Abstract
Acoustic trauma often results in permanent damage to the cochlea, triggering changes in processing within central auditory structures such as the inferior colliculus (IC). The serotonergic neuromodulatory system, present in the IC, is responsive to chronic changes in the activity of sensory systems. The current study investigated whether the density of serotonergic innervation in the IC is changed following acoustic trauma. The trauma stimulus consisted of an 8 kHz pure tone presented at a level of 113 dB SPL for six consecutive hours to anesthetized CBA/J mice. Following a minimum recovery period of three weeks, serotonergic fibers were visualized via histochemical techniques targeting the serotonin reuptake transporter (SERT) and quantified using stereologic probes. SERT-positive fiber densities were then compared between the traumatized and protected hemispheres of unilaterally traumatized subjects and those of controls. A significant effect of acoustic trauma was found between the hemispheres of unilaterally traumatized subjects such that the IC contralateral to the ear of exposure contained a lower density of SERT-positive fibers than the IC ipsilateral to acoustic trauma. No significant difference in density was found between the hemispheres of control subjects. Additional dimensions of variability in serotonergic fibers were seen among subdivisions of the IC and with age. The central IC had a slightly but significantly lowered density of serotonergic fibers than other subdivisions of the IC, and serotonergic fibers also declined with age. Overall, the results indicate that acoustic trauma is capable of producing modest but significant decreases in the density of serotonergic fibers innervating the IC.
1. Introduction
The main target of acoustic trauma is the cochlea. This has been demonstrated by chronic reductions in both cochlear potentials and the compound action potential (CAP) (Melichar et al., 1980; Sohmer et al., 1980; Sugisawa et al., 1994), as well as decreased spontaneous firing rate of the auditory nerve (Liberman and Dodds, 1984; Liberman and Kiang, 1978; Eldredge et al., 1973). However, damage to the auditory periphery also induces functional changes at multiple levels of the central auditory system (CAS). These include increased spontaneous activity, tonotopic map reorganization, broadened tuning of response fields, increased response amplitudes of suprathreshold auditory evoked potentials and changes in the balance of inhibition and excitation near frequency regions related to damage (Barsz et al., 2007; Davis et al., 1989; Ma et al., 2006; Komiya and Eggermont, 2000; Noreña and Eggermont, 2003; Tan et al., 2007; Rachel et al., 2002; Wang et al., 2002; Vale and Sanes, 2002; Szczepaniak and Mǿller, 1996; Bledsoe et al., 1995; Milbrandt et al., 2000; Suneja et al., 1998; Michler and Illing, 2002). While some CAS changes occur immediately as a result of loss of input from the periphery, others suggest the induction of long term plastic compensatory mechanisms. For example, recordings made in the dorsal cochlear nucleus (DCN) following acoustic trauma indicate that spontaneous activity levels are initially decreased, reflecting immediate loss of peripheral input. However, two to five days after exposure these levels are found to be elevated, and the level of spontaneous activity can continue to increase for up to six months following trauma (Kaltenbach et al., 2000). Similarly, in subjects with induced selective inner hair cell loss, the compound response amplitude measured in the inferior colliculus (IC) is initially below normal, but increases slowly, reaching maximal output no less than two weeks following inner hair cell destruction (Salvi et al., 2000). This increase in response gain can still be recorded up to 6 months later, indicating a long term compensatory response. The overarching conclusion is that the CAS is capable of adjusting the gain of cells in both cortical and subcortical areas in order to compensate for the chronic reduction in input from damaged cochlear regions. However, the mechanisms regulating these changes in response to acoustic trauma are largely unknown.
The neuromodulatory serotonergic system is a potential candidate involved in regulating CAS plasticity following chronic peripheral damage. Serotonergic involvement in plasticity has been demonstrated in multiple sensory systems, including the visual (Vetencourt et al., 2008; Normann et al., 2007), somatosensory (Esaki et al., 2005; Okamoto et al., 2002), and olfactory systems (Lombion et al., 2007), where it regulates the ability to adapt to chronic changes in sensory input. Many levels of the central auditory pathway are densely innervated with serotonergic axonal projections originating from the raphe nuclei located within the brainstem (Klepper and Herbert, 1991; Thompson et al., 1994; Hurley and Thompson, 2001; Thompson and Hurley, 2004). Furthermore, serotonin is involved in regulating the response gain and other properties of auditory responses at cortical and subcortical levels. For example, serotonin regulates the gain of auditory cortical responses to changes in stimulus intensity (Nathan et al., 2006) and to the temporal order of consecutive stimuli (Johnson et al., 1998). Serotonin also regulates the gain of neural responses in some subcortical auditory nuclei such as the IC. Increased levels of serotonin in the IC, induced by either exogenous application (Hurley and Pollak, 2001) or endogenous release (Hall and Hurley, 2007), have a generally inhibitory effect on the majority of IC cells resulting in a reduction in driven firing rates and a decrease in the frequency range of response. Response gain and frequency tuning at this level may be mediated both via direct stimulation of specific postsynaptic serotonergic receptors (Hurley, 2006, 2007), and indirectly by stimulation of receptors located on inhibitory GABAergic neurons (Hurley et al., 2008). At a perceptual level, it has been demonstrated that administration of serotonin into the central ventricular system leads to a depression of the acoustic startle response (Davis et al., 1980), and dysfunction within the serotonergic system has been implicated in various disorders affecting auditory perception such as hyperacusis (Marriage and Barnes, 1995), tinnitus (Salvinelli et al., 2003; Simpson and Davies, 2000), and schizophrenia (Johnson et al., 1998). For these reasons, it is plausible that serotonin is capable of regulating homeostatic gain control in the auditory system in response to chronic damage to the auditory periphery by altering the density of serotonergic fibers within auditory nuclei.
Because serotonin has a predominantly inhibitory effect in the IC (Hall and Hurley, 2007; Hurley and Pollak, 2001) and activity within the IC is upregulated following acoustic trauma (Salvi et al., 2000; Szczepaniak and Mǿller, 1996; Willott and Lu, 1982), we hypothesized that acoustic trauma would lead to a reduction in the density of serotonergic fibers innervating the IC. To investigate this hypothesis, we systematically quantified the density of serotonergic fibers throughout the IC in two groups of mice: those undergoing unilateral acoustic trauma, and a non-traumatized control group. Density results were then compared within unilaterally traumatized and control animals.
2. Methods
All procedures used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Indiana University at Bloomington.
2.1. Acoustic trauma
Subjects were adult male CBA/J mice. Ten mice received unilateral acoustic trauma, and eight served as control subjects. All subjects were anesthetized via intraperitoneal injections of 120 mg/kg ketamine and 5 mg/kg xylazine. Supplemental doses of anesthesia were administered during the induction of acoustic trauma as needed based on reflexive response to tail and toe pinches. No more than five mice were acoustically traumatized concurrently. The acoustic trauma stimulus consisted of an 8000 Hz tone presented at a level of 113 dB SPL for 6 h via a Selenium ST300 SLF Super Tweeter speaker. The speaker was placed directly above the mouse enclosure. The stimulus was calibrated against a Brüel and Kjær 1/8th inch microphone positioned in the location of the subjects’ head in the mouse enclosure with the speaker located directly above. In subjects undergoing unilateral acoustic trauma, the left ear was exposed to the acoustic stimulus while the right ear was plugged via application of petroleum jelly (Coles et al., 1982; Kelly and Reger, 1937). Petroleum jelly was removed immediately following exposure to the stimulus. Control subjects were anesthetized in a manner and duration identical to those of experimental subjects, but were not exposed to an acoustic trauma stimulus. Following trauma induction and/or administration of anesthesia (depending upon subject group), mice were returned to animal quarters for a minimum of three weeks and maximum of 16 weeks. The purpose of the recovery period was to allow any changes in the auditory CNS resulting from acoustic exposure to stabilize. Experimental and control subjects were processed concurrently to control for effects of age and duration of recovery period.
This stimulus significantly increased auditory brainstem response (ABR) thresholds in a group of mice subjected to bilateral trauma (n = 7). ABRs were measured in anesthetized mice through silver wire electrodes placed subcutaneously with one reference located just caudal to the pinna along the midline, another reference located at the vertex, and a ground electrode located at the nape of the neck. Stimuli were tone bursts of 1 ms duration with 0.1 ms rise/fall times presented at a rate of 20 tone bursts/second, and ABR waveforms were based on a minimum of 1024 stimulus repetitions. Thresholds were measured after recovery periods ranging from 21 to 81 days (median = 41 days) and compared between control and bilaterally traumatized subject groups at frequencies of 4, 8, 16, and 20 kHz. Thresholds between the two groups were significantly different at 16 kHz (ANOVA, F(1,10) = 5.639, p = 0.039) with the thresholds of control mice averaging 14.5 dB better than those of bilaterally traumatized mice. This relatively modest threshold shift above the frequency of trauma is characteristic of tonal trauma, in which large threshold shifts immediately after trauma recover substantially after 2–4 weeks, and are often observed at frequencies above the trauma frequency (Dong et al., 2010a; Li, 1992; Salvi et al.,1990; Willott and Henry, 1974).
2.2. Visualization of serotonergic innervation
Subjects were perfused (phosphate buffered 4% paraformaldehyde in 0.1 M PBS with pH of 7.0), brains were extracted, and a pin was placed in the left hemisphere in a location that did not interfere with the IC to facilitate differentiation of hemispheres. Brains were then incubated in 15% sucrose solution followed by 30% sucrose solution for 24 h each. Brains were sliced into 50 µm sections along the coronal axis using a sliding microtome and placed in a phosphate buffered saline (PBS) solution (0.1 M, pH 7.4). Slices were quenched in 0.5% hydrogen peroxide for 30 min, followed by a 1 h incubation in a general blocking solution (1% bovine serum albumin + 5% normal goat serum + PBS-Tx) and a 20 min incubation in 1:5 avidin:PBS-Tx and then 1:5 biotin: PBS-Tx (avidin/biotin blocking kit, Vector Labs). Slices were then rinsed five times for 5 min each in PBS-Tx. The primary rabbit antibody targeted the serotonin reuptake transporter (SERT) (Immunostar, #24330, Hudson, WI), a transporter found only on serotonergic cells. Visualization of serotonergic axons via targeting SERT has been shown to be comparable to visualization using antibodies targeting serotonin and may reduce non-specific background staining (Mamounas et al., 2000). Samples were incubated in 1:5000 primary antibody + 1% normal goat serum in PBS for 48 h at a temperature of 4 °C on a rotator plate. Following rinses in PBS-Tx, samples were incubated in 1:500 biotinylated goat anti-rabbit antibody + 2% normal goat serum in PBS-Tx for 1 h. Samples were rinsed, and the secondary antibody was amplified for visualization via a 45 min treatment with ABComplex (Vectastain Elite ABC kit, Vector Labs, Burlingame, CA) in PBS. Following a rinse in PBS, the samples were then incubated in a DAB solution containing nickel (DAB kit, Vector Labs). Samples were then rinsed in distilled water, plated on chromium gel coated slides, allowed to air dry, and cover-slipped using DPX Mounting Medium (Sigma–Aldrich, St. Louis, MO). Control subjects not exposed to trauma were processed concurrently with experimental subjects in order to mitigate differences in immunohistochemical staining due to daily variance in ambient conditions.
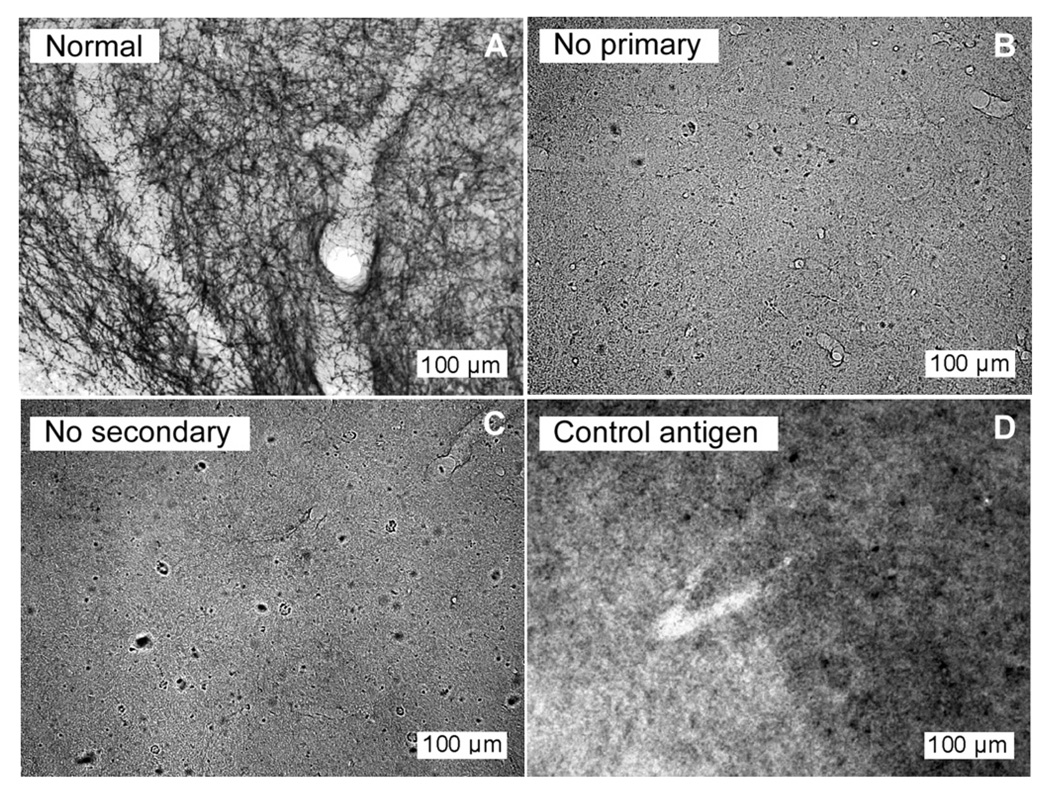
In addition, controls for the immunohistochemical staining procedure consisted of the omission of primary antibody, the omission of secondary antibody, and the preadsorption of the primary antibody with a control peptide (Immunostar, #24332, Hudson, WI) for 36 h. Controls were performed in parallel with routine antibody staining for SERT in two separate subjects. In both subjects, all three control treatments abolished labeling of serotonergic fibers in the IC (Fig. 1).
Fig. 1.
Control treatments remove SERT labeling in the IC. Sections at 20× processed in parallel from a single brain illustrating (A) normal staining of SERT fibers using primary antibody at 1:5000, (B) lack of fiber staining with omission of the primary antibody, (C) lack of fiber staining with omission of the secondary antibody, and (D) lack of fiber staining following preadsorption of the primary antibody at 1: 5000 with the control peptide. All images were normalized to the range of pixel values present.
2.3. Stereological quantification of fiber length density
Serotonergic fiber length density (SFLD) was estimated using the space balls method (Kreczmanski et al., 2009; Calhoun and Mouton, 2001) provided by StereoInvestigator software (MBF Bioscience, Williston, VT) at 60× magnification on a Nikon E800 light microscope. Space ball probes consisted of virtual spheres centered within the thickness of a tissue slice (average thickness of 15–20 µm following immunohistochemical processing). This probe configuration allows for unbiased estimation of linear anatomical structures by avoiding issues of tissue slice and/or structure orientation, and has been used previously to estimate length density of linear neural structures (Calhoun and Mouton, 2001; Shamy et al., 2007). Spheres with a radius of 10 µm (generated by StereoInvestigator while focusing through the section thickness) were systematically and randomly placed every 150 µm within the tissue sections through each IC region of interest with a guard zone of 2.5 µm both between the upper surface of the section and the upper surface of the space ball sphere and between the lower surface of the section and the lower surface of the sphere. This spacing ensured that a minimum of 20 space ball probes were placed within each region of each hemisphere of each tissue sample, and most samples contained many more probes depending upon the area of the IC region within a given tissue slice. SFLD for each IC region was obtained from the total number of intersections between SERT-positive fibers and space ball spheres using the following formula derived from the description of the space balls method in the literature (Kreczmanski et al., 2009; Calhoun and Mouton, 2001):
| (1) |
In which SFLD is serotonergic fiber length density, ∑i represents the sum of intersections between spheres and SERT-positive fibers, Dx and Dy represent the distance between the center of the sphere and the x- axis and y- axes, respectively (75 µm for each), t is the actual average section thickness after histologic processing, r represents the radius of each sphere, and V is the total volume of sampled tissue.
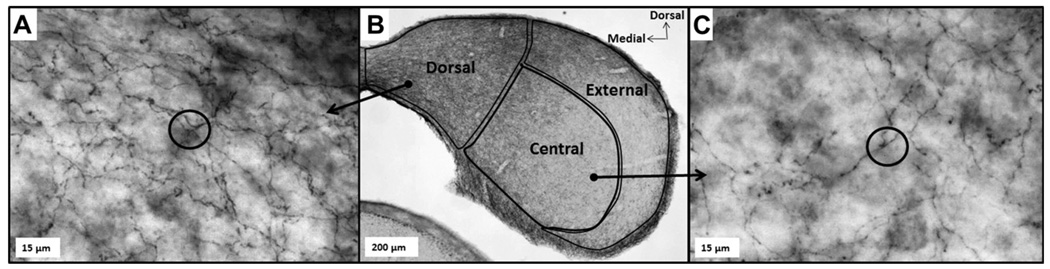
Within each hemisphere, the inferior colliculus (IC) of each brain was divided into three regions for analysis based on stereotaxic mouse brain coordinates (Paxinos and Franklin, 2004). These regions, demonstrated in the center panel of Fig. 2, included the dorsal IC (ICd), the external IC (ICe), and the central nucleus of the IC (ICc). The central nucleus of the IC was further divided into roughly equal regions corresponding to low- and high-frequency responsiveness based on tonotopic maps in mice (Romand and Ehret, 1990). Estimates of SFLD within each IC region were calculated separately for each hemisphere of unilaterally exposed subjects. A demonstration of fiber density analysis using space balls probes is shown in the left and right panels of Fig. 2 for the ‘ICd’ and ‘ICc’ subdivisions, respectively. Space ball probes are represented in each panel as two-dimensional circles seen at one tissue depth. Individual regional density measurements were calculated by averaging the serotonin fiber length density measurements from a minimum of six and a maximum of eight slices for each individual. Samples were evenly spaced from the rostral to caudal pole of each IC to ensure even sampling throughout. Researchers obtaining density measurements were blind as to which subject group each sample belonged. An error analysis, conducted by counting SERT-positive fibers in six of the sample regions twice with random placement of the space ball probes by Stereo-Investigator, gave variation in fiber density estimates of less than 15%.
Fig. 2.
Serotonergic fiber staining in the inferior colliculus (IC). Center panel shows a transverse section of a typical mouse IC demonstrating SERT-positive fiber staining at 4× magnification. The three subdivisions of the IC (dorsal (ICd), external (ICe), and central (ICc)) are superimposed. Notice that the ICd as well as dorsomedial portions of the ICe and ICc regions contain darker staining, indicating a greater density of serotonergic fibers in these areas compared with more ventrolateral areas. Panels on the left and right show SERT-positive fiber staining in areas of the ICd and ICc subdivisions, respectively, at a magnification of 40×. A ‘space ball’ probe has been superimposed in each, appearing as a black 2D circle. Notice that at this plane of focus, SERT-positive fibers cross the perimeter of the space ball probe six times in the area of the ICd, but only three times in the area of the ICc.
2.4. Data analysis
Hemispheric differences in SFLD in unilaterally acoustically traumatized subjects and control subjects were assessed using a repeated measures analysis of variance (ANOVA), treating IC subdivision and hemisphere as within-subjects variables and treatment (trauma or control) as a between-subjects factor. Pearson’s correlations were used to determine whether serotonergic fiber density corresponded to age. Multiple regression was used to assess whether with the effects of trauma correlated with ages at the time of perfusion or trauma. All statistics were performed using SPSS (IBM, Armonk, NY).
3. Results
3.1. Serotonergic fibers in the IC
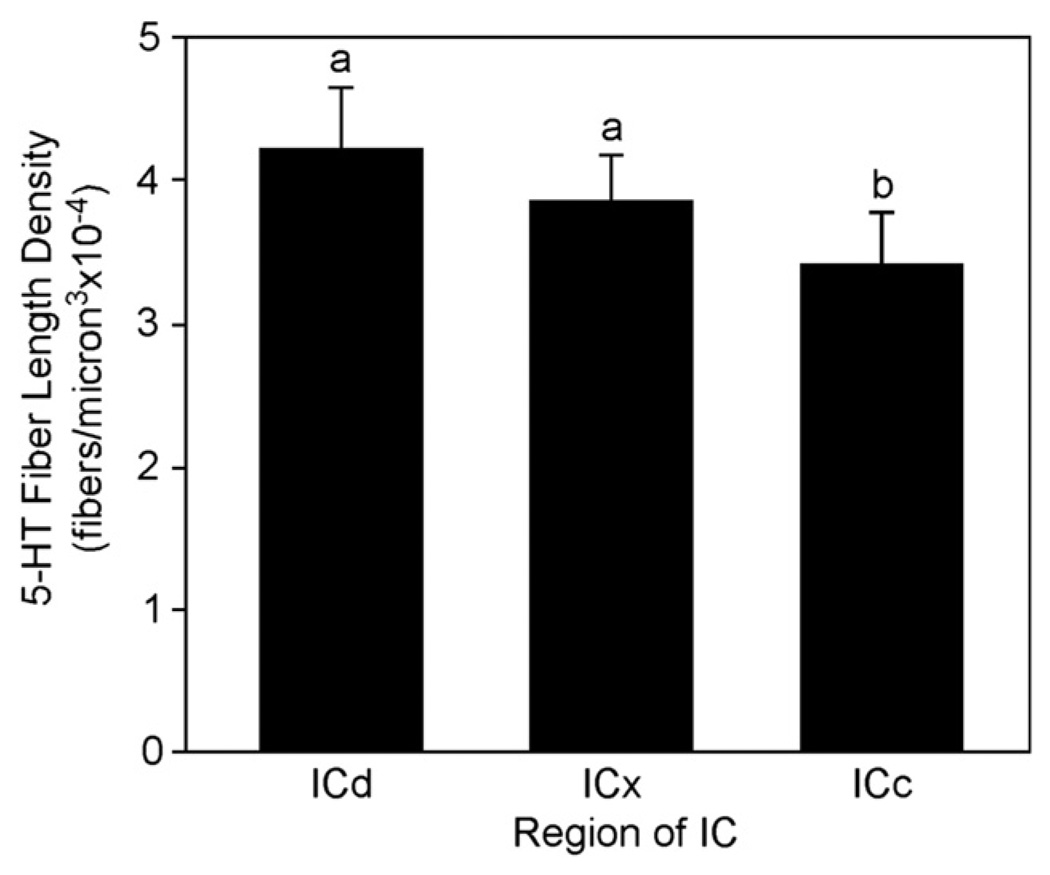
Previous research has provided qualitative evidence that the density of serotonergic innervation varies along a general gradient within the mammalian IC. Although all regions of the IC contain abundant serotonergic fibers, more fibers innervate shell regions, including the dorsal and external cortices, than the central IC (Hurley and Thompson, 2001; Kaiser and Covey, 1997; Klepper and Herbert, 1991; Zeng et al., 2007). Within the central IC, dorsolateral regions have reduced fiber density toward the ventromedial region (Hurley and Thompson, 2001; Kaiser and Covey, 1997; Klepper and Herbert, 1991). The results of the present study quantitatively support these findings. The dorsal region of the IC demonstrated the greatest SFLD, followed by the external region and finally the central region. In control mice, differences in SFLD were significant among all regions (ANOVA with repeated measures: F(2,14) = 6.10, p < 0.05; Bonferroni post-hoc tests, p < 0.05). When both control and traumatized mice were included, the dorsal and external regions were each significantly different from the central region, but not from each other (Fig. 3; ANOVA with repeated measures F(2,34) = 13.51, Bonferroni post-hoc tests p < 0.05).
Fig. 3.
Mean SFLD in the dorsal cortex (ICd), external cortex (ICe), and central IC (ICc) subdivisions in both traumatized and control groups of mice. Densities in ICd and ICx were each significantly different from the central IC, but not from each other. Letters designate the outcome of a repeated measures ANOVA.
3.2. SFLD and unilateral acoustic trauma
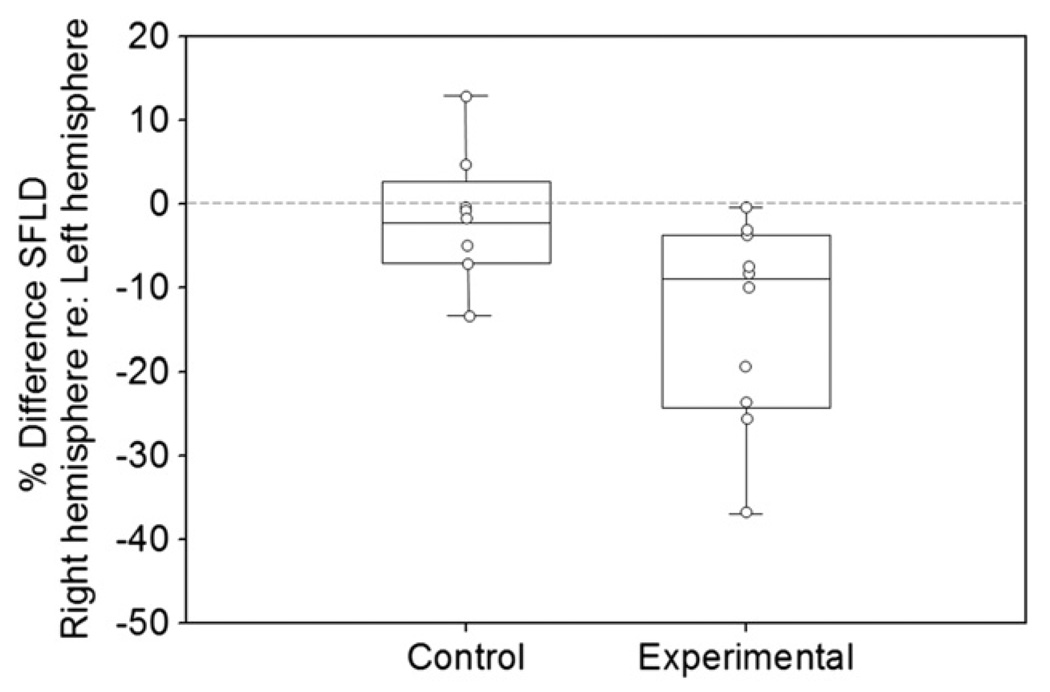
Unilateral trauma caused a significant difference in serotonergic fiber density. SFLD is plotted in Fig. 4 in terms of the percent difference in the right IC compared to the left IC of each individual, with control group data presented on the left and unilaterally traumatized group data shown on the right. A percent difference value of 0 would indicate equal SFLD between the hemispheres, while positive values indicate greater SFLD in the right compared to the left and negative values indicate reduced SFLD in the right compared to the left. Boxes represent the 25th and 75th percentiles, and whiskers represent the 5th and 95th percentiles. Individual data points are displayed as circles within each group. Notice that the SFLD in the right hemisphere of unilaterally traumatized subjects averages approximately 8% lower than the SFLD in the individual’s left IC. Every individual in the experimental subject group demonstrated at least some reduction in SFLD in the right IC compared to the left IC. The hemispheric difference for each individual varied widely, however, ranging from a less than 1% to almost 40% difference between the contralateral and ipsilateral sides (Fig. 4, Table 1). The data were submitted to a repeated-measures ANOVA treating hemisphere and IC region as within-subjects variables and treatment (control vs trauma) as a between-subjects factor. This confirmed that differences in SFLD between hemispheres were greater for the traumatized group than the control group (interaction of side × treatment, F(1,16) = 5.90, Bonferroni post-hoc test p < 0.05). Subdivisions of the IC did not differ from each other in the effect of trauma on SFLD (interaction of region × side × treatment, F(2,15) = 0.216, Bonferroni post-hoc test p > 0.05).
Fig. 4.
Comparison of SFLD between the hemispheres within unilaterally traumatized and control subject groups. Individuals in the unilateral acoustically traumatized group had significantly lower SFLD in the right hemisphere (contralateral to acoustic trauma) compared to the left hemisphere. No significant differences were found between SFLD of the right and left hemispheres of control subjects. Circles represent individual subject values within each group. Boxes represent 25th and 75th percentiles. Whiskers represent 5th and 95th percentiles.
Table 1.
Comparison of hemispheric difference in SFLD between traumatized and control subjects.
| Dorsal (ICd) |
External (ICe) |
Central (ICc) |
Individual Average |
|
|---|---|---|---|---|
| El | −32.90% | −25% | −20.50% | −26.13% |
| E2 | −37.50% | 0% | −33.40% | −23.63% |
| E3 | −9.70% | −19.70% | −0.50% | −9.97% |
| E4 | −38.90% | 0.30% | −18.30% | −18.97% |
| E5 | −38.20% | −31.90% | 44.20% | −38.10% |
| E6 | −1.70% | 1.10% | 0.20% | −0.13% |
| E7 | −1.80% | −7.80% | −2.50% | −4.03% |
| E8 | −11.10% | 0.40% | 1.70% | −3.00% |
| E9 | −1.00% | −11.50% | −11.70% | −8.07% |
| E10 | 0.40% | −9.90% | −10.70% | −6.73% |
| Experimental Group Average | −17.24% | −10.40% | −13.99% | −13.88% |
| Cl | −30.70% | 21.80% | −13.70% | −7.53% |
| C2 | 5.40% | 19.20% | 12.00% | 12.20% |
| C3 | 5.80% | 0.10% | −10.40% | −1.50% |
| C4 | −23.40% | 1.60% | −17.50% | −13.10% |
| C5 | 1.50% | −7.20% | 2.60% | −1.03% |
| C6 | 4.80% | −2.30% | 9.00% | 3.83% |
| C7 | 3.10% | −23.20% | 10.40% | −3.23% |
| C8 | −15.50% | 0.30% | −3.00% | −6.07% |
| Control Group Average | −6.13% | 1.29% | −1.33% | −2.05% |
Since our acoustic traumawas a tone at 8 kHz and mice hear well into the ultrasonic range (Portfors et al., 2009), we assessed whether the trauma differentially affected fiber densities in low- versus high-frequency regions of the central IC in our control versus unilaterally traumatized groups. We did this by dividing the central nucleus into a low- and high-frequency regions based on electrophysiologically derived tonotopic maps in mice (Romand and Ehret, 1990). We found no evidence of interhemispheric differences that diverged between low- and high-frequency regions (repeated measures ANOVA: F(1,16) = 0.289, p = 0.599 for 3-way interaction among treatment group, frequency region, and hemisphere). We conclude that the acoustic trauma did not cause differential effects on fiber densities in low- and high-frequency regions of the central IC.
3.3. Effects of age on SFLD and susceptibility to trauma
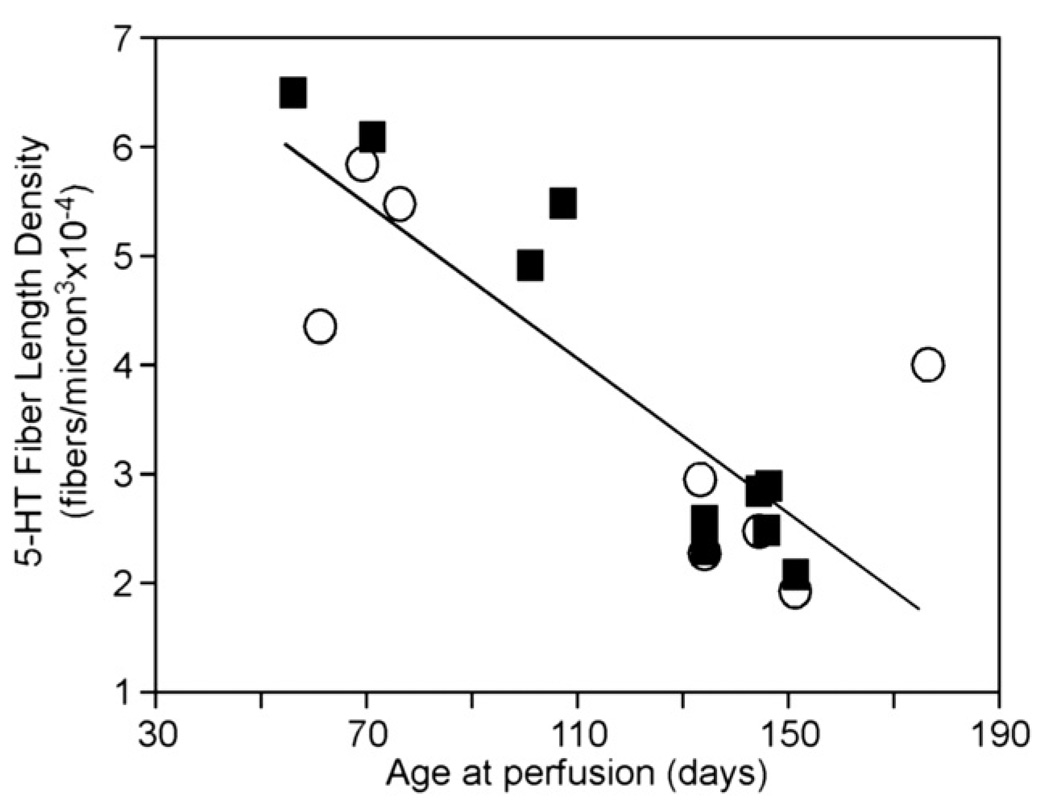
Previous work in our lab has indicated that the serotonergic system is influenced by age, with socially evoked increases in serotonin diminishing up to several months in age (Hall et al., 2011). In the present study, we likewise found that subject age had a strong effect on SFLD (Pearson’s correlation, p < 0.001, r = −0.83). Over a range of age of 55–175 days at the time of perfusion, SFLD diminished by approximately threefold across the traumatized and control groups of mice (Fig. 5). The factor of age thus accounts for a substantial degree of interindividual variability in the density of serotonergic fibers in the mice in our study.
Fig. 5.
Age-related decline in serotonergic fiber density. Age is represented in days at the time of perfusion. Circles represent untraumatized controls, and squares represent unilaterally traumatized animals. Serotonin fiber length density is averaged for all subdivisions of both colliculi.
Because the serotonergic fiber system was sensitive to age, we also assessed whether sensitivity to trauma also varied with age by comparing the ratio of contralateral versus ipsilateral SFLD in the mice with unilateral trauma to their ages at the time of trauma and the time of perfusion (Table 2). These comparisons were not significant (multiple regression, p > 0.05), suggesting that the serotonergic system is capable of change in both the youngest and oldest subjects in our study. Another factor which may have affected bilateral differences in SFLD was the duration of subject recovery following acoustic exposure (Table 2). However, no significant relationship existed (Pearson’s correlation, p > 0.05), indicating that bilateral differences in SFLD in the unilaterally traumatized subjects were present at three weeks post-trauma and persisted for up to sixteen weeks.
Table 2.
Comparison of age and SFLD in all mice.
| Subject ID | Age at trauma (days) |
Recovery (days) |
Age at perf (days) |
Avg SFLD (fibers/ µm3 × 10−4 |
|---|---|---|---|---|
| Trauma | ||||
| E1 | 95 | 38 | 133 | 2.64 |
| E2 | 95 | 38 | 133 | 2.40 |
| E3 | 95 | 48 | 143 | 2.91 |
| E4 | 95 | 55 | 150 | 2.14 |
| E5 | 65 | 80 | 145 | 2.96 |
| E6 | 65 | 80 | 145 | 2.57 |
| E7 | 42 | 28 | 70 | 6.15 |
| E8 | 42 | 13 | 55 | 4.98 |
| E9 | 57 | 43 | 100 | 6.55 |
| E10 | 43 | 63 | 106 | 5.54 |
| Control | ||||
| C1 | 95 | 38 | 133 | 2.39 |
| C2 | 95 | 48 | 143 | 2.54 |
| C3 | 95 | 55 | 150 | 1.98 |
| C4 | 65 | 80 | 175 | 4.08 |
| C5 | 60 | 0 | 60 | 4.44 |
| C6 | 35 | 33 | 68 | 5.91 |
| C7 | 48 | 27 | 75 | 5.55 |
| C8 | 60 | 72 | 132 | 3.03 |
4. Discussion
We have shown that when mice are subjected to unilateral acoustic trauma, the IC contralateral to the traumatized ear has a lowered density of serotonergic projections than the ipsilateral IC. To our knowledge, this is the first study indicating that serotonergic projections within the CAS are capable of plasticity following damage to the auditory periphery. The lateralized change in serotonergic fibers that we observed is comparable to lateralized changes in other neurotransmitter systems in the IC following unilateral trauma, including multiple features of inhibitory neurotransmitter systems (Dong et al., 2010b; Vale et al., 2004). In the following discussion we address the potential implications of our findings for serotonergic involvement in auditory plasticity and dysfunction.
4.1. Regional differences in serotonergic fiber density in the IC
The densities of serotonergic fibers were significantly different among subregions of the IC, confirming previous qualitative reports of a higher density of fibers in the peripheral regions of the IC compared to the central nucleus in a wide range of species (reviewed in Hurley and Hall, 2011). In the current study, the dorsal cortex contained the highest fiber density, followed by the external cortex and the central nucleus of the IC. Although differences among subregions were significant, they were relatively small (Fig. 3), supporting a widespread influence of serotonin in all subdivisions of the IC.
Despite the differences in fiber density among subdivisions of the IC, acoustic trauma did not have different effects among subdivisions. Within the central nucleus of the IC, we separately analyzed the effects of trauma on two broad tonotopic regions, based on the rationale that we used a tonal acoustic trauma. However, there was no difference in the effect of trauma in relatively low-frequency and relatively high-frequency regions. The lack of a tonotopic difference is not especially surprising, given that there is no evidence for tonotopic organization of serotonergic projections from their origin in raphe nuclei to the inferior colliculus.
4.2. Age-related changes in serotonergic fiber density
An additional and previously unreported dimension of variability in the densities of serotonergic fibers in the IC related to age. A substantial decline in fiber density on the order of threefold occurred over a range of age at the time of perfusion from approximately 8–25 weeks. We have previously observed an interesting parallel in a functional measure of the serotonergic system. In male mice presented with an intruder, serotonin increases locally within the IC (Hall et al., 2011). This serotonergic response is significantly related to age, declining over the course of 7–18 weeks. Previous measurements of the tissue content of serotonin and its metabolite in another auditory nucleus, the anteroventral cochlear nucleus (AVCN) have shown an increase in rats of 21–24 months of age relative to younger rats (Cransac et al., 1996), so the serotonergic system may not stabilize with adulthood, but instead continue to change with increasing age.
Although we demonstrated age-related plasticity, it did not interact with plasticity due to trauma in our modestly sized group of 10 mice undergoing trauma. The size of the disparity in fiber density did not significantly correlate with the age at trauma or at perfusion. Moreover, the effect of trauma on fiber density did not correspond to the amount of time allowed for recovery, suggesting that the plasticity we observed in serotonergic fiber density had stabilized by the time we measured it. This reflects the timecourse of other types of plasticity induced by acoustic trauma such as threshold or the expression of GABA receptor subunits. These changes stabilize several weeks after the exposure to acoustic trauma (Dong et al., 2010a; Li, 1992; Salvi et al., 1990).
4.3. Features of plasticity following unilateral trauma
Although we demonstrated a significant alteration in serotonergic fiber density in the IC following trauma, several features of this difference, particularly those related to the lateralization of changes in fiber density, remain unexplored. Most directly, the proximate cause of differences we observed between contralateral colliculi following unilateral trauma are unclear. Differences may have resulted from a decrease in innervation in the IC contralateral to trauma, an increase in innervation in the IC ipsilateral to trauma, or a combination of both. In comparison, investigations of plasticity within other sensory systems have suggested that serotonin is downregulated in response to chronic deprivation of sensory input (Qu et al., 2000). A second set of issues relates to the extent of the trauma-evoked serotonergic changes. It is possible, for example, that similar changes occur in other auditory regions. Whether events at the level of raphe neurons play a role in the changes we observed, or whether alterations in serotonergic projections occur in nonauditory brain regions innervated by raphe neurons, also remain to be explored. Finally, we did not investigate functional correlates of changes in serotonergic fiber density in this study. Although the lateralized plasticity in fiber density followed from acoustic trauma, we did not measure changes in threshold unilaterally. Thus, we cannot assess whether the severity of change in fiber density is directly related to the degree of change in threshold.
4.4. Functional implications for the role of serotonin in sensory plasticity
A change in the density of serotonergic fibers has the potential to alter auditory processing in a number of ways. A change in the release of serotonin could directly modulate intrinsic excitability and synaptic properties in the IC, interact with trauma-evoked changes in other neurotransmitter systems, or trigger upstream gating of plasticity. Here we briefly discuss these possibilities.
In the IC, serotonin influences the rate of spontaneous firing and both the rate and timing of evoked responses of single neurons in a range of ways. The effect of serotonin on a given neuron is determined in part by the type of receptor mediating its effects. Different types of serotonin receptors in the IC may regulate intrinsic excitability (Hurley, 2006, 2007), alter the release of GABA (Hurley et al., 2008), or contribute to activity-dependent plasticity (Bohorquez and Hurley, 2009; Hall et al., 2010). However, a major effect of serotonin is to decrease the responsiveness of IC neurons, reducing both spontaneous and evoked activity (Hall and Hurley, 2007; Hurley and Pollak, 1999). If serotonin availability corresponds to fiber density, a consequence of decreased serotonergic fiber density could therefore be an increased level of spontaneous and evoked neural activity following acoustic trauma. Since serotonin levels are relatively elevated in the IC of awake animals and during stressful events (Hall et al., 2010), the effects of altered fiber density could be highest in these situations.
The density changes in the serotonergic system we observed may also interact with other responses to acoustic trauma, including those in additional neurotransmitter systems as well as modulation of the serotonergic system at the receptor level. For example, bilateral deafening yields persistent downregulation of 5-HT5B receptor expression and upregulation of the 5-HT2C receptor in the mouse IC (Holt et al., 2005). In addition, the altered fiber density we observed suggests that the availability of serotonin transporters were altered, something that could also influence the amplitude or timing of serotonin availability. Peripheral damage leads to changes in other neurotransmitter systems within the IC such as the GABAergic and dopaminergic systems (Milbrandt et al., 2000; Mossop et al., 2000; Tong et al., 2005). The serotonergic system exerts mutual influence on each of these systems (Hurley et al., 2008; Peruzzi and Dut, 2004; Prisco et al., 1994; Smith et al., 1997), raising the possibility that interactions among neurotransmitter systems may influence the effect of the serotonergic system on auditory processing following acoustic trauma.
A final possibility is that serotonin may gate plasticity following trauma. In the visual system, exogenously increasing serotonin levels, or providing sensorimotor enrichment that leads to an increase in serotonin, can activate ocular dominance plasticity following monocular deprivation in adults (Baroncelli et al., 2010; Vetencourt et al., 2008;). Previous authors have proposed that serotonin similarly acts by triggering plasticity in the adult auditory system via multiple mechanisms (Tadros et al., 2007). Thus, serotonin could potentially play an upstream role in regulating plasticity in other neurotransmitter systems in the IC, but this possibility has been poorly explored in the auditory system.
4.5. Serotonin and tinnitus
Our findings are consistent with an existing hypothesis on the involvement of serotonin in the generation of tinnitus following acoustic trauma (Marriage and Barnes, 1995). Most tinnitus etiologies involve loss of input from the auditory periphery to the CAS, such as found in cases of ototoxicity, auditory nerve or cochlear ablation, acoustic trauma, noise-induced hearing loss, and presbycusis (Eggermont and Roberts, 2004). In these cases, loss of normal excitation provided by input from the periphery leads to a reduction of inhibitory neurotransmission in corresponding CAS areas (Szczepaniak and Mǿller, 1995; Abbott et al., 1999). This, in turn, is believed to cause hyperexcitability in CAS structures (Salvi et al., 2000; Mǿller, 2006; Bartels et al., 2007; Schaette and Kempter, 2006) which is perceived as tinnitus. Serotonin has been proposed to play an intermediary role in this process, expressing plasticity following acoustic trauma and regulating the balance between excitation and inhibition in central auditory circuits (Marriage and Barnes, 1995). The results we have presented here support the hypothesis that the serotonergic systemdoes express plasticity even in response to relatively narrow-band acoustic trauma.
Support for this model is found in another uniquely reversible type of tinnitus is that arising from high doses of sodium salicylate. In the CAS, sodium salicylate reduces release of inhibitory neurotransmitters such as GABA (Wang et al., 2006), thereby increasing spontaneous firing rates in the IC (Basta and Ernst, 2005) and secondary auditory cortex (Eggermont and Kenmochi, 1998) and increasing the amplitude of local field potentials recorded from the auditory cortex (Yanget al., 2007). Such effects are reminiscent of the long term effects of peripheral damage on CAS activity. In the case of sodium salicylate, the normal increase in GABAergic transmission found in response to the presence of serotonin in the IC is suppressed following administration of sodium salicylate (Wang et al., 2008). This finding implies that at least a portion of the effects of sodium salicylate and resulting tinnitus are controlled via modulation of serotonin and its effects on GABAergic neurotransmission.
5. Summary and conclusions
In summary, the results of the present study indicate that pure tone acoustic trauma is capable of producing significant changes in the density of serotonergic fibers innervating the IC contralateral to the ear of damage. This finding helps to bolster the conclusion of previous studies that serotonin is involved in modulating the activity of sensory cells following persistent changes in sensory input. Serotonin may therefore play a role in the generation of pathologies related to auditory plasticity such as tinnitus. It remains to be seen whether similar changes in serotonergic innervation occur in response to other types of insults to the auditory system, such as exposure to ototoxic drugs or auditory nerve transection. If serotonin is involved in modulating auditory plasticity in response to damage, further study of its effects may lead to improved understanding of central auditory response to peripheral damage including the potential for treatment of auditory disorders such as tinnitus and hyperacusis.
Acknowledgments
The authors would like to thank Abby Howenstein and Katherine Knisely for their help in running experiments and with data analysis. We would also like to thank Robert Withnell and William Shofner their time, expertise, and helpful comments on the manuscript. This project is supported by a grant from Indiana University’s Faculty Research Support Program.
Abbreviations
- CAS
central auditory system
- IC
inferior colliculus
- ABR
auditory brainstem response
- SERT
serotonin reuptake transporter
- SFLD
serotonergic fiber length density
References
- Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience. 1999;93(4):1375–1381. doi: 10.1016/s0306-4522(99)00300-0. [DOI] [PubMed] [Google Scholar]
- Baroncelli L, Sale A, Viegi A, Vetencourt JFM, De Pasquale R, Baldini S, Maffei L. Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp. Neurol. 2010;226(1):100–109. doi: 10.1016/j.expneurol.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Barsz K, Wilson WW, Walton JP. Reorganization of receptive fields following hearing loss in inferior colliculus neurons. Neuroscience. 2007;147(2):532–545. doi: 10.1016/j.neuroscience.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels H, Staal MJ, Albers FWJ. Tinnitus and neural plasticity of the brain. Otol. Neurotol. 2007;28:178–184. doi: 10.1097/MAO.0b013e31802b3248. [DOI] [PubMed] [Google Scholar]
- Basta D, Ernst A. Erratum to “Noise-induced changes of neuronal spontaneous activity in mice inferior colliculus brain slices. Neurosci. Lett. 2005;374:74–79. doi: 10.1016/j.neulet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system. Neuroreport. 1995;7:225–229. [PubMed] [Google Scholar]
- Bohorquez A, Hurley LM. Activation of serotonin 3 receptors changes in vivo auditory responses in the mouse inferior colliculus. Hear. Res. 2009;251(1–2):29–38. doi: 10.1016/j.heares.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Mouton PR. Length measurement: new developments in neurostereology and 3D imagery. J. Chem. Neuroanat. 2001;21:257–265. doi: 10.1016/s0891-0618(01)00093-x. [DOI] [PubMed] [Google Scholar]
- Coles RB, Gower DM, Boyd PJ, Lewis DB. Acoustic transmission through the head of the common mole, Talpa europaea. J. Exp. Biol. 1982;101:337–341. doi: 10.1242/jeb.101.1.337. [DOI] [PubMed] [Google Scholar]
- Cransac H, Peyrin L, Cottet-Emard JM, Farhat F, Pequignot JM, Reber A. Aging effects on monoamines in rat medial vestibular and cochlear nuclei. Hear Res. 1996;100(1–2):150–156. doi: 10.1016/0378-5955(96)00116-5. [DOI] [PubMed] [Google Scholar]
- Davis RI, Ahroon WA, Hamernik RP. The relation among hearing loss, sensory cell loss and tuning characteristics in the chinchilla. Hear Res. 1989;41:1–14. doi: 10.1016/0378-5955(89)90173-1. [DOI] [PubMed] [Google Scholar]
- Davis M, Strachan DI, Kass E. Excitatory and inhibitory effects of serotonin on sensorimotor reactivity measured with acoustic startle. Science. 1980;209(4455):521–523. doi: 10.1126/science.7394520. [DOI] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity in gene expression in guinea-pig auditory brainstem. Eur. J. Neurosci. 2010a;31:16–28. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Dong S, Rodger J, Mulders WH, Robertson G. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res. 2010b;1342:24–32. doi: 10.1016/j.brainres.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Kenmochi M. Salicylate and quinine selectively increase spontaneous firing rates in secondary auditory cortex. Hear Res. 1998;117(1–2):149–160. doi: 10.1016/s0378-5955(98)00008-2. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ, Roberts LE. The neuroscience or tinnitus. Trends Neurosci. 2004;27(11):676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Eldredge DH, Mills JH, Bohne BA. Anatomical, behavioral, and electrophysiological observations on chinchillas after long exposures to noise. Adv. Otorhinolaryngol. 1973;20:64–81. doi: 10.1159/000393089. [DOI] [PubMed] [Google Scholar]
- Esaki T, Cook M, Shimoji K, Murphy DL, Sokoloff L, Holmes A. Developmental disruption of serotonin transporter function impairs cerebral responses to whisker stimulation in mice. Proc. Natl. Acad. Sci. U S A. 2005;102(15):5582–5587. doi: 10.1073/pnas.0501509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Rebec GV, Hurley LM. Serotonin in the inferior colliculus fluctuates with behavioral state and environmental stimuli. J. Exp. Biol. 2010;213:1009–1017. doi: 10.1242/jeb.035956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Sell GL, Hurley LM. Social regulation of serotonin in the auditory midbrain. Behav. Neurosci. 2011;125:501–511. doi: 10.1037/a0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall IC, Hurley LM. The serotonin releaser fenfluramine alters the auditory responses of inferior colliculus neurons. Hear Res. 2007;228(1–2):82–94. doi: 10.1016/j.heares.2007.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt AG, Asako M, Lomax CA, MacDonald JW, Tong L, Lomax MI, Altschuler RA. Deafness-related plasticity in the inferior colliculus: gene expression profiling following removal of peripheral activity. J. Neurochem. 2005;93:1069–1086. doi: 10.1111/j.1471-4159.2005.03090.x. [DOI] [PubMed] [Google Scholar]
- Hurley LM. Different serotonin receptor agonists have distinct effects on sound-evoked responses in inferior colliculus. J. Neurophysiol. 2006;96:2177–2188. doi: 10.1152/jn.00046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM. Activation of the serotonin 1A receptor alters the temporal characteristics of auditory responses in the inferior colliculus. Brain Res. 2007;1181:21–29. doi: 10.1016/j.brainres.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Hall IC. Context-dependent modulation of auditory processing by serotonin. Hear. Res. 2011;279(1–2):74–84. doi: 10.1016/j.heares.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J. Neurosci. 1999;9(18):8071–8082. doi: 10.1523/JNEUROSCI.19-18-08071.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J. Neurophysiol. 2001;85:828–842. doi: 10.1152/jn.2001.85.2.828. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Thompson AM. Serotonergic innervation of the auditory brainstem of the Mexican free-tailed, bat, Tadarida brasiliensis. J. Comp. Neurol. 2001;435(1):78–88. doi: 10.1002/cne.1194. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Tracy JA, Bohorquez A. Serotonin 1B receptor modulates frequency response curves and spectral integration in the inferior colliculus by reducing GABAergic inhibition. J. Neurophysiol. 2008;100(3):1656–1667. doi: 10.1152/jn.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Stevens KE, Rose GM. 5-Hydroxytryptamine2 receptors modulate auditory filtering in the rat. J. Pharmacol. Exp. Ther. 1998;285(2):643–650. [PubMed] [Google Scholar]
- Kaiser A, Covey E. 5-HT innervation of the auditory pathway in birds and bats. Acoustical Signal processing in the central auditory system, Syka. J. Science, New York. 1997:71–78. [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kelly NH, Reger SN. The effect of binaural occlusion of the external auditory meati on the sensitivity of the normal ear for bone conducted sound. J. Exp. Psychol. 1937;21:211–221. [Google Scholar]
- Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res. 1991;557(1–2):190–201. doi: 10.1016/0006-8993(91)90134-h. [DOI] [PubMed] [Google Scholar]
- Komiya H, Eggermont JJ. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120:750–756. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- Kreczmanski P, Heinsen H, Mantua V, Woltersdorf F, Masson T, Ulfig N, Schmidt-Kastner R, Korr H, Steinbusch HWM, Hof PR, Schmitz C. Microvessel length density, total length, and length per neuron in five subcortical regions in schizophrenia. Acta Neuropathol. 2009;117:409–421. doi: 10.1007/s00401-009-0482-7. [DOI] [PubMed] [Google Scholar]
- Li HS. Influence of genotype and age on acute acoustic trauma and recovery in CBA/Ca and C57BL/6J mice. Acta Otolaryngol. 1992;112:956–967. doi: 10.3109/00016489209137496. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW. Single-neuron labeling and chronic cochlear pathology. II. Stereocilia damage and alterations of spontaneous discharge rates. Hear Res. 1984;6(1):43–53. doi: 10.1016/0378-5955(84)90024-8. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NYS. Acoustic trauma in cats: cochlear pathology and auditory-nerve activity. Acta Otolaryngol. 1978;358:5–63. [PubMed] [Google Scholar]
- Lombion S, Morand-Villeneuve N, Millot J. Effects of anti-depressants on olfactory sensitivity in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2007;32:629–632. doi: 10.1016/j.pnpbp.2007.10.021. [DOI] [PubMed] [Google Scholar]
- Ma WD, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF Promotes the Regenerative Sprouting, but not Survival, of Injured serotonergic axons in the adult rat brain. J. Neurosci. 2000;20(2):771–782. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriage J, Barnes NM. Is central hyperacusis a symptom of 5-hydroxytryptamine 5-HT dysfunction? J. Laryngol. Otol. 1995;109:915–921. doi: 10.1017/s0022215100131676. [DOI] [PubMed] [Google Scholar]
- Melichar I, Syka J, Ulehlová L. Recovery of the endocochlear potential and the K+ concentrations in the cochlear fluids after acoustic trauma. Hear Res. 1980;2(1):55–63. doi: 10.1016/0378-5955(80)90016-7. [DOI] [PubMed] [Google Scholar]
- Michler SA, Illing R. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein GAP-43 in the rat auditory brainstem. J. Comp. Neurol. 2002;451(3):250–266. doi: 10.1002/cne.10348. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147(1–2):251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Mǿller A. Neural plasticity in tinnitus. Prog. Brain Res. 2006;157:365–372. doi: 10.1016/S0079-6123(06)57022-0. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147(1–2):183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Nathan PJ, Segrave R, Phan KL, O’Neill B, Croft RJ. Direct evidence that acutely enhancing serotonin with the selective serotonin reuptake inhibitor citalopram modulates the loudness dependence of the auditory evoked potential (LDAEP) marker of central serotonin function. Hum. Psychopharmacol. Clin. Exp. 2006;21(1):47–52. doi: 10.1002/hup.740. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183(1–2):137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Normann C, Schmitz D, Fürmaier A, Döing C, Bach M. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol. Psychiatry. 2007;62:373–380. doi: 10.1016/j.biopsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Imbe H, Morikawa Y, Itch M, Sekimoto M. 5-HT2A receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain. 2002;99:133–143. doi: 10.1016/s0304-3959(02)00070-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. New York: Elsevier Science; 2004. [Google Scholar]
- Peruzzi D, Dut A. GABA, serotonin and serotonin receptors in the rat inferior colliculus. Brain Res. 2004;998(2):247–250. doi: 10.1016/j.brainres.2003.10.059. [DOI] [PubMed] [Google Scholar]
- Prisco S, Pagannone S, Esposito E. Serotonin-dopamine interaction in the rat ventral tegmental area: an electrophysiological study in vivo. J. Pharmacol. 1994;271:83–90. [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009;162:486–500. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Qu Y, Eyse UT, Vandesande F, Arckens L. Effect of partial sensory deprivation on monoaminergic neuromodulators in striate cortex of adult cat. Neuroscience. 2000;101(4):863–868. doi: 10.1016/s0306-4522(00)00441-3. [DOI] [PubMed] [Google Scholar]
- Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. 2002;164:206–214. doi: 10.1016/s0378-5955(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Romand R, Ehret G. Development of tonotopy in the inferior colliculus. I. Electrophysiological mapping in house mice. Dev. Brain Res. 1990;54(2):221–234. doi: 10.1016/0165-3806(90)90145-o. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Wang J, Ding D. Auditory plasticity and hyperactivity following cochlear damage. Hear Res. 2000;147:261–274. doi: 10.1016/s0378-5955(00)00136-2. [DOI] [PubMed] [Google Scholar]
- Salvi RJ, Saunders SS, Gratton MA, Arehole S, Powers N. Enhanced evoked response amplitudes in the inferior colliculus of the chinchilla following acoustic trauma. Hear Res. 1990;50:245–257. doi: 10.1016/0378-5955(90)90049-u. [DOI] [PubMed] [Google Scholar]
- Salvinelli F, Casale M, Paparo F, Persico AM, Zini C. Subjective tinnitus, temporomandibular joint dysfunction, and serotonin modulation of neural plasticity: causal or casual triad? Med. Hypotheses. 2003;61:446–448. doi: 10.1016/s0306-9877(03)00194-4. [DOI] [PubMed] [Google Scholar]
- Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur. J. Neurosci. 2006;23:3124–3138. doi: 10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- Shamy JL, Buckmaster CA, Amaral DG, Calhoun ME, Rapp PR. Reactive plasticity in the dentate gyrus following bilateral entorhinal cortex lesions in cynomolgus monkeys. J. Comp. Neurol. 2007;502(2):192–201. doi: 10.1002/cne.21313. [DOI] [PubMed] [Google Scholar]
- Simpson JJ, Davies WE. A review of evidence in support of a role for 5-HT in the perception of tinnitus. Hear Res. 2000;145:1–7. doi: 10.1016/s0378-5955(00)00093-9. [DOI] [PubMed] [Google Scholar]
- Smith GS, Dewey SL, Brodie JD, Logan J, Vitkun SA, Simkowitz P, Schloesser R, Alexoff DA, Hurley A, Cooper T, Volkow ND. Serotonergic modulation of dopamine measured with [11C]raclopride and PET in normal human subjects. Am. J. Psychiatry. 1997;154:490–496. doi: 10.1176/ajp.154.4.490. [DOI] [PubMed] [Google Scholar]
- Sohmer H, Kinarti R, Gafni M. The source along the basilar membrane of the cochlear microphonic potential recorded by surface electrodes in man. Electroencephalogr. Clin. Neurophysiol. 1980;49:506–514. doi: 10.1016/0013-4694(80)90393-4. [DOI] [PubMed] [Google Scholar]
- Sugisawa T, Ishida A, Hotta S, Yamamura K. The effect of 6 kHz tone exposure on inner ear function of the guinea pig: relation to changes in cochlear microphonics, action potential, endocochlear potential and chemical potentials of K+-ions and Na+-ions, using a double-barrel glass electrode. Eur. Arch. Otorhinolaryngol. 1994;251(3):154–159. doi: 10.1007/BF00181827. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Potashner SJ, Benson CG. Plastic changes in Glycine and GABA release and uptake in adult brain Stem auditory nuclei after unilateral Middle ear Ossicle removal and cochlear ablation. Exp. Neurol. 1998;151:273–288. doi: 10.1006/exnr.1998.6812. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Mǿller AR. Evidence of neuronal plasticity within the inferior colliculus after noise exposure: a study of evoked potentials in the rat. Electroencephalogr. Clin. Neurophysiol. 1996;100(2):158–164. doi: 10.1016/0013-4694(95)00234-0. [DOI] [PubMed] [Google Scholar]
- Szczepaniak WS, Mǿller AR. Evidence of decreased GABAergic influence on temporal integration in the inferior colliculus following acute noise exposure: a study of evoked potentials in the rat. Neurosci. Lett. 1995;196(1–2):77–80. doi: 10.1016/0304-3940(95)11851-m. [DOI] [PubMed] [Google Scholar]
- Tadros S, D’Souza M, Zettel M, Zhu X, Lynch-Erhardt M, Frisina R. Serotonin 2B receptor: upregulated with age and hearing loss in mouse auditory system. Neurobiol. Aging. 2007;28:1112–1123. doi: 10.1016/j.neurobiolaging.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Tan J, Rüttiger L, Panford-Walsh A, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Köpschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Thompson AM, Hurley LM. Dense serotonergic innervation of principal nuclei of the superior olivary complex in mouse. Neurosci. Lett. 2004;356:179–182. doi: 10.1016/j.neulet.2003.11.052. [DOI] [PubMed] [Google Scholar]
- Thompson GC, Thompson AM, Garrett KM, Britton BH. Serotonin and serotonin receptors in the central auditory system. Otolaryngol. Head Neck Surg. 1994;110:93–102. doi: 10.1177/019459989411000111. [DOI] [PubMed] [Google Scholar]
- Tong L, Altschuler RA, Holt AG. Tyrosine hydroxylase in rat auditory midbrain: distribution and changes following deafness. Hear Res. 2005;206:28–41. doi: 10.1016/j.heares.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur. J. Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Juíz JM, Moore DR, Sanes DH. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. Eur. J. Neurosci. 2004;20:2133–2140. doi: 10.1111/j.1460-9568.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo B, Zhou K, Xu T, Chen L. Sodium salicylate reduces inhibitory postsynaptic currents in neurons of rat auditory cortex. Hear Res. 2006;215:77–83. doi: 10.1016/j.heares.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo B, Huang Y, Zhou K, Chen L. Sodium salicylate suppresses serotonin-induced enhancement of GABAergic spontaneous inhibitory postsynaptic currents in rat inferior colliculus in vitro. Hear Res. 2008;236:42–51. doi: 10.1016/j.heares.2007.11.015. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Salvi RJ. Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage. Hear Res. 2002;168:238–249. doi: 10.1016/s0378-5955(02)00360-x. [DOI] [PubMed] [Google Scholar]
- Willott JF, Henry KR. Auditory evoked potentials: developmental changes of threshold and amplitude following early acoustic trauma. J. Comp. Physiol. Psychol. 1974;86:1–7. doi: 10.1037/h0035922. [DOI] [PubMed] [Google Scholar]
- Willott JF, Lu SM. Noise-induced hearing loss can alter neural coding and increase excitability in the central nervous system. Science. 1982;216:1331–1334. doi: 10.1126/science.7079767. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Zeng S, Li J, Zhang X, Zuo M. Distinction of neurochemistry between the cores and their shells of auditory nuclei in tetrapod species. Brain Behav. Evol. 2007;70:1–20. doi: 10.1159/000101066. [DOI] [PubMed] [Google Scholar]