Abstract
Patients with human immunodeficiency virus (HIV) infection are at increased risk of developing coronary heart disease (CHD). Although factors potentially contributing to this elevated risk include traditional CHD risk factors and antiretroviral medications, more recent data support a role for inflammatory and immunologic factors as central to a complex mechanism. Decreasing CHD risk among HIV-infected patients is likely to involve modification of inflammatory and immunologic factors through antiretroviral therapy or other novel strategies as well as targeted treatment of traditional CHD risk factors. This review will highlight epidemiologic data investigating the association between HIV and CHD outcomes. An overview of potential mechanistic factors associated with CHD in HIV infection and of strategies for managing CHD risk in HIV-infected patients is also included. Specific cardiovascular and metabolic risk factors, CHD risk prediction, and the immunologic basis for CHD in HIV-infected patients will be discussed in separate reviews.
Treatment for human immunodeficiency virus (HIV) infection has become increasingly accessible and effective in many clinical settings, with reductions in mortality rates attributable to the treatment of infectious complications of advanced HIV disease [1–3] and increased life expectancy [4, 5]. However, as HIV-related mortality has decreased, there has been a concomitant relative increase in the proportion of deaths attributable to noninfectious complications, including cardiovascular disease (CVD) [3, 6–9]. Non-AIDS events have specifically been shown to be associated with increased mortality rates relative to AIDS events [10, 11]. Moreover, CVD has been shown to impact morbidity, as demonstrated by a trend in the rise of CVD hospitalization rates [12]. The World Health Organization predicts that HIV/AIDS and ischemic heart disease will be in the top 3 causes for both global mortality and global disability-adjusted life-years in the year 2030, suggesting that an interdependency of these 2 diseases will pose a major global clinical and public health challenge [13].
CORONARY HEART DISEASE OUTCOMES COMPARING HIV-INFECTED PATIENTS WITH CONTROL PATIENTS
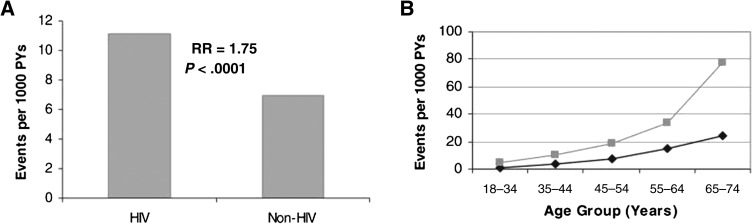
Studies investigating coronary heart disease (CHD) outcomes comparing HIV-infected patients with control patients have consistently shown HIV-infected patients to be at increased risk over time and across varied clinical settings. Coronary heart disease hospitalization rates were significantly higher in HIV-infected patients than in non-HIV-infected patients in an ongoing observational study of the Kaiser Permanente Medical Care Program of Northern California [14], with recently updated data showing a significantly increased adjusted rate ratio for CHD (rate ratio, 1.2; 95% confidence interval [CI], 1.1–1.4) and for acute myocardial infarction (AMI) (rate ratio, 1.4; 95% CI, 1.3–1.7) [15]. In a study of California Medicaid claims data including >3 million patients, CHD incidence was significantly increased in HIV-infected patients versus non-HIV-infected patients for men aged ≤34 years and women aged ≤44 years [16]. Data from the Partners HealthCare System in Boston have also demonstrated increased AMI incidence rates in HIV-infected patients versus non-HIV-infected patients, with a relative risk (RR) for AMI of 1.75 (95% CI, 1.51–2.02) in multivariate modeling adjusting for demographics and common CHD risk factors [17] (Figure 1). Comparing rates of first hospitalization for ischemic heart disease in Danish patients infected with HIV versus a population-based control group, HIV-infected patients had a significantly increased risk (adjusted RR, 2.12; 95% CI, 1.62–2.76) [18]. The incidence of AMI was increased in the French Hospital Database on HIV (FHDH) cohort compared with sex- and age-standardized rates from the general French population, with a standardized morbidity ratio of 1.5 (95% CI, 1.3–1.7) [19] and was similarly increased in a Quebec HIV cohort compared with a matched control group, with an adjusted incidence ratio of 2.11 (95% CI, 1.69–2.63) [20]. Finally, recent data from over 28 000 patients in the Veterans Administration (VA) system showed a significantly increased risk of AMI in HIV-infected patients versus non-HIV-infected patients, with an adjusted hazard ratio of 1.94 (95% CI, 1.58–2.37) [21]. Taken together, these data suggest that HIV infection confers a heightened risk of CHD that is independent of demographic characteristics or traditional vascular risk factors.
Figure 1.
A, Myocardial infarction rates and corresponding adjusted relative risk (RR). Bars indicate crude rates of acute myocardial infarction events per 1000 person-years (PYs) as determined by International Classification of Diseases, Ninth Revision (ICD-9) coding. The RR and associated P value are shown above the bars. The RR was determined from Poisson regression analysis adjusting for age, gender, race, hypertension, diabetes, and dyslipidemia. The associated 95% confidence interval for the RR shown is 1.51–2.02. B, Myocardial infarction rates by age group. Light line indicates patients diagnosed with human immunodeficiency virus (HIV) disease. Dark line indicates patients not diagnosed with HIV disease. Data shown include both sexes. Rates represent number of events per 1000 PYs as determined by ICD coding. From Triant et al [17], by permission of The Endocrine Society.
CHD IN SPECIFIC HIV POPULATIONS
Women
Mortality trends in HIV-infected women parallel those of the general HIV population, with decreasing AIDS-related mortality and increasing CVD-related mortality [22]. Several studies suggest that the impact of HIV infection on CHD is relatively greater for women compared with men. In the Partners cohort, the adjusted RR for AMI comparing HIV-infected patients with non-HIV-infected patients was 3.0 for women versus 1.4 for men [17]. Findings from the FHDH cohort closely paralleled these results, with standardized morbidity ratios for AMI comparing HIV-infected patients with non-HIV-infected patients greater for women (2.7) compared with men (1.4) [19]. Investigation of cardiovascular trends in HIV-infected women, however, is limited by the fact that women are traditionally underrepresented in both HIV clinical trials [23] and in cohort studies of HIV and CVD, with the proportion of female patients in several major studies ranging from 0% to 30% [14, 16, 17, 24, 25].
Hepatitis C Virus–Coinfected Patients
Whether coinfection with hepatitis C virus (HCV) alters CVD risk for HIV-infected patients is also an area of active investigation. In a study of the Veterans Aging Cohort Study Virtual Cohort, patients coinfected with HIV and HCV had a significantly higher risk of CHD compared with those who were HIV-infected alone or those who had neither infection [26]. Similarly, another study showed rates of CVD to be significantly higher among HIV/HCV coinfected patients versus HIV monoinfected patients in adjusted analyses, with a trend for an increase in AMI rates [27]. However, no association between HCV status and AMI was observed in another large HIV cohort [28].
POTENTIAL MECHANISTIC FACTORS CONTRIBUTING TO HIV-ASSOCIATED CHD
The mechanism of CHD among HIV-infected patients is likely to reflect a complex interplay of factors, including traditional CHD risk factors, antiretroviral drug effects, and HIV-related parameters, including inflammatory and immunologic changes. A multifactorial etiology is supported by the association of HIV with multiple vascular indices reflecting discrete and progressive stages of atherosclerosis, ranging from endothelial dysfunction [29–31] to increased carotid intima media thickness (a validated marker of preclinical atherosclerosis) [32–36] to coronary artery calcification [37, 38] to coronary plaque itself [39].
Traditional CHD Risk Factors
Coronary heart disease risk factors established in the general population—including smoking, dyslipidemia, diabetes, hypertension, and visceral adiposity—have been shown to be increased and to confer CHD risk in HIV populations [17, 40–50]. Metabolic factors associated with HIV infection are described in detail in reviews by Stanley et al and Harris et al (this supplement). Despite a plausible role for traditional CHD risk factors to be important contributors among HIV-infected patients, they are not likely to be solely responsible for elevated CHD risk in this group, as significant levels of risk have been shown to remain after adjustment for traditional CHD risk factors in multivariate analyses [17, 32]. The remaining risk might be explained in part by both antiretroviral medications and novel CHD risk factors including inflammation and immune dysfunction.
Antiretroviral Medications
Potential associations between antiretroviral drugs and AMI have been thought to explain increased CHD risk among HIV-infected patients, with early reports demonstrating an association between AMI and the protease inhibitor (PI) class of medications [51–53]. The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study group, examining the effects of antiretroviral therapy (ART) exposure on CHD outcomes in an observational cohort dating back to 1999, demonstrated an adjusted relative risk of AMI of 1.16 per year of ART exposure, largely driven by the PI class and not fully explained by elevated lipid indices [24]. In contrast, a large study from the VA system showed no effect between any ART class and CHD or cerebrovascular event outcomes [54]. The conflicting results might be attributable to differing stages of disease in the cohorts, with ongoing viral replication and consequent inflammation possibly obscuring adverse medication effects.
More recent analyses have focused on individual antiretroviral drugs. Following the finding that adjustment for the nucleoside reverse transcriptase inhibitor (NRTI) class of medications attenuated AMI risk in the D:A:D cohort, the group demonstrated recent use of abacavir to be associated with a relative risk of AMI of 1.90, an unanticipated finding given the absence of known metabolic effects [55]. Acute myocardial infarction rates were higher for abacavir users versus nonusers in each predicted CHD risk category, with the highest relative risk of AMI in the lowest predicted risk category (yet, as anticipated, higher absolute rates in the higher predicted risk categories for both abacavir users and nonusers) [55]. Subsequent investigations have shown significant associations of AMI with abacavir, didanosine, lopinavir/ritonavir, and indinavir in a follow-up D:A:D analysis [56] and with lopinavir/ritonavir, amprenavir/ritonavir, and fosamprenavir/ritonavir in an analysis from the FHDH cohort [57].
Multiple studies examining the potential abacavir-AMI association using alternate data sources have been presented or published since the initial D:A:D finding in 2008, with conflicting results (Table 1). Although some studies have confirmed the initial finding [20, 58–61], others, including 3 studies each pooling randomized controlled trial (RCT) data [62–64], have failed to demonstrate an association [57, 65, 66]. However, the RCTs included were not designed to specifically test the hypothesis that abacavir is related to AMI and may have been underpowered or lacked adjudicated outcome events. Furthermore, observational studies can be limited by confounding by indication, or channeling bias, which occurs when a risk factor for the outcome (eg, renal dysfunction) is also a factor that influences the primary exposure (eg, decision to treat with abacavir), thereby placing patients with the primary exposure at increased risk of the outcome [67]. Although directed investigation has supported several possible mechanisms for an association between abacavir and AMI, including platelet reactivity [68], endothelial dysfunction [30], inflammation [69, 70], T-cell activation [71], atherogenic lipid profiles [72], arterial stiffness [73], and leukocyte adhesion [74], additional studies have failed to demonstrate an association between the drug and biomarkers of inflammation or thrombosis [75–79]. The initial abacavir data prompted, in part, a Department of Health and Human Services (DHHS) guideline change removing abacavir as a first-line NRTI in 2008 [64], but intense study since that time has not provided clear resolution to the issue, and clinical decision making remains individualized.
Table 1.
Studies Investigating the Association of Abacavir With Cardiovascular Disease
| Study | No. (HIV) | Design | No. (Outcome) a | Effect | Effect Size (95% CI) |
| D:A:D [55] | 33 347 | Observational cohort | 517 | Yes | RR, 1.90 (1.47–2.45) |
| SMART [58] | 2752 | Observational RCT | 19 | Yes | HR, 4.3 (1.4–13.0) |
| GSK Repository [65] | 14 174 | Pooled RCTs | 27 | No | RR, 0.81 (.38–1.75) |
| STEAL [60] | 357 | RCT | 9 | Yes | HR, 0.12 (.02–.98) for TDF vs ABC use |
| Danish HIV Cohort [61] | 2952 | Prospective cohort | 67 | Yes | IRR, 2.00 (1.10–3.64) |
| FHDH [57] | 1173 | Nested case-control | 289 | No | OR, 1.27b (.64–2.49) |
| VHA CCR [66] | 19 424 | Observational cohort | 278 | No | HR, 1.18 (.92–1.50) |
| RAMQ, Med-Echo [20] | 7053 | Nested case-control | 139 | Yes | OR, 1.79 (1.16–2.76) |
| Meta-analysis [62] | 9233 | 28 RCT meta-analysis | 79 | No | RR, 0.73 (.39–1.35) |
| FDA meta-analysis [64] | 9832 | 26 RCT meta-analysis | 47 | No | OR, 1.02 (.56–1.84) |
| ALLRT [63] | 5056 | ACTG RCTs | 36 | No | HR, 0.7 (.2–2.4) |
| VA HIV CCR [59] | 10 931 | Observational cohort | 501 | Yes | HR, 1.48 (1.08–2.04) |
Abbreviations: ABC, abacavir; ACTG, AIDS Clinical Trials Group; ALLRT, AIDS Clinical Trials Group Longitudinal Linked Randomized Trials; CI, confidence interval; CCR, Clinical Case Registry; D:A:D, Data Collection on Adverse Events of Anti-HIV Drugs; FDA, Food and Drug Administration; FHDH, French Hospital Database on HIV; GSK, GlaxoSmithKline; HIV, human immunodeficiency virus; HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio; RAMQ, Regie de l’assurance-maladie du Quebec; RCT, randomized controlled trial; RR, relative rate (RR, risk ratio for Cruciani et al); SMART, Strategies for Management of Antiretroviral Therapy; STEAL, Simplification with Tenofovir-Emtricitabine or Abacavir-Lamivudine; TDF, tenofovir; VA, Veterans Administration; VHA, Veterans Health Administration.
No. (outcome) refers to total no. of outcome events in abacavir-exposed and -unexposed patients. All outcomes are acute myocardial infarction with exception of STEAL and VA HIV CCR, for which the outcome was combined cardiovascular event.
OR is after exclusion of patients who did not use cocaine or intravenous drugs.
Inflammation
Accruing data suggest that inflammation—implicated in CHD risk for general populations [80, 81]—may be in part driving the association between HIV and CHD and may explain the persistent risk seen after adjustment for traditional CHD risk factors. Inflammatory indices are elevated in HIV-infected patients versus non-HIV-infected patients [82], increase with escalating levels of HIV RNA [83, 84], and predict mortality in HIV-infected patients [70, 85]. Initial interest in the role of inflammation in HIV-related CHD was generated by the Strategies for Management of Antiretroviral Therapy (SMART) study, a trial investigating the effect of episodic ART guided by CD4+ T-cell count (drug conservation [DC]) compared with continuous therapy (viral suppression [VS]) on the outcome of opportunistic disease or death [86]. Contrary to expectation if hypothesizing that cumulative drug exposure increases CHD risk, the rate of major cardiovascular events was increased in the drug conservation group, with a hazard ratio of 1.57 (DC vs VS, 95% CI, 1.00–2.46; P = .05) [87] (Supplementary Figure). Of note, when specifically examined, no significant relationship was found between the most recent viral load value and CVD events [87]. Subsequent analyses have demonstrated the biomarkers interleukin 6 (IL-6) and d-dimer to be increased a month after treatment interruption [70] and IL-6 to be correlated and high-density lipoprotein inversely correlated with HIV RNA after treatment interruption [87]. Multiple recent studies have added support to the hypothesis that elaboration of inflammatory and coagulation biomarkers in the setting of increasing HIV viremia might fuel CHD among HIV-infected patients; [6, 88–93] this topic is discussed in depth in the review by Hsue et al (this supplement).
Immune Dysfunction
Immune dysfunction has also been proposed to confer CHD risk among HIV-infected patients. A study from the Partners cohort showed a CD4+ T-cell count of <200/μL to be associated with AMI, with an adjusted odds ratio of 1.74, a risk comparable to traditional CHD risk factors [94]. In an analysis from the HIV Outpatient Study cohort, a CD4+ T-cell count of <500/μL was associated with a combined cardiovascular endpoint independent of CHD risk factors or ART and had an attributable risk comparable to that of smoking or nonoptimized low-density lipoprotein cholesterol [95]. Additional data have supported the association of low CD4+ T-cell count with CHD outcome events [15] and with non-AIDS events [96, 97], although further studies have failed to demonstrate a significant relationship [7, 21]. Immune activation has also been specifically assessed in relation to vascular outcomes. A study of the Women’s Interagency Health Study cohort showed increased immune activation of CD4+ and CD8+ T cells in HIV-infected women compared with controls and demonstrated higher frequencies of activated T cells to be associated with carotid artery lesions [98]. Finally, studies demonstrating a decrease in non-AIDS events following receipt of ART [99, 100] and an increase in CVD events in patients with incomplete immune recovery following ART initiation [101] (as demonstrated by a CD4+ T-cell count <200/μL at 2 years) lend further support to the hypothesis that a robust immune system mitigates CHD risk. The potential association between immunosenescence and CHD is discussed in the review by Hsue et al (this supplement).
Persistent CHD Risk in the Setting of Virologic Suppression
Although suppressing viral replication with ART reduces inflammation and immune activation, it does not fully normalize these processes [82, 102], and data suggest that even residual levels can result in adverse clinical outcomes. A recent study demonstrated a specific association between immune activation markers and carotid artery plaque in patients virologically suppressed on ART, suggesting that persistent immunologic and inflammatory changes remain important in mediating CHD [98]. Further supporting a role for chronic HIV-related effects in CHD risk are data that showed carotid intima media thickness to be elevated in all HIV groups versus controls, including in elite controllers (HIV-infected patients who maintain an undetectable HIV RNA by standard assay in the absence of ART), independent of ART exposure, viremia, or advanced immunodeficiency [33]. Taken together, available data suggest that the mechanism of HIV-related CHD is indeed complex, with important etiologic factors beyond traditional CHD risk factors and antiretroviral medications. It is likely that the inflammatory and immunologic consequences of a chronic infectious process—even when controlled on medications—underlie the development of premature atherosclerosis and a clinical scenario of accelerated aging.
STRATEGIES FOR CHD RISK REDUCTION AMONG HIV-INFECTED PATIENTS
Predicting CHD Risk
Commonly used CHD risk prediction tools have been applied but not formally validated for use in HIV-infected populations [103] that may differ from the population for which the rules were derived with respect to demographic composition and the presence of nontraditional risk factors. Coronary heart disease risk estimation for HIV-infected patients is discussed in detail in the review by D’Agostino (this supplement).
Role of HIV Therapy
Recent data have prompted a major shift in thinking about the role of ART in relation to CHD risk. Although it was previously thought that virologic suppression came at the expense of possible proatherogenic side effects of antiretroviral drugs, current data suggest that the overall benefits of treatment—in terms of virologic suppression with concomitant reduction in inflammatory markers and improvement of immune function—are likely to be cardioprotective. Furthermore, improved toxicity profiles of first-line antiretroviral agents over time may have led to decreased CHD risk, which has yet to be captured in large cohort studies. In a notable reflection of these changes, the 2010 International AIDS Society–USA treatment guidelines cited high baseline cardiovascular risk as an indication to initiate ART [104]. Whether initiating HIV treatment at higher CD4+ T-cell counts, an intervention shown in observational studies to improve mortality [105] and AIDS-free survival [106], will provide benefit from a cardiovascular standpoint is being actively investigated in the Strategic Timing of Antiretroviral Therapy trial. Despite the probable benefit of treating HIV infection in terms of mitigating CHD risk, individual drugs vary in terms of atherogenic potential. Although there is no clear consensus on the clinical use of abacavir with respect to CHD risk, some HIV clinicians might favor avoiding abacavir use in patients at high underlying CHD risk if an alternative treatment is available yet continue the medication for low-risk patients who have undetectable HIV RNA. Components of the current first-line antiretroviral drugs per the DHHS HIV treatment guidelines [107] have not been shown to have significant adverse effects from a cardiovascular standpoint. Specific antiretroviral drug selection should be tailored to the individual patient with balanced consideration of potential risks and benefits.
Managing Traditional CHD Risk Factors
Established modifiable CHD risk factors—smoking, dyslipidemia, diabetes, and hypertension—play a significant role in HIV-associated CHD and merit aggressive management. Whether the same interventions proven effective for general populations can be applied to HIV-infected patients, however, is not always known. The lack of data on CHD preventative measures tailored to HIV-infected patients has been a topic of an American Heart Association State-of-the-Science conference [108], and many studies suggest that management of CHD risk is in fact different for HIV-infected patients. For example, the Framingham Risk Score for predicted 10-year CHD risk underpredicted risk for HIV-infected patients on ART [103]; hemoglobin A1C, recently recommended as a screening test for diabetes in general populations [109], underestimated glucose in HIV-infected patients [110]; and lipid-lowering therapy with statins or fibrates was slightly less effective for HIV-infected patients [111]. Many cardiovascular interventions have been shown to be underused in HIV populations, including both aspirin [112] and lipid-lowering therapy [113]. Furthermore, whether evidence-based public health strategies targeting traditional CHD risk factors for the general population, such as the “Million Hearts” initiative [114], need to be further tailored to HIV-infected patients is unknown.
In terms of specific management principles, smoking cessation is perhaps the most important intervention given the impact of smoking among HIV-infected patients, and the major tobacco clinical practice guideline for general populations has cited HIV as an area of importance [115]. Acute myocardial infarction rates among HIV-infected patients have been shown to decrease with increased time since quitting smoking, with an incidence rate ratio of 3.73 within 1 year since quitting versus 2.07 within 3 years since quitting [116]. Routine screening, intensive counseling, referral to smoking cessation groups, and pharmacologic interventions proven safe for HIV-infected patients [117] should be routinely practiced and prioritized. HIV-specific dyslipidemia guidelines have been published [118], and the management of dyslipidemia and metabolic abnormalities are discussed in the review by Stanley et al (this supplement). Vigilance about drug interactions is critical because PIs interact to varying degrees with statins via inhibition of cytochrome P450. Fewer data exist regarding newer antiretroviral agents and newer statins; studies suggest that rosuvastatin is safe and effective in HIV-infected patients [119, 120] but might need to be dose-adjusted with concurrent atazanavir/ritonavir use [121]. Blood pressure should be monitored at HIV diagnosis and annually thereafter, and elevated blood pressure should be managed according to Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) guidelines [122].
Managing Novel CHD Risk Factors
Modulating inflammation and immune activation that persist despite virologic suppression is likely to be an important approach to prevent CHD in HIV-infected patients. Several strategies are being explored, as discussed in depth by Hsue et al (this supplement). In preliminary studies, treatment intensification has been investigated to reduce low-level viral replication thought to be associated with inflammation and immune activation, with varying results [123–125]. Additionally, statins might have a role beyond lipid lowering in reducing inflammation. Statins have been specifically shown to decrease C-reactive protein in HIV-infected patients on boosted PIs [126], and a recent study showed overall mortality to be decreased with statin therapy in an HIV cohort, although CHD mortality was not able to be assessed specifically due to the small number of outcome events [127]. Finally, microbial translocation in the gut is postulated to fuel persistent immune activation, and immunomodulatory therapies are being explored to target this process [128].
FUTURE DIRECTIONS AND IMPLICATIONS
The intersection of HIV infection and CHD poses significant challenges from a clinical standpoint for those providing care to HIV-infected patients and from a public health standpoint in light of the global impact of both diseases. Although recent investigations have enhanced our knowledge of this complex area, important questions remain unanswered: What is the role of HIV treatment and its timing in decreasing CHD risk? Which HIV-infected patients should be prescribed aspirin? Is there a role for statins for HIV-infected patients beyond that of lipid lowering? What is the clinical significance of inflammatory and immunologic changes that persist after virologic suppression and how should they be managed? Will novel markers of CHD risk (eg, biomarkers or imaging studies) enhance risk prediction and stratification for HIV-infected patients? Should HIV infection itself be considered a CHD risk factor? Targeting HIV and CHD in a coordinated manner has the potential to significantly impact the long-term clinical care of HIV-infected patients and the development of public health strategies for cardiovascular prevention in this at-risk population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number K01AI073109). This supplement was supported by the Harvard Center for AIDS Research and an educational grant from Bristol Myers-Squibb.
Potential conflicts of interest.
Author certifies no potential conflicts of interest.
The author has submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Lewden C, Chene G, Morlat P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72–7. doi: 10.1097/QAI.0b013e318134257a. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 4.van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 5.Lodwick RK, Sabin CA, Porter K, et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–5. doi: 10.1016/S0140-6736(10)60932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antiretroviral Therapy Cohort Collaboration. Causes of death in HIV-1–infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–96. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achhra AC, Amin J, Law MG, et al. Immunodeficiency and the risk of serious clinical endpoints in a well studied cohort of treated HIV-infected patients. AIDS. 2010;24:1877–86. doi: 10.1097/QAD.0b013e32833b1b26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnet F, Chene G, Thiebaut R, et al. Trends and determinants of severe morbidity in HIV-infected patients: the ANRS CO3 Aquitaine Cohort, 2000–2004. HIV Med. 2007;8:547–54. doi: 10.1111/j.1468-1293.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 9.Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS. 2010;24:1537–48. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 10.Neuhaus J, Angus B, Kowalska JD, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24:697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocroft A, Reiss P, Gasiorowski J, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262–70. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 12.Crum-Cianflone NF, Grandits G, Echols S, et al. Trends and causes of hospitalizations among HIV-infected persons during the late HAART era: what is the impact of CD4 counts and HAART use? J Acquir Immune Defic Syndr. 2010;54:248–57. doi: 10.1097/qai.0b013e3181c8ef22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein D, Hurley LB, Quesenberry CP, Jr, Sidney S. Do protease inhibitors increase the risk for coronary heart disease in patients with HIV-1 infection? J Acquir Immune Defic Syndr. 2002;30:471–7. doi: 10.1097/00126334-200208150-00002. [DOI] [PubMed] [Google Scholar]
- 15.Klein D, Leyden W, Xu L, et al. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections (Boston, MA) Alexandria, VA: CROI: 2011. Contribution of immunodeficiency to CHD: cohort study of HIV+ and HIV– Kaiser Permanente members. [Google Scholar]
- 16.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 17.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obel N, Thomsen HF, Kronborg G, et al. Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis. 2007;44:1625–31. doi: 10.1086/518285. [DOI] [PubMed] [Google Scholar]
- 19.Lang S, Mary-Krause M, Cotte L, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 20.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec’s public health insurance database. J Acquir Immune Defic Syndr. 2011;57:245–53. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 21.Freiberg M, McGinnis K, Butt A, et al. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections (Boston, MA) Alexandria, VA: CROI: 2011. HIV is associated with clinically confirmed myocardial infarction after adjustment for smoking and other risk factors. [Google Scholar]
- 22.French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Struble K, Soon G, Min M, Chan-Tack K, Murray J, Birnkrant D. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections (Montreal, Canada) Alexandria, VA: CROI: 2009. Meta-analysis of efficacy outcomes for treatment-naive and treatment-experienced HIV-infected women in randomized controlled clinical trials: 2000 to 2008. [Google Scholar]
- 24.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 25.Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–10. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 26.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4:425–32. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11:462–8. doi: 10.1111/j.1468-1293.2009.00815.x. [DOI] [PubMed] [Google Scholar]
- 28.Weber R, Sabin C, Reiss P, et al. HBV or HCV coinfections and risk of myocardial infarction in HIV-infected individuals: the D:A:D cohort study. Antivir Ther. 2010;15:1077–86. doi: 10.3851/IMP1681. [DOI] [PubMed] [Google Scholar]
- 29.Francisci D, Giannini S, Baldelli F, et al. HIV type 1 infection, and not short-term HAART, induces endothelial dysfunction. AIDS. 2009;23:589–96. doi: 10.1097/QAD.0b013e328325a87c. [DOI] [PubMed] [Google Scholar]
- 30.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS. 2009;23:2021–7. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–32. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunfeld C, Delaney JA, Wanke C, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–9. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsue PY, Hunt PW, Schnell A, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. AIDS. 2009;23:1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–83. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 35.Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz MW, Stephan C, Harmjanz A, et al. Both long-term HIV infection and highly active antiretroviral therapy are independent risk factors for early carotid atherosclerosis. Atherosclerosis. 2008;196:720–6. doi: 10.1016/j.atherosclerosis.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–7. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Kingsley LA, Cuervo-Rojas J, Munoz A, et al. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: multicenter AIDS Cohort Study. AIDS. 2008;22:1589–99. doi: 10.1097/QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients—association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 41.Gritz ER, Vidrine DJ, Lazev AB, Amick BC, 3rd, Arduino RC. Smoking behavior in a low-income multiethnic HIV/AIDS population. Nicotine Tob Res. 2004;6:71–7. doi: 10.1080/14622200310001656885. [DOI] [PubMed] [Google Scholar]
- 42.Mamary EM, Bahrs D, Martinez S. Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDS. 2002;16:39–42. doi: 10.1089/108729102753429389. [DOI] [PubMed] [Google Scholar]
- 43.Saves M, Chene G, Ducimetiere P, et al. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292–8. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 44.Carr A, Samaras K, Burton S, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [See comment] [DOI] [PubMed] [Google Scholar]
- 45.Periard D, Telenti A, Sudre P, et al. Atherogenic dyslipidemia in HIV-infected individuals treated with protease inhibitors. The Swiss HIV Cohort Study. Circulation. 1999;100:700–5. doi: 10.1161/01.cir.100.7.700. [DOI] [PubMed] [Google Scholar]
- 46.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 47.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 48.Bergersen BM, Sandvik L, Dunlop O, Birkeland K, Bruun JN. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naive and HIV-negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22:731–6. doi: 10.1007/s10096-003-1034-z. [DOI] [PubMed] [Google Scholar]
- 49.Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7:10–15. doi: 10.1111/j.1468-1293.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 50.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–60. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.