Abstract
Complications of atherosclerosis, including myocardial infarction and stroke, are the leading cause of death and disability worldwide. Recent data strongly implicate cardiovascular death as a contributor to mortality among patients with human immunodeficiency virus (HIV) infection, with evidence suggesting increased incidence of atherosclerosis among these patients. Therefore, greater understanding of atherosclerotic mechanisms and how these responses may be similar or distinct in HIV-infected patients is needed. Key concepts in atherosclerosis are reviewed, including the evidence that inflammation and abnormal metabolism are major drivers of atherosclerosis, and connected to the current literature regarding atherosclerosis in the context of HIV.
Atherosclerosis—the leading cause of death worldwide—has received much attention as to its origins, mechanisms, treatment, and distinct patterns in specific populations [1]. The fact that our perspective on atherosclerosis has undergone extensive evolution may surprise those outside this field. A century ago, atherosclerosis was considered a degenerative disease of the elderly, not one that cut so deeply into life expectancy or its quality [2, 3]. Since then, over time, multiple fundamental shifts in perspective have occurred, each of which has broadened our understanding of the atherosclerotic process (Figure 1).
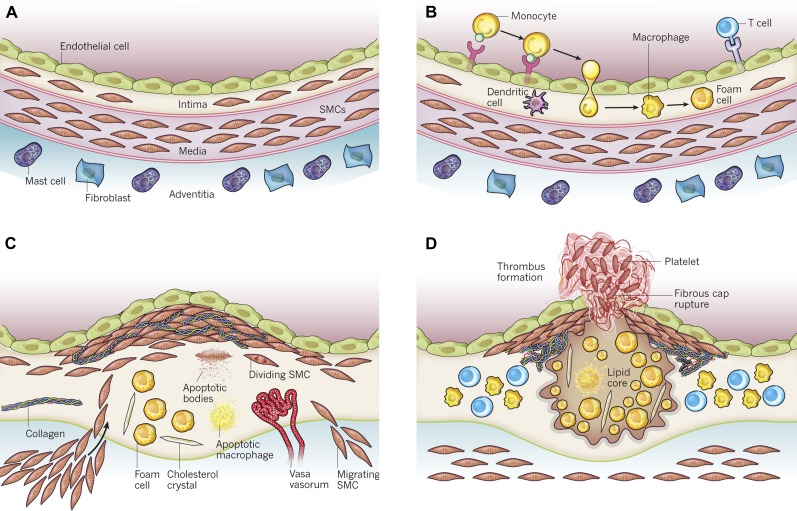
Figure 1.
Stages in the development of atherosclerotic lesions. The normal muscular artery and the cell changes that occur during disease progression to thrombosis are shown. A, Normal artery, which contains 3 layers. The inner layer, the tunica intima, is lined by a monolayer of endothelial cells that is in contact with blood overlying a basement membrane. The human intima contains resident smooth muscle cells (SMCs). The middle layer, or tunica media, contains SMCs embedded in a complex extracellular matrix. Arteries affected by obstructive atherosclerosis generally have the structure of muscular arteries. The adventitia, the outer layer of arteries, contains mast cells, nerve endings, and microvessels. B, Initial steps of atherosclerosis, including adhesion of blood leukocytes to the activated endothelial monolayer, directed migration of the bound leukocytes into the intima, maturation of monocytes (the most numerous of the leukocytes recruited) into macrophages, and their uptake of lipid, yielding foam cells. C, Lesion progression, which involves the migration of SMCs from the media to the intima, the proliferation of resident intimal SMCs and media-derived SMCs, and the heightened synthesis of extracellular matrix macromolecules such as collagen, elastin, and proteoglycans. Plaque macrophages and SMCs can die in advancing lesions, some by apoptosis. Extracellular lipid derived from dead and dying cells can accumulate in the central region of a plaque, often denoted as the lipid or necrotic core. Advancing plaques also contain cholesterol crystals and microvessels. D, Thrombosis, the ultimate complication of atherosclerosis, which often complicates a physical disruption of the atherosclerotic plaque. Shown is a fracture of the plaque’s fibrous cap, which has enabled blood coagulation components to come into contact with tissue factors in the plaque’s interior, triggering the thrombus that extends into the vessel lumen, where it can impede blood flow. Modified from Figure 1 of Libby et al [1]. Reprinted with permission of Nature Publishing Group.
The role of thrombosis, including platelet action and coagulation forces, is important in atherosclerotic complications because myocardial infarction (MI) is an event caused by acute plaque rupture and not a gradual loss of the arterial lumen [4]. Specific roles for the cellular components of the arterial wall—namely, endothelial cells (ECs) [5] and vascular smooth muscle cells (VSMCs) [6]—have come into play. Pathologic studies have revealed that atherosclerosis begins early, in young adulthood if not sooner, even if clinical complications are decades away [7]. The success, and confusion, around the impact of various cholesterol- and lipid-modulating medications on cardiovascular events has continued to force attention on the relationship between lipid metabolism and atherosclerosis. A central modern tenet of atherosclerosis is that of inflammation as a key driver in the process, a realization that made the biology of monocytes, macrophages, B cells, and T cells relevant to clinical issues such as MI [8]. The global epidemic of obesity and diabetes has coupled with the demonstration of increased cardiovascular risk in these metabolic problems to draw attention to how processes such as adipogenesis and insulin sensitivity may contribute to cardiovascular disease [9, 10].
The knowledge that individuals infected with human immunodeficiency virus (HIV) have an increased incidence of atherosclerosis and its complications adds this subgroup of individuals to the list of those in whom the nature of atherosclerosis has become relevant. Indeed, each of the new insights into atherosclerosis noted above, as well as others, may bear particular relevance to patients infected with HIV. In this review, we provide an overview of current concepts in atherosclerosis, with a focus on more recent findings, before considering how these issues influence atherosclerosis and its complications in the context of HIV.
THE VESSEL WALL IN ATHEROSCLEROSIS
More antiquated notions of atherosclerosis envisioned a pipe in which plaque buildup ultimately led to the cessation of blood flow and ischemic infarction of the distal tissue. In fact, although atherosclerosis does progress through the well-established phases of fatty streaks to more complex plaque, in its latter stages, the atherosclerotic lesion moves abluminally, preserving the lumen and blood flow [11, 12]. In many ways, the process of atherosclerosis can be understood as a classic response to injury, with the insulting forces in this case being cardiovascular risk factors such as elevated cholesterol levels, smoking, diabetes, and hypertension. The endothelium is the first interface between risk factors and mediators that appear in the circulation and organismal responses. We now understand the endothelium to be a biologically active organ that plays a key role in atherosclerosis [5]. For example, ECs are a source of nitric oxide, which has many effects including vasomotor function. A critical first step in atherogenesis is activation of ECs in response to injurious factors and inflammatory mediators that alter the Teflon-like properties of ECs through their expression of chemoattractant cytokines, known as chemokines, and their induction of adhesion molecules, such as vascular cell adhesion molecule 1 (VCAM-1) [13]. Chemokines such as monocyte chemoattractant protein 1 (MCP-1) have been strongly linked to atherosclerosis. Through these and other steps, ECs help recruit and foster the entry of inflammatory cells such as monocytes into the arterial wall. Once in the wall, monocytes differentiate into macrophages as they engage in steps involved in wound healing, ingesting low-density lipoproteins (LDLs), which may have become oxidized, as well as cellular debris. VSMCs present in the arterial media can migrate to the subintimal space, proliferate, and engage in forming a fibrous cap. This process essentially seals off the plaque, and its highly thrombotic nature, from the circulation [1]. Signaling between macrophages and T cells can promote release of matrix-degrading enzymes known as matrix metalloproteinases (MMPs), another response to injury that in other settings of wound healing is essential but in this context may be maladaptive. MMPs can degrade the fibrous cap, resulting in plaque destabilization, rupture, and acute MI, events more likely to happen when the cap is thinner [8]. Superficial erosion of the endothelium is another mechanism through which these processes may occur [14].
INFLAMMATION IN ATHEROSCLEROSIS
Even this brief synopsis makes clear the importance of inflammation in atherosclerosis. The evidence for this extends from autopsy studies, which showed inflammatory cells such as macrophages and T cells to be among the most prevalent cell types in the shoulder regions of ruptured lesions, to in vitro studies in which proatherosclerotic forces induce pathogenic responses in ECs and VSMCs, to population studies suggesting that measures of inflammatory responses, from white blood cell counts to levels of C-reactive protein (CRP), predict cardiovascular risk [1]. Indeed, studies suggest that CRP level predicts cardiovascular events independent of other risk factors and to an extent similar to that of elevated LDL levels [15]. How to best integrate CRP measurements into clinical practice remains under debate and may well be addressed in the next iteration of the National Cholesterol Education Program’s Adult Treatment Panel guidelines, expected to be released soon.
Monocyte differentiation into macrophages, an essential step in atherosclerosis, is driven by various forces, including the presence of oxidized LDL, and specific mediators, such as macrophage colony-stimulating factor [16]. Macrophage uptake of oxidized LDL promotes their further evolution into foam cells. Dendritic cells are also part of this process [17]. T cells are particularly important players in atherosclerosis even if they are present in lower numbers than are macrophages, whereas B cells and mast cells are also players in these processes [8]. As such, it becomes apparent that both innate and adaptive immunity are integral in the origins and natural history of atherosclerosis and its complications.
Innate immune responses in atherosclerosis involve both ECs and macrophages, with activation of pattern recognition receptors that control important cellular responses [18]. Oxidized LDL uptake can induce expression of proximal potent proinflammatory mediators such as nuclear factor κB (NF-κB), activator protein 1, and interferon regulatory factor 1. Toll-like receptors (TLRs) are pattern recognition receptors implicated in many aspects of atherosclerosis, with myeloid differentiation primary response gene 88 being especially important as a mediator in distal effects exerted by the TLR family. Activation of these pathways can promote inflammation and atherosclerosis [19].
Adaptive immunity is also relevant to atherosclerosis, with data indicating that antigen presentation is part of the process [20]. Mouse studies demonstrate that deficiency in either T cells or B cells, as well as specific mediators from such cells, is associated with less atherosclerosis. An extensive body of literature continues to evolve in regard to how specific aspects of T-cell biology comes into play in atherosclerotic complications [21]. T cells expressing CD4 are commonly found in plaque, as are CD8-expressing cells, although to a lesser extent [22]. The αβ T-cell antigen receptor is also abundantly expressed in plaque T cells. T-helper type 1 cells and their products such as interferon γ (IFN-γ) can promote atherosclerosis, as do multiple other interleukin factors. Details of these T-cell mechanisms continue to be worked out and suggest a very complex network of inflammatory cell responses involved in atherosclerosis.
One more recent advance in this area has been the recognition that some inflammatory cells may play a protective role, limiting inflammation and atherosclerosis. This concept of inflammatory cell heterogeneity forces reappraisal of existing data regarding inflammation and atherosclerosis. For example, regulatory T cells have been suggested to decrease atherosclerosis, perhaps through release of cytokines such as transforming growth factor β and interleukin 10 [22]. Likewise, monocytes have also been subclassified into proinflammatory/proatherosclerotic (M1) type versus the more atheroprotective (M2) type [23]. Ly-6 is a family of glycoproteins expressed by cells of the hematopoietic lineage, and Ly-6c is one of 7 known serotypes. Proinflammatory monocytes having higher levels of Ly-6c may correlate with increased release of proinflammatory cytokines.
METABOLIC DRIVERS OF INFLAMMATION AND ATHEROSCLEROSIS
Interestingly, many of these same core mechanisms of inflammation have also been implicated in adipose tissue, whether through changes in adipocytes and their release of inflammatory mediators and modulators, or through evidence that inflammatory cells take residence in adipose tissue where they can exert systemic effects [24, 25]. These observations seem especially germane given the increased risk found in individuals with diabetes and even prediabetes and the increasing prevalence of both adiposity and diabetes and its presence in increasingly younger segments of the population.
Obesity involves expansion of adipocytes and their storage of triglycerides, which contain high-energy-yielding fatty acids [26]. Fatty acid trafficking, for example through the fatty acid transporter CD36, could modify cellular responses [27, 28]. Extensive data suggest that different depots of fat may have distinct biologic properties, with visceral fat suggested as being more associated with diabetes and atherosclerosis [29]. Other lines of evidence argue that the real issue is increased adiposity, with deposition of triglycerides/lipids in liver, as in nonalcoholic steatohepatitis, or in skeletal muscle, causing the cascade of responses associated with cardiovascular disease [29, 30]. Regardless of these findings, increased adiposity is closely associated with a constellation of abnormalities that increase inflammation and atherosclerosis. Specific mediators released by adipocytes can be identified as contributing to all the key aspects of cardiometabolic disease. At the same time, inflammatory cells are present in adipose tissue and bear hallmarks of central inflammatory responses such as NF-κB activation. Given the extent to which adipose tissue is vascularized, inflammatory cells present in fat would have ready access to the circulation and the potential for systemic effects.
CARDIOVASCULAR DISEASE IN HIV-INFECTED PATIENTS
Increased incidence of cardiovascular disease may occur in HIV-infected patients through a number of the mechanisms outlined above [31, 32]. Infection has been raised as a potential contributor to many chronic illnesses, including atherosclerosis, which may be a result of a generalized increase in inflammatory pathways. Given the specific cellular players involved in atherosclerosis discussed above and the unique effects of HIV on the immune system, it is plausible to consider that HIV infection itself could either directly or indirectly promote atherosclerosis via monocyte or T-cell activation. Similarly, the concomitant infections experienced by HIV-infected patients, including cytomegalovirus (CMV) infection and others, could also promote atherosclerosis, as reviewed by Hsue et al in this supplement. Alternatively, changes associated with HIV infection could alter risk factors and mediators that then promote atherosclerosis. For example, cytokine dysregulation associated with HIV infection has been shown to contribute to dyslipidemia. In addition, therapies to treat HIV infection could influence atherosclerosis by altering vascular function and metabolism, for example, by promoting insulin resistance, impaired fibrinolysis, dyslipidemia, platelet dysfunction, and visceral fat accumulation. As the HIV-infected individuals live longer and become progressively older, these various issues become even more relevant.
Early coronary atherosclerosis has been reported in young HIV-infected patients [33, 34]. Both traditional and nontraditional cardiovascular risk factors likely contribute to increased cardiovascular disease in the HIV-infected patient population, and these processes may impact on different aspects of the atherosclerotic process. (For a review of potential effects of dyslipidemia, insulin resistance, and abdominal adiposity in HIV infection and contributions to cardiovascular risk, see Stanley et al in this supplement.) For example, Lo and colleagues [35, 36] have demonstrated that traditional risk factors contribute to calcified plaque, whereas as nontraditional factors, including monocyte and macrophage activation, are associated with noncalcifed plaque, which may be more vulnerable and prone to rupture.
POTENTIAL EFFECTS OF ANTIRETROVIRAL THERAPY AND TREATMENT INTERRUPTION ON THE ATHEROSCLEROTIC PROCESS
Antiretroviral medications may contribute to atherosclerotic heart disease development in patients with HIV infection. In the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study that included 23 437 HIV-infected patients, the adjusted relative rate of MI was 1.16 per year of exposure to combination antiretroviral therapy (95% confidence interval [CI], 1.09–1.23 MIs per year) [37]. In this study, the investigators also found that higher total serum cholesterol and triglyceride levels and presence of diabetes were associated with increased incidence of MI. The D:A:D study further showed increased MI rates in patients treated with protease inhibitors [37, 38]. Protease inhibitors may cause atherosclerosis through dyslipidemia or possibly through foam cell formation because HIV protease inhibitors can upregulate scavenger receptor CD36 in LDL receptor null mice and increase CD36-dependent cholesteryl ester accumulation in macrophages [39]. Protease inhibitors have also been associated with increased plasminogen activator inhibitor 1 levels and increased fibrinogen levels [40].
On the other hand, HIV drug treatment interruption has also been associated with increased cardiovascular event rates. In the Strategies for Management of Anti-Retroviral Therapy (SMART) trial, patients in the drug conservation group showed a trend of increased cardiovascular events compared with those in the viral suppression group, with a hazard ratio of 1.57 (95% CI, 1.00–2.46; P = .05) [41, 42]. Patients in the drug conservation group had a reduction in total cholesterol, LDL cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels after treatment interruption, and the ratio of total cholesterol to HDL cholesterol increased unfavorably. Data from the SMART trial suggest that activation of inflammatory and coagulation pathways induced by HIV infection may be further worsened by treatment interruption and thus further increase the risk of death among HIV-infected patients [43].
HISTOPATHOLOGIC STUDIES IN HIV INFECTION
Autopsy studies in young patients with HIV infection showed thickening of the intima composed of VSMCs, macrophages, and rare lymphocytes in all cases; 60% of these cases had atherosclerosis with the pattern of coronary lesions involving all vessels circumferentially without any healthy space, with a pattern resembling the accelerated atherosclerosis associated with cardiac transplant recipients [34].
Micheletti et al [44] also examined histopathology of coronary arteries of young to middle-aged HIV-infected patients who had died of advanced AIDS and found evidence of coronary luminal narrowing, calcification, and high plaque lipid content in a high proportion of the HIV-infected patients. These histopathologic studies strongly support a connection between HIV infection and atherosclerosis.
HIV INFECTION, VIRAL PROTEINS, AND ATHEROSCLEROSIS
HIV infection in itself may cause detrimental changes in the vascular endothelium. For example, HIV viral load has been found to be associated with endothelial dysfunction [45, 46]. Wolf et al [47] found markers of endothelial activation, including soluble VCAM-1, soluble Intercellular Adhesion Molecule 1, and von Willebrand factor, to be higher in untreated HIV-infected patients than in healthy control patients in the Swiss HIV Cohort Study. Levels of these endothelial activation markers decreased with antiretroviral therapy, and soluble VCAM-1 and von Willebrand factor levels correlated with viral load in this study.
HIV-1 Tat protein can cause endothelial dysfunction in porcine coronary arteries [48]. HIV-1 Tat can also promote the secretion of the chemokine MCP-1, thus promoting migration of monocytes into the vascular intima [49, 50]. HIV-infected patients with the MCP-1 2518G allele are at increased risk for atherosclerosis [51]. The MCP-1 2518G allele has also been associated with severity of coronary artery disease in patients without HIV infection [52]. Circulating MCP-1 levels have also been associated with increased coronary atherosclerosis seen on coronary computed tomography (CT) angiography in HIV-infected men [35]. In addition, HIV Nef protein has been demonstrated to impair efflux of cholesterol from macrophages by downregulating adenosine triphosphate binding cassette transporter A1, and therefore increasing the promotion of foam cell formation [53].
ATHEROSCLEROSIS AND HIV INFECTION ARE INFLAMMATORY DISEASES INVOLVING THE IMMUNE SYSTEM
Inflammatory and immunologic factors may contribute to increased cardiovascular disease in the population of HIV-infected individuals. CRP has been found to be associated with increased acute MI risk in HIV-infected patients [54]. Furthermore, several studies have also demonstrated that acute MI rates are related to low CD4 cell counts [55, 56]. Recently, HIV-associated T-cell activation has also been found to be associated with subclinical carotid atherosclerosis, as Kaplan et al [57] demonstrated that HIV-associated T-cell activation and senescence are related to subclinical carotid atherosclerosis using T-cell phenotyping and carotid ultrasound in 115 HIV-infected women in the Women’s Interagency HIV study.
Because atherosclerosis is an inflammatory process in which monocytes, T cells, B cells, and their associated mediators play key roles, as reviewed elsewhere [4, 58, 59], the state of chronic immune activation experienced by HIV-infected patients likely predisposes them to atherosclerosis development. Tilton et al [60] demonstrated that HIV-infected patients, regardless of antiretroviral therapy status, had elevated spontaneous production of monocyte inflammatory cytokines tumor necrosis factor α, interleukin 1β, and interleukin 6 compared with uninfected control participants.
Concomitant infections in the HIV-infected patient could also contribute to inflammation and atherosclerosis. For example, CMV infection may play a possible role in atherogenesis [61]. Hsue et al [62] have previously shown HIV-infected patients to have higher levels of CRP, higher levels of CD4 and CD8 T-cell activation, and higher CMV-specific T-cell response, and that CMV-specific T-cell response (measured by expression of IFN-γ after exposure to CMV pp65 peptide antigens) was independently related to higher carotid intima-media thickness in patients with HIV infection.
SUBCLINICAL CORONARY ATHEROSCLEROSIS AND SOLUBLE CD163, A MONOCYTE/MACROPHAGE-SPECIFIC ACTIVATION MARKER, IN HIV-INFECTED PATIENTS
With the use of coronary CT angiography, patients with HIV infection were reported to have a higher prevalence of subclinical coronary atherosclerosis and greater burden of coronary atherosclerotic plaque, particularly noncalcified plaque, than were HIV-seronegative subjects with similar cardiovascular risk factors, even those patients with low Framingham risk score and no symptoms of cardiovascular disease [35]. In this study, coronary plaques in the HIV-infected patients were more likely to be noncalcified rather than calcified plaque lesions. Plaques with inflammatory, necrotic, lipid-rich cores are more vulnerable to rupture, are typically noncalcified, and have low attenuation on CT imaging [63, 64].
CD163 is a scavenger receptor expressed on the surface of macrophages and monocytes specifically [65]. Soluble CD163 is the proteolytic cleavage product shed by activated monocytes/macrophages. Soluble CD163 was recently shown to be a marker of HIV disease activity in early infected and chronically infected patients [66] and was demonstrated to be positively associated with noncalcified coronary plaque burden, independent of traditional cardiovascular risk factors, among asymptomatic men with chronic HIV infection and even among those with low or undetectable levels of HIV RNA [36]. Levels of soluble CD163 have also been shown to be elevated in non-HIV-infected patients with coronary artery disease [67]. Thus, the activation of monocytes and macrophages in HIV infection might contribute to the formation of vulnerable atherosclerotic plaques.
PLATELET FUNCTION IN HIV INFECTION
Because coronary arterial thrombosis, important in acute MIs, is mediated by platelets, abnormalities in platelet function may potentially play a role in the pathogenesis of increased cardiac events in patients living with HIV infection. Satchell et al [68] measured levels of time-dependent platelet aggregation in response to platelet agonists in 20 HIV-infected individuals and 20 age- and sex-matched HIV-negative individuals and found significant differences in platelet responses between the 2 groups, suggesting possible defects in platelet reactivity in patients with HIV infection. More studies are needed to further study platelet function in HIV-infected patients and the possible role of HIV in acute thrombotic events.
CONCLUSIONS
Cardiovascular disease is an emerging cause of death among HIV-infected patients. Multiple potential mechanisms exist by which HIV infection and its therapy may contribute to the development of atherosclerotic disease, including known traditional cardiovascular risk factors as well as inflammatory and immunologic mechanisms. Given the rapid and ongoing evolution of our thinking about atherosclerosis and its complications in the general population, the need to understand how these issues relate to atherosclerosis in patients with HIV infection is apparent. Such insights will no doubt guide therapy in the future.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported by the National Institutes of Health (grant numbers K23 HL092792 to J. L. and R01 HL048743 to J. P.). This supplement was supported by the Harvard University Center for AIDS Research and an educational grant from Bristol-Myers Squibb.
Potential conflicts of interest.
J. P. has consulted for Bristol-Myers Squibb. J. L. reports no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–25. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 2.McMillan GC. Historical review of research on atherosclerosis. Adv Exp Med Biol. 1995;369:1–6. doi: 10.1007/978-1-4615-1957-7_1. [DOI] [PubMed] [Google Scholar]
- 3.Mayerl C, Lukasser M, Sedivy R, Niederegger H, Seiler R, Wick G. Atherosclerosis research from past to present—on the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch. 2006;449:96–103. doi: 10.1007/s00428-006-0176-7. [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 5.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009;335:191–203. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 6.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–9. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGill HC, Jr, McMahan CA, Gidding SS. Preventing heart disease in the 21st century: implications of the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) study. Circulation. 2008;117:1216–7. doi: 10.1161/CIRCULATIONAHA.107.717033. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am. 2011;95:919–37. doi: 10.1016/j.mcna.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–76. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 11.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: hemodynamic and biochemical mechanisms underlying Glagov’s phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–8. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 12.Choi SY, Mintz GS. What have we learned about plaque rupture in acute coronary syndromes? Curr Cardiol Rep. 2010;12:338–43. doi: 10.1007/s11886-010-0113-x. [DOI] [PubMed] [Google Scholar]
- 13.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 14.Shah PK. Inflammation and plaque vulnerability. Cardiovasc Drugs Ther. 2009;23:31–40. doi: 10.1007/s10557-008-6147-2. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–38. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 16.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manthey HD, Zernecke A. Dendritic cells in atherosclerosis: functions in immune regulation and beyond. Thromb Haemost. 2011;106:772–8. doi: 10.1160/TH11-05-0296. [DOI] [PubMed] [Google Scholar]
- 18.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–45. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michelsen KS, Wong MH, Shah PK, et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–84. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–58. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 21.Taleb S, Tedgui A, Mallat Z. Adaptive T cell immune responses and atherogenesis. Curr Opin Pharmacol. 2010;10:197–202. doi: 10.1016/j.coph.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Ketelhuth DF, Hansson GK. Cellular immunity, low-density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost. 2011;106:779–86. doi: 10.1160/TH11-05-0321. [DOI] [PubMed] [Google Scholar]
- 23.Johnson JL, Newby AC. Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol. 2009;20:370–8. doi: 10.1097/MOL.0b013e3283309848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res. 2011;108:1002–16. doi: 10.1161/CIRCRESAHA.110.226860. [DOI] [PubMed] [Google Scholar]
- 27.Plutzky J. Expansion and contraction: the mighty, mighty fatty acid. Nat Med. 2009;15:618–9. doi: 10.1038/nm0609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Berkel TJ, Out R, Hoekstra M, Kuiper J, Biessen E, van Eck M. Scavenger receptors: friend or foe in atherosclerosis? Curr Opin Lipidol. 2005;16:525–35. doi: 10.1097/01.mol.0000183943.20277.26. [DOI] [PubMed] [Google Scholar]
- 29.Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. Circ Res. 2007;101:27–39. doi: 10.1161/CIRCRESAHA.107.151621. [DOI] [PubMed] [Google Scholar]
- 30.Bieghs V, Rensen PC, Hofker MH, Shiri-Sverdlov R. NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis. 2012;220:287–9.3. doi: 10.1016/j.atherosclerosis.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 31.Grinspoon S. Diabetes mellitus, cardiovascular risk, and HIV disease. Circulation. 2009;119:770–2. doi: 10.1161/CIRCULATIONAHA.108.835710. [DOI] [PubMed] [Google Scholar]
- 32.Hakeem A, Bhatti S, Cilingiroglu M. The spectrum of atherosclerotic coronary artery disease in HIV patients. Curr Atheroscler Rep. 2010;12:119–24. doi: 10.1007/s11883-010-0089-4. [DOI] [PubMed] [Google Scholar]
- 33.Henry K, Melroe H, Huebsch J, et al. Severe premature coronary artery disease with protease inhibitors. Lancet. 1998;351:1328. doi: 10.1016/S0140-6736(05)79053-X. [DOI] [PubMed] [Google Scholar]
- 34.Tabib A, Leroux C, Mornex JF, Loire R. Accelerated coronary atherosclerosis and arteriosclerosis in young human-immunodeficiency-virus-positive patients. Coron Artery Dis. 2000;11:41–6. doi: 10.1097/00019501-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 38.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 39.Dressman J, Kincer J, Matveev SV, et al. HIV protease inhibitors promote atherosclerotic lesion formation independent of dyslipidemia by increasing CD36-dependent cholesteryl ester accumulation in macrophages. J Clin Invest. 2003;111:389–97. doi: 10.1172/JCI16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koppel K, Bratt G, Schulman S, Bylund H, Sandstrom E. Hypofibrinolytic state in HIV-1-infected patients treated with protease inhibitor-containing highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;29:441–9. doi: 10.1097/00042560-200204150-00003. [DOI] [PubMed] [Google Scholar]
- 41.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 42.Stein JH. Cardiovascular risks of antiretroviral therapy. N Engl J Med. 2007;356:1773–5. doi: 10.1056/NEJMe078037. [DOI] [PubMed] [Google Scholar]
- 43.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Micheletti RG, Fishbein GA, Fishbein MC, et al. Coronary atherosclerotic lesions in human immunodeficiency virus-infected patients: a histopathologic study. Cardiovasc Pathol. 2009;18:28–36. doi: 10.1016/j.carpath.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blum A, Hadas V, Burke M, Yust I, Kessler A. Viral load of the human immunodeficiency virus could be an independent risk factor for endothelial dysfunction. Clin Cardiol. 2005;28:149–53. doi: 10.1002/clc.4960280311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis. 2006;42:1325–32. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf K, Tsakiris DA, Weber R, Erb P, Battegay M. Antiretroviral therapy reduces markers of endothelial and coagulation activation in patients infected with human immunodeficiency virus type 1. J Infect Dis. 2002;185:456–62. doi: 10.1086/338572. [DOI] [PubMed] [Google Scholar]
- 48.Paladugu R, Fu W, Conklin BS, et al. Hiv Tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38:549–55. doi: 10.1016/s0741-5214(03)00770-5. discussion 55–6. [DOI] [PubMed] [Google Scholar]
- 49.Gosling J, Slaymaker S, Gu L, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103:773–8. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park IW, Wang JF, Groopman JE. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood. 2001;97:352–8. doi: 10.1182/blood.v97.2.352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.