Abstract
Background
The risk for relapse of child bipolar I disorder (BP-I) is highly correlated with environmental factors. Immediate early genes of the Early Growth Response (EGR) Gene family are activated at high levels in the brain in response to environmental events, including stress, and mediate numerous neurobiological processes that have been associated with mental illness risk. The objective of this study is to evaluate whether single nucleotide polymorphisms (SNPs) in EGR genes are associated with the risk to develop child bipolar I disorder.
Methods
To investigate whether EGR genes may influence susceptibility to child Bipolar I disorder (BP-I), we used Family Based Association Tests to examine whether SNPs in each of the EGR genes were associated with illness in 49 families.
Results
Two SNPs in EGR3 displayed nominally significant associations with child BP-I (p=0.027 and p=0.028); though neither was statistically significant following correction for multiple comparisons. Haplotype association analysis indicated these SNPs are in linkage disequilibrium (LD). None of the SNPs tested in EGR1, EGR2, or EGR4 was associated with child BP-I.
Limitations
This study was limited by small sample size, which resulted in it being underpowered to detect a significant association after correction for multiple comparisons.
Conclusions
Our study revealed a preliminary finding suggesting that EGR3, a gene that translates environmental stimuli into long-term changes in the brain, warrants further investigation for association with risk for child BP-I disorder in a larger sample. Such studies may help reveal mechanisms by which environment can interact with genetic predisposition to influence this severe mental illness.
Keywords: Child bipolar I disorder, Early Growth Response Gene 3, Environment, Family Based Association Test, Immediate early gene, Stress
Introduction
Bipolar I disorder (BP-I) affects approximately 5% of the population of 12 to 20 year-olds in the United States (Grant et al., 2005). Like other major mental illnesses, the risk to develop child BP-I is multifactorial; influenced by both familial and psychosocial factors (Geller et al., 2008; Geller et al., 2006; Geller et al., 2004b; Wozniak et al., 2009). These include external stressors, such as the presence of bipolar disorder in a family member, and putative protective factors, including level of maternal warmth (Geller et al., 2008; Geller et al., 2006; Wozniak et al., 2009). In particular, low maternal warmth is one of the leading risk factor associated with the risk of mania relapse after recovery in child BP-I subjects (Geller et al., 2008; Geller et al., 2004b). This strong environmental influence on the susceptibility to recurrent child BP-I led us to investigate whether single nucleotide polymorphisms (SNPs) in genes that play critical roles in the response and adaptation to stress (Fish et al., 2004; Gallitano-Mendel et al., 2007), may be associated with risk for child BP-I.
The Early Growth Response (EGR) Genes: EGR1, EGR2, EGR3, and EGR4, make up a family of immediate-early gene (IEG) zinc-finger transcription factors. By definition, IEGs are rapidly activated in response to a stimulus; showing high levels of messenger ribonucleic acid (mRNA) transcription within 30 to 45 minutes (Senba and Ueyama, 1997). As transcription factors, the EGR proteins bind to DNA to regulate a host of downstream genes. These target genes presumably mediate the known roles of the EGRs in processes such as growth factor regulation, myelination, vascularization, inflammation, synaptic plasticity, and memory formation and reconsolidation (Fahmy et al., 2003; Gallitano-Mendel et al., 2007; Jessen and Mirsky, 2002; Jones et al., 2001; Liebermann and Hoffman, 2002; Milbrandt, 1987; O’Donovan et al., 1999; Poirier et al., 2008). Dysfunction in many of these processes has been implicated in psychiatric illness pathogenesis (Hanson and Gottesman, 2005; Jones et al., 2005; Moises et al., 2002; O’Donovan et al., 1999). The EGRs are thus poised to translate environmental events into long-term changes in the brain that may influence psychiatric illness susceptibility.
The functions of EGR-family genes have been extensively studied in animals where they are known to be activated throughout the brain in response to a wide range of stressful stimuli (Senba and Ueyama, 1997). Furthermore, disruption of their function produces defects in the response and adaptation to stress (Gallitano-Mendel et al., 2007; Weaver et al., 2004), including: heightened stress-reactivity, elevated release of corticosterone (the rodent equivalent of cortisol), altered anxiety-related behaviors, and hyperactivity (Fish et al., 2004; Gallitano-Mendel et al., 2007).
Of particular relevance to child BP-I, are studies showing that EGR1 (A.K.A. NGFI-A, zif-268) plays a critical role in animal models of early maternal behavior that result in long-lasting changes in hypothalamic-pituitary-adrenal (HPA) axis regulation, and abnormal anxiety-related behaviors later in life (Fish et al., 2004; Weaver et al., 2004). The mechanism underlying this effect involves EGR-mediated regulation of glucocorticoid receptor expression (Fish et al., 2004; Weaver et al., 2007). Recent studies in postmortem brains suggest that similar mechanisms may occur in humans (McGowan et al., 2009). These studies together indicate that at least one member of the EGR gene family plays a critical role translating early maternal behavior into long-term changes in HPA-axis function and anxiety-related behavior later in life, in a manner that appears to be conserved across species.
Of particular interest is EGR3, which has strong biological rationale for a role in risk for psychotic illnesses. This includes that EGR3 is regulated by multiple candidate proteins implicated in risk for schizophrenia, bipolar disorder, or both psychotic illnesses, such as neuregulin 1 (NRG1), calcineurin (CN), N-methyl D-aspartate (NMDA) receptors, serotonin 2A receptors (5-HT2ARs), and brain-derived neurotrophic factor (BDNF) (Buckley et al.; Geller et al., 2004a; Gerber et al., 2003; Gonzalez-Maeso et al., 2003; Hippenmeyer et al., 2002; Kato, 2007; Mittelstadt and Ashwell, 1998; Roberts et al., 2006; Stefansson et al., 2002; Yamagata et al., 1994). Notably, both BDNF and HTR2A (which encodes the 5-HT2AR) have revealed suggestive associations with early onset BP-I and child BP-I (Geller et al., 2004a; Manchia et al.; Tang et al., 2008). Additional support linking EGR3 with these genes comes from studies in gene-targeted mice that show shared phenotypes between EGR3-deficient mice, NRG1-deficient mice, and CN-deficient mice (Gallitano-Mendel et al., 2007; Hippenmeyer et al., 2002; Miyakawa et al., 2003) suggested shared biological roles for these proteins in vivo. Although many of these studies addressed associations with schizophrenia, a growing body of literature suggests there is overlap in genetic vulnerabilities across psychotic disorders (Lichtenstein et al., 2009; Maier, 2008). For example, both NRG1 and CN-subunit gene (PPP3CC) were originally identified as associated with schizophrenia, and have now also shown associations with adult BP-I (Maier, 2008; Mathieu et al., 2008).
Together these findings suggest the possibility of a biological pathway, disruption at any point of which can increase risk for these mental illnesses. Such a pathway addresses the recent recognition that the genetic contribution to psychiatric illnesses is influenced by many genes, each of small effect size (Purcell et al., 2009).
Finally, each of the human EGR genes also maps to a chromosomal locus implicated in psychotic disorder susceptibility. SNPs in one member of the EGR gene family, EGR3, are associated with schizophrenia, and EGR3 mRNA expression is reduced in the forebrain and hippocampus of postmortem schizophrenia patients compared with controls (Kim et al.; Mexal et al., 2005; Yamada et al., 2007). Recently, in a study investigating circadian genes and adult bipolar disorder, EGR3 was the only gene that showed a nominally significant association (Mansour et al., 2009). For these reasons, we decided to explore whether SNPs in any of the human EGR genes (EGR1, EGR2, EGR3, or EGR4), are associated with the risk for child BP-I.
Subjects and Methods
Subject Ascertainment
Probands were a subset of the sample described in detail in Geller et al. (Geller et al., 2008) and were enrolled in the NIMH-funded “Phenomenology and Course of Pediatric Bipolar Disorders” study between September 25, 1995 and December 15, 1998. Subjects were obtained from designated outpatient child psychiatric and pediatric sites by consecutive new case ascertainment. The facilities that agreed to participate in the consecutive new case ascertainment were largely only available to families with health insurance (Geller et al., 2002b).
Inclusion and Exclusion Criteria
Subjects met the inclusion criteria of 7 to 16 years old, males and females, good physical health, and a diagnosis of current DSM-IV BP-I (manic or mixed phase) for at least 2 weeks with at least 1 cardinal symptom of mania (i.e., elation and/or grandiosity) (Geller et al., 2008). A Children’s Global Assessment Scale (CGAS) score of ≤60 was required to establish clinically significant impairment (Bird et al., 1987; Shaffer et al., 1983).
Exclusion criteria were IQ less than 70, adopted status, pervasive developmental disorders, schizophrenia, epilepsy or other major medical or neurologic disorder, baseline substance dependency or pregnancy, and manic symptoms only while taking potential mania-inducing medications (e.g., antidepressants, stimulants). Subjects who developed substance use disorders or became pregnant during the follow-up study were continued on the protocol (Geller et al., 2008). There were no family psychopathology exclusions (Geller et al., 2006).
Rationales for these criteria were as follows. The duration criterion was similar to conservative duration in multiple nosologic schemas and was longer than the duration criterion of DSM-IV. Because this was a phenomenology study of child mania, current episodes of mania, or mixed mania, were required (Geller et al., 2002b). The cardinal symptom criterion avoided diagnosing mania only by symptoms that overlapped with those of attention-deficit/hyperactivity disorder (ADHD) (e.g., distractibility, hyperactivity). A lower age of 7.0 years was selected for credibility of interview assessments, and an upper age of 16.11 years was chosen so that subjects would still be teenagers at the 2-year follow-up assessment (Geller et al., 2002a). A CGAS score of ≤60 was chosen to ensure clinical impairment. Presence of substance use disorders and/or pregnancy at baseline was an exclusion criterion to avoid confounding the diagnosis of child BP-I with mental status effects of substance use or gestational state. This exclusion did not alter entrance into the study, however, because of the prepubertal age of the subjects (Geller et al., 2002b). Subjects could not be adopted, because of concurrent family and molecular genetic studies (Geller et al., 2002b).
Assessment
Comprehensive assessments were obtained by highly experienced research nurses with established interrater reliability (Geller et al., 2001). These included the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS), a semistructured interview that was administered separately to mothers about their children and to children about themselves(Geller et al., 1996). To avoid bias, different raters were used for the mother and child within each family. Mother and child responses on the WASH-U-KSADS were combined by using the most severe, as described by Bird et al. (Bird et al., 1992).
Consensus conferences were held to establish DSM-IV consensus diagnoses. In these meetings, a child psychiatrist (B.G.) and the research nurses who administered the assessments reviewed all assessment instruments, videotapes, school reports, agency records, and pediatrician records.
Family Study Assessments
Methods for assessing siblings under age 19 were exactly the same as those used to assess the probands. The Schedule for Affective Disorders and Schizophrenia–Lifetime Bipolar Version modified for DSM-IV was administered to parents and siblings aged 19.0 years and older by experienced research nurses blind to any information about the probands. The Global Assessment Scale (the adult counterpart of the GAS) was also completed.
Each DSM-IV diagnostic category was scored on a 6-point scale. Scores were as follows: 1= relative had no psychopathology in that diagnostic area; 2= relative had doubtful pathology; 3= relative fit criteria for the diagnosis but had no clinical impairment; 4= relative had all but 1 symptom of a diagnosis and there was significant clinical impairment; 5= relative met all criteria with definite clinical impairment; and 6= relative met all criteria with very severe impairment. Only diagnoses with scores of 4, 5, or 6, which signified definite clinical impairment, were counted for the analyses in this communication (Geller et al., 2006). No DSM-IV hierarchies were used in assigning relative diagnoses. In relatives, BP-I was diagnosed using the same criteria used for probands.
After complete description of the study, parents and adult siblings signed informed consent forms and probands and siblings under the age of 18.0 signed assent forms. Procedures were approved by the Office for Human Research Protection, Human Studies Committee, at Washington University in Saint Louis, and are in accordance with the Helsinki Declaration of 1975.
DNA specimen collection and Genotyping
DNA samples were previously collected, and isolated from whole blood, from a cohort of families of child BP-I patients (Geller et al., 2002b). 33 SNPs spanning the EGR1, EGR2, EGR3 and EGR4 genes were selected from the National Center for Biotechnology Information (NCBI) website databases: Map Viewer and the Database of Single Nucleotide Polymorphisms (dbSNP). High-density genotyping of SNPs was performed using the SNPLEX™ 48-plex system (Applied Biosystems Inc., Foster City, CA), blind to sample identification. The SNPlex probe (Applied Biosystems, w0605102411) was designed to genotype 33 known SNPs spanning the entire EGR1, 2, 3, and 4 genes, (which are all small genes, ranging between 2.2–2.8 kb) and proximal upstream and downstream regions of each gene (exact genomic locations of SNPs are provided in Table 2). Preference was given to SNPs within the coding region and intron of each gene. The total number of SNPs selected was based upon the SNPLex genotyping methodology used, and were the subset from a larger, selected group which were predicted to work as an ensemble in the assay based upon the company’s proprietary computational analysis. Of the 33 SNPs, 28 were successful in the SNPlex probe and produced bi-allelic genotypes. The remaining 5 failed in the assay or produced mono-allelic results (i.e. there was no variation at this genomic location in our samples).
Table 2. SNPs genotyped in child BP-I families and reliability of individual SNP results.
The two EGR3 SNPs with nominally significant p values (rs10104039 and rs10095121) are indicated in bold.
| SNP | Chromosome | Position | Gene | N Unreliable Genotypes |
|---|---|---|---|---|
| rs3813224 | 2 | 73370272 | EGR4 | 0 |
| rs2229294 | 2 | 73372041 | EGR4 | 0 |
| rs6718289 | 2 | 73374674 | EGR4 | 0 |
| rs6747506 | 2 | 73375390 | EGR4 | 1 |
| rs1522928 | 2 | 73379045 | EGR4 | 0 |
| rs1548587 | 5 | 137820638 | EGR1 | 0 |
| rs7729723 | 5 | 137825279 | EGR1 | 1 |
| rs1800778 | 5 | 137828912 | EGR1 | 0 |
| rs11743810 | 5 | 137830303 | EGR1 | 0 |
| rs6914 | 5 | 137832534 | EGR1 | 0 |
| rs11741807 | 5 | 137833551 | EGR1 | 0 |
| rs2051861 | 5 | 137834686 | EGR1 | 0 |
| rs17088536 | 8 | 22593172 | EGR3 | 0 |
| rs10104039 | 8 | 22594329 | EGR3 | 0 |
| rs10095121 | 8 | 22594371 | EGR3 | 0 |
| rs1996147 | 8 | 22600103 | EGR3 | 2 |
| rs3750192 | 8 | 22604735 | EGR3 | 0 |
| rs1008949 | 8 | 22609566 | EGR3 | 3 |
| rs4535741 | 8 | 22612369 | EGR3 | 1 |
| rs4872533 | 8 | 22615638 | EGR3 | 0 |
| rs4520160 | 8 | 22617829 | EGR3 | 12 |
| rs4295666 | 8 | 22618037 | EGR3 | 2 |
| rs2090052 | 8 | 22619040 | EGR3 | 1 |
| rs11775096 | 8 | 22623299 | EGR3 | 0 |
| rs10128333 | 10 | 64240044 | EGR2 | 0 |
| rs1509963 | 10 | 64240417 | EGR2 | 0 |
| rs2295814 | 10 | 64241282 | EGR2 | 0 |
| rs2297488 | 10 | 64245251 | EGR2 | 0 |
N = number of events.
Genotyping was performed according to the manufacturer’s protocol. Briefly, 120ng samples of genomic DNA were used for oligonucleotide ligation assay (OLA). OLA product was exonuclease-purified and PCR-amplified. Biotinylated strands were captured onto streptavidin-coated plates. Fluorescently labeled ZipChute™ probes bound to single stranded DNA SNPs were eluted and detected by capillary electrophoresis. Amplifications and sequencing were performed using Gene Amp 9700 PCR cyclers and an ABI 3730XL DNA analyzer. Genotype-calling was performed using the GeneMapper ®4.0 program from Applied Biosystems and confirmed by a second investigator. All plates contained positive and negative control wells. For quality control (QC), all DNA samples were genotyped with the SNPlex probe twice (two separate plates), and genotype-calling was performed blind to sample identification. Mismatched replicates were reviewed by eye. If the inconsistency was not easily explained then the SNP was removed from analysis. Additional heritability QC was performed by confirming that genotypes of the offspring were compatible with those of the parents. The number of trios (families) found to produce incompatible (“unreliable”) results for each SNP in the probe is listed in the last column of table 2. Data from the trios that produced an incompatible result was removed from analysis for the SNP that produced the incompatible result.
Statistical Analyses
Genotype data were analyzed using Family Based Association Test (FBAT) (http://www.biostat.harvard.edu/~fbat/fbat.htm) software (Laird and Lange, 2006). The FBAT is an extension of the Transmission Disequilibrium Test (TDT), a genetic analysis conducted on family trios (affected child and both parents) which assesses whether one allele is inherited in affected children at a rate greater than that predicted by Mendelian inheritance patterns (Laird and Lange, 2006). The FBAT allows inclusion of data that cannot be analyzed in the strict TDT, such as families that are missing a parent. Advantages of these methodologies over other population-based approaches include that they test for both association and linkage, and are not susceptible to population stratification, which can confound case-control studies (Laird and Lange, 2006). SNPs that showed significant preferential transmission (p<0.05) of the minor allele (that which occurs with lower frequency in the population) were then included in haplotype analyses. The Bonferroni test was used to control for multiple comparisons. PBAT (GoldenHelix) (Laird and Lange, 2006) was used to estimate power, using the “MOI (Model of inheritance), p (disease allele frequency), K (disease prevalence), AF (genetic attributable factor)” mode with MOI = additive, K = 0.05 and AF = 0.1 and p ranging from 0.1 to 0.9.
Linkage Disequilibrium Analyses
Haploview (Barrett et al., 2005) was used to estimate Linkage Disequilibrium between SNPs for the EGR3 region using study genotype data and CEU Hapmap (Frazer et al., 2007) data release 28. The EGR3 region on chromosome 8, from 22,593,172 to 22,623,299, included 12 SNPs genotyped in this study and 35 SNPs in the CEU Hapmap individuals. Hapmap Genome Browser release 28 (http://hapmap.ncbi.nlm.nih.gov/) with Copy Number Variation Region Track enabled was used to identify known CNVs in the EGR3 region on chromosome 8.
Results
To test the hypothesis that polymorphisms in EGR-family IEGs are associated with child BP-I, we genotyped SNPs across each of the four EGR genes in 49 child BP-I probands from 49 independent families. Genotype data were available for both parents for all but 2 of the families. Characteristics of the sample are presented in Table 1. Table 2 lists the SNPs tested, their genomic location, and the number of unreliable genotype results by SNP. A genotyping result is determined to be unreliable for a family when the genotype of the offspring is incompatible with those of the parents (e.g., if the child was C-C, but the mother was C-C and the father was T-T). All but 7 of the 28 SNPs listed in Table 1 produced reliable genotype results in all 49 families. One SNP, rs4520160, produced a high percentage of unreliable results (12/49 = 24.5%) indicating that this particular SNP was not well assayed by our probe.
Table 1.
Subject Characteristics
| Characteristic | Mean | SD |
|---|---|---|
| Age | 10.9 | 2.8 |
| Age of current mania episode onset | 7.7 | 3.7 |
| Duration of current mania episode | 3.2 | 2.7 |
| CGAS score | 43.9 | 8.0 |
| % | N | |
| Sex | ||
| Female | 32.7 | 16 |
| Male | 67.3 | 33 |
| Pubertal status | ||
| Prepubertal | 59.2 | 29 |
| Pubertal | 40.8 | 20 |
| Race | ||
| Caucasian | 87.8 | 43 |
| African American | 2.0 | 1 |
| Hispanic | 2.0 | 1 |
| Other | 8.2 | 4 |
| Psychosis | 57.1 | 28 |
| Mixed mania (MDD) | 63.3 | 31 |
| Suicidality | 20.4 | 10 |
| Elated mood | 79.6 | 39 |
| Grandiosity | 75.5 | 37 |
| Mother BP-I | 22.4 | 11 |
| Father BP-I | 36.7 | 18 |
| Both parents BP-I | 6.1 | 3 |
| Sibling BP-I* | 27.5 | 11 |
N=40 subjects had siblings
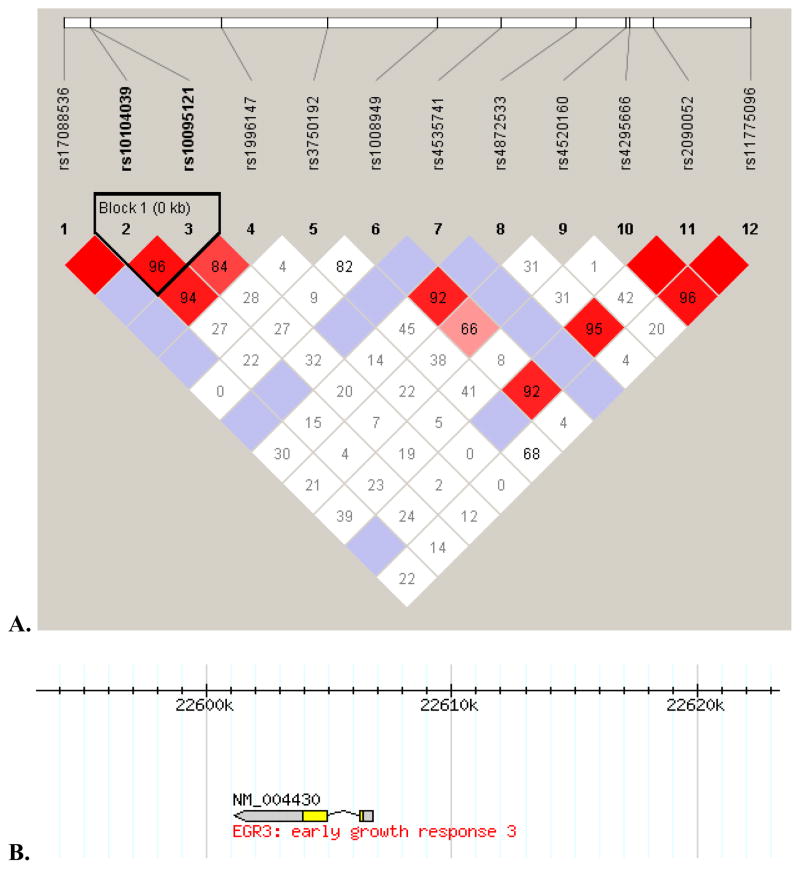
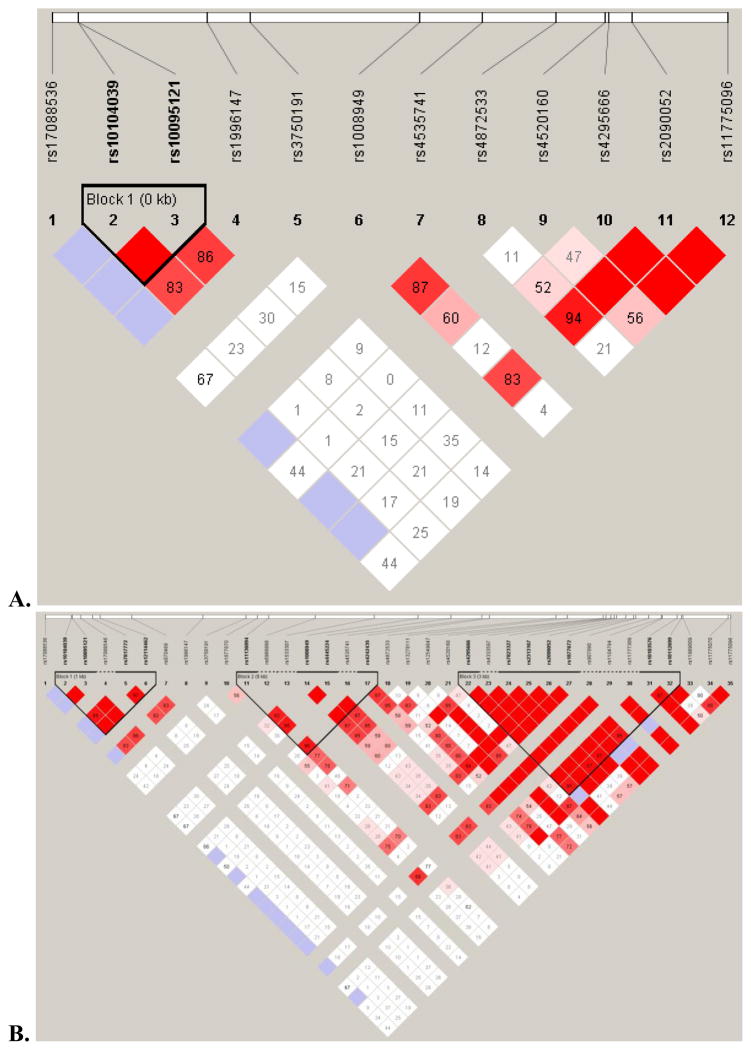
The genotypes of the affected proband and one or both parents were analyzed for preferential inheritance of specific alleles for each SNP using FBAT. The frequency of parental rs10095121 T alleles was 76.6%, and the frequency of parental rs10104039 C alleles was 71.1%. Proband alleles were in Hardy-Weinberg equilibrium for both SNPs (rs10095121: χ2=0.41, p=0.817; rs10104039: χ2=0.04, p=0.982). Two SNPs in EGR3 demonstrated preferential inheritance of the minor allele with significance values p=0.027 and p=0.028 (Table 3a). These values were not significant following Bonferroni correction for multiple comparisons (which would require p<0.0018). We then analyzed haplotypes for association with child BP-I using the Family-Based Haplotype Test (Table 3b). In 22 informative families, the rs10095121 C allele and the rs10104039 T allele were preferentially transmitted, and in 24 informative families, the rs10095121 T allele and the rs10104039 C allele were preferentially transmitted, indicating that rs10095121 and 10104039 are in LD. Figures 1 and 2 highlight the strong LD pattern between the rs10104039 and rs10095121 SNPs found in proximity to EGR3. The LD pattern observed in the study genotype data (Figure 1) is similar to the LD pattern for the same region in the Hapmap CEU individuals (Figure 2).
Table 3.
Single Marker and Haplotype Association Findings with P values <0.05 in Analyses of Child BP-I-Affected Families
| a. | ||||
|---|---|---|---|---|
| Family-Based Association Test of rs10095121 in 26 Informative Families | ||||
| SNP | Allele | Allele Frequency | χ2 | p |
| rs10095121 | C | 0.234 | 4.83 | 0.028 |
| rs10095121 | T | 0.766 | ||
| Family-Based Association Test of rs10104039 in 28 Informative Families | ||||
| SNP | Allele | Allele Frequency | χ2 | p |
| rs10104039 | C | 0.711 | 4.90 | 0.027 |
| rs10104039 | T | 0.289 | ||
| b. | |||||
|---|---|---|---|---|---|
| Family-Based Haplotype Test of rs10095121 and rs10104039 | |||||
| rs10095121 | rs10104039 | Haplotype Frequency | N Families | χ2 | p |
| C | C | 0.006 | 7 | 0.75 | 0.387 |
| C | T | 0.250 | 22 | 6.06 | 0.014 |
| T | C | 0.682 | 24 | 4.95 | 0.026 |
| T | T | 0.061 | 10 | 0.00 | 0.966 |
χ2, Chi-square test
Figure 1. Linkage Disequilibrium in the region of EGR3.
A. LD plot for the 30kbp region on chromosome 8 (22,593,172 to 22,623,299) that encompasses the 12 EGR3 SNPs studied in this article. Strong LD shown between the two EGR3 SNPs (rs10104039 and rs10095121) highlighted in this article. B. EGR3 overlayed on top of chromosome 8: 22,593,172 to 22,623,299 region.
Figure 2. A similar LD pattern is observed in CEU data as in the study sample.
A. The same 12 SNPs in the CEU data. B. All 35 CEU Hapmap SNPs for the same region on chromosome 8 (22,593,172 to 22,623,299).
Power was calculated using estimates for unknown parameters, (such as disease prevalence, the inheritance model, and the genetic attributable fraction,) and it was assumed that the disease risk allele was analyzed in our sample. Assuming values of 0.05 for disease prevalence (K), 0.1 for genetic attributable fraction (AF), and an additive model of inheritance (which may better reflect the etiology of complex diseases such as bipolar disorder) then the estimated power to detect a significant association ranges from 0.05 to 0.15 (for disease allele frequencies ranging from 0.9 to 0.1) for a sample size of N = 50 families with 1 affected proband. These parameter ranges indicate that our sample size of 49 families is underpowered to detect an association in the SNPs, should one truly exist. Increasing the number of families to N = 100, 200 and 500 would increase the power to detect a positive association to 0.27, 0.49 and 0.87, respectively, for disease allele frequencies of 0.1.
Discussion
The objective of the current study was to test the hypothesis that polymorphisms in the EGRs, genes involved the response and adaptation to stress in animal studies (Fish et al., 2004; Gallitano-Mendel et al., 2007; Senba and Ueyama, 1997; Weaver et al., 2004; Weaver et al., 2007), may show associations with child BP-I, a mental illness influenced by stress (Geller et al., 2008; Geller et al., 2004b). Interestingly, although each of the EGR genes maps to a chromosomal region relevant for mental illness, and EGR1, EGR2, and EGR3 each map to loci that have specifically been implicated in bipolar disorder, only SNPs in EGR3 demonstrated statistically significant associations. Several studies have examined the EGR genes themselves for genetic associations with psychotic disorders. Notably, only EGR3 has produced positive findings. Yamada and colleagues (Yamada et al., 2007) examined SNPs across all four EGR genes for association with schizophrenia in a Japanese population, and reported that EGR3 demonstrated significant associations. Kim and colleagues (Kim et al.) assessed SNPs in EGR2 and EGR3 for association with schizophrenia in a Korean population and found a positive association with EGR3. A recent study examining whether circadian-regulated genes may influence the risk for bipolar disorder in adults, found that, of the 21 genes tested, only EGR3 displayed a nominally significant association result (Mansour et al., 2009). However, as seen with studies on other candidate genes, other studies have failed to identify associations between EGR3 (or other EGRs) and schizophrenia; one in a Chinese population and another in a second, independent Japanese population (Kyogoku et al.; Liu et al.). The SNP which showed nominal association in the BP-I circadian rhythm study (rs1996147) (Mansour et al., 2009) did not produce a significant association in our sample. The EGR3 SNP showing association in the schizophrenia studies (rs35201266) was not included in our assay probe, synthesis of which preceded publication of these studies.
Over the last several years a number of Genome-Wide Association Studies (GWAS) have been performed in BP-I disorder (reviewed in (Gershon et al., 2011; O’Donovan et al., 2009; Sullivan, 2010)). While initial hopes were that this approach would reveal a small number of genes that were responsible for the majority of risk for the illness, this has not been the case for BP-I, or for any of the psychiatric illnesses. In fact, genes identified in GWAS fail to account for the major portion of heritable variance for these illnesses (Gershon et al., 2011).
The fact that EGR3 has not appeared as a significant gene in these GWASs is not surprising due to its small size (2.5 kb), and consequent few SNPs represented in GWAS compared with larger genes. It is also consistent with the current consensus in the field that the contribution of any individual gene toward risk for psychotic illnesses is likely to be small (Gershon et al.; O’Donovan et al., 2009; Sullivan).
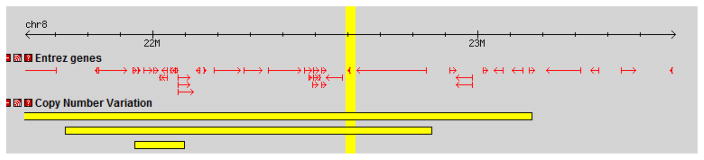
Another type of genomic variation, copy number variation (CNVs), can now also be investigated in GWAS, and is a current area of focus in the field (Tam et al., 2009)(Ref Gershon, 2011). CNVs have shown highly significant associations in schizophrenia studies, though their relative rare occurrence limits the percentage of illness risk that they may account for (Tam et al., 2009). It will be of great interest to see whether these associations are also found for BP-I. This is of particular relevance to the current study as our investigation indicated that EGR3 resides in a CNV region of the genome (Figure 3), suggesting an area for future study.
Figure 3. EGR3 resides in a region of Copy Number Variation.
The EGR3 region on chromosome 8, highlighted as a vertical band in the middle of the figure, is known to be part of a Copy Number Variant (CNV) region (horizontal bars). The small arrow within the vertical yellow band is EGR3. The entire figure (left to right) spans approximately 2 Mbp on chromosome 8.
A recent study analyzed the results of GWAS studies in BP-I using a “convergent functional genomics” approach, and concluded that both BDNF and NRG1 were leading candidate genes for the illness (Patel et al.). Since EGR3 is regulated by these proteins, this suggests the possibility that dysfunction in these proteins may each influence risk based on their roles in a shared biological pathway, the function of which would presumably be critical for mental health. As further support for this hypothesis, a recent biomedical informatics study investigating a network of transcription factors and micro RNAs implicated in schizophrenia risk identified EGR3 as the central gene in the network (Guo et al.). They also highlighted that EGR3 activation is the final common element in several pathways associated with illness risk, including those downstream of NRG1, NMDA receptor activation, and neurotrophins such as BDNF (Guo et al.). Indeed the field of psychiatry is now following the lead of other fields, such as cancer biology and immunology, in focusing on biological pathways, which may help account for the fact that numerous genes of small effect-size appear to influence risk for these illnesses.
Limitations
Our study has several limitations. The first of these is that testing for numerous SNPs requires statistical testing to control for multiple comparisons; the nominally significant associations we have identified do not survive this significance testing. Therefore these results must be considered with caution, and will need to be replicated in one or more independent, and ideally larger, samples. The first limitation is directly related to the second, which is the small size of our test sample. The power analyses we conducted using the PBAT indicate that this sample size is underpowered to detect a significant risk allele across a broad range of potential frequencies of occurrence of this allele in the populations (ranging from 10% to 90%) under our model assumptions. The fact that we were still able to identify two SNPs (which are in LD with one another) with nominally significant associations underscores the interest of EGR3 for further investigation. Increasing the sample size would increase the power to detect significant associations that could possibly survive the stringent Bonferroni correction for multiple comparisons.
The genotyping methodology we employed, SNPlex, carries additional limitations. Although advances in genomic and computational technologies allow us to predict characteristics that may enhance or decrease the potential for amplifying a specific region of DNA, we are still far from the ideal of having 100% success rate in multiplex probe assays, such as SNPlex. Factors that may have contributed to error in the function of our SNPlex probe include GC-rich DNA regions and epigenetic events including methylation and histone acetylation which influence how available the DNA strands are to primer binding and amplification. These may account for some of the problems encountered with the SNPlex probe designed for our study. In particular, 5 of the SNP assays included in the probe either failed to produce results or indicated no variation in the population (i.e. the DNA nucleotide was not polymorphic).
One of the advantages of our family-based study was that it allowed us to identify additional errors by analyzing inherited genotypes for incompatibility. Events in which an inherited genotype is incompatible with those of the parents are labeled as “unreliable” events in table 2. One SNP in particular, rs4520160, had a 24.5% unreliable rate, indicating that our assay is not accurate for this SNP. The fact that the majority of SNPs (21/28) produced no unreliable genotypes in any of the 49 families tested, is encouraging. Unreliable rates of 1/49–3/49 (2% – 6%) in 6 SNPs (see Table 2) suggest that the results of these SNPs should be considered with caution. Also encouraging is our finding of correlation between adjacent markers (SNPs rs10095121 and rs10104039 appear to be in LD), as it decreases the likelihood that the finding results from assay error.
Conclusion
The ability to detect a preliminary association for EGR3 in our study of limited sample size makes this gene of particular interest for further investigation in a larger follow-up association study of child BP-I families.
Acknowledgments
We are grateful to Edwin H. Cook, Jr., M.D., University of Illinois, Chicago, for prior isolation of DNA from blood samples, En-Tan Zhang, M.D., Ph.D., Leela Muppana, M.S., and Mohammad Torabi, Ph.D., and Amber Monib, University of Arizona College of Medicine–Phoenix, for technical and statistical assistance, and Jeffrey Sutherland, B.S., NIDDK, for assistance with the SNPlex protocol. This work was supported by the Pfizer Fellowship in Biological Psychiatry Grant (to ALG), a NARSAD/Sidney R Baer Jr. Foundation Young Investigator Award (to ALG), an ASU/Mayo Clinic Seed Grant (to VD), NIMH MH-53063 (to BG), MH-57451 (to BG), and the Theodore and Vada Stanley Foundation (to BG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Bird HR, Canino G, Rubio-Stipec M, Ribera JC. Further measures of the psychometric properties of the Children’s Global Assessment Scale. Arch Gen Psychiatry. 1987;44:821–824. doi: 10.1001/archpsyc.1987.01800210069011. [DOI] [PubMed] [Google Scholar]
- Bird HR, Gould MS, Staghezza B. Aggregating data from multiple informants in child psychiatry epidemiological research. J Am Acad Child Adolesc Psychiatry. 1992;31:78–85. doi: 10.1097/00004583-199201000-00012. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Pillai A, Howell KR. Brain-derived neurotrophic factor: findings in schizophrenia. Curr Opin Psychiatry. 24:122–127. doi: 10.1097/YCO.0b013e3283436eb7. [DOI] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- Fahmy RG, Dass CR, Sun LQ, Chesterman CN, Khachigian LM. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med. 2003;9:1026–1032. doi: 10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, Meaney MJ. Epigenetic Programming of Stress Responses through Variations in Maternal Care. Ann N Y Acad Sci. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW, Boudreau A, Hardenbol P, Leal SM, Pasternak S, Wheeler DA, Willis TD, Yu F, Yang H, Zeng C, Gao Y, Hu H, Hu W, Li C, Lin W, Liu S, Pan H, Tang X, Wang J, Wang W, Yu J, Zhang B, Zhang Q, Zhao H, Zhao H, Zhou J, Gabriel SB, Barry R, Blumenstiel B, Camargo A, Defelice M, Faggart M, Goyette M, Gupta S, Moore J, Nguyen H, Onofrio RC, Parkin M, Roy J, Stahl E, Winchester E, Ziaugra L, Altshuler D, Shen Y, Yao Z, Huang W, Chu X, He Y, Jin L, Liu Y, Shen Y, Sun W, Wang H, Wang Y, Wang Y, Xiong X, Xu L, Waye MM, Tsui SK, Xue H, Wong JT, Galver LM, Fan JB, Gunderson K, Murray SS, Oliphant AR, Chee MS, Montpetit A, Chagnon F, Ferretti V, Leboeuf M, Olivier JF, Phillips MS, Roumy S, Sallee C, Verner A, Hudson TJ, Kwok PY, Cai D, Koboldt DC, Miller RD, Pawlikowska L, Taillon-Miller P, Xiao M, Tsui LC, Mak W, Song YQ, Tam PK, Nakamura Y, Kawaguchi T, Kitamoto T, Morizono T, Nagashima A, Ohnishi Y, Sekine A, Tanaka T, Tsunoda T, Deloukas P, Bird CP, Delgado M, Dermitzakis ET, Gwilliam R, Hunt S, Morrison J, Powell D, Stranger BE, Whittaker P, Bentley DR, Daly MJ, de Bakker PI, Barrett J, Chretien YR, Maller J, McCarroll S, Patterson N, Pe’er I, Price A, Purcell S, Richter DJ, Sabeti P, Saxena R, Schaffner SF, Sham PC, Varilly P, Altshuler D, Stein LD, Krishnan L, Smith AV, Tello-Ruiz MK, Thorisson GA, Chakravarti A, Chen PE, Cutler DJ, Kashuk CS, Lin S, Abecasis GR, Guan W, Li Y, Munro HM, Qin ZS, Thomas DJ, McVean G, Auton A, Bottolo L, Cardin N, Eyheramendy S, Freeman C, Marchini J, Myers S, Spencer C, Stephens M, Donnelly P, Cardon LR, Clarke G, Evans DM, Morris AP, Weir BS, Tsunoda T, Mullikin JC, Sherry ST, Feolo M, Skol A, Zhang H, Zeng C, Zhao H, Matsuda I, Fukushima Y, Macer DR, Suda E, Rotimi CN, Adebamowo CA, Ajayi I, Aniagwu T, Marshall PA, Nkwodimmah C, Royal CD, Leppert MF, Dixon M, Peiffer A, Qiu R, Kent A, Kato K, Niikawa N, Adewole IF, Knoppers BM, Foster MW, Clayton EW, Watkin J, Gibbs RA, Belmont JW, Muzny D, Nazareth L, Sodergren E, Weinstock GM, Wheeler DA, Yakub I, Gabriel SB, Onofrio RC, Richter DJ, Ziaugra L, Birren BW, Daly MJ, Altshuler D, Wilson RK, Fulton LL, Rogers J, Burton J, Carter NP, Clee CM, Griffiths M, Jones MC, McLay K, Plumb RW, Ross MT, Sims SK, Willey DL, Chen Z, Han H, Kang L, Godbout M, Wallenburg JC, L’Archeveque P, Bellemare G, Saeki K, Wang H, An D, Fu H, Li Q, Wang Z, Wang R, Holden AL, Brooks LD, McEwen JE, Guyer MS, Wang VO, Peterson JL, Shi M, Spiegel J, Sung LM, Zacharia LF, Collins FS, Kennedy K, Jamieson R, Stewart J. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148:633–643. doi: 10.1016/j.neuroscience.2007.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH., Jr Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004a;161:1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- Geller B, Craney JL, Bolhofner K, Nickelsburg MJ, Williams M, Zimerman B. Two-year prospective follow-up of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2002a;159:927–933. doi: 10.1176/appi.ajp.159.6.927. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Bolhofner K, Zimerman B. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B, Tillman R, Bolhofner K, Zimerman B, Strauss NA, Kaufmann P. Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: morbid risk, age at onset, and comorbidity. Arch Gen Psychiatry. 2006;63:1130–1138. doi: 10.1001/archpsyc.63.10.1130. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004b;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- Geller B, Williams M, Zimerman B, Frazier J. Washington University in St Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) Washington University; St. Louis, MO: 1996. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo C. Reliability of the Washington University in St. Louis Kiddie Schedule for Affective Disorders and Schizophrenia (WASH-U-KSADS) mania and rapid cycling sections. J Am Acad Child Adolesc Psychiatry. 2001;40:450–455. doi: 10.1097/00004583-200104000-00014. [DOI] [PubMed] [Google Scholar]
- Geller B, Zimerman B, Williams M, Delbello MP, Bolhofner K, Craney JL, Frazier J, Beringer L, Nickelsburg MJ. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002b;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- Gerber DJ, Hall D, Miyakawa T, Demars S, Gogos JA, Karayiorgou M, Tonegawa S. Evidence for association of schizophrenia with genetic variation in the 8p21.3 gene, PPP3CC, encoding the calcineurin gamma subunit. Proc Natl Acad Sci U S A. 2003;100:8993–8998. doi: 10.1073/pnas.1432927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon ES, Alliey-Rodriguez N, Liu C. After GWAS: searching for genetic risk for schizophrenia and bipolar disorder. Am J Psychiatry. 2011;168:253–256. doi: 10.1176/appi.ajp.2010.10091340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23:8836–8843. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, Ruan WJ, Huang B. Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2005;66:1205–1215. doi: 10.4088/jcp.v66n1001. [DOI] [PubMed] [Google Scholar]
- Guo AY, Sun J, Jia P, Zhao Z. A novel microRNA and transcription factor mediated regulatory network in schizophrenia. BMC Syst Biol. 4:10. doi: 10.1186/1752-0509-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson DR, Gottesman II. Theories of schizophrenia: a genetic-inflammatory-vascular synthesis. BMC Med Genet. 2005;6:7. doi: 10.1186/1471-2350-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer S, Shneider NA, Birchmeier C, Burden SJ, Jessell TM, Arber S. A role for neuregulin1 signaling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Signals that determine Schwann cell identity. J Anat. 2002;200:367–376. doi: 10.1046/j.1469-7580.2002.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AL, Mowry BJ, Pender MP, Greer JM. Immune dysregulation and self-reactivity in schizophrenia: do some cases of schizophrenia have an autoimmune basis? Immunol Cell Biol. 2005;83:9–17. doi: 10.1111/j.1440-1711.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, Charnay P, Bozon B, Laroche S, Davis S. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. 2007;61:3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Song JY, Joo EJ, Lee KY, Ahn YM, Kim YS. EGR3 as a potential susceptibility gene for schizophrenia in Korea. Am J Med Genet B Neuropsychiatr Genet. 153B:1355–1360. doi: 10.1002/ajmg.b.31115. [DOI] [PubMed] [Google Scholar]
- Kyogoku C, Yanagi M, Nishimura K, Sugiyama D, Morinobu A, Fukutake M, Maeda K, Shirakawa O, Kuno T, Kumagai S. Association of calcineurin A gamma subunit (PPP3CC) and early growth response 3 (EGR3) gene polymorphisms with susceptibility to schizophrenia in a Japanese population. Psychiatry Res. 185:16–19. doi: 10.1016/j.psychres.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Laird NM, Lange C. Family-based designs in the age of large-scale gene-association studies. Nat Rev Genet. 2006;7:385–394. doi: 10.1038/nrg1839. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebermann DA, Hoffman B. Myeloid differentiation (MyD)/growth arrest DNA damage (GADD) genes in tumor suppression, immunity and inflammation. Leukemia. 2002;16:527–541. doi: 10.1038/sj.leu.2402477. [DOI] [PubMed] [Google Scholar]
- Liu BC, Zhang J, Wang L, Li XW, Wang Y, Ji J, Yang FP, Wan CL, Gao LH, Xu YF, Feng GY, He L, Zhao XZ, He G. No association between EGR gene family polymorphisms and schizophrenia in the Chinese population. Prog Neuropsychopharmacol Biol Psychiatry. 34:506–509. doi: 10.1016/j.pnpbp.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Maier W. Common risk genes for affective and schizophrenic psychoses. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):37–40. doi: 10.1007/s00406-008-2008-z. [DOI] [PubMed] [Google Scholar]
- Manchia M, Zai CC, Squassina A, Vincent JB, De Luca V, Kennedy JL. Mixture regression analysis on age at onset in bipolar disorder patients: investigation of the role of serotonergic genes. Eur Neuropsychopharmacol. 20:663–670. doi: 10.1016/j.euroneuro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Talkowski ME, Wood J, Chowdari KV, McClain L, Prasad K, Montrose D, Fagiolini A, Friedman ES, Allen MH, Bowden CL, Calabrese J, El-Mallakh RS, Escamilla M, Faraone SV, Fossey MD, Gyulai L, Loftis JM, Hauser P, Ketter TA, Marangell LB, Miklowitz DJ, Nierenberg AA, Patel J, Sachs GS, Sklar P, Smoller JW, Laird N, Keshavan M, Thase ME, Axelson D, Birmaher B, Lewis D, Monk T, Frank E, Kupfer DJ, Devlin B, Nimgaonkar VL. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disord. 2009;11:701–710. doi: 10.1111/j.1399-5618.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu F, Miot S, Etain B, El Khoury MA, Chevalier F, Bellivier F, Leboyer M, Giros B, Tzavara ET. Association between the PPP3CC gene, coding for the calcineurin gamma catalytic subunit, and bipolar disorder. Behav Brain Funct. 2008;4:2. doi: 10.1186/1744-9081-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D’Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexal S, Frank M, Berger R, Adams CE, Ross RG, Freedman R, Leonard S. Differential modulation of gene expression in the NMDA postsynaptic density of schizophrenic and control smokers. Brain Res Mol Brain Res. 2005;139:317–332. doi: 10.1016/j.molbrainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987;238:797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Mittelstadt PR, Ashwell JD. Cyclosporin A-sensitive transcription factor Egr-3 regulates Fas ligand expression. Mol Cell Biol. 1998;18:3744–3751. doi: 10.1128/mcb.18.7.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moises HW, Zoega T, Gottesman I. The glial growth factors deficiency and synaptic destabilization hypothesis of schizophrenia. BMC Psychiatry. 2002;2:8. doi: 10.1186/1471-244X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends in Neuroscience. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- O’Donovan MC, Craddock NJ, Owen MJ. Genetics of psychosis; insights from views across the genome. Hum Genet. 2009;126:3–12. doi: 10.1007/s00439-009-0703-0. [DOI] [PubMed] [Google Scholar]
- Patel SD, Le-Niculescu H, Koller DL, Green SD, Lahiri DK, McMahon FJ, Nurnberger JI, Jr, Niculescu AB., 3rd Coming to grips with complex disorders: genetic risk prediction in bipolar disorder using panels of genes identified through convergent functional genomics. Am J Med Genet B Neuropsychiatr Genet. 153B:850–877. doi: 10.1002/ajmg.b.31087. [DOI] [PubMed] [Google Scholar]
- Poirier R, Cheval H, Mailhes C, Garel S, Charnay P, Davis S, Laroche S. Distinct functions of egr gene family members in cognitive processes. Front Neurosci. 2008;2:47–55. doi: 10.3389/neuro.01.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, Brynjolfsson J, Gunnarsdottir S, Ivarsson O, Chou TT, Hjaltason O, Birgisdottir B, Jonsson H, Gudnadottir VG, Gudmundsdottir E, Bjornsson A, Ingvarsson B, Ingason A, Sigfusson S, Hardardottir H, Harvey RP, Lai D, Zhou M, Brunner D, Mutel V, Gonzalo A, Lemke G, Sainz J, Johannesson G, Andresson T, Gudbjartsson D, Manolescu A, Frigge ML, Gurney ME, Kong A, Gulcher JR, Petursson H, Stefansson K. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF. The psychiatric GWAS consortium: big science comes to psychiatry. Neuron. 2010;68:182–186. doi: 10.1016/j.neuron.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam GW, Redon R, Carter NP, Grant SG. The role of DNA copy number variation in schizophrenia. Biol Psychiatry. 2009;66:1005–1012. doi: 10.1016/j.biopsych.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Tang J, Xiao L, Shu C, Wang G, Liu Z, Wang X, Wang H, Bai X. Association of the brain-derived neurotrophic factor gene and bipolar disorder with early age of onset in mainland China. Neurosci Lett. 2008;433:98–102. doi: 10.1016/j.neulet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J, Faraone SV, Mick E, Monuteaux M, Coville A, Biederman J. A controlled family study of children with DSM-IV bipolar-I disorder and psychiatric co-morbidity. Psychol Med. 2009:1–10. doi: 10.1017/S0033291709991437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Gerber DJ, Iwayama Y, Ohnishi T, Ohba H, Toyota T, Aruga J, Minabe Y, Tonegawa S, Yoshikawa T. Genetic analysis of the calcineurin pathway identifies members of the EGR gene family, specifically EGR3, as potential susceptibility candidates in schizophrenia. Proc Natl Acad Sci U S A. 2007;104:2815–2820. doi: 10.1073/pnas.0610765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Andreasson KI, Worley PF. Egr3/Pilot, a zinc finger transcription factor, is rapidly regulated by activity in brain neurons and colocalizes with Egr1/zif268. Learn Mem. 1994;1:140–152. [PubMed] [Google Scholar]