi: 10.1002/ajpa.1330210204. [DOI] [PubMed] [Google Scholar]
- Salmon TN, Blakeslee AF. Genetics of sensory thresholds: variations within single individuals in taste sensitivity for PTC. Proceedings of the National Academy of Sciences. 1935;21:78–83. doi: 10.1073/pnas.21.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger R, Race RR. The MNSs blood group system. American Journal of Human Genetics. 1951;3:332–343. [PMC free article] [PubMed] [Google Scholar]
- Sanghvi LD, Khanolkar VR. Data relating to seven genetical characters in six endogamous groups in Bombay. Annals of Eugenics. 1949–50;15:62–76. doi: 10.1111/j.1469-1809.1949.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Sato T, Okada Y, Miyamoto T, Fujiyama R. Distribution of non-tasters for phenylthiocarbamide and high sensitivity to quinine hydrochloride of the non-tasters in Japanese. Chemical Senses. 1997;22:547–551. doi: 10.1093/chemse/22.5.547. [DOI] [PubMed] [Google Scholar]
- Say B, Kiran O, Altay C, Berkel I. The incidence of PTC taste sensitivity in the population of Ankara. Turkish Journal of Pediatrics. 1966;8:171–175. [PubMed] [Google Scholar]
- Schelling J-L, Tetreault L, Lasagna L, Davis M. Abnormal taste threshold in diabetes. Lancet. 1965;1:508–512. doi: 10.1016/s0140-6736(65)92017-9. [DOI] [PubMed] [Google Scholar]
- Schiffman SS, Gatlin LA, Frey AE, Heiman SA, Stagner WC, Cooper DC. Taste perception of bitter compounds in young and elderly persons: relation to lipophilicity of bitter compounds. Neurobiology of Aging. 1994;15:743–750. doi: 10.1016/0197-4580(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Schlosberg A, Baruch I. Phenylthiocarbamide (PTC) tasting in paranoid and nonparanoid schizophrenic patients. Perception Motor Skills. 1992;74:383–386. doi: 10.2466/pms.1992.74.2.383. [DOI] [PubMed] [Google Scholar]
- Scott TR, Giza BK, Yan J. Electrophysiological responses to bitter stimuli in primate cortex. Annals of the New York Academy of Science. 1998;855:498–501. doi: 10.1111/j.1749-6632.1998.tb10613.x. [DOI] [PubMed] [Google Scholar]
- Scott-Emuakpor AB, Uviovo JE, Warren ST. Genetic variation in Nigeria. I. The genetics of phenylthiourea tasting ability. Human Hereditary. 1975;25:360–369. doi: 10.1159/000152747. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Dutta MN. Genetic investigations among the Ahom of Assam. Journal of the Indian Medical Association. 1991;89:13–15. [PubMed] [Google Scholar]
- Set PK. Genetical study of Angami Nagas (Nagaland, India): A1A2B0 MN Rh blood groups, ABO(H) secretion, PTC taste sensitivity and colour blindness. Human Biology. 1973;45:457–468. [PubMed] [Google Scholar]
- Seth PK. PTC taste threshold distributions among the betel chewers, non-vegetarians and smokers. Eastern Anthropologist. 1962;15:36–49. [Google Scholar]
- Seth PK, Seth S. Genetical study of Angami Nagas (Nagaland, India): A1A2BO, MN, Rh blood groups, ABO(H) secretion, PTC taste sensitivity and colour-blindness. Human Biology. 1973;45:457–468. [PubMed] [Google Scholar]
- Setterfield W, Schott RG, Snyder LH. Studies in human inheritance XV. The bimodality of the threshold curve for the taste of phenyl-thio-carbamide. Ohio Journal of Science. 1936;36:231–235. [Google Scholar]
- Shah GM, Sattar NA. The incidence of phenyl thiocarbamide (P.T.C.) taste threshold and sensitivity in Iraqi Arabs. Medical Journal of Basrah University. 1981;4:121–131. [Google Scholar]
- Sharma I, Bhalla V. PTC taste sensitivity and colour vision in Dogras of Tawi Valley (Jammu and Kashmir) Indian Journal of Physical Anthropology & Human Genetics. 1981;7:47–51. [Google Scholar]
- Sharma JC. Blood and P.T.C. taste studies in Pajabis and the effect of age and certain eating habits on taste threshold. Anthropologist. 1962;6:40–46. [Google Scholar]
- Sharma JC. Taste sensitivity to phenylthiocarbamide among three Mongoloid populations of the Indian border. Acta Genetica Medicae et Gemellogoiae. 1967;16:317–324. doi: 10.1017/s1120962300013111. [DOI] [PubMed] [Google Scholar]
- Sheba C. Taste sensitivity to phenylthiourea among the Jewish population groups in Israel. American Journal of Human Genetics. 1962;14:44–51. [PMC free article] [PubMed] [Google Scholar]
- Shepard TH. Phenylthiocarbamide non-tasting among congenital athyrotic cretins: further studies in an attempt to explain the increased incidence. Journal of Clinical Invest. 1961;40:1751–1757. doi: 10.1172/JCI104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard TH, Gartler SM. Increased incidence of nontasters of phenylthiocarbamide among congenital athyreotic cretins. Science. 1960;131:929. doi: 10.1126/science.131.3404.929. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ. A blood group genetical survey in eastern and central Polynesians. American Journal of Physical Anthropology. 1957;15:357–366. doi: 10.1002/ajpa.1330150315. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ, Semple NM, D’Sena GWL. A genetical survey in Chenchu, South India: blood, taste and secretion. The Medical Journal of Australia. 1953a;I:497–503. [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ, Semple NM, Kodama S. A collaborative genetical survey in Ainu: Hidaka, island of Hokkaido. American Journal of Physiology Anthropology. 1953b;11:47–82. doi: 10.1002/ajpa.1330110115. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ, Semple NM. A blood group genetical survey in Australian aborigines. American Journal of Physical Anthropology. 1954a;12:599–606. doi: 10.1002/ajpa.1330120409. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ, Sringam S. A blood group genetical survey in Thais, Bangkok. American Journal of Physiology Anthropology. 1954b;12:407–412. doi: 10.1002/ajpa.1330120314. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ, Semple NM, Fry EI. A blood group genetical survey in Cook islanders, Polynesia, and comparisons with American Indians. American Journal of Physiology Anthropology. 1955;13:667–690. doi: 10.1002/ajpa.1330130409. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Graydon JJ, Semple NM, Swindler DR. A blood group genetical survey in West Nakanai, New Britian. American Journal of Physiology Anthropology. 1956;14:275–286. doi: 10.1002/ajpa.1330140220. [DOI] [PubMed] [Google Scholar]
- Simmons RT, Semple NM, Cleland JB, Casley-Smith JR. A blood group genetical survey in Australian aborigines at Hasst’s Bluff, central Australia. Anthropologist. 1957;15:547–553. doi: 10.1002/ajpa.1330150406. [DOI] [PubMed] [Google Scholar]
- Singh L. Effect of some social habits on PTC taste distribution among the Brahmins of Bhimtal (U.P.) Journal of Anthropological Society Nippon. 1975;83:244–252. [Google Scholar]
- Singh N, Bagga A. Defective colour vision and taste sensitivity to PTC in the Maharashtrian population. International Journal of Anthropology. 1994;9:317–320. [Google Scholar]
- Sirajuddin SM. Blood groups A1 A2 BO MN, Rh(D), PTC and colour blindness studies among Chenchus of Andhra Pradseh. Anthropologist—Delhi. 1977;24:14–20. [Google Scholar]
- Sirsat S. Effect of migration on some genetical characters in six endohamous groups in India. Annals of Human Genetics, London. 1956;21:145–154. doi: 10.1111/j.1469-1809.1971.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Skude G. Sweet taste perception for phenythiourea (P.T.C.) Hereditas. 1959;45:597–622. [Google Scholar]
- Skude G. Complexities of human taste variation. Journal of Hereditary. 1960;51:259–263. [Google Scholar]
- Skude G. Studies in sweet taste perception for phenylthiourea (P.T.C.) Hereditas. 1963;50:196–202. [Google Scholar]
- Smith GS, Lorey FW, Small MF. Taste sensitivity to phenylthiourea (PTC) in the Rhesus monkey (Macaca mulatta) Primates. 1981;22:404–408. [Google Scholar]
- Smith SE. Taste thresholds in drug addicts and alcoholics. British Journal of Addict Alcohol Other Drugs. 1972;67:317–321. doi: 10.1111/j.1360-0443.1972.tb01214.x. [DOI] [PubMed] [Google Scholar]
- Snyder LH. Inherited taste deficiency. Science. 1931;74:151–152. doi: 10.1126/science.74.1910.151. [DOI] [PubMed] [Google Scholar]
- Soltan HC, Bracken SE. The relation of sex to taste reactions. Journal of Heredity. 1958;49:280–284. [Google Scholar]
- Spence MA, Falk CT, Neiswanger K, Field LL, Marazita ML, Allen FH, Jr, Siervogel RM, Roche AF, Crandall BF, Sparkes RS. Estimating the recombination frequency for the PTC-Kell linkage. Human Genetics. 1984;67:183–186. doi: 10.1007/BF00272997. [DOI] [PubMed] [Google Scholar]
- Srivastava AC. PTC taste sensitivity in the high-rank Muslims of Uttar Pradesh. Human Heredity. 1974;24:379–382. doi: 10.1159/000152674. [DOI] [PubMed] [Google Scholar]
- Srivastava AC. PTC taste variation in relation to age. Anthropologist—Delhi. 1976;23:52–54. [Google Scholar]
- Srivastava AC, Tyagi D. A study of taste sensitivity to phenylthiourea (P.T.C.) among various population groups in Uttar Pradesh. Bulletin Department Anthropology. 1967;16:99–109. [Google Scholar]
- Srivastava RP. Measurement of taste sensitivity to phenythiourel [sic] (P.T.C.) in Uttar Pradesh. Eastern Anthropologist. 1959;12:267–272. [Google Scholar]
- Srivastava RP. Frequency of non-tasters among the Danguria Tharu of Uttar Pradesh. Eastern Anthropologist. 1961;14:258–259. [Google Scholar]
- Srivastava RP. Further data on non-tasters among the Tharus of Uttar Pradesh. Eastern Anthropologist. 1964;17:19–22. [Google Scholar]
- Srivastava RP. A study of ABO blood groups and P.T.C. taste in a multi-caste village of Karnataka. Indian Anthropologist. 1980;10:61–66. [Google Scholar]
- Stanchev I, Tsonev K, Minchev M. Duodenal ulcer: genetic analysis by the phenylthiocarbamide test. Folia Medica. 1985;27:13–16. [PubMed] [Google Scholar]
- Stefano GFD, Molieri JJ. P.T.C. tasting among three Indian groups of Nicaragua. American Journal of Physiology Anthropology. 1976;44:371–374. doi: 10.1002/ajpa.1330440218. [DOI] [PubMed] [Google Scholar]
- Sudhakar G, Babu BV, Padma V. Variation in tasting ability for PTC and colour blindness among Chakali, an Andhra Caste. Journal of Human Ecology. 1992;3:237–238. [Google Scholar]
- Sunderland E. The tasting of phenylthiocarbamide in selected populations in the United Kingdom. The Eugenics Review. 1966;58:143–148. [PMC free article] [PubMed] [Google Scholar]
- Sunderland E, Rosa PJ. The ability to taste phenylthiocarbamide among the Tripolitanians, Cyrenaicans, and Fezzanites of Libya and the Kikuyu, Kamba, and Taita of Kenya. Human Biology. 1975;47:473–481. [PubMed] [Google Scholar]
- Sunderland E, Ryman R. P.T.C. thresholds, blood factors, colour vision and fingerprints of Jivaro Indians in Eastern Ecuador. American Journal of Physical Anthropology. 1968;28:339–344. doi: 10.1002/ajpa.1330280320. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Taste blindness of Japanese. Nature (London) 1949;163:177. doi: 10.1038/163177a0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Takeuchi T, Kitazawa Y. Phenylthiourea taste testing in primary glaucomas. Acta Society Opthamology Japan. 1966;70:88–91. [PubMed] [Google Scholar]
- Swinson RP. Phenylthiocarbamide taste sensitivity in alcoholism. British Journal of Addict Alcohol Other Drugs. 1973;68:33–36. doi: 10.1111/j.1360-0443.1973.tb01219.x. [DOI] [PubMed] [Google Scholar]
- Swinson RP. Genetic markers and alcoholism. Recent Developments Alcohol. 1983;1:9–24. doi: 10.1007/978-1-4613-3617-4_2. [DOI] [PubMed] [Google Scholar]
- Taylor C. A note on differential taste responses to P.T.C. (phenyl-thio-carbamide) Human Biology. 1961;31:220–222. [PubMed] [Google Scholar]
- Tepper BJ. 6-n-Propylthiouracil: a genetic marker for taste, with implications for food preference and dietary habits. American Journal of Human Genetics. 1998;63:1271–1276. doi: 10.1086/302124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiology Behaviour. 1997;61:949–954. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- Terry MC. Taste-blindness and diabetes in the colored population of Jamaica. Journal of Heredity. 1950;41:306–307. [Google Scholar]
- Terry MC, Segall G. The association of diabetes and taste-blindness. Journal of Heredity. 1947;38:135–138. doi: 10.1093/oxfordjournals.jhered.a105709. [DOI] [PubMed] [Google Scholar]
- Thambipillai V. Taste thresholds for phenyl-thio-urea in Malay school children. Annals of Human Genetics. 1955–56;21:232–238. doi: 10.1111/j.1469-1809.1956.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Than-Sint F, Mya-Tu M. Taste sensitivity to phenylthiocarbamide in a Burmese population sample. Human Hereditary. 1974;24:554–557. doi: 10.1159/000152693. [DOI] [PubMed] [Google Scholar]
- Thieme FP. The geographic and racial distribution of ABO and Rh blood types and tasters of PTC in Puerto Rico. American Journal of Human Genetics. 1952;4:94–112. [PMC free article] [PubMed] [Google Scholar]
- Tills D, Kopec AC, Tills RE. The Distribution of the Human Blood Groups and other Polymorphisms. 1 edn. Supplement. Oxford: Oxford University Press; 1983. [Google Scholar]
- Tiwari SC. Taste threshold for phenylthiourea among the Tibetans. Anthropologist. 1966;13:57–64. [Google Scholar]
- Tiwari SC, Bhasin MK. Taste deficiency for phenylthiourea in the Garhwali Brahmins and Rajputs. The Eastern Anthropologist. 1967;20:243–256. [Google Scholar]
- Tobach E, Bellin JS, Das DK. Differences in bitter taste perception in three strains of rats. Behavior Genetics. 1974;4:405–410. doi: 10.1007/BF01066160. [DOI] [PubMed] [Google Scholar]
- Tripathy KC. Taste, mid-digital hair and occipital hair whorls of Kondhs Orissa. Adibasi. 1966;7:29–36. [Google Scholar]
- Tripathy KC. PTC taste sensitivity in some Orissan Castes. Man in India—Ranchi. 1969;49:64–70. [Google Scholar]
- Tsuji T. Individual differences and inheritance of taste-ability for phenyl-thio-carbamide and related compounds. Japanese Journal of Human Genetics. 1957;2:96–117. [Google Scholar]
- Umansky I, Reid J, Corcoran PA, Bolling D, Schulze J, Renwick JH, Moorrees CF, Allen FH., Jr Genetics of blood groups. Linkage analysis of 207 pedigrees. Vox Sanguinis. 1966;11:450–459. doi: 10.1111/j.1423-0410.1966.tb04241.x. [DOI] [PubMed] [Google Scholar]
- Very PS, Iacono C. Phenylthiourea taste blindness and MMPI personality patterns in adult Caucasian females. Journal of Clinical Psychology. 1968;24:187–188. doi: 10.1002/1097-4679(196804)24:2<187::aid-jclp2270240212>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Vyas GN, Bhatia HM, Banker DD, Purandare NM. Study of blood groups and other genetical characters in sex Gujarati endogamous groups in Western India. Annals of Human Genetics, London. 1958;22:185–189. doi: 10.1111/j.1469-1809.1958.tb01413.x. [DOI] [PubMed] [Google Scholar]
- Vyas GN, Bhatia HM, Sukumaran PK, Balkrishnan V, Sanghvi LD. Study of blood groups, abnormal hemoglobins and other genetical characters in some tribes of Gujarat. American Journal of Physiology Anthropology. 1962;20:255–265. doi: 10.1002/ajpa.1330200310. [DOI] [PubMed] [Google Scholar]
- Wheatcroft PE, Thornburn CC. Toxicity of the taste testing compound phenylthiocarbamide. Nature New Biology. 1972;235:93–94. doi: 10.1038/newbio235093a0. [DOI] [PubMed] [Google Scholar]
- Whissell-Buechy D. Effects of age and sex on taste sensitivity to phenylthiocarbamide (PTC) in the Berkeley Guidance sample. Chemical Senses. 1990a;15:39–57. [Google Scholar]
- Whissell-Buechy D. Genetic basis of the phenylthiocarbamide polymorphism. Chemical Senses. 1990b;15:27–37. [Google Scholar]
- Whissell-Buechy D, Wills C. Male and female correlations for taster (P.T.C.) phenotypes and rate of adolescent development. Annals of Human Biology. 1989;16:131–146. doi: 10.1080/03014468700006982. [DOI] [PubMed] [Google Scholar]
- Whitney G, Harder DB. Phenylthiocarbamide (PTC) preference among laboratory mice: understanding of a previously ‘unreplicated’ report. Behaviour Genetic. 1986;16:605–610. doi: 10.1007/BF01066287. [DOI] [PubMed] [Google Scholar]
- Whittemore PB. Phenylthiocarbamide (PTC) tasting and reported depression. Journal of Clinical Psychology. 1986;42:260–263. doi: 10.1002/1097-4679(198603)42:2<260::aid-jclp2270420206>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Whittemore PB. Phenylthiocarbamide (PTC) tasting, genetics, and depression. Journal of Clinical Psychology. 1990;46:262–272. doi: 10.1002/1097-4679(199005)46:3<262::aid-jclp2270460303>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Xiong M, Guo SW. Fine-scale genetic mapping based on linkage disequilibrium: theory and applications. American Journal of Human Genetics. 1997a;60:1513–1531. doi: 10.1086/515475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Guo SW. Fine-scale mapping of quantitative trait loci using historical recombinations. Genetics. 1997b;145:1201–1218. doi: 10.1093/genetics/145.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M, Guo SW. The power of linkage detection by the transmission/disequilibrium tests. Human Heredity. 1998;48:295–312. doi: 10.1159/000022821. [DOI] [PubMed] [Google Scholar]
- Yanagida I. Frequency of PTC non tasters in dental patients. Journal of Osaka University Dental Society. 1988;33:479–489. [PubMed] [Google Scholar]
- Zhang R, Yan L, Peng B. Studies of taste competence of phenylthicarbamide [sic] (PTC) of ten various nationalities, Gansu and Qinghai. Acta Anthropologic a Sincia. 1988;7:353–358. [Google Scholar]