Abstract
Cardiovascular disease (CVD) risk assessment tools such as the Framingham Risk Functions, often called Framingham Risk Scores, are common in the evaluation of the CVD risk among individuals in the general population. These functions are multivariate risk algorithms that combine data on CVD risk factors, such as sex, age, systolic blood pressure, total cholesterol level, high-density lipoprotein cholesterol level, smoking behavior, and diabetes status, to produce an estimate (or risk) of developing CVD or a component of it (such as coronary heart disease, stroke, peripheral vascular disease, and heart failure) over a fixed period (eg, the next 10 years). These estimates of CVD risk are often major inputs in recommending drug treatments, such as agents to reduce cholesterol level. The Framingham Risk Functions are valid in diverse populations, at times requiring a calibration adjustment for proper applicability. With the realization that individuals with human immunodeficiency virus (HIV) infection often have elevated CVD risk factors, the evaluation of CVD risk for these individuals becomes a serious concern. Researchers have recently developed new CVD risk functions specifically for HIV-infected patients and have also examined the extension of existing Framingham Risk Functions to the HIV-infected population. This article first reviews briefly the Framingham Study and risk functions, covering their objectives, their components, evaluation of their performance, and transportability and validity on non-Framingham populations. It then reviews the development of CVD risk functions for HIV-infected individuals and comments on the usefulness of extending the Framingham risk equation to the HIV-infected population and the need to develop more-specific risk prediction equations uniquely tailored to this population.
EPIDEMIOLOGIC BACKGROUND OF THE FRAMINGHAM STUDY
The background of the Framingham Study has been described in detail [1–7]. Prior to World War II, major public health efforts were directed at control of infectious diseases. As effective antibiotic therapies were developed, the scourge of infectious diseases was replaced in the 1940s and 1950s by the mounting epidemic of cardiovascular disease (CVD). By the 1950s, 1 in 3 men in the United States developed CVD before the age of 60 years. While CVD was less prevalent in females, the development of CVD in women had debilitating and often fatal consequences [1, 2]. CVD had become the leading cause of death and a major factor limiting the increase in life expectancy beyond the age of 45 years. Further, there were no known treatments for CVD and no strategies available to prolong life, even in those who managed to survive an attack.
A critical need existed to treat and reverse the process of CVD, but strategies in this regard developed slowly, and most remained in the conceptual and development stages. In the face of this, there were many who believed a primary-prevention approach would be promising, possibly even more important than a search for cures [1]. The logic for the development of preventive strategies for CVD was powerful, given the frequency of and significant morbidity and mortality associated with CVD. If the onset of CVD could be delayed by preventive approaches, life expectancy would significantly increase. To develop such approaches, the preventable and modifiable predisposing factors had to be identified. Further, CVD was recognized to be multifactorial and develop over time, making a longitudinal study necessary. To study CVD appropriately, it was necessary to identify people without CVD; note their lifestyle and other potential risk factors, such as age and sex; follow them over time; and relate these factors to the development of CVD. A longitudinal cohort–based epidemiological study was deemed necessary to identify factors and relate them to the development of CVD [1, 3]. This approach was described as one that explores “certain relationships in health and disease which, with present technological methods, cannot be observed directly” [3]. The factors that did relate to the development of CVD were later labeled by Dr William B. Kannel as CVD risk factors.
These efforts led to the initiation of the Framingham Heart Study [1–5]. To achieve the goals of the study, a systematic sample of 2 of every 3 families in the town of Framingham Massachusetts was selected. People in those families who were aged 30–59 years were invited to participate in the study. Ultimately, 5209 individuals (2336 men and 2873 women) joined the study. The major aim of the study was to secure epidemiological data on CVD. This encompassed the establishment of the relation of risk factors (eg, clinical parameters such as age, sex, blood pressure, cholesterol level, and body weight, and lifestyle parameters, such as smoking behavior, physical activity, and alcohol consumption) to CVD. Biennial examinations were administered in which the risk factors were evaluated. Continuous surveillance methods identified when a CVD event occurred. The Framingham Study was very successful, with an Offspring Cohort (with 2489 males and 2646 females) initiated in 1971 and a Third-Generation Cohort (with >4000 subjects) initiated in 2001 [6, 7].
FROM INDIVIDUAL RISK FACTORS TO MULTIVARIABLE FRAMINGHAM RISK FUNCTIONS
Originally, the Framingham Study examined the relation of individual risk factors to the development of CVD. This approach then shifted to the question of whether the individual risk factors could be combined into a multivariate function to give an assessment (ie, the probability risk) of developing a CVD event over a specific period (eg, 10 years). The original hypothesis of the Framingham Study was that the cause of CVD is multifactorial. A multivariable assessment was a logical consequence of this hypothesis. Further, the attention focused on the development of a primary CVD event (eg, the first CVD event in a person who had no history of CVD at the time of enrollment). As Dr Kannel stated, “Multivariable risk formulations [called Framingham Risk Functions or the Framingham Risk Score] for estimating the probability of CVD conditional on the burden of a number of specified risk factors have been produced to facilitate evaluation of candidates for CVD in need of preventive management” [8]. Major Framingham Risk Functions exist and have had widespread use for coronary heart disease (angina, myocardial infarction [MI], and coronary death), hard coronary events (MI and coronary deaths), stroke, and global CVD (including CVD deaths, coronary disease, and stroke [including transient ischemic attack]) [8–14]. The cholesterol treatment guideline for the Adult Treatment Panel III is based on a Framingham Risk Function [14–16].
DEVELOPMENT, EVALUATION, AND TRANSPORTABILITY OF THE FRAMINGHAM RISK FUNCTIONS
The development of the Framingham Risk Functions has been well documented [8–18]. Of equal importance, the evaluation of the performance of the functions and their applicability beyond the setting in which they were developed has also been well studied and documented [11, 19, 20]. The Framingham Risk Functions have been shown to be valid for white individuals and people of other races and ethnicities in the United States beyond Framingham [11] and, with a calibration adjustment, to be valid for Japanese-American males in Honolulu [11], for individuals in Spain [21], and for individuals in China [22].
The following components are important in the development and evaluation of the functions: (1) a clearly defined set of individuals who are the at-risk population (eg, people free of CVD), (2) clearly defined CVD outcomes that some subjects will develop (eg, MI or coronary death), (3) a selected follow-up time (eg, 10 years), (4) a well-defined and obtainable set of CVD risk factors (eg, systolic blood pressure, total cholesterol level, and smoking history), and (5) a mathematical model to relate the CVD risk factors to the development of the disease.
Often, the mathematical model is a time-to-event model, such as the Cox proportional hazard model [9, 12, 13, 23] or an accelerated failure model [10, 24]. The coefficients of these models relate to the hazard ratios or relative risks of the CVD risk factors. The performance of the function is evaluated by discrimination and calibration measures [19, 20, 23]. The area under the receiver operating characteristic curve is usually used for discrimination (eg, for ranking risk), and a χ2 test is reserved for calibration (ie, predicting the number of events and comparing this to the number of events observed). For applying the Framingham Risk Functions to populations beyond Framingham, a calibration adjustment is needed to adjust for differences in underlying risk [11, 21, 22]. The various Framingham Risk Functions are available on the Framingham Web site [25]. The functions are based on mathematical models, and the Web site allows one to compute the risks, using Excel (Microsoft) programs. The Supplementary Figure displays a score sheet that is an approximation to the function and can be used to calculate risk if one does not have access to the Web-based function. While the score sheet does not produce biases, it is not as precise as the Excel programs, and the Framingham Study encourages the direct use of the mathematical function rather than the approximation in the score sheet.
APPLICATION AND EXTENSION OF RISK PREDICTION TO HIV-INFECTED POPULATIONS
Increased CVD rates are seen among HIV-infected patients, compared with age-matched non–HIV-infected patients [26]. The increased burden of CVD among HIV-infected patients is likely a consequence of increased traditional risk factors, including dyslipidemia and insulin resistance, and nontraditional risk factors, such as immune activation and inflammation, that may contribute to an accelerated aging process characterized by higher-than-anticipated rates of noninfectious comorbidities. The ability to accurately predict the degree of cardiovascular risk is therefore an essential element of this population’s future care, and its importance is reflected by an American Heart Association–sponsored State-of-the-Science conference that focused on the topic [27, 28].
Traditional cardiovascular risk prediction algorithms developed in non–HIV-infected populations may not accurately predict risk for HIV-infected patients because of potential differences in the etiology of CVD in the HIV population. Indeed, risk prediction tools such as the Framingham Risk Score were not designed for use in populations with HIV infection and were developed for patients with different demographic and clinical characteristics [9, 13]. Studies in non–HIV-infected populations have demonstrated the Framingham Risk Scores to perform differently in subgroups with different demographic [11] or geographic characteristics [29]. To be applicable to HIV-infected populations, a recalibration [11, 30] may be needed to adjust for underprediction or overprediction. Also, factors unique to HIV infection may influence the performance of a standard risk prediction tool. For example, a change in treatment regimens may impact cardiovascular risk, differential impact of traditional cardiovascular risk factors, and potential contributions to cardiovascular risk of inflammatory and immunologic parameters.
Early investigations of CVD risk in HIV-infected patients used the Framingham data and existing Framingham functions directly to evaluate CVD risk. Two case-control studies [31, 32] in which HIV-infected patients (cases) were matched to Framingham participants (controls) demonstrated that (1) fat redistribution (increased waist circumference and/or reduced hip and extremity circumferences) in HIV was associated with elevated metabolic CVD risk factors; (2) the Framingham Risk Score [9] was elevated in HIV-infected patients with fat redistribution; (3) controls matched on the basis of age, sex, body mass index, and waist and hip circumferences were at the same risk; and (4) HIV-infected subjects without fat redistribution were not at elevated coronary heart disease risk. The studies provided useful insights into CVD risk among HIV-infected subjects and used the Framingham equations to estimate the risk contributed by the increased prevalence of traditional risk factors for CVD in the HIV-infected population.
Subsequent cross-sectional studies assessed the degree of agreement of predicted risk probabilities or risk predictions (but not the actual 10-year outcomes) for different cardiovascular risk equations among HIV-infected patients. Three studies have assessed the degree of correlation among the Framingham, Systematic Coronary Risk Evaluation (SCORE), and Prospective Cardiovascular Münster (PROCAM) equations. There was significant concordance among the scores in a cross-sectional Spanish HIV-infected cohort, with observed agreement of 84% between Framingham and PROCAM (κ = 0.36), 83% between Framingham and SCORE (κ = 0.32), and 93% between PROCAM and SCORE (κ = 0.46) [33]. In a similar study from Brazil, there was moderate agreement between the Framingham and PROCAM equations (κ = 0.43) and between the PROCAM and SCORE equations (κ = 0.48), but less agreement between the Framingham and SCORE equations (κ = 0.22) [34]. A comparison of the Framingham and PROCAM equations at 2 HIV referral centers in Brazil showed good agreement between the scores (κ = 0.64) [35]. In another cross-sectional study of a Thai HIV-infected cohort, predicted 10-year risk of coronary heart disease was higher for the Framingham risk equation, compared with both the Ramathibodi-Electricity Generating Authority of Thailand (Rama-EGAT) equation and the Data Collection on Adverse Effects of Anti-HIV Drugs (D:A:D) equation (see below), with differences between the 3 scores more pronounced in patients with a higher predicted risk [36]. These studies compare different risk functions with one another but give no real answer as to how well the functions will do in actual risk prediction. The agreement of the order of magnitude of 80% or so indicates good agreement. However, the low κ values of 0.30–0.40 demonstrated in some of the comparisons highlight the dangers of trying to understand the usefulness of the Framingham Risk Function in such cross-sectional comparisons. Furthermore, given that the comparisons were not of the actual outcomes (events), the usefulness of this approach in general is questionable [36].
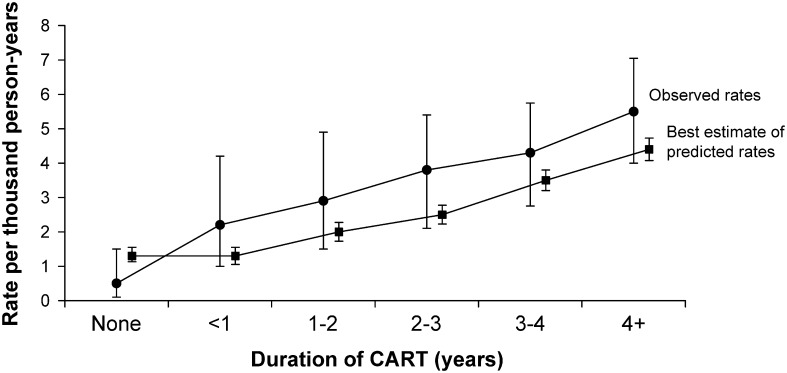
While there is relatively good concordance among risk prediction tools for HIV-infected patients, it is not clear that these tools accurately predict risk. The D:A:D study applied the Framingham risk equation [10] to an HIV-positive observational cohort of >23 000 patients to compare observed versus predicted events on the basis of duration of antiretroviral therapy [37] (Figure 1). Several methods were employed in order to compare event rates: predictions were extrapolated to provide 10-year risk estimates; missing covariates were imputed; all patients were assumed not to have left ventricular hypertrophy, owing to the absence of data for this variable; and patients with prior CVD were included but considered to be at already increased risk. For patients receiving antiretroviral therapy, observed acute MI events were higher than predicted, yet in patients not receiving antiretroviral therapy, observed acute MI events were lower than predicted (although there were only 3 observed events in this group). For all groups, however, the confidence limits overlapped, suggesting that the Framingham equation did not significantly underpredict events in patients receiving antiretroviral therapy. However, the study was too small, with only 129 myocardial infarctions, to be definitive and did not resolve whether the Framingham equation is useful for predicting CVD events in the HIV-infected population.
Figure 1.
Observed and predicted rates of myocardial infarction by duration of combination antiretroviral treatment (CART). Observed rates are observed number of myocardial infarctions (MIs) divided by person-years of follow-up. Predicted rates are the sum of estimated individual predicted probabilities of MI. See Methods for details. Error bars are 95% confidence intervals, based on the Poisson distribution for observed rates and bootstrap resampling for the best estimate of the predicted rates. Reprinted from Law et al. [37] with permission of John Wiley & Sons.
Additionally, several studies have explored the association between the Framingham risk equation and measures of subclinical atherosclerosis among HIV-infected patients [36, 37]. It should be noted that the Framingham functions do not attempt to predict subclinical disease. Therefore, assessment of the usefulness of the Framingham risk equation for predicting subclinical atherosclerotic disease in HIV-infected persons goes beyond the intended purpose of the Framingham equation and does not reflect appropriate use of the equation.
In light of the uncertain data, illustrated above, concerning the usefulness of traditional cardiovascular risk prediction tools to accurately predict risk for HIV-infected patients, several groups have developed cardiovascular risk prediction models tailored to HIV-infected populations. On the basis of 5 cohorts of non–HIV-infected men, investigators developed a prognostic model for the outcome of coronary heart disease (defined by International Classification of Diseases code) tailored to changes in risk factors typically observed in patients starting antiretroviral therapy [38]. The model included variables such as body mass index and fasting blood glucose level, traditional cardiovascular risk factors that are commonly seen among HIV-infected patients. Hazard ratios for the model, however, were derived from non–HIV-infected men and extrapolated to HIV-infected patients. Moreover, the prognostic model was limited to traditional cardiovascular risk factors and did not include factors specific to HIV infection, including indices of inflammation and fat redistribution, that are increasingly recognized to play a pivotal role in HIV-associated CVD.
More recently, the D:A:D group took an important step toward addressing the issue of HIV-specific risk prediction by developing a cardiovascular risk equation on the basis of covariates derived from a large HIV-infected cohort [39]. By use of data from >20 000 patients in a prospective observational cohort, most of whom were in developed countries, models that included exposure to HIV medications (indinavir, lopinavir/ritonavir, and abacavir), as well as traditional cardiovascular risk factors, were developed to predict several cardiovascular outcomes. CD4 cell count and HIV RNA load were considered as covariates but did not achieve statistical significance. The performance of the models was assessed by discrimination (the area under the receiver operating curve was 0.78, 0.78, and 0.77 for acute myocardial infarction, coronary heart disease, and CVD end points, respectively) and calibration (the ratio of predicted to observed events was 0.97, 0.96, and 0.95 for acute MI, coronary heart disease, and CVD end points, respectively) and was found to be similar to the Framingham equation [10] in terms of ordering patient risk (discrimination) but superior to the Framingham equation in terms of accurately predicting risk (calibration). Importantly, the risk equation was validated in the same data set from which it was derived rather than in an independent data set, although the investigators employed an internal-external cross-validation (ie, “test and hold”) technique, in which models were developed from one subcohort and validated in another, to mimic external validation. Further limitations of the analysis comparing the D:A:D-derived and Framingham equations include shorter follow-up times for D:A:D patients, necessitating extrapolation to 5-year predicted risks; differing end point definitions; exclusion of patients without a complete risk factor profile; and inclusion of a limited number of outcomes in women, precluding the development of sex-specific equations. Also, the Framingham equation used for comparison to the D:A:D was not ideal for the comparison. For example, it did not contain a variable for hypertension medication, an important predictor of outcomes, and its outcome did not match the outcomes used in the D:A:D analysis. The latter had cardiac procedures in the outcome, whereas the Framingham function did not, and the Framingham function had silent MIs as one of its outcomes, whereas the D:A:D did not.
WHERE WE STAND IN TERMS OF CARDIAC RISK PREDICTION IN THE HIV POPULATION
HIV-infected patients confront an escalating epidemic of CVD that is comparable to that faced by the general population more than half a century ago. Stratifying risk among HIV-infected patients and devising cardiovascular preventive strategies are priorities for the ongoing care of HIV-infected patients. While the Framingham risk function can be used to give a general estimation of risk and should be considered for this purpose, the development of more-specific tools tailored to HIV-infected patients is necessary. An HIV-specific risk prediction tool has been developed, but its evaluation is limited, and it is unclear whether it can be generalized to HIV-infected patients in diverse settings until it has been validated in an external data set. Furthermore, it is likely that cardiovascular risk prediction for HIV-infected patients might need to incorporate a component reflecting altered inflammatory and immunologic profiles. Adding these variables to the existing Framingham Risk Functions seems appropriate. The development of cardiovascular risk prediction tools specific to HIV-infected patients is likely to have a significant impact on future cardiovascular preventive strategies for this at-risk group.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
I thank Dr. Virginia Triant for her thoughtful contributions and review of the manuscript.
Financial support.
R. B. D. is the co-principal investigator of the Framingham contract from the National Heart, Lung, and Blood Institute’s Framingham Heart Study, National Institutes of Health. Framingham Heart Study research is supported by the NIH/NHLBI (contract N01-HC-25195). This supplement was supported by the Harvard Center for AIDS Research and an educational grant from Bristol -Myers-Squibb.
Potential conflicts of interest.
R. B. D. certifies no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dawber TR. The Framingham study: the epidemiology of atherosclerotic disease. Cambridge, MA: Harvard University Press; 1980. [Google Scholar]
- 2.D'Agostino Sr RB, Kannel WB. In: Proceedings of the American Statistical Association sesquicentennial invited paper sessions. Alexandria, VA: American Statistical Association; 1989. Epidemiological background and design: the Framingham Study. [Google Scholar]
- 3.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–56. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 5.Dawber TR, Moore FE. In: Papers presented at the 1951 Annual Conference of the Milbank Memorial Fund. New York, NY: Milbank Memorial Fund; 1952. Longitudinal study of heart disease in Framingham, Massachusetts: an interim report; research in public health; pp. 241–7. [Google Scholar]
- 6.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 7.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 8.Kannel WB. Multivariate evaluation of candidates for cardiovascular disease. In: Rosendorff C, editor. Essential cardiology principles and practice. 2nd ed. Totowa, NJ: Humana Press; 2006. 3. [Google Scholar]
- 9.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 10.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–62. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 11.D'Agostino R, Grundy S, Sullivan L, Wilson P. Validation of the Framingham coronary heart disease prediction scores: Results of a multiple ethnic group investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 12.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–18. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 13.D'Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM. Primary prevention of coronary heart disease: integrating risk assessment with intervention. Circulation. 1999;100:988–98. doi: 10.1161/01.cir.100.9.988. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. 2004;23:1631–0. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- 18.Abbott RD, McGee D National Heart L, Blood I, US Department of Commerce. National Technical Information Service N. The Framingham Study: an epidemiological investigation of cardiovascular disease: Section 37: the probability of developing certain cardiovascular diseases in eight years specified values of some characteristics. Springfield, VA: National Technical Information Service; 1987. [Google Scholar]
- 19.D'Agostino RB, Nam B-H. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, Rao CR, editors. Amsterdam: Handbook of statistics. Vol 23. Elsevier; 2003. pp. 1–25. [Google Scholar]
- 20.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–23. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 21.Marrugat J, D'Agostino R, Sullivan L, et al. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J Epidemiol Community Health. 2003;57:634–8. doi: 10.1136/jech.57.8.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Hong Y, D'Agostino RB, Sr, et al. Predictive value for the Chinese population of the Framingham CHD risk assessment tool compared with the Chinese Multi-Provincial Cohort Study. JAMA. 2004;291:2591–9. doi: 10.1001/jama.291.21.2591. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 25. Risk score profiles. Framingham Heart Study Web site. Available at: http://www.framinghamheartstudy.org/risk/index.html. Accessed 30 November 2011.
- 26.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schambelan M, Wilson PW, Yarasheski KE, et al. Development of appropriate coronary heart disease risk prediction models in HIV-infected patients. Circulation. 2008;118:e48–53. doi: 10.1161/CIRCULATIONAHA.107.189627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grinspoon SK, Grunfeld C, Kotler DP, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation. 2008;118:198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bastuji-Garin S, Deverly A, Moyse D, et al. The Framingham prediction rule is not valid in a European population of treated hypertensive patients. J Hypertens. 2002;20:1973–80. doi: 10.1097/00004872-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Brindle PM, McConnachie A, Upton MN, Hart CL, Davey Smith G, Watt GC. The accuracy of the Framingham risk-score in different socioeconomic groups: a prospective study. Br J Gen Pract. 2005;55:838–45. [PMC free article] [PubMed] [Google Scholar]
- 31.Hadigan C, Meigs J, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in Adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. doi:10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 32.Hadigan C, Meigs JB, Wilson PW, et al. Prediction of coronary heart disease risk in HIV-infected patients with fat redistribution. Clin Infect Dis. 2003;36:909–16. doi: 10.1086/368185. [DOI] [PubMed] [Google Scholar]
- 33.Knobel H, Jerico C, Montero M, et al. Global cardiovascular risk in patients with HIV infection: concordance and differences in estimates according to three risk equations (Framingham, SCORE, and PROCAM) AIDS Patient Care STDS. 2007;21:452–7. doi: 10.1089/apc.2006.0165. [DOI] [PubMed] [Google Scholar]
- 34.Moreira Guimaraes MM, Bartolomeu Greco D, Ingles Garces AH, et al. Coronary heart disease risk assessment in HIV-infected patients: a comparison of Framingham, PROCAM and SCORE risk assessment functions. Int J Clin Pract. 2010;64:739–45. doi: 10.1111/j.1742-1241.2009.02248.x. [DOI] [PubMed] [Google Scholar]
- 35.Barros ZM, de Alencar Ximenes RA, Miranda-Filho DB, et al. Comparison between the Framingham and prospective cardiovascular of Munster scores for risk assessment of coronary heart disease in human immunodeficiency virus-positive patients in Pernambuco, Brazil. Metab Syndr Relat Disord. 2010;8:489–97. doi: 10.1089/met.2009.0100. [DOI] [PubMed] [Google Scholar]
- 36.Edwards-Jackson N, Kerr S, Tieu H, et al. Cardiovascular risk assessment in persons with HIV infection in the developing world: comparing three risk equations in a cohort of HIV-infected Thais. HIV Med. 2011;12:510–5. doi: 10.1111/j.1468-1293.2011.00916.x. [DOI] [PubMed] [Google Scholar]
- 37.Law MG, Friis-Moller N, El-Sadr WM, et al. The use of the Framingham equation to predict myocardial infarctions in HIV-infected patients: comparison with observed events in the D: A:D Study. HIV Med. 2006;7:218–30. doi: 10.1111/j.1468-1293.2006.00362.x. [DOI] [PubMed] [Google Scholar]
- 38.May M, Sterne JA, Shipley M, et al. A coronary heart disease risk model for predicting the effect of potent antiretroviral therapy in HIV-1 infected men. Int J Epidemiol. 2007;36:1309–18. doi: 10.1093/ije/dym135. [DOI] [PubMed] [Google Scholar]
- 39.Friis-Moller N, Thiebaut R, Reiss P, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.