Abstract
Background
Atrial fibrillation (AF) is an increasingly common problem in primary care, but little is known about its prevalence and the distribution of AF risk factors in the general population.
Methods
We determined the prevalence of AF and the distribution of known AF risk factors among persons participating in the population-based Gutenberg Health Study. To this end, we used interview data about the medical diagnosis of AF and electrocardiograms (ECGs) that were performed for the study in 5000 persons aged 35 to 74. The response rate was 60.4%.
Results
There were 5000 persons in the study sample (age 52.2 ± 11 years; 50.6% were women). The prevalence of AF, weighted for the age and sex distribution of the general population, was 2.5%. AF was found to be more common in older persons, with a more pronounced increase in men: whereas its prevalence was 0.7% in 35- to 44-year-old men, the corresponding figure for the age group 65- to 74 was as high as 10.6%. Twenty five participants (15.5% of AF cases) received their initial diagnosis of AF on the basis of the study ECG. Compared to persons without AF, persons with AF were older and more commonly male, and they had a higher burden of cardiovascular risk factors. 14.3% of persons with AF had none of the well-established risk factors for AF (systolic blood pressure, antihypertensive medication, increased body-mass-index, heart failure). 42.7% of persons with AF were not taking either anticoagulants or platelet inhibitors.
Conclusion
These data indicate that the prevalence of AF in the middle-aged general population is 2.5% overall, and higher in the elderly. AF is thus a significant public health problem, and greater awareness of it is needed.
Atrial fibrillation (AF) is an often underestimated disease, and its prevalence in the Western world is increasing (1, 2). The lifetime risk of developing AF is approximately 25%, and it mainly affects older persons (3). Major complications include thromboembolic events up to and including fatal stroke (4), heart failure (5), and increased morbidity and mortality not explained by cardiovascular comorbidity alone (6).
The reasons for the increase in prevalence are not fully clear. The main reasons are an aging population and longer survival times with cardiovascular diseases. The increasing awareness of AF, resulting in more frequent diagnosis, may also play a role (3, e1).
Data on AF are obtained mainly from registries or hospital databases. For example, the Euro Heart Survey on Atrial Fibrillation and the German Competence Network on Atrial Fibrillation (AFNET) contribute to the understanding of diagnostic measures, type of AF from paroxysmal to permanent, concomitant diseases, and treatment according to guidelines for patients diagnosed with AF (7). However, primary AF prevention mechanisms need to be implemented earlier. Few data are available on AF in the German general population. We have therefore investigated the prevalence of AF and the presence of known risk factors for AF and thromboembolic complications in the German population on the basis of the Gutenberg Health Study (GHS), an ongoing, population-based study.
Methods
Study participants
Since 2007, persons from the city of Mainz and the region of Mainz-Bingen have been selected at random via the registration office and invited to take part in a five-hour examination at the University Medical Center of the Johannes Gutenberg University Mainz. This is the population-based GHS. Those invited to take part are aged between 35 and 74 and stratified in 10-year age groups. The main aim of the GHS is to investigate cardiovascular diseases and their risk factors. It has been designed to determine the incidence of cardiovascular morbidity and cardiovascular diseases such as myocardial infarction and atrial fibrillation. Its response rate is 60.4%.
While they are at the study site, patients undergo a detailed computer-assisted interview covering cardiovascular risk factors, lifestyle, socioeconomic status, and other areas. The prevalence of cardiovascular diseases such as myocardial infarction, cardiac failure, and stroke is determined by history taking. The definition of heart failure used is left ventricular ejection fraction (LVEF) below 55%, treated heart failure (determined by history taking), and/or shortness of breath (New York Heart Association [NYHA] Classification ≥1).
AF is diagnosed on the basis of a history of AF diagnosed by a physician and/or evidence of AF on the 12-lead resting ECG performed during the study, which lasts for 10 seconds. For this evaluation, all ECGs were interpreted by cardiologists, and the results generated automatically by the machine (GE Healthcare, CardioSoft v6) were verified. ECG-based diagnosis of AF was confirmed by at least two independent cardiologists. AF diagnosed on the basis of surface ECG is defined as absolutely irregular R peak intervals and an absence of P waves with a clear beginning and end (8). Thirty-eight individuals did not undergo ECG at the study site and/or had no history of AF. Extensive details of initial examination and the determination of cardiovascular risk factors have been published (9). Blood biomarkers were measured using standard techniques.
The GHS was approved by the Ethics Committee of the Rhineland-Palatinate State Medical Association. Every participant provides his/her written consent before beginning the study. Responsibility for data accuracy lies with the authors.
Statistics
As the population sample used in the GHS is stratified according to sex, age group, and urban versus suburban/rural origin, statistics describing the prevalence of AF and risk factors were calculated by age group (35 to 44, 45 to 54, 55 to 64, and 65 to 74 years) and weighted for the actual sex and age distribution of the city of Mainz and the region of Mainz-Bingen (n = 210 867, data of the German Federal Statistical Office, Wiesbaden 2011, as of December 31, 2007). They were also standardized for age according to the 1976 European standard population (WHO), in order to allow comparability with other studies.
The risk factors of the Framingham risk prediction algorithm for the occurrence of AF were used to determine the prevalence of classic AF risk factors (10). Risk of AF was assessed using the beta coefficient of the Framingham algorithm for 5-year AF prediction (11). CHADS2 score (cardiac failure, hypertension, age, diabetes, stroke [doubled]) was calculated to assess the risk of stroke in individuals with AF, and CHA2DS2-VASc score (cardiac failure or dysfunction, hypertension, age ≥75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74, sex category) was calculated to estimate the risk of stroke in the next 12 months without anticoagulation therapy (12, 13). The data used to calculate these scores are shown in the eBox.
eBox. Further explanation of methods.
In the Gutenberg Health Study, standard anthropometric data are measured to determine classic cardiovascular risk factors. Participants’ medication history is obtained from information they provide and from drug packaging. Drugs are classified according to the Anatomical Therapeutic Chemical (ATC) Classification System.
Classic cardiovascular risk factors are defined as follows:
Smoking divides participants into nonsmokers (people who have never smoked or no longer smoke) and current smokers.
Diagnosis of diabetes mellitus is based on a medical history taken by a physician, or fasting blood glucose ≥126 mg/dL, or blood glucose ≥200 mg/dL in nonfasting participants.
Dyslipidemia is defined as diagnosis by a physician or LDL/HDL ratio >3.5.
Antihypertensive medication or mean systolic blood pressure ≥140 mm Hg or mean diastolic blood pressure ≥90 mm Hg results in a diagnosis of hypertension.
The data used to calculate CHADS2 score are shown in eBox Table 1. Those used to calculate CHA2DS2-VASc score are shown in eBox Table 2.
In addition, the relative and absolute risk reduction achieved using appropriate warfarin treatment for anticoagulation was calculated for persons with a potential indication for anticoagulation therapy (participants with AF not receiving antithrombotic therapy, i.e. not taking heparin, oral anticoagulants, and/or platelet aggregation inhibitors) (14, 15).
The publicly available program R Software, version 2.14.0 (R Development Core Team, 2011) was used for data analysis (e2).
Results
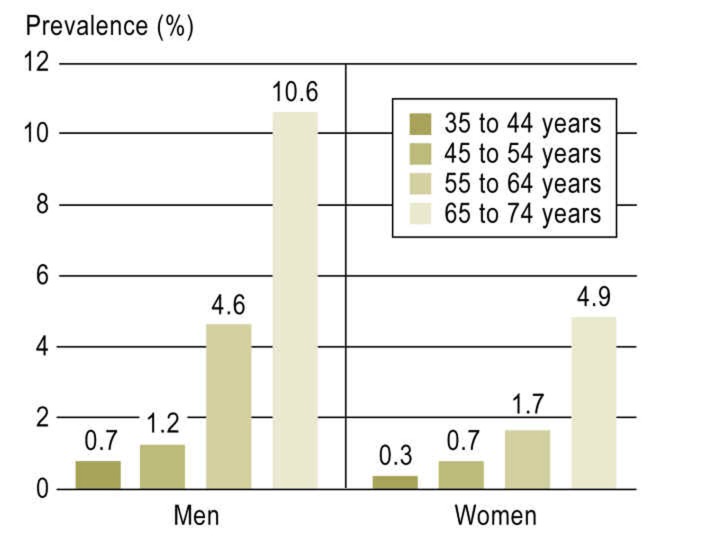
For the first 5000 GHS participants, the frequency of AF was 3.2% (n = 161). Men were more frequently affected than women, with a frequency of 4.6% (n = 115) versus 1.9% (n = 46). The weighted prevalence for the population of Mainz/Mainz-Bingen was 2.5%. There was a nonlinear increase with age in both men and women. In men aged 35 to 44 the prevalence was 0.7%, rising to 10.6% in those aged 65 to 74. In women there was an increase from 0.3% to 4.9% (Figure 1).
Figure 1.
Prevalence of atrial fibrillation in Gutenberg Health Study (GHS) patients by age group and sex
The distribution in the subgroup containing participants with AF shown on the current ECG is similar (data not shown). The weighted characteristics of the first 5000 GHS participants are reproduced in eTable 1 and data standardized for age according to the European standard population are shown in eTable 2. Study participants with AF were older, and fewer than one-third of them were women. The cardiovascular risk profile was less favorable, and the prevalence of cardiovascular diseases such as heart attack and heart failure was higher. In particular, almost 10% of participants with AF reported a previous stroke, versus 1.5% in the other cohort.
eTable 1. Characterstics of the study sample for the total cohort and by prevalence of atrial fibrillation (nonweighted distribution in the Gutenberg Health Study).
| Variable | Total population, n = 5000 | Participants without atrial fibrillation, n = 4801 | Participants with atrial fibrillation, n = 161 |
| Age, years | 55.5±10.9 | 55.2±10.9 | 64.9±8.3 |
| Women, n (%) | 2460 (49.2; 47.8 to 50.6) | 2390 (49.8; 48.4 to 51.2) | 46 (28.6; 21.5 to 35.6) |
| Smokers, n (%) | 959 (19.2; 18.1 to 20.3) | 935 (19.5; 18.4 to 20.6) | 18 (11.2; 6.3 to 16.1) |
| Body mass index, kg/m2 | 26.5 (23.9 to 29.8) | 26.5 (23.8 to 29.7) | 28.2 (25.8 to 32.1) |
| Systolic blood pressure, mmHg | 132.8±17.7 | 132.8±17.7 | 133.8±17.8 |
| Diastolic blood pressure, mmHg | 83.2±9.5 | 83.2±9.4 | 82.4±11.1 |
| Heart rate, bpm | 68.9±10.9 | 68.8±10.8 | 69.5±13.0 |
| Total cholesterol, mg/dL | 223±41 | 224±41 | 211±44 |
| Total/HDL cholesterol | 4.02 (3.30 to 4.92) | 4.02 (3.30 to 4.90) | 4.50 (3.63 to 5.20) |
| Diabetes, n (%) | 374 (7.5; 6.8 to 8.2) | 349 (7.3; 6.5 to 8.0) | 21(13.0; 7.8 to 18.3) |
| Hypertension, n (%) | 2 564 (51.3; 49.9 to 52.7) | 2 427 (50.6; 49.1 to 52.0) | 118 (73.3; 66.4 to 80.2) |
| Cardiac failure, n (%) | 965 (19.4; 18.3 to 20.5) | 883 (18.4; 17.3 to 19.5) | 78 (48.8; 40.9 to 56.6) |
| Myocardial infarction, n (%) | 156 (3.1; 2.6 to 3.6) | 134 (2.8; 2.3 to 3.3) | 22 (13.8; 8.4 to 19.3) |
| Stroke, n (%) | 95 (1.9; 1.5 to 2.3) | 78 (1.6; 1.3 to 2.0) | 17 (10.6; 5.8 to 15.4) |
| PQ interval, ms | 160±24 | 160±24 | 171±26* |
| Biomarker | |||
| Creatinine, mg/dL | 0.88 (0.79 to 0.98) | 0.88 (0.79 to 0.97) | 0.95 (0.83 to 1.06) |
| C-reactive protein, mg/L | 1.70 (0.50 to 3.30) | 1.60 (0.50 to 3.20) | 2.50 (1.37 to 4.93) |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 61.9 (28.5 to 124.4) | 60.1 (27.7 to 118.2) | 290.6 (90.4 to 977.7) |
The data shown are mean±standard deviation for continuous variables and number, percentage, and 95% confidence interval for categorical variables.
*The PQ interval is shown for persons with sinus rhythm in the study ECG (n = 96 participants with a history of atrial fibrillation)
eTable 2. Characterstics of the study sample for the total cohort and by prevalence of atrial fibrillation (standardized for age according to the 1976 European standard population*1).
| Variable | Total population, n = 5000 | Participants without atrial ‧fibrillation, n = 4801 | Participants with atrial fibrillation, n = 161 |
| Age, years | 51.9±10.6 | 51.6±10.5 | 62.2±9.5 |
| Women, % | 50 | 50.4 | 30.1 |
| Smokers, % | 21.4 | 21.6 | 12.7 |
| Body mass index, kg/m2 | 26.3 (23.6 to 29.6) | 26.2 (23.6 to 29.5) | 28.2 (25.5 to 32.2) |
| Systolic blood pressure, mmHg | 130.6±16.9 | 130.5±16.9 | 133.0±17.6 |
| Diastolic blood pressure, mmHg | 83.1±9.4 | 83.1±9.3 | 82.8±10.9 |
| Heart rate, bpm | 69.0±10.7 | 69.0±10.6 | 69.3±12.4 |
| Total cholesterol, mg/dL | 222±41 | 222±41 | 212±43 |
| Total/HDL cholesterol | 4.01 (3.28 to 4.92) | 4.00 (3.28 to 4.91) | 4.54 (3.64 to 5.21) |
| Diabetes, % | 5.9 | 5.7 | 11.7 |
| Hypertension, % | 45 | 44.4 | 70 |
| Cardiac failure, % | 17.4 | 16.8 | 43.1 |
| Myocardial infarction, % | 2.1 | 1.9 | 12.2 |
| Stroke, % | 1.4 | 1.2 | 9.2 |
| PQ interval, ms | 158±23 | 158±23 | 169±25*2 |
| Biomarker | |||
| Creatinine, mg/dL | 0.87 (0.78 to 0.97) | 0.87 (0.78 to 0.97) | 0.94 (0.82 to 1.05) |
| C-reactive protein, mg/L | 1.60 (0.50 to 3.10) | 1.60 (0.50 to 3.10) | 2.30 (1.30 to 4.68) |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 52.6 (23.1 to 104.9) | 51.5 (22.9 to 102.0) | 234.5 (60.9 to 884.4) |
The data shown are mean±standard deviation for continuous variables and percentage for categorical variables.
*1The data in this table were standardized for age according to the 1976 European standard population (WHO), in order to allow comparability with other studies.
*2The PQ interval is shown for persons with sinus rhythm in the study ECG (n = 96 participants with a history of atrial fibrillation)
Creatinine, a renal function parameter; C-reactive protein, a marker for inflammation; and N-terminal pro-B-type natriuretic peptide, an indicator of cardiac stress, were all higher in the group with AF than in the total cohort (Table 1).
Table 1. Characterstics of the study sample for the total cohort and by prevalence of atrial fibrillation (weighted for the population of Mainz and the region of Mainz-Bingen).
| Variable | Total population, n = 5000 | Participants without atrial ‧fibrillation, n = 4801 | Participants with atrial ‧fibrillation, n = 161 |
| Age, years | 52.2±11.1 | 51.9±11.0 | 63.2±9.5 |
| Women, % | 50.1*1 | 50.5 (50.3 to 50.7) | 31.8 (24.3 to 39.3) |
| Smokers, % | 20.9 (19.7 to 22.2) | 21.2 (19.9 to 22.5) | 11.6 (6.5 to 16.8) |
| Body mass index, kg/m2 | 26.3 (23.6 to 29.6) | 26.2 (23.6 to 29.5) | 28.3 (25.7 to 32.1) |
| Systolic blood pressure, mm Hg | 130.8±17.0 | 130.7±17.0 | 133.0±17.7 |
| Diastolic blood pressure, mm Hg | 82.9±9.4 | 83.0±9.3 | 82.5±11.0 |
| Heart rate, bpm | 69.0±10.7 | 69.0±10.6 | 69.4±12.7 |
| Total cholesterol, mg/dL | 222±41 | 222±41 | 212±44 |
| Total/HDL cholesterol | 4.00 (3.28 to 4.91) | 4.00 (3.28 to 4.90) | 4.53 (3.63 to 5.20) |
| Diabetes, % | 6.0 (5.4 to 6.6) | 5.8 (5.2 to 6.4) | 12.2 (7.2 to 17.2) |
| Hypertension, % | 45.4 (44.0 to 46.7) | 44.7 (43.3 to 46.1) | 71.6 (64.2 to 79.1) |
| Cardiac failure, % | 17.7 (16.7 to 18.8) | 17.1 (16.0 to 18.2) | 46.3 (38.3 to 54.3) |
| Myocardial infarction, % | 2.3 (1.9 to 2.6) | 2.0 (1.7 to 2.4) | 12.7 (7.6 to 17.8) |
| Stroke, % | 1.5 (1.2 to 1.8) | 1.3 (1.0 to 1.6) | 9.8 (5.3 to 14.4) |
| PQ interval, ms | 158±23 | 158±23 | 170±26*2 |
| Biomarker | |||
| Creatinine, mg/dL | 0.87 (0.78 to 0.97) | 0.87 (0.78 to 0.97) | 0.93 (0.82 to 1.05) |
| C-reactive protein, mg/L | 1.60 (0.50 to 3.20) | 1.60 (0.50 to 3.10) | 2.30 (1.30 to 4.71) |
| N-terminal pro-B-type natriuretic peptide, pg/mL | 53.7 (23.5 to 108.0) | 52.4 (23.0 to 104.4) | 258.0 (65.0 to 931.7) |
The data shown are mean±standard deviation for continuous variables, median and 25th to 75th percentile for skewed variables, and percentage and confidence ?interval for categorical variables.
*1For sex, confidence intervals have no informative value for the total population, because sex is a weight variable.
*2The PQ interval is shown for persons with sinus rhythm in the study ECG (n = 96 participants with a history of atrial fibrillation)
In 40% (n = 65), AF was recorded in the study ECG. AF was detected in the study ECG in 29.4% (n = 40) of the 136 participants who reported that they had already been diagnosed with AF by a physician. Twenty-five participants, 0.5% of the total cohort and 15.5% of the participants with a GHS diagnosis of AF, were not aware that they had AF before the study and were first diagnosed during examination at the study site. According to the Framingham risk score for AF, these individuals had a mean risk of developing AF in the next five years of 2.0%.
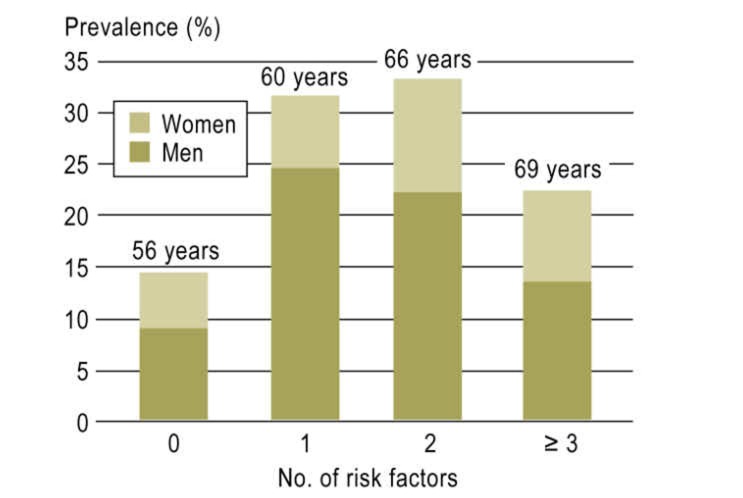
The risk factor distribution of the Framingham risk score in participants with AF was similar for both sexes except for a trend towards a higher prevalence of cardiac failure in women (weighted distribution shown in Table 2, nonweighted distribution in eTable 3). Figure 2 shows the cumulative number of risk factors in participants with AF. 14.3% of patients presented none of the risk factors measured other than age (13.0% of men, 17.1% of women). Only approximately 21.7% of participants presented three or more risk factors.
Table 2. Prevalence of classic risk factors for atrial fibrillation* in men and women (weighted for the population of Mainz and the region of Mainz-Bingen).
| Variable | Men, n = 115 | Women, n = 46 |
| Age, years | 66.0 (57.0 to 70.0) | 66.0 (56.8 to 71.6) |
| Systolic blood pressure, mmHg | 133.8±17.4 | 131.3±18.6 |
| Antihypertensive medication, % | 70.9 (61.4 to 80.4) | 67.0 (53.2 to 80.9) |
| Body mass index, kg/m2 | 28.2 (26.2 to 32.1) | 28.3 (23.9 to 33.1) |
| Cardiac failure, % | 40.3 (31.1 to 49.5) | 59.4 (44.5 to 74.3) |
| PQ interval, ms | 170±25 | 171±27 |
*Based on the Framingham risk score (10) The data shown are mean±standard deviation for continuous variables, median and 25th to 75th percentile for skewed variables, and percentage and 95% confidence interval for categorical variables. The PQ interval is shown for persons with sinus rhythm in the study ECG and a history of atrial fibrillation
eTable 3. Prevalence of classic risk factors for atrial fibrillation (e5) in men and women (nonweighted distribution in the Gutenberg Health Study).
| Variable | Men, n = 115 | Women, n = 46 | ||
| Age, years | 67.0 (60.3 to 71.8) | 67.0 (60.0 to 72.0) | ||
| Systolic blood pressure, mmHg | 134.5±17.5 | 132.0±18.5 | ||
| Antihypertensive medication, n (%) | 88 (76.5) | 31 (67.4) | ||
| Body mass index, kg/m2 | 29.2±4.7 | 29.6±6.7 | ||
| Cardiac failure, n (%) | 50 (43.5) | 28 (62.2) | ||
| PQ interval, ms | 170.8±25.1 | 170.8±27.2 |
The data shown are mean±standard deviation for continuous variables and number and percentage for categorical variables. The PQ interval is shown for persons with sinus rhythm in the study ECG and a history of atrial fibrillation
Figure 2.
Distribution of risk factors according to the Framingham risk score (11) for atrial fibrillation in Gutenberg Health Study (GHS) participants with atrial fibrillation. The bars indicate percentages by sex. The median age of participants is stated above the bars. Prevalences are weighted for the population of Mainz and the region of Mainz-Bingen
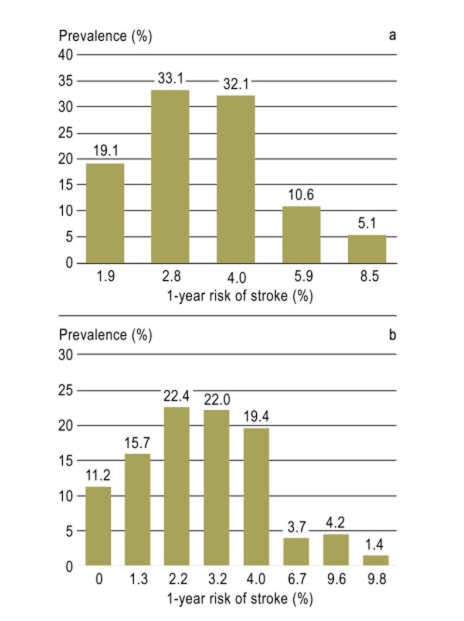
The risk stratification algorithms CHADS2 and CHA2DS2-VASc scores were used to determine the risk of stroke in the next year for participants with AF if they did not receive suitable treatment. Figure 3 shows the number of participants in each category. More than 80% of patients with AF had a risk of stroke of more than 2% if left untreated according to their CHADS2 scores (Figure 3a), and almost three-fourths of participants according to CHA2DS2-VASc scores (Figure 3b). According to CHA2DS2-VASc scores, only 11.2% had a negligible risk. In the rest of the study population, the median CHADS2 and CHA2DS2-VASc score was 1.0. The mean CHADS2 and CHA2DS2-VASc scores of participants first diagnosed with AF on the basis of the study ECG were 2.0 and 3.0 respectively, with a mean 1-year risk of a thromboembolic event of 4.0% and 3.2% respectively.
Figure 3.
Risk of stroke according to a) CHADS2 score (adjusted for aspirin intake); and b) CHA2DS2-VASc score (adjusted for warfarin intake) (12, 13) in Gutenberg Health Study (GHS) participants with atrial fibrillation. Prevalences are weighted for the population of Mainz and the region of Mainz-Bingen
Table 3 provides an overview of medication relevant to AF taken by all study participants and participants with AF (the nonweighted distribution is shown in eTable 4).
Table 3. Medication of the study sample for the total cohort and participants with atrial fibrillation (weighted for the population of Mainz and the region of Mainz-Bingen).
| Variable | Total population, n = 5000 | Participants with atrial fibrillation, n = 161 |
| Antithrombotic therapy | ||
| Heparin, % | 0.1 (0.0 to 0.2) | 0.5 (0.0 to 1.5) |
| Oral anticoagulants, % | 1.6 (1.3 to 1.9) | 37.1 (29.5 to 44.7) |
| Platelet inhibitors, % | 8.1 (7.4 to 8.8) | 23.1 (16.7 to 29.6) |
| None of the above, % | 90.3 (89.6 to 91.0) | 42.7 (34.6 to 50.8) |
| Antiarrhythmic medication | ||
| Class I, % | 0.1 (0.0 to 0.2) | 2.8 (0.3 to 5.2) |
| Class III, % | 0.3 (0.2 to 0.5) | 8.8 (4.4 to 13.1) |
| Class IV, % | 0.9 (0.7 to 1.2) | 8.1 (3.2 to 12.9) |
| Digitalis, % | 0.5 (0.4 to 0.7) | 16.4 (10.8 to 22.1) |
| Other medication | ||
| ACE inhibitors, % | 12.0 (11.2 to 12.9) | 36.4 (28.9 to 43.9) |
| AT-II antagonists, % | 7.2 (6.5 to 7.8) | 18.4 (12.2 to 24.5) |
| Beta-blockers, % | 13.5 (12.6 to 14.4) | 56.1 (48.0 to 64.2) |
| Dihydropyridine, % | 5.5 (4.9 to 6.1) | 17.8 (11.7 to 23.9) |
| Diuretics, % | 13.4 (12.5 to 14.2) | 43.0 (35.1 to 50.9) |
| Nitrates, % | 0.7 (0.5 to 0.9) | 6.5 (2.7 to 10.3) |
| Renin inhibitors, % | 0.1 (0.0 to 0.2) | 0.7 (0.0 to 2.2) |
| Statins, % | 9.8 (9.0 to 10.5) | 34.9 (27.3 to 42.5) |
| Insulin, % | 2.1 (1.7 to 2.5) | 5.0 (1.6 to 8.3) |
| Oral antidiabetic medication, % | 3.6 (3.2 to 4.1) | 6.5 (2.7 to 10.3) |
| Thyroid hormone therapy, % | 11.4 (10.5 to 12.3) | 16.5 (10.5 to 22.5) |
The data shown are percentage and 95% confidence interval for medication intake; ACE: angiotensin-converting enzyme
eTable 4. Medication of the study sample for the total cohort and participants with atrial fibrillation (nonweighted distribution in the Gutenberg Health Study).
| Variable | Total population, n = 5000 | Participants with atrial fibrillation, n = 161 |
| Antithrombotic therapy | ||
| Heparin, n (%) | 6 (0.1) | 1 (0.6) |
| Oral anticoagulants, n (%) | 104 (2.1) | 63 (39.1) |
| Platelet inhibitors, n (%) | 516 (10.3) | 41 (25.5) |
| None of the above, n (%) | 4 370 (87.6) | 62 (38.5) |
| Antiarrhythmic medication | ||
| Class I, n (%) | 7 (0.1) | 5 (3.1) |
| Class III, n (%) | 24 (0.5) | 15 (9.3) |
| Class IV, n (%) | 56 (1.1) | 12 (7.5) |
| Digitalis, n (%) | 37 (0.7) | 29 (18.0) |
| Other medication | ||
| ACE inhibitors, n (%) | 746 (15.0) | 65 (40.4) |
| AT-II antagonists, n (%) | 441 (8.8) | 30 (18.6) |
| Beta-blockers, n (%) | 821 (16.5) | 94 (58.4) |
| Dihydropyridine, n (%) | 344 (6.9) | 29 (18.0) |
| Diuretics, n (%) | 832 (16.7) | 75 (46.6) |
| Nitrates, n (%) | 49 (1.0) | 11 (6.8) |
| Renin inhibitors, n (%) | 6 (0.1) | 1 (0.6) |
| Statins, n (%) | 610 (12.2) | 58 (36.0) |
| Insulin, n (%) | 130 (2.6) | 8 (5.0) |
| Oral antidiabetic medication, n (%) | 230 (4.6) | 11 (6.8) |
| Thyroid hormone therapy, n (%) | 600 (12.0) | 25 (15.5) |
The data shown are number and percentage for medication intake; ACE: angiotensin-converting enzyme.
As expected, medication for cardiovascular diseases is taken more frequently by participants with AF. 42.7% of participants with AF reported that they took no anticoagulants or platelet inhibitors. The median CHADS2 and CHA2DS2-VASc scores of these participants were 1.0 and 2.0 respectively. 58.7% had a CHADS score ≥1. According to study data (14, 15), appropriate warfarin treatment for anticoagulation would reduce these individuals’ risk of a thromboembolic event in the next year from 2.1% to 0.75%. This represents a relative risk reduction of 65% and an absolute risk reduction of 1.4%.
Antiarrhythmic therapy other than beta-blockers was reported by approximately 20% of participants with AF, and 16% took digitalis medication. Also, thyroid hormone therapy was slightly more frequent in AF patients than in the study population as a whole.
Discussion
In a population-based German cohort, we observed an AF prevalence of 2.5% of participants aged between 35 and 74, with a significant rise in the number of cases with increasing age. The distribution of AF risk factors was similar for both sexes. The mean risk of stroke was 2.8% to 3.2% annually in participants with AF. The study ECG identified new-onset AF in 25 participants, with a mean 1-year risk of stroke (CHADS2) of approximately 4.0%.
The weighted frequencies observed in this German cohort are in line with the data recently published in an Icelandic study (2). The prevalence reported, slightly less than 3%, may at first glance seem relatively low. However, this is due to the age distribution of the members of the cohort. AF occurs mainly in older persons, a fact also illustrated by the rise in AF with increasing age that was observed in this population. For the sake of comparison, the prevalence of myocardial infarction was 2.3%, and that of stroke 1.5%. This makes AF a relatively common disease. The cost of AF to the healthcare system is substantial (16). In addition, 83% of AF patients are symptomatic and it has a negative impact on quality of life (17).
It is also worth remembering that other cardiovascular diseases, such as coronary heart disease, are becoming less common as causes of death (18). The prevalence and therefore the total mortality of AF and its sequelae, however, are increasing, despite a slight improvement in survival times of individual patients with AF (1, 2).
It is interesting that 14.3% of participants with AF presented none of the known, repeatedly validated AF risk factors of the Framingham risk score other than age (10, 19, 20), that almost half the individuals with AF had no more than one risk factor, and that only around one-fifth presented three or more risk factors. This indicates that other risk factors also play a role in the development of AF. The Framingham risk factors can only explain up to 60% of the risk in the population (11, 19), which means that there is considerable room for improvement in risk prediction.
As expected, the values of easy-to-measure biomarkers for inflammation (C-reactive protein) and cardiac stress (N-terminal pro-B-type natriuretic peptide) are higher in the group of participants with AF. However, it was also shown that additional measurement of both biomarkers only slightly increases the predictive value of the known risk factors (21). The search for specific biomarkers and clinical risk factors for AF continues. While research and the development of guidelines have seen major efforts regarding risk and the prevention of AF sequelae, knowledge of predisposition towards AF and primary prevention is still at an early stage.
AF patients’ main contacts are primary care and hospital physicians (17). The Outpatient Registry Upon Morbidity of Atrial Fibrillation (ATRIUM) shows that most AF patients are treated by primary care physicians (22). This makes it even more important to raise awareness of AF among these categories of physicians. The percentage of persons first diagnosed during the GHS was 0.5% of the total study population, or 15.5% of participants with AF. CHADS2 and CHA2DS2-VASc scores showed a mean 1-year risk of a thromboembolic stroke event of 3.6%. This means that the risk in this subgroup is considerable. The presented data show how important a simple ECG can be for at-risk patients. Data reported in the literature suggest that fewer than half of initial diagnoses of AF are based on AF-specific symptoms. Most AF diagnoses are made during visits to doctors for other complaints or, in almost one-fourth of cases, during routine examinations (17).
Stroke prevention therapy for AF patients according to guidelines has reduced the incidence of this serious sequela (23). Oral anticoagulation therapy is also increasingly being recommended for patients with a moderate risk of stroke, i.e. scores of 1.0 or higher (8, 24). In our cohort, more than one-third of participants with AF were not receiving anticoagulation therapy, although their mean CHA2DS2-VASc score was 2.0. Of all participants with AF, 14.3% had a negligible risk of stroke, with a CHA2DS2-VASc score of 0, meaning that according to current recommendations they did not require anticoagulation therapy. It remains to be seen how new anticoagulants will alter anticoagulation strategies in the near future.
Limitations
As expected, the number of cases of AF in a population-based cohort of middle-aged persons is small, so the conclusions presented here must be interpreted with caution. A study involving older persons would be needed to estimate the prevalence of AF in the general population more precisely, because AF is a disease of old age.
As AF is often asymptomatic and can be intermittent, the prevalence of AF found in this research should be treated as an underestimate, as a result of underdiagnosis. Even in patients with symptomatic episodes of AF, asymptomatic episodes of AF can be detected in up to 62% of cases (25). The extent to which more intensive screening for AF in primary prevention, for example by ECG, leads to a higher AF detection rate and the consequences of this in allowing early intervention and individual treatment strategies remain to be seen (e1).
This systematic, detailed research (including research into cardiovascular risk factors and patients’ medication history) into a random, population-based sample and analysis of cases of AF in the study have allowed us to describe up-to-date, population-based data on AF and on the distribution of stroke risk factors in the studied cohort. Follow-up lasting several years will provide valuable information on AF risk factors and incidence.
At present, we can demonstrate that AF is a disease significant for the general population, and that its prevalence rises substantially with increasing age. Screening using 12-lead ECG can reveal cases not yet diagnosed. Knowledge of how AF develops and of the distribution of risk factors in the general population is essential if long-term preventive measures are to be developed and gaps in care are to be closed.
eBox Table 1. CHADS2 score and risk of stroke adapted according to Gage et al. (e3).
| CHADS2 score | Patients (n = 1733) | Adjusted stroke rate (% per year) (95% confidence interval) |
| 0 | 120 | 1.9 (1.2 to 3.0) |
| 1 | 463 | 2.8 (2.0 to 3.8) |
| 2 | 523 | 4.0 (3.1 to 5.1) |
| 3 | 337 | 5.9 (4.6 to 7.3) |
| 4 | 220 | 8.5 (6.3 to 11.1) |
| 5 | 65 | 12.5 (8.2 to 1..5) |
| 6 | 5 | 18.2 (10.5 to 27.4) |
CHADS2: cardiac failure, hypertension, age, diabetes, stroke (doubled)
eBox Table 2. Adjusted stroke rate, corresponding to CHA2DS2-VASc score according to Lip et al. (e4).
| CHA2DS2-VASc score | Patients (n = 7329) | Adjusted stroke rate (% per year) |
| 0 | 1 | 0 |
| 1 | 422 | 1.3 |
| 2 | 1230 | 2.2 |
| 3 | 1730 | 3.2 |
| 4 | 1718 | 4 |
| 5 | 1159 | 6.7 |
| 6 | 679 | 9.8 |
| 7 | 294 | 9.6 |
| 8 | 82 | 6.7 |
| 9 | 14 | 15.2 |
CHA2DS2-VASc: cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74, sex category (female)
Acknowledgments
Translated from the original German by Caroline Devitt, MA.
The Gutenberg Health Study is funded by the government of the German federal state of Rhineland-Palatinate (Rhineland-Palatinate Trust for Innovation, contract no. AZ 961–386261/733), Mainz University Medical Center’s Science Program Wissen schafft Zukunft and its Vascular Prevention Research Division, Boehringer Ingelheim, and Philips Medical Systems.
PD Dr. Schnabel is sponsored by the German Research Foundation (Deutsche Forschungsgemeinschaft), Emmy Noether Program SCHN 1149/3–1.
Footnotes
Dr. Wild has received research fees from Boehringer, Daiichi Sankyo, Sanofi-Aventis, Bayer Vital, and Portavita BV.
Prof. Munzel has received consultancy fees from Servier, Actavis, and Boehringer. He received reimbursement of conference fees from Abbott, Servier, and Boehringer; travel expenses from Abbott, Servier, Boehringer, and Actavis; lecture fees from Servier, Boehringer, and Actavis; and research fees from Actavis and Servier.
Prof. Blankenberg has received research fees from Boehringer Ingelheim and Philips Medical Systems.
PD Dr. Schnabel and Ms Wilde (B.A.) declare that no conflict of interest exists.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Stefansdottir H, Aspelund T, Gudnason V, Arnar DO. Trends in the incidence and prevalence of atrial fibrillation in Iceland and future projections. Europace. 2011;13:1110–1117. doi: 10.1093/europace/eur132. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- 4.Currie CJ, Jones M, Goodfellow J, et al. Evaluation of survival and ischaemic and thromboembolic event rates in patients with non-valvar atrial fibrillation in the general population when treated and untreated with warfarin. Heart. 2006;92:196–200. doi: 10.1136/hrt.2004.058339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Wolf PA, D’Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 7.Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11:423–434. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 9.Schnabel RB, Schulz A, Wild PS, et al. Non-invasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4:348–350. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 10.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schnabel RB, Aspelund T, Li G, et al. Validation of an atrial fibrillation risk algorithm in whites and african americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 13.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–2738. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 15.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 16.Holstenson E, Ringborg A, Lindgren P, et al. Predictors of costs related to cardiovascular disease among patients with atrial fibrillation in five European countries. Europace. 2011;13:23–30. doi: 10.1093/europace/euq325. [DOI] [PubMed] [Google Scholar]
- 17.Aliot E, Breithardt G, Brugada J, et al. An international survey of physician and patient understanding, perception, and attitudes to atrial fibrillation and its contribution to cardiovascular disease morbidity and mortality. Europace. 2010;12:626–633. doi: 10.1093/europace/euq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in US. deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 20.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 21.Schnabel RB, Larson MG, Yamamoto JF, et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation. 2010;121:200–207. doi: 10.1161/CIRCULATIONAHA.109.882241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P. Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol. 2011;100:897–905. doi: 10.1007/s00392-011-0320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieuwlaat R, Olsson SB, Lip GY, et al. Guideline-adherent antithrombotic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fibrillation. The Euro Heart Survey on Atrial Fibrillation. Am Heart J. 2007;153:1006–1012. doi: 10.1016/j.ahj.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Lip GY. Anticoagulation therapy and the risk of stroke in patients with atrial fibrillation at ’moderate risk’ [CHADS2 score=1]: simplifying stroke risk assessment and thromboprophylaxis in real-life clinical practice. Thromb Haemost. 2010;103:683–685. doi: 10.1160/TH10-01-0038. [DOI] [PubMed] [Google Scholar]
- 25.Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation. 2005;112:307–313. doi: 10.1161/CIRCULATIONAHA.104.518837. [DOI] [PubMed] [Google Scholar]
- e1.Dormann H, Diesch K, Ganslandt T, Hahn EG, et al. Kennzahlen und Qualitätsindikatoren einer medizinischen Notaufnahme. Dtsch Arztebl Int. 2010;107(15):261–267. doi: 10.3238/arztebl.2010.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.R A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-08-9. www.R-project.org [Google Scholar]
- e3.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- e4.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- e5.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e6.Trappe JH. Atrial Fibrillation: Established and innovative methods of evaluation and treatment. Dtsch Artzebl Int. 2012;109(1-2):1–7. doi: 10.3238/arztebl.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]