Abstract
Surgery is the most effective therapy for cancer in the United States, but disease still recurs in more than 40% of patients within 5 years after resection. Chemotherapy is given postoperatively to prevent relapses; however, this approach has had marginal success. After surgery, recurrent tumors depend on rapid neovascular proliferation to deliver nutrients and oxygen. Phosphatidylserine (PS) is exposed on the vascular endothelial cells in the tumor microenvironment but is notably absent on blood vessels in normal tissues. Thus, PS is an attractive target for cancer therapy after surgery. Syngeneic mice bearing TC1 lung cancer tumors were treated with mch1N11 (a novel mouse chimeric monoclonal antibody that targets PS), cisplatin (cis), or combination after surgery. Tumor relapses and disease progression were decreased 90% by combination therapy compared with a 50% response rate for cis alone (P = .02). Mice receiving postoperative mch1N11 had no wound-related complications or added systemic toxicity in comparison to control animals. Mechanistic studies demonstrated that the effects of mch1N11 were associated with a dense infiltration of inflammatory cells, particularly granulocytes. This strategy was independent of the adaptive immune system. Together, these data suggest that vascular-targeted strategies directed against exposed PS may be a powerful adjunct to postoperative chemotherapy in preventing relapses after cancer surgery.
Introduction
Surgery is the best option for cure in most solid tumors in the United States; however, this treatment has a 40% to 60% recurrence rate depending on the type of cancer [1]. For example, in non-small cell lung cancer (NSCLC), more than 50% of patients with resectable stage I to IIIA cancer will have recurrence after surgery. Many of these recurrences occur from residual tumor deposits in the resection margins, which rapidly proliferate after removal of the primary tumor. As a consequence, cisplatin (cis) chemotherapy is typically administered as an adjuvant after surgery to prevent relapses [2]. However, this approach improves the 5-year survival rate by a marginal 4% [3].
Most clinically effective adjuvant anticancer therapies are based on their ability to directly kill dividing tumor cells as they begin to proliferate. However, therapies that target other cells within the proliferating tumor deposits in the local wound after surgery are now being developed. For example, compared with the normal tissue beds, the vasculature in tumors exhibits an increased rate of proliferation, structural differences, and expression of unique genes and gene products [4,5]. The tumor endothelium has been successfully targeted by drugs that inhibit angiogenesis. The efficacy of an anti-vascular endothelial growth factor (VEGF) antibody to inhibit tumor angiogenesis has been shown in colon and lung cancer [6–8]. Another category of drugs classified as vascular-disrupting agents have also been developed that can specifically affect established tumor vessels [9,10]. The agent used in this study, a phosphatidylserine (PS)-targeting antibody, also binds to and destroys the tumor vasculature.
PS is the most abundant anionic phospholipid of the plasma membrane and is tightly segregated to the inner leaflet of the plasma membrane in most mammalian cells. However, PS expression is significantly increased on tumor-associated blood vessels and is externalized in 15% to 40% of tumor vessel endothelia [10]. PS is notably absent from the vasculature of normal tissues [11,12]. The tumor microenvironment has hypoxic and acidic conditions that are rich in cytokines, leukocytes, thrombin, metabolites, and reducing/oxidizing factors that may explain the high expression of PS on vessels penetrating carcinomas [10–12]. These stresses elicit the production of reactive oxygen species, which may oxidize membrane phospholipids and generate calcium fluxes that inhibit the ATP-dependent transporter aminophospholipid translocase and/or activate PS-exporting enzymes [13]. In this study, we investigate the use of PS as a target on the endothelial cells of new vessels that develop in recurrent tumors as they begin to proliferate after surgery.
Mch1N11 is a novel mouse chimeric immunoglobulin G 2A antibody that binds to and stabilizes complexes of PS and the PS-binding plasma protein, β2-glycoprotein I. The antibody only binds to cell surfaces on which PS is exposed. One of the functions of exposed PS is to silence unwanted inflammatory responses and immune responses against normal cells undergoing apoptosis at the end of their natural life span. However, in tumors, exposed PS has anti-inflammatory and immunosuppressive actions that dampen the host's ability to control tumor growth. Treatment with PS-targeting antibodies thus evokes an antitumor inflammatory response. Previous studies have shown that PS-targeting antibodies are effective against pancreatic, breast, and lung cancers [13–15]. Furthermore, it has been shown in murine models that PS-targeting antibodies are most effective when combined with radiation and/or chemotherapy (i.e., docetaxel, gemcitabine) [13–15]. The PS-targeting antibody, bavituximab, has completed phase 1 studies [10,16] and is currently in randomized phase 2 trials in patients with lung and pancreatic cancers. To our knowledge, the present study is the first to use a PS-targeting antibody in an adjuvant setting when there is a rapidly changing vascular bed around tumor deposits after surgery.
In this project, we examined whether a PS-targeting antibody can be combined with cis after surgery to improve outcomes. We postulated that this combination of cis/mch1N11 would be uniquely potent after surgery owing to several mechanistic advantages. First, cis is the standard of care for lung cancer after surgery and is known to enhance PS exposure in the tumor microenvironment [17,18]. Second, mch1N11 and cis are complementary after surgery when tumor deposits in the local wound begin to proliferate. Mch1N11 attacks the hypoxic core of a new rapidly expanding tumor, whereas cis attacks the rapidly dividing tumor cells of the oxygenated tumor periphery [15]. Third, surgery is known to create a unique environment for new blood vessel development owing to changes in angiogenic factors (e.g., VEGF) [4]. Thus, we postulated that this postoperative environment would be particularly susceptible to vascular-targeting strategies such as mch1N11. Finally, a major concern of using other antivascular strategies (such as commercially available bevacizumab) is wound healing complications. We did not find this toxicity with our novel agent. In summary, our data demonstrate that, when combined with cis, the PS-targeting antibody enhances tumor killing, prevents relapses, and slows the progression of tumor recurrences after surgery.
Materials and Methods
Animals
Female C57BL/6 (B6, Thy1.2) mice were purchased from Charles River Laboratories (Malvern, PA). All mice were maintained in pathogen-free conditions and used for experiments at ages 8 weeks or older. The Animal Use Committees of the Children's Hospital of Philadelphia, The Wistar Institute, and the University of Pennsylvania approved all protocols in compliance with the Guide for the Care and Use of Laboratory Animals.
Cell Lines
TC1 cells were derived from mouse lung epithelial cells of a C57BL/6 mouse, immortalized with human papillomavirus type 16 E6 and E7, and transformed with the c-Ha-ras oncogene [19]. The TC1 cell line was maintained in RPMI medium supplemented with 10% heat-inactivated fetal bovine serum, 1% glutamine, and 1% penicillin and streptomycin. Cells were cultured at 37°C in a humidified incubator containing 5% CO2.
Chemotherapy
Cis was purchased from APP Pharmaceuticals, LLC (Schaumburg, IL). Cis was administered intraperitoneally (i.p.) once weekly to mice at 6 mg/kg for C57BL/6 mice. Cis was suspended in 200 µl of normal saline for i.p. injections.
Antibodies
The mouse chimeric antibody, mch1N11, is a mouse immunoglobulin G 2A version of a fully human PS-targeting antibody (PGN635) directed against PS-β2-glycoprotein I complexes. The specificity of PGN635 is similar to that of the chimeric antibody, bavituximab. PGN635 was generated by Peregrine Pharmaceuticals Inc (Tustin, CA) in conjunction with Affitech AG (Oslo, Norway). C44 is an isotype-matched control antibody for mch1N11. Both C44 and mch1N11 were purified as previously described [20].
Animal Flank Tumor Models
C57BL/6 mice were inoculated subcutaneously on the right flank with 1.2 x 106 TC1 cells. When the tumors reached approximately 250 mm3, mice were randomized and grouped into four cohorts: 1) C44 antibody only, 2) cis + C44 antibody, 3)mch1N11 antibody only, and 4) cis + mch1N11 antibody. A weekly treatment cycle was set up on day fourteen when the tumors approached 300 mm3: mice from groups 1 and 3 were treated with 100 µg of antibody, whereas mice from groups 2 and 4 were treated with 100 µg of antibody and 3 or 6 mg/kg of cis. Antibodies were injected biweekly, whereas cis was injected weekly. A total of five antibody and three cis injections were completed during the course of the experiment. Tumor dimensions were measured at the time of injection with calipers and tumor size was determined by the equation: π/6 x length x width x width. All experiments had at least five mice per group and were repeated at least once.
Surgery
Surgery was performed on mice bearing flank tumors using an established partial resection model [21]. Partial resection was performed on C57BL/6 mice inoculated subcutaneously with 1.2 x 106 TC1 cells. Surgery was performed when tumors reached approximately 500 mm3. Mice were anesthetized with intramuscular ketamine (80 mg/kg) and xylazine (10 mg/kg) and shaved with hair clippers before surgery. A 1- to 2-cm incision was made adjacent to the tumor, and 90% of the tumor was removed using standard blunt dissection technique. The incision was closed using sterile silk 4-0 sutures. Buprenorphine (0.2 mg/kg) was administered at the time of surgery and 6 hours after as postoperative analgesia. Preoperative treatment was unknown to the investigator performing surgery and making tumor measurements.
In Vivo Depletion of CD8 T Cells
To deplete CD8 T cells during treatment with mch1N11 and cis in our TC1 model, mice received i.p. injections of 300 µg of monoclonal antibodies purified from the anti-CD8 hybridoma 53-6.7 (obtained from the American Type Culture Collection, Manassas, VA). Injections were administered 1 day before beginning mch1N11 and cis therapies. Subsequently, mice received biweekly doses throughout the experimental period to ensure depletion of CD8 T cells. Depletion was confirmed by flow cytometry of splenocytes.
Immunohistochemical Stains
Animals treated with either C44 control antibody or PS-targeting antibody were killed when tumor volume curves began to diverge. Their tumors were harvested and bisected with one half placed either in Tissue-Tek OCT (Sakura Inc, United Kingdom) and stored at -80°C or in formalin for paraffin sectioning.
To detect apoptosis, TUNEL staining and cleaved caspase 3 staining were used. TUNEL staining was performed using ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (S7101; Chemicon International, Temecula, CA), following the manufacturer's instructions. Cleaved caspase 3 staining was performed using rabbit anti-cleaved caspase 3 antibody (no. 9661; Cell Signaling, Danvers, MA). Tissue sections were treated in heated citrated buffer for antigen unmasking followed by treatment with 3% hydrogen peroxide per the manufacturer's recommendations.
To detect endothelial cells, monoclonal anti-CD31 (mAB390) [22] was raised from hybridoma supernatant and purified. Frozen tumor sections were prepared as previously described [23].
Flow Cytometric Analysis
For flow cytometric analysis, tumors were removed from killed mice and minced into fine pieces in digestion buffer containing 0.1 mg/ml DNase I and 2.0 mg/ml collagenase type IV (Sigma, St Louis, MO). Samples were incubated in digestion buffer at 37°C for 1 hour, filtered through a 70-µm filter, and washed twice with R10. Fc receptors were blocked with antimouse CD16/CD32 antibodies (BD Biosciences PharMingen, San Diego, CA). After one wash with PBS plus 2% fetal bovine serum (staining buffer), cells were incubated for 30 minutes at 4°C with appropriate antibodies obtained from BD Biosciences PharMingen. Samples were then washed and resuspended in staining buffer or fixed in 2% paraformaldehyde. Flow cytometry was completed using a Becton Dickinson FACS Calibur flow cytometer (San Jose, CA) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Splenocytes and tumor cells were studied by flow cytometric analysis as previously described [24]. Cells were stained with the following antibodies against the following antigens: Ly6G, Ly6C, CD11b, CD45, CD8, CD4, F4/80, CD206, 4-1BB, CD137, and GR1. All previously listed fluorescently labeled antibodies used were purchased from BD Biosciences, except for CD206-phycoerythrin (PE; obtained from AbD Serotec, Raleigh, NC), 4-1BB (CD137)-PE (obtained from Abcam, Boston, MA), and GR1-FITC (obtained from eBioscience, San Diego, CA).
Statistical Analyses
For flow cytometry, immunohistochemistry, reverse transcription-polymerase chain reaction, and flank tumor volume studies comparing differences between two groups, we used unpaired Student's t tests. For studies comparing more than two groups, ANOVA with appropriate post hoc testing was implemented. Kaplan-Meier curves were used to determine the postoperative median survival. Postoperative survival rates (defined as the time from surgery to the time which flank tumor volume reached 1500 mm3) for treatment groups were compared using the log-rank statistic. Differences were considered significant when P < .05. Data are presented as mean (SE), unless otherwise noted.
Results
Efficacy of PS-Targeting Antibody and Concomitant Chemotherapy in the TC1 Model
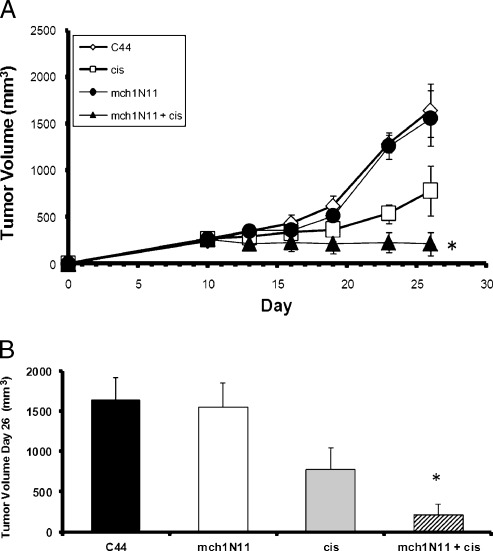
We first evaluated the efficacy of the PS-targeting antibody mch1N11 alone or in combination with cis chemotherapy in a mouse model of lung cancer (TC1 tumors). Treatment was started 7 days after tumor cell inoculation. Mice were treated i.p. with mch1N11, C44, cis, or the combination of mch1N11 and cis (Figure 1). In this model, cis slowed tumor growth to some extent, but mch1N11 had little effect when used alone. Combination therapy was superior to mch1N11 or cis alone. At day 26, tumor growth was inhibited 87% by combination therapy and 52%by cis in comparison with the control group (Figure 1, A and B). The mean tumor volume of the combination group was 216 mm3, and mean the tumor volume of the group receiving C44 alone was 1643 mm3 (P = .02). The group receiving mch1N11 alone demonstrated little difference from the group receiving C44 antibody (P > .1). These results show that mch1N11 significantly improves the therapeutic efficacy of cis for the treatment of the murine NSCLC cell line TC1 in mice.
Figure 1.
TC1 tumor growth is slowed by cis and mch1N11 in vivo. (A) Mice bearing TC1 tumor were treated with cis and mch1N11 once tumors reached approximately 200 mm3. The group receiving both treatments demonstrated a dramatic decrease in tumor growth in comparison to the groups receiving C44 alone, cis alone, and mch1N11 alone. (B) Tumor volume of tumor bearing mice at day 26. Mice were treated with C44, cis, mch1N11, or combination therapy (mch1N11 and cis). *P < .05.
Cytotoxic Chemotherapy and Targeted PS Therapy Inhibits Recurrence of Tumor after Surgery
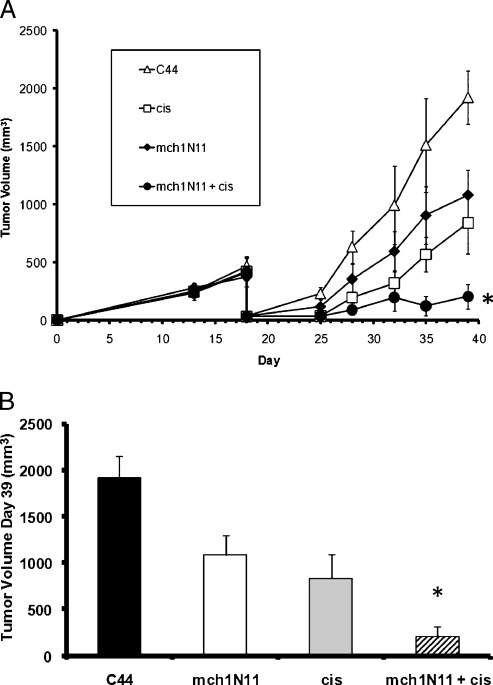
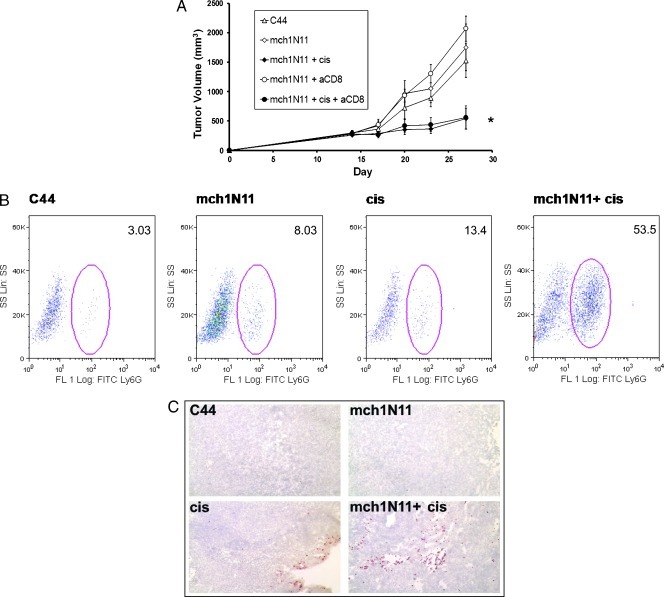
We next determined whether cis and the PS-targeting antibody could prevent local recurrences after surgery. Tumors that recur after surgical resection are known to proliferate rapidly and develop neovasculature. Mice bearing flank TC1 tumor measuring approximately 500 mm3 underwent partial resection of 90% of the tumor [21]. On postoperative day 3, mice were treated with C44, mch1N11, cis, or combination therapy. Antibody was given biweekly, and cis was given weekly. Under these circumstances, mchN11 had a significant antitumor effect in the postoperative tumor microenvironment. At postoperative day 21, mch1N11 monotherapy reduced the tumor by 44% (Figure 2, A and B; P < .05). As expected, cis alone inhibited tumor growth after surgery by 62% (P < .05). The combination of cis and mchN11 had a strong reduction of recurrent tumors, with mean tumor volume 90% smaller than control animals (P < .01; Figure 2, A and B). The mean tumor volume of the combination group at postoperative day 21 was 206 mm3 in comparison to the control tumor volume of 1921 mm3 (P = .002). Mice receiving mch1N11 and cis demonstrated increased postoperative survival in comparison to the other groups as measured by time to reach a recurrent tumor volume of 1500 mm3 (P = .03; data not shown). Animals were killed after this owing to ethical considerations. The surgical wounds of the groups receiving mch1N11 healed normally and were indistinguishable from the surgical wounds of the mice receiving C44 or cis.
Figure 2.
Surgery effectively combines with cis and mch1N11 to control tumor growth. (A) Mice underwent cytoreduction of tumor when growth reached approximately 500 mm3. After surgery, mice received C44, cis, mch1N11, or cis and mch1N11 combined. (B) Tumor volume at day 39 of mice that underwent surgery and treatment of TC1 tumor. *P < .01.
Mechanisms of Efficacy: Expression of PS on Tumor Cells Is Minimal
Cis therapy could potentially induce the expression of PS on either the tumor cells or the associated tumor vasculature. We thus evaluated whether subtoxic concentrations of cis can induce exposure of PS in murine NSCLC (TC1) in vitro. TC1 cells were treated with low dose and high dose of cis for 24 hours at 37°C to determine the expression of PS. The drug was removed, and cells were stained for annexin V and 7-AAD. Flow cytometry was used to detect PS expression and apoptosis. TC1 cells treated with PBS alone demonstrated 4.4% expression of PS. Treated cells at both concentrations of cis demonstrated less than 10% apoptosis and similar levels of PS expression (26.3% vs 25.1%), indicating that cis is effective in increasing PS expression at low and high doses.
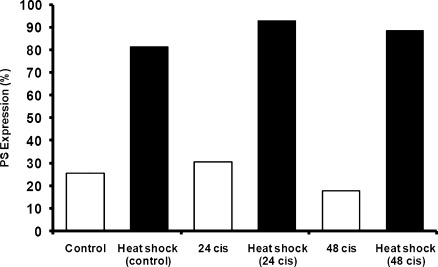
Next, we evaluated the externalization of PS in tumor cells in vivo after injection of cis (5 mg/kg) into immunocompetent mice bearing established TC1 tumors (250 mm3). The tumors were harvested 24 hours after the cis injection, were digested, and were stained for annexin V. Flow cytometry demonstrated a marginal increase in PS exposure in vivo at 24 hours in comparison to control tumor (33% vs 28.5%; Figure 3). Repeat experiments confirmed a small increase in PS expression in established flank tumors. The PS expression decreased 48 hours after injection, demonstrating that cis transiently increased PS expression. Our studies demonstrate that cis induces only a slight increase in the exposure of PS in NSCLC in mice as detected by annexin V, making direct binding of the PS-targeting antibody to tumor cells an unlikely explanation for efficacy.
Figure 3.
Cis induces little change in PS exposure on TC1 tumor cells. Mice bearing TC1 tumor (∼200 mm3) were treated with 5 mg/kg cis, and PS expression on tumors was measured 24 and 48 hours after treatment.
PS-Targeting Antibody and Cis Induce Apoptosis of Vascular Endothelial Cells in the Tumor Microenvironment
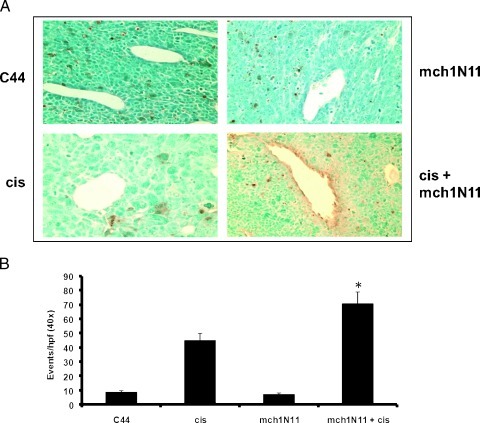
We next evaluated the tumor vasculature. The ability of combination mch1N11 and cis to induce apoptosis of tumor cells was analyzed using a TUNEL assay and cleaved caspase 3 antibody. Mice bearing TC1 tumor (∼200 mm3) were treated i.p. with C44 (100 µg/mouse), mch1N11 (100 µg/mouse), cis (6 mg/kg), or combination therapy for 2 weeks. Tumors were harvested 4 days after the last dose of mch1N11 and analyzed for positively stained apoptotic cells. Tumors from the combination therapy group demonstrated endothelial cells that positively stained for apoptosis as demonstrated by the TUNEL assay (Figure 4A). Furthermore, cleaved caspase 3 staining demonstrated a significant increase in positively stained cells in the combination therapy group in comparison to all other groups (P = .02; Figure 4B). These data indicate that cis and mch1N11 are able to induce apoptosis in tumor endothelial cells.
Figure 4.
Mch1N11 and cis induce apoptosis in tumor endothelial cells. (A) Mice bearing TC1 tumor were treated with C44, mch1N11, cis, or a combination of mch1N11 and cis. Tumors were harvested, fixed in formalin, sectioned in paraffin, and stained for apoptosis using TUNEL staining kit. Only the combination therapy group had apoptotic tumor endothelial cells. (B) Mice bearing TC1 tumor were treated with C44, mch1N11, cis, or a combination of mch1N11 and cis. Tumors were harvested and frozen in OCT blocks. Tumors were stained for cleaved caspase 3 antibody. Tumors treated with cis or mch1N11/cis demonstrated significantly increased numbers of positively stained cells compared with the tumors treated with C44 or mch1N11 alone.
Mch1N11 Functions in a CD8 T Cell Independent Manner and Produces an Increase in Intratumoral Neutrophils
To determine whether the antitumor mechanism of mch1N11 is mediated by the adaptive immune system, we examined the role of lymphocyte populations on the efficacy of mch1N11. The combination therapy for mch1N11 and cis on TC1 tumors was repeated in mice depleted of CD8 T cells. Mice were depleted of CD8 T cells using a monoclonal rat antimouse antibody. Mice were randomized to five treatment groups at day 14: C44, mch1N11, combination therapy (mch1N11 and cis), mch1N11 and CD8 antibody, and combination therapy plus CD8 antibody. At day 27 after tumor cell inoculation, mice receiving combination therapy and CD8-depleting antibody demonstrated no significant difference in tumor size to mice receiving combination therapy (P > .1; Figure 5A). Furthermore, the two groups receiving combination therapy demonstrated significant decrease in tumor volume in comparison to all other groups (P = .02). These data show that the antitumor efficacy of mch1N11 and cis is independent of CD8 T cells.
Figure 5.
Mch1N11 functions in a CD8 T-cell-independent manner with intratumoral neutrophil infiltration. (A) Mice were randomized into five groups once tumor size reached approximately 200 mm3: C44, mch1N11, mch1N11/Cis, mch1N11 and anti-CD8 antibody, and mch1N11/Cis and anti-CD8 antibody. The group receiving mch1N11/cis and anti-CD8 antibody demonstrated no difference in tumor growth compared with the group receiving mch1N11/Cis alone. This indicates mch1N11 inhibits tumor growth in the absence of CD8 T cells. (B) Mice bearing recurrent TC1 tumor were treated with C44, mch1N11, cis, or a combination of mch1N11 and cis. Tumors were harvested and digested for flow cytometry analysis revealing significant neutrophil (Ly6G+) infiltration in mice treated with combination therapy. (C) Treated tumor sections were stained for Ly6G+ and confirmed the neutrophil infiltration in the perivascular tissues.
Because CD8 T cells did not play a role in the therapeutic efficacy, we next analyzed recurrent TC1 tumors using flow cytometry and immunohistochemistry to further investigate the tumor microenvironment for other immune cells. Mice bearing large TC1 tumors (∼500 mm3) underwent partial resection of tumor and were treated i.p. with C44, mch1N11, cis, or combination therapy for 2 weeks. Tumors were resected from all four groups 24 hours after the fourth dose of therapy and analyzed using flow cytometry and immunohistochemistry. There was an increase in CD11b cells infiltrating the tumors after combination therapy (1.6% of entire tumor population) compared with untreated animals (0.4% of entire tumor; P < .01). We specifically evaluated for the presence of granulocytes and macrophages.
Intratumoral flow analysis of tumors revealed an infiltration of neutrophils of those mice which received mch1N11, cis, and combination therapy (Figure 5B). To confirm this finding, immunohistochemistry was performed on frozen sections of tumor. The staining validated this finding and agrees with previous work that attributes mch1N11's efficacy to an increased inflammatory response driven by host cell infiltration into the perivascular tumor tissues (Figure 5C) [13].
We also evaluated the presence of intratumoral macrophages after combination cis and mch1N11. There was a significant increase of leukocytes expressing Ly6C after combination therapy (Table 1). In addition to the increase of macrophages, the infiltrating macrophages expressed a predominately M1 phenotype (F4/80hiCD206lo) after combination therapy. In controls, the ratio of M2 macrophages (F4/80hiCD206hi) to M1 macrophages suggested a leaning toward a protumor phenotype (Table 1). However, after combination therapy, there were twice as many M1 macrophages as M2 macrophages (18.3% vs 38.3%, respectively). These data suggest that the inflammatory infiltrate that follows combination therapy is partially responsible for increased antitumor responses.
Table 1.
Tumors Harvested and Digested for Flow Cytometry Were Analyzed for CD11b, Ly6C, F4/80, and CD206 Expression.
| Intratumoral Macrophages | F4-80hi/CD206hi | F4-80hi/CD206lo | Ratio | |
| Control | 15.2% (1.3) | 41.2% | 26.9% | 0.7 |
| Cis | 31.1% (3.0) | 33.5% | 22.5% | 0.7 |
| PS | 23.8% (2.8) | 24.7% | 31.2% | 1.3 |
| Combination | 46.5% (6.9) | 18.3% | 38.3% | 2.1 |
F4/80 is a macrophage activation marker, CD206 is the macrophage mannose receptor, and their expression suggests a protumor phenotype.
Values in parentheses are SDs.
Discussion
The objective of this study was to determine whether using targeted vascular therapy after surgery could inhibit local tumor recurrences without causing the toxicity associated with other vascular-targeting agents. Our major finding from this study is that combining mch1N11 with cis slows tumor recurrences after surgery. Our data suggest that the mechanism is that cis, either directly or indirectly (i.e., by killing tumor cells that then activates their associated vasculature), increases PS expression of the tumor vasculature. This vascular PS presents a target for the mch1N11 antibody that binds and induces intense neutrophil infiltration, leading to vascular disruption and tumor death. This process seems independent of the adaptive immune system.
In our studies, we saw some efficacy of the PS-targeting antibody mch1N11 when used alone in the postsurgical setting (Figure 2A) but little effect in “unstressed” TC1 tumors (Figure 1A). This is consistent with the idea that the postoperative setting “stimulates/stresses” the vasculature in the remaining tumor, whereas there is relatively little baseline expression of PS in unstressed TC1 tumors. One potential explanation is that tumor relapses occur in tumor microenvironments that are rich in cytokines (VEGF), leukocytes, metabolites, and reducing/oxidizing factors during their early development. This rapid proliferation that occurs in a recurrent tumor likely affects the neovasculature of a developing tumor and may explain the ability of mch1N11 to inhibit these cancer deposits. This may be a unique feature of recurrent tumors that can be exploited by PS-targeting therapy. Importantly, the use of the chemotherapy drug cis markedly enhanced the efficacy of mch1N11 in both the presurgery and postsurgery setting and leading to tumor endothelial cell death (Figure 4).
The results here demonstrate that targeting PS on tumor vasculature can enhance the efficacy of cis and surgery without major toxicity. The unique postoperative environment provides an ideal time to target rapidly dividing cells with cis and developing vessels with mch1N11. Bevacizumab (trade name Avastin) is a drug that blocks angiogenesis and is currently in clinical use for a wide variety of tumors. However, this agent has several contraindications surrounding surgery owing to the increased risk of bleeding and wound healing complications [4,25]. Although only a small animal model of surgery, we did not have any of these morbidities with mch1N11. PS-targeting therapy specifically targets and disrupts tumor vessels as opposed to bevacizumab, which is an anti-VEGF antibody. This present study contributes to the previous preclinical and clinical studies showing the promising nature of PS as a target molecule in recurrent cancers [26].
One of the future studies will be to optimize the pharmacokinetics of cis with mch1N11. We typically gave mch1N11 24 and 72 hours after each cis dose. However, this sequence may not be ideal. For example, we may not require the second dose of antibody for any benefit. Also, the dose of cis (3 mg/kg) may be reduced owing to the improved efficacy of the combination, which would lower the toxicity of this agent. Further investigation is warranted to establish the pharmacodynamics and pharmacokinetics of cis when vascular endothelial cells are targeted and damaged after mch1N11 treatment.
The observed decrease in tumor volume in our studies was accompanied by an inflammatory infiltrate (CD11b+). Immunostaining and flow cytometry showed that there was a significant increase in neutrophils (Ly6G+) into the perivascular tissues (Figure 5, B and C). Infiltrating neutrophils in tumors have been associated with either protumor or antitumor immune responses depending on the context [27]. Our treatment seemed to attract large numbers of antitumor, N1-type neutrophils. A similar type of response has been noted after treatment of tumors with photodynamic therapy—another therapy that may affect tumor vasculature [28]. Macrophages have also been observed to infiltrate tumors in mice treated with PS-targeting antibodies [12,13,15]. In our study, we postulated that the increased apoptosis and innate immune response may have stimulated antigenic cell killing, danger signals, and the induction of antitumor T cells (as is the case with photodynamic therapy) [28]. However, when we depleted CD8 T cells, there was no evidence of an adaptive immune effect. Others have reported that treatment with a PS-targeting antibody plus irradiation induced T-cell tumor immunity in a rat glioma model [14].
One caveat of these data is the experiments that incorporate surgery depend on a flank tumor model. Flank tumor models have inherent limitations due to rapid growth kinetics that lack complex host-tumor interactions and have more acute immune responses [29,30]. In addition, the orthotopic position does not permit intricate networks that may develop in a native microenvironment. However, with the exception of breast and skin cancers, orthotopic tumors (e.g., lung, pancreas, liver, brain, colon, or the prostate tumors) are not conducive to surgical resection in mice. Our goal was to determine whether PS-targeted therapy would affect the postoperative tumor milieu; thus, this model was appropriate and informative.
In conclusion, using a vascular endothelial-targeted therapy after surgery shows significant promise to prevent lung cancer recurrences after surgery. This approach is likely due to the unique window of opportunity after surgery when there is a rapidly proliferating tumor with significant capillary development and dependence on a source of oxygen and nutrients. PS-targeting therapy during this time can incite a strong inflammatory response, partially driven by neutrophils, which can result in tumor cell killing and improved clinical outcomes.
Abbreviations
- cis
cisplatin
- PS
phosphatidylserine
- VEGF
vascular endothelial growth factor
Footnotes
J.D.P. was supported by a grant from the American Medical Association Foundation. L.A.A. was supported from the Lavin Family Supporting Foundation. S.S. was supported by the National Lung Cancer Partnership (Young Investigator Award) and Society of University Surgeons (Clinical Scholar Award). P.E.T. is a consultant to and has equity interests in Peregrine Pharmaceuticals, Inc, which produced the phosphatidylserine-targeting antibody, bavituximab.
References
- 1.Aliperti LA, Predina JD, Vachani A, Singhal S. Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol. 2011;18:603–607. doi: 10.1245/s10434-010-1442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pisters KM, Le Chevalier T. Adjuvant chemotherapy in completely resected non-small-cell lung cancer. J Clin Oncol. 2005;23:3270–3278. doi: 10.1200/JCO.2005.11.478. [DOI] [PubMed] [Google Scholar]
- 3.Stuschke M, Pottgen C. Chemotherapy: effectiveness of adjuvant chemotherapy for resected NSCLC. Nat Rev Clin Oncol. 2010;7:613–614. doi: 10.1038/nrclinonc.2010.165. [DOI] [PubMed] [Google Scholar]
- 4.Kong B, Michalski CW, Friess H, Kleeff J. Surgical procedure as an inducer of tumor angiogenesis. Exp Oncol. 2010;32:186–189. [PubMed] [Google Scholar]
- 5.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 6.Holmgren L, O'Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–153. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 7.Hadj Tahar A. Bevacizumab for advanced colorectal cancer. Issues Emerg Health Technol. 2004:1–4. [PubMed] [Google Scholar]
- 8.Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, Blumenschein G, Jr, Lee JJ, Liu DD, Truong MT, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005;23:2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 9.Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
- 10.Derose P, Thorpe PE, Gerber DE. Development of bavituximab, a vascular targeting agent with immune-modulating properties, for lung cancer treatment. Immunotherapy. 2011;3:933–944. doi: 10.2217/imt.11.87. [DOI] [PubMed] [Google Scholar]
- 11.Ran S, Downes A, Thorpe PE. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002;62:6132–6140. [PubMed] [Google Scholar]
- 12.Ran S, He J, Huang X, Soares M, Scothorn D, Thorpe PE. Antitumor effects of a monoclonal antibody that binds anionic phospholipids on the surface of tumor blood vessels in mice. Clin Cancer Res. 2005;11:1551–1562. doi: 10.1158/1078-0432.CCR-04-1645. [DOI] [PubMed] [Google Scholar]
- 13.Beck AW, Luster TA, Miller AF, Holloway SE, Conner CR, Barnett CC, Thorpe PE, Fleming JB, Brekken RA. Combination of a monoclonal anti-phosphatidylserine antibody with gemcitabine strongly inhibits the growth and metastasis of orthotopic pancreatic tumors in mice. Int J Cancer. 2006;118:2639–2643. doi: 10.1002/ijc.21684. [DOI] [PubMed] [Google Scholar]
- 14.He J, Yin Y, Luster TA, Watkins L, Thorpe PE. Antiphosphatidylserine antibody combined with irradiation damages tumor blood vessels and induces tumor immunity in a rat model of glioblastoma. Clin Cancer Res. 2009;15:6871–6880. doi: 10.1158/1078-0432.CCR-09-1499. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Bennett M, Thorpe PE. A monoclonal antibody that binds anionic phospholipids on tumor blood vessels enhances the antitumor effect of docetaxel on human breast tumors in mice. Cancer Res. 2005;65:4408–4416. doi: 10.1158/0008-5472.CAN-05-0031. [DOI] [PubMed] [Google Scholar]
- 16.Gerber DE, Stopeck AT, Wong L, Rosen LS, Thorpe PE, Shan JS, Ibrahim NK. Phase I safety and pharmacokinetic study of bavituximab, a chimeric phosphatidylserine-targeting monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17:6888–6896. doi: 10.1158/1078-0432.CCR-11-1074. [DOI] [PubMed] [Google Scholar]
- 17.Drucker L, Ciobotaro P, Kimchi O, Tohami T, Yarkoni S, Radnay J, Shapira H, Lishner M. Initial exposed phosphatidylserine levels correlate with cellular response to cytotoxic drugs. Eur J Haematol. 2003;70:98–105. doi: 10.1034/j.1600-0609.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 18.Mochizuki T, Kuge Y, Zhao S, Tsukamoto E, Hosokawa M, Strauss HW, Blankenberg FG, Tait JF, Tamaki N. Detection of apoptotic tumor response in vivo after a single dose of chemotherapy with 99mTc-annexin V. J Nucl Med. 2003;44:92–97. [PubMed] [Google Scholar]
- 19.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- 20.Ran S, Gao B, Duffy S, Watkins L, Rote N, Thorpe PE. Infarction of solid Hodgkin's tumors in mice by antibody-directed targeting of tissue factor to tumor vasculature. Cancer Res. 1998;58:4646–4653. [PubMed] [Google Scholar]
- 21.Broomfield S, Currie A, van der Most RG, Brown M, van Bruggen I, Robinson BW, Lake RA. Partial, but not complete, tumor-debulking surgery promotes protective antitumor memory when combined with chemotherapy and adjuvant immunotherapy. Cancer Res. 2005;65:7580–7584. doi: 10.1158/0008-5472.CAN-05-0328. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120:2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- 23.Yan HC, Juhasz I, Pilewski J, Murphy GF, Herlyn M, Albelda SM. Human/severe combined immunodeficient mouse chimeras. An experimental in vivo model system to study the regulation of human endothelial cell-leukocyte adhesion molecules. J Clin Invest. 1993;91:986–996. doi: 10.1172/JCI116320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, Albelda SM. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- 25.Bose D, Meric-Bernstam F, Hofstetter W, Reardon DA, Flaherty KT, Ellis LM. Vascular endothelial growth factor targeted therapy in the perioperative setting: implications for patient care. Lancet Oncol. 2010;11:373–382. doi: 10.1016/S1470-2045(09)70341-9. [DOI] [PubMed] [Google Scholar]
- 26.He J, Luster TA, Thorpe PE. Radiation-enhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin Cancer Res. 2007;13:5211–5218. doi: 10.1158/1078-0432.CCR-07-0793. [DOI] [PubMed] [Google Scholar]
- 27.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy enhancement of antitumor immunity is regulated by neutrophils. Cancer Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber K, Rowley DA, Riethmuller G, Schreiber H. Cancer immunotherapy and preclinical studies: why we are not wasting our time with animal experiments. Hematol Oncol Clin North Am. 2006;20:567–584. doi: 10.1016/j.hoc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Stewart TJ, Greeneltch KM, Lutsiak ME, Abrams SI. Immunological responses can have both pro- and antitumour effects: implications for immunotherapy. Expert Rev Mol Med. 2007;9:1–20. doi: 10.1017/S1462399407000233. [DOI] [PubMed] [Google Scholar]