Abstract
As antiretroviral therapy has decreased human immunodeficiency virus (HIV)–associated mortality, cardiometabolic abnormalities have become increasingly apparent in HIV-infected individuals. Many patients treated for HIV infection exhibit body composition changes, including peripheral fat atrophy and visceral lipohypertrophy. In addition, HIV-infected individuals demonstrate a higher prevalence of dyslipidemia, insulin resistance and diabetes, and cardiovascular risk, compared with the general population. Although antiretroviral therapy appears to contribute to some of the cardiometabolic abnormalities in HIV infection, HIV itself, immunologic factors, and lifestyle factors are also important mediators of cardiovascular risk. Treatment strategies for body composition changes and cardiometabolic abnormalities in HIV infection include lifestyle modification, lipid-lowering agents, insulin sensitizers, and treatments to reverse endocrine abnormalities in HIV, including growth hormone–releasing hormone. None of these strategies has comprehensively addressed the abnormalities experienced by this population, however, and further research is needed into combined strategies to improve body composition and ameliorate cardiovascular risk.
A large percentage of individuals with human immunodeficiency virus (HIV) infection, particularly those receiving antiretroviral therapy, experience peripheral fat atrophy, visceral fat accumulation, and cardiometabolic comorbidities, including dyslipidemia, impaired glucose homeostasis, and increased risk for cardiovascular disease. As antiretroviral therapy continues to increase life expectancy for HIV-infected individuals, management of these issues becomes increasingly important. This review will describe changes in body composition, lipid profile, glucose homeostasis, and cardiovascular health in individuals with HIV infection, concluding with a review of existing strategies and research goals to address these issues.
PERIPHERAL LIPOATROPHY AND VISCERAL ADIPOSE TISSUE ACCUMULATION IN HIV INFECTION
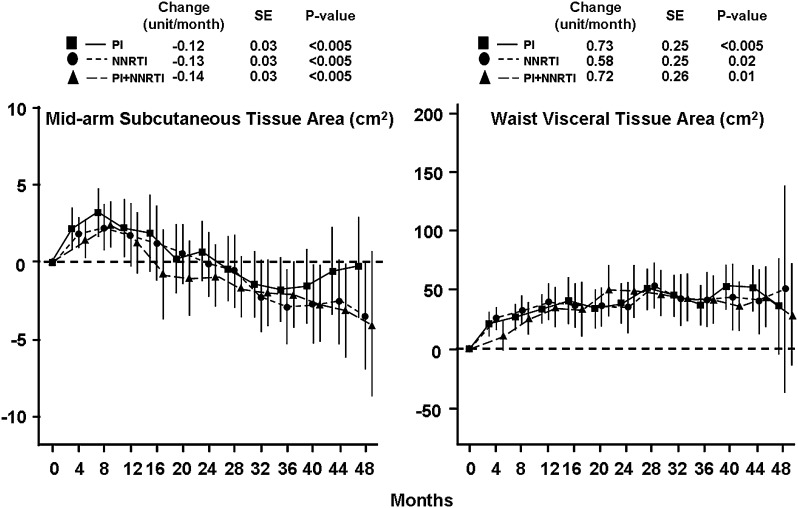
Abnormal fat distribution occurs in more than half of HIV-infected patients treated with antiretroviral therapy [1, 2]. Patients may experience lipoatrophy in the face and limbs; lipohypertrophy of visceral, breast, cervical, and/or dorsocervical adipose tissue; or a combination of lipoatrophy and lipohypertrophy. In addition to lipohypertrophy of existing fat depots, HIV-infected individuals also exhibit increased prevalence of ectopic fat distribution in the liver, muscles, and dorsocervical area [3–5]. Although certain antiretroviral medications appear more highly associated with abnormalities in fat redistribution than others [6], changes in body composition may occur to some degree with any antiretroviral strategy. Studies of antiretroviral-naive patients beginning treatment show clear increases in visceral adipose tissue and trunk fat (Figure 1) even with contemporary regimens (Supplementary Figure 1) [7, 8].
Figure 1.
Changes in mid-arm subcutaneous fat and visceral adipose tissue area in antiretroviral-naive patients beginning treatment with regimens involving protease inhibitors (PIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), or PIs plus NNRTIs. Mean change from baseline and 95% confidence intervals are shown. For each strategy, the rates of change beginning at the 4-month follow-up visit are shown at the top of each panel, with P values for comparison to the null hypothesis. Adapted from [7] by permission of Lippincott Williams & Wilkins, Inc.
Decreases in limb subcutaneous fat are also prevalent in regimens including many antiretrovirals, including zidovudine and stavudine (Figure 1) [7], whereas in patients who are using newer agents such as abacavir, lamivudine, tenofovir, efavirenz, and atazanavir, lipoatrophy may be less common and limb fat may instead increase (Supplementary Figure 1) [8]. The mechanisms of lipoatrophy are numerous and include the inhibition of adipocyte differentiation by protease inhibitors [9, 10] and the impairment of mitochondrial function by nucleoside reverse-transcriptase inhibitors, particularly thymidine analogues [11, 12]. The etiology of lipohypertrophy and ectopic fat accumulation is less clear. The phenotypic similarity of HIV-associated lipohypertrophy and Cushing syndrome has prompted detailed investigation of cortisol dynamics. Although a minority of individuals with HIV-associated lipohypertrophy have demonstrated elevated serum and urine cortisol concentrations, HIV-infected lipodystrophic individuals show appropriate suppression of cortisol levels in response to dexamethasone and also demonstrate normal diurnal variation in cortisol levels, arguing against true Cushing syndrome [13, 14]. Rather, lipohypertrophy may be related to elevated levels of inflammatory cytokines [15] or to “overflow” storage of surplus energy, particularly triglyceride and free fatty acid, that cannot be stored in atrophic subcutaneous depots [16]. Lipohypertrophy does not always occur in conjunction with lipoatrophy, however, and further research is necessary to determine the etiology of abnormal fat accumulation during HIV infection.
Although HIV-infected individuals with abnormal fat distribution generally maintain a body mass index in the normal or overweight range, they often experience cardiometabolic complications similar to those seen in frank obesity. Hadigan et al [17] demonstrated significantly increased prevalence of dyslipidemia and impaired glucose homeostasis in HIV-infected individuals with abnormal fat distribution, compared with HIV-negative controls matched for age and body mass index. Samaras et al [18] recently demonstrated that HIV-infected men with lipodystrophy had levels of C-reactive protein, adiponectin, tumor necrosis factor α, and interleukin 6 similar to those in obese HIV-negative men, despite significantly lower body mass index and body fat. Both peripheral fat atrophy and visceral fat accumulation are associated with an increase in cardiometabolic risk factors. Wohl et al [19] have shown that both increased visceral fat and decreased subcutaneous leg fat are associated with elevated triglyceride levels (Supplementary Figure 2) and decreased high-density lipoprotein cholesterol (HDL-C) levels. Likewise, both decreased subcutaneous leg fat and increased visceral fat are strongly associated with decreased insulin sensitivity in this population [20, 21], as well as in the general population [22]. Albu et al [21] demonstrated that insulin sensitivity increases in proportion to the percentage of total body fat stored in the lower extremities in both HIV-infected and noninfected women. In the Fat Redistribution and Metabolic Change in HIV (FRAM) cohort, HIV-infected individuals in the highest tertiles of upper trunk subcutaneous adipose tissue and visceral adipose tissue had a significantly increased risk of insulin resistance (odds ratios, 2.1 for upper trunk subcutaneous adipose tissue and 3.1 for visceral adipose tissue), compared with those in the lowest tertiles [20]. Data from FRAM also have demonstrated that both peripheral lipoatrophy and visceral lipohypertrophy are associated with increased Framingham Risk Score, which is predictive of myocardial infarction, in HIV-infected individuals [23]. Scherzer et al [24] have recently shown an association between visceral adiposity and overall mortality in individuals with HIV infection, such that individuals in the highest tertile of visceral adipose tissue had an odds ratio of mortality of 2.1, compared with those in the lowest tertile. Importantly, both lipoatrophy and visceral fat accumulation are associated with subclinical atherosclerosis as measured by coronary artery calcium score from computed tomography angiography [25], and visceral fat is associated with progression of subclinical atherosclerosis in HIV-infected patients [26].
INSULIN RESISTANCE AND DIABETES MELLITUS IN HIV INFECTION
Although antiretroviral-naive individuals appear to have a risk of diabetes that is similar to [27, 28] or modestly greater than [29] that for the general population, individuals treated with antiretroviral therapy show a marked increase in impaired glucose tolerance and type 2 diabetes mellitus [17, 29]. In a cohort of HIV-infected individuals with abnormal fat distribution, Hadigan et al [17] demonstrated that approximately one-third of patients had abnormal glucose tolerance and that 7% had previously undiagnosed diabetes, according to a 2-hour glucose following oral glucose tolerance test. More recently, Brown et al [29] demonstrated that HIV-infected men receiving antiretroviral therapy had a baseline prevalence of diabetes of 14%, compared with 5% in controls, and had an approximately 4-fold risk of developing diabetes during follow-up.
One of the primary contributors to impaired glucose homeostasis in the HIV-infected population is abnormal fat distribution, particularly visceral adiposity and lower extremity fat atrophy. Intramyocellular lipid levels, which are generally increased in HIV-infected patients compared with those in controls, are also strongly associated with insulin resistance [3]. In addition, numerous other factors also appear to play important roles in the high prevalence of insulin resistance and diabetes in this population. Brown et al [30] have demonstrated that systemic inflammatory markers, including high sensitivity c-reactive protein and tumor necrosis factor receptors 1 and 2, are associated with increased risk of developing diabetes in HIV infection, suggesting a role of chronic inflammation in impaired glucose metabolism. In support of this, protease inhibitors are known to induce suppressor of cytokine signaling 1, which upregulates tumor necrosis factor α and other inflammatory cytokines [31]. Antiretrovirals also appear to contribute to an increased risk of diabetes. In the Data Collection on Adverse Events of Anti-HIV Drugs (D.A.D.) study, the incidence of diabetes increased with increasing cumulative exposure to antiretrovirals, even after control for other risk factors [32]. Moreover, Capeau et al [33] recently demonstrated that the incidence of diabetes may be related more specifically to the use of indinavir, stavudine, and didanosine, as well as to increased waist-to-hip ratio and peripheral lipoatrophy, and it may be decreasing with the use of newer, more contemporary agents. The mechanisms by which antiretrovirals may impair glucose homeostasis are numerous. Many protease inhibitors block the glucose transporter GLUT4, impairing insulin-stimulated glucose use [34]. Protease inhibitors also appear to directly affect glucose sensing by β cells, causing impaired insulin release [35]. Nucleoside reverse-transcriptase inhibitors, particularly the thymidine-containing analogues stavudine and zidovudine, may also contribute to insulin resistance through adverse effects on mitochondria [12, 36]. Fleischman et al [36] have demonstrated that administration of stavudine to non-HIV–infected healthy controls for 1 month significantly reduced insulin sensitivity and decreased mitochondrial DNA levels, compared with placebo administration. Finally, hepatitis C virus coinfection may be associated with a higher risk of diabetes in HIV-infected individuals [28].
DYSLIPIDEMIA IN HIV INFECTION
Early in the course of untreated HIV infection, both HDL-C and low-density lipoprotein cholesterol (LDL-C) levels decrease and triglyceride levels increase [37, 38]. Following treatment with antiretroviral therapy, triglyceride levels often increase further [39], and HDL-C levels typically remain low. LDL-C levels commonly increase, often beyond the baseline level before HIV acquisition [37]. Thus, many patients with HIV infection, particularly those with associated body composition abnormalities, have a dyslipidemic profile of decreased HDL-C and increased triglyceride and LDL-C levels [17]. An increased triglyceride level is often the most pronounced lipid abnormality in HIV-infected patients and appears to contribute independently to increased cardiovascular risk in this population. In the D.A.D. study, each 2-fold increase in triglyceride levels was associated with a 17% increase in the risk of myocardial infarction (relative risk, 1.17; 95% confidence interval 1.06–1.29), after control for other traditional risk factors [40]. Moreover, Janiszewski et al [41] reported that, in a cohort of >2000 men and women with HIV infection, hypertriglyceridemia, together with increased waist circumference, was highly predictive of metabolic syndrome, insulin resistance, and cardiovascular risk as measured by the Framingham Risk Score. In addition to abnormal levels of HDL-C, triglycerides, and LDL-C, Riddler et al [42] have also demonstrated that HIV-infected individuals receiving antiretroviral therapy have altered particle size, compared with HIV-negative controls, with increased amounts of very low-density lipoprotein cholesterol and small LDL-C particles and decreased amounts of HDL-C and large LDL-C particles [42]. These abnormalities were not seen in antiretroviral-naive individuals with HIV infection, who exhibited a low HDL-C level but also a decreased number of small LDL-C particles, compared with controls [42].
Both increased visceral fat and decreased subcutaneous adipose tissue are associated with increased circulating free fatty acid and elevated triglyceride levels [19, 43]. In addition, detailed studies of lipid handling during HIV infection have shown a number of abnormalities. Rates of basal lipolysis are significantly higher in HIV-infected individuals, compared with uninfected controls [43, 44]. Moreover, HIV-infected individuals demonstrate a significantly higher free fatty acid level following an oral glucose challenge, reflecting reduced ability of insulin to suppress lipolysis at the level of the adipocyte [43]. Hepatic de novo lipogenesis is also increased [45], and peripheral fatty acid trapping is impaired [46]. Together, these abnormalities lead to increased circulating free fatty acid, very low-density lipoprotein cholesterol, and triglyceride levels. The etiology of these abnormalities appears in part to be due to HIV infection itself, as changes in lipid handling are seen in individuals naive to antiretroviral therapy, potentially in relation to elevated levels of circulating inflammatory cytokines, particularly interferon α [45]. In addition, HIV-infected individuals may have increased saturated fat intake compared with controls, exacerbating lipid abnormalities [47]. Antiretrovirals, particularly protease inhibitors, also contribute to impaired lipid handling. Many protease inhibitors impair adipocyte differentiation and decrease triglyceride accumulation in adipocytes, leading to increased circulating triglyceride levels [9, 48]. In addition, a common protease inhibitor, ritonavir, has been shown to increase basal lipolysis in vitro [49], and ritonavir-boosted lopinavir, darunavir, and atazanavir decrease fatty acid oxidation in skeletal muscle cells in vitro [50]. Both of these effects, if consistent in vivo, would increase circulating free fatty acid levels. Different protease inhibitors have varying effects on lipid handling, with atazanavir typically showing less effect on adipocyte differentiation, triglyceride storage, and fatty acid oxidation than other protease inhibitors, such as lopinavir or ritonavir [9, 50].
TREATMENT STRATEGIES TO AMELIORATE CARDIOMETABOLIC RISK IN HIV-INFECTED INDIVIDUALS
Since HIV-infected individuals demonstrate multiple cardiovascular risk factors, including central adiposity, insulin resistance, dyslipidemia, and increased systemic inflammation, a comprehensive strategy will likely be necessary to address cardiometabolic risk in this population.
Overall Cardiometabolic Risk
Lifestyle modification should be the first-line approach, with a focus on smoking cessation in addition to exercise and diet modification. Formalized exercise programs in individuals with HIV infection have demonstrated benefit to reduce cholesterol and triglyceride levels [51, 52] and decrease waist circumference [51, 53]. In addition, Fitch et al demonstrated reductions in systolic blood pressure and hemoglobin A1c (HbA1c) level with a 6-month lifestyle intervention modeled after the National Cholesterol Education Project (NCEP) recommendations for diet and activity [53].
In many patients, however, lifestyle modification may not be adequate to thoroughly address cardiovascular risk, and in such patients more specific strategies to reduce insulin resistance, dyslipidemia, and central fat accumulation may be useful.
Insulin Resistance
Metformin treatment in patients with central fat accumulation has been shown to significantly improve insulin levels, waist circumference, and diastolic blood pressure [54], as well as tissue plasminogen activator antigen and plasminogen activator inhibitor 1 levels [55]. A recent study suggests metformin may reduce coronary artery calcium progression in HIV-infected patients with the metabolic syndrome [56]. Importantly, however, metformin appears to decrease both visceral and subcutaneous adipose tissue and may not be appropriate for individuals with severe lipoatrophy [54]. In contrast, thiazolidinediones, particularly pioglitazone, may increase limb fat while also improving insulin sensitivity and increasing HDL-C levels [57, 58]. Of interest, a recent pilot study involving HIV-infected men with lipoatrophy used a combination of pioglitazone and leptin, demonstrating that the addition of leptin further decreased insulin resistance, increased adiponectin levels, and decreased postprandial glucose levels, compared with pioglitazone alone [59]. Leptin is an investigational drug, and further study is needed regarding its effects in HIV-associated body composition changes. Addition of exercise training to pioglitazone therapy also augments the effects of pioglitazone in reducing insulin resistance [60]. Rosiglitazone has also been studied in this population, but effects on limb fat are not consistent, and rosiglitazone may have adverse cardiovascular effects [58, 61, 62].
Dyslipidemia
For individuals with dyslipidemia, both statin and fibrate therapy have shown a benefit [63–65]. Statins may be slightly less effective in HIV-infected patients than in the general population but have demonstrated a reduction of approximately 25% in LDL-C levels [64]. Doses may need to be reduced because of interactions with many protease inhibitors, which are metabolized by CYP3A4 and may increase serum concentrations of certain statins [66]. In patients with elevated triglyceride levels, both gemfibrozil [64, 67] and fenofibrate [68, 69] lower triglyceride levels significantly, although gemfibrozil appears to be somewhat less effective in HIV-infected individuals, compared with non–HIV-infected individuals. For individuals with elevations in both triglyceride and LDL-C levels, combination therapy with fibrates and statins is effective [69]. Niacin and fish oil have also been investigated in the HIV-infected population. Wohl et al [70] demonstrated that fish oil lowers triglyceride levels by 20%–25% and is well tolerated in HIV-infected individuals receiving antiretroviral therapy. Niacin, alone or in combination with a fibrate, also effectively reduces triglyceride levels and may have favorable effects on HDL-C levels, as well [68, 71, 72]. In addition, niacin may improve endothelial function [73] and increase adiponectin levels [68]. Niacin may have adverse effects on glycemia [68, 72], however, and may not be appropriate for patients with impaired glucose homeostasis. The effects of niacin, particularly the extended-release formulation, on insulin resistance may be transient [71], and the long-term effects of extended-release niacin on glucose homoeostasis remain under investigation. Finally, acipimox has also been investigated and demonstrates both reduction in triglyceride levels and improved insulin sensitivity, but acipimox remains investigational in the United States [74].
Central Fat Accumulation
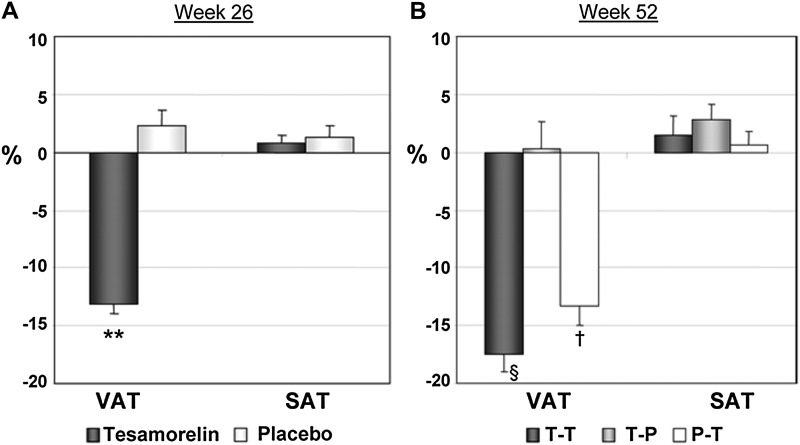
A number of agents have been proposed to reduce central fat accumulation. Both lifestyle modification and metformin have been shown to reduce waist circumference in HIV-infected patients, but they are not selective in this regard, and they tend to cause weight loss and reduce subcutaneous and visceral fat. Growth hormone secretion is reduced in HIV-infected individuals in association with increased visceral adiposity [75, 76], and both exogenous growth hormone and growth hormone–releasing hormone effectively reduce visceral fat and decrease triglyceride levels in HIV-infected individuals [77–80]. Exogenous growth hormone per se, as distinct from growth hormone–releasing hormone, the antecedent secretagogue hormone, exacerbates insulin resistance [79, 80], however, and thus remains investigational in this population predisposed to insulin resistance at baseline. A growth hormone–releasing hormone analogue, tesamorelin, was recently approved by the US Food and Drug Administration to reduce visceral fat in HIV-infected individuals with increased central fat accumulation. This strategy is known to increase endogenous growth hormone pulsatility [81] and may thus be a more rational and better tolerated strategy than use of exogenous growth hormone, which increases growth hormone levels in a nonpulsatile fashion. Indeed, treatment for 26–52 weeks with tesamorelin effectively reduces visceral adiposity by approximately 15% without significantly affecting subcutaneous adiposity in HIV-infected patients (Figure 2) [82, 83]. In addition, tesamorelin significantly reduced triglyceride levels by approximately 40 mg/dL over 6 months and did not affect fasting glucose or insulin levels or results of 2-hour glucose or insulin following oral glucose tolerance testing [83]. Tesamorelin did have a modest effect on HbA1c levels, with an increase of 0.1% ± 0.4% over 6 months, but there was no effect on HbA1c levels after 52 weeks of treatment, potentially because the beneficial effects of visceral fat reduction counterbalanced any hyperglycemic effects of tesamorelin [83]. In association with decreases in visceral fat, tesamorelin also has beneficial effects to increase levels of adiponectin, an adipokine associated with increased insulin sensitivity, and to reduce tissue plasminogen activator antigen levels, which appear to be associated with increased cardiovascular risk [84]. Further research is needed to determine whether reductions in visceral fat with tesamorelin may be associated with other cardiometabolic benefits. Finally, tesamorelin improved psychological distress related to abdominal fat accumulation and may thus increase well-being and quality of life.
Figure 2.
Percentage change from baseline in abdominal visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) at 26 weeks (a) and 52 weeks (b). Data are mean ± standard error of the mean. T-T indicates the group receiving tesamorelin for 52 weeks. T-P indicates the group receiving tesamorelin from week 0–26 followed by placebo from week 27–52. P-T indicates the group receiving placebo from week 0–26 followed by tesamorelin from week 27–52. **, P < .001 vs placebo; §, P < .001 vs baseline and vs T-P; †, P < .001 vs baseline. From Falutz et al [83]. Copyright 2010, The Endocrine Society.
Overall, tesamorelin was well tolerated, but 3% of people who received it had local and distant hypersensitivity reactions requiring discontinuation of the drug. Moreover, the effect of tesamorelin did not last beyond treatment, with reaccumulation of visceral fat occurring within 3–6 months of discontinuation, and the optimal duration of treatment remains unknown. Patients receiving tesamorelin should be carefully monitored to ensure that IGF-I levels remain within the normal range, as supraphysiologic levels of IGF-I may cause side effects, and there is concern that, in theory, increased IGF-I levels may be associated with malignancy.
In summary, treatment of HIV-associated cardiometabolic and body composition abnormalities requires a comprehensive approach, including lifestyle modification, smoking cessation, and use of lipid-lowering and/or insulin sensitizing agents when appropriate (see Table 1). For patients with central adiposity, tesamorelin may beneficially augment endogenous growth hormone secretion, selectively reducing visceral fat and lowering triglyceride levels. These currently available agents do not completely reverse the cardiometabolic abnormalities in many patients, however, and further research is needed into novel and combination agents to address cardiovascular and metabolic risk in this population.
Table 1.
Summary of Treatment Strategies for Human Immunodeficiency Virus–Associated Cardiometabolic Abnormalities
| Variable | Lifestyle | Metformin | TZD | Lipid-Lowering Agents | GHRH Analogue |
| Lipid levels | May improve | No effect | May increase HDL-C level | Improve | Improve |
| Glucose homeostasis | Improves | Improves | Improves | No effect | Minimal effecta |
| Central obesity | Improves | May improve | No effect | No effect | Improves |
| Peripheral lipoatrophy | No effect | May Worsen | May Improve | No effect | No effect |
| Safety | Excellent | Good | Fair | Good | Good |
Abbreviations: GHRH, growth hormone–releasing hormone; HDL-C, high-density lipoprotein cholesterol; TZD, thiazolidinedione.
GHRH analogue therapy may modestly increase hemoglobin A1c levels over 6 months of treatment but shows no effect over 12 months.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (grants K24 DK064545 and R01 DK063639 to S. G. and grant K23 DK089910 to T. S.) and the Mary Fisher Clinical AIDS Research and Education Fund. This supplement was supported by the Harvard Center for AIDS Research and an educational grant from Bristol-Myers Squibb.
Potential conflicts of interest.
S. G. has received research funding from Theratechnologies, Amgen, and Bristol Myers Squibb and has served as a consultant for EMD Serono and for Theratechnologies. T. S. certifies no potential conflicts of interest.
All authors have submitted the ICJME Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grunfeld C, Saag M, Cofrancesco J, Jr, et al. Regional adipose tissue measured by MRI over 5 years in HIV-infected and control participants indicates persistence of HIV-associated lipoatrophy. AIDS. 2010;24:1717–26. doi: 10.1097/QAD.0b013e32833ac7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005;40:1837–45. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 3.Gan SK, Samaras K, Thompson CH, et al. Altered myocellular and abdominal fat partitioning predict disturbance in insulin action in HIV protease inhibitor-related lipodystrophy. Diabetes. 2002;51:3163–9. doi: 10.2337/diabetes.51.11.3163. [DOI] [PubMed] [Google Scholar]
- 4.Torriani M, Hadigan C, Jensen ME, Grinspoon S. Psoas muscle attenuation measurement with computed tomography indicates intramuscular fat accumulation in patients with the HIV-lipodystrophy syndrome. J Appl Physiol. 2003;95:1005–10. doi: 10.1152/japplphysiol.00366.2003. [DOI] [PubMed] [Google Scholar]
- 5.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–7. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 6.Shlay JC, Sharma S, Peng G, Gibert CL, Grunfeld C. The effect of individual antiretroviral drugs on body composition in HIV-infected persons initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;51:298–304. doi: 10.1097/QAI.0b013e3181aa1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shlay JC, Bartsch G, Peng G, et al. Long-term body composition and metabolic changes in antiretroviral naive persons randomized to protease inhibitor-, nonnucleoside reverse transcriptase inhibitor-, or protease inhibitor plus nonnucleoside reverse transcriptase inhibitor-based strategy. J Acquir Immune Defic Syndr. 2007;44:506–17. doi: 10.1097/QAI.0b013e31804216cf. [DOI] [PubMed] [Google Scholar]
- 8.McComsey GA, Kitch D, Sax PE, et al. Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis. 2011;53:185–96. doi: 10.1093/cid/cir324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RJ, Wilson CG, Wabitsch M, Lazar MA, Steppan CM. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity (Silver Spring) 2006;14:994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, MacNaul K, Szalkowski D, Li Z, Berger J, Moller DE. Inhibition of adipocyte differentiation by HIV protease inhibitors. J Clin Endocrinol Metab. 1999;84:4274–7. doi: 10.1210/jcem.84.11.6234. [DOI] [PubMed] [Google Scholar]
- 11.Mallon PW, Unemori P, Sedwell R, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–96. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 12.Nolan D, Hammond E, Martin A, et al. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS. 2003;17:1329–8. doi: 10.1097/00002030-200306130-00007. [DOI] [PubMed] [Google Scholar]
- 13.Miller KK, Daly PA, Sentochnik D, et al. Pseudo-Cushing's syndrome in human immunodeficiency virus-infected patients. Clin Infect Dis. 1998;27:68–72. doi: 10.1086/514638. [DOI] [PubMed] [Google Scholar]
- 14.Yanovski JA, Miller KD, Kino T, et al. Endocrine and metabolic evaluation of human immunodeficiency virus- infected patients with evidence of protease inhibitor-associated lipodystrophy. J Clin Endocrinol Metab. 1999;84:1925–31. doi: 10.1210/jcem.84.6.5740. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JA, Albu JB, Engelson ES, et al. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. 2003. Am J Physiol Endocrinol Metab. 2004;286:E261–271. doi: 10.1152/ajpendo.00056.2003. [DOI] [PubMed] [Google Scholar]
- 16.Huang-Doran I, Sleigh A, Rochford JJ, O'Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207:245–55. doi: 10.1677/JOE-10-0272. [DOI] [PubMed] [Google Scholar]
- 17.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–9. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 18.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obes (Silver Spring) 2009;17:53–9. doi: 10.1038/oby.2008.500. [DOI] [PubMed] [Google Scholar]
- 19.Wohl D, Scherzer R, Heymsfield S, et al. The associations of regional adipose tissue with lipid and lipoprotein levels in HIV-infected men. J Acquir Immune Defic Syndr. 2008;48:44–52. doi: 10.1097/QAI.0b013e31816d9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grunfeld C, Rimland D, Gibert CL, et al. Association of upper trunk and visceral adipose tissue volume with insulin resistance in control and HIV-infected subjects in the FRAM study. J Acquir Immune Defic Syndr. 2007;46:283–90. doi: 10.1097/qai.0b013e31814b94e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albu JB, Kovera AJ, Allen L, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82:1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–60. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lake JE, Wohl D, Scherzer R, et al. Regional fat deposition and cardiovascular risk in HIV infection: the FRAM study. AIDS Care. 2011;23:929–38. doi: 10.1080/09540121.2010.543885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherzer R, Heymsfield SB, Lee D, et al. Decreased limb muscle and increased central adiposity are associated with 5-year all-cause mortality in HIV infection. AIDS. 2011;25:1405–14. doi: 10.1097/QAD.0b013e32834884e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guaraldi G, Stentarelli C, Zona S, et al. Lipodystrophy and anti-retroviral therapy as predictors of sub-clinical atherosclerosis in human immunodeficiency virus infected subjects. Atherosclerosis. 2010;208:222–7. doi: 10.1016/j.atherosclerosis.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Guaraldi G, Zona S, Orlando G, et al. Progression of coronary artery calcium in men affected by human immunodeficiency virus infection. Int J Cardiovasc Imaging. 2011 doi: 10.1007/s10554-011-9898-y. [DOI] [PubMed] [Google Scholar]
- 27.Brar I, Shuter J, Thomas A, Daniels E, Absalon J. A comparison of factors associated with prevalent diabetes mellitus among HIV-infected antiretroviral-naive individuals versus individuals in the National Health and Nutritional Examination Survey cohort. J Acquir Immune Defic Syndr. 2007;45:66–71. doi: 10.1097/QAI.0b013e318031d7e3. [DOI] [PubMed] [Google Scholar]
- 28.Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23:1227–34. doi: 10.1097/QAD.0b013e32832bd7af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown TT, Cole SR, Li X, et al. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179–84. doi: 10.1001/archinte.165.10.1179. [DOI] [PubMed] [Google Scholar]
- 30.Brown TT, Tassiopoulos K, Bosch RJ, Shikuma C, McComsey GA. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33:2244–9. doi: 10.2337/dc10-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carper MJ, Cade WT, Cam M, et al. HIV-protease inhibitors induce expression of suppressor of cytokine signaling-1 in insulin-sensitive tissues and promote insulin resistance and type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2008;294:E558–67. doi: 10.1152/ajpendo.00167.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D: A:D) study. Diabetes Care. 2008;31:1224–9. doi: 10.2337/dc07-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1,046 HIV-infected patients started on a combination antiretroviral treatment: the ANRS CO8 APROCO-COPILOTE cohort. AIDS. 2011;26:303–14. doi: 10.1097/QAD.0b013e32834e8776. [DOI] [PubMed] [Google Scholar]
- 34.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J Biol Chem. 2000;275:20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 35.Koster JC, Remedi MS, Qiu H, Nichols CG, Hruz PW. HIV protease inhibitors acutely impair glucose-stimulated insulin release. Diabetes. 2003;52:1695–700. doi: 10.2337/diabetes.52.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischman A, Johnsen S, Systrom DM, et al. Effects of a nucleoside reverse transcriptase inhibitor, stavudine, on glucose disposal and mitochondrial function in muscle of healthy adults. Am J Physiol Endocrinol Metab. 2007;292:E1666–73. doi: 10.1152/ajpendo.00550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riddler SA, Smit E, Cole SR, et al. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–82. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 38.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–52. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 39.Friis-Moller N, Weber R, Reiss P, et al. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179–93. doi: 10.1097/01.aids.0000060358.78202.c1. [DOI] [PubMed] [Google Scholar]
- 40.Worm SW, Kamara DA, Reiss P, et al. Elevated triglycerides and risk of myocardial infarction in HIV-positive persons. AIDS. 2011;25:1497–504. doi: 10.1097/QAD.0b013e32834917c6. [DOI] [PubMed] [Google Scholar]
- 41.Janiszewski PM, Ross R, Despres JP, et al. Hypertriglyceridemia and waist circumference predict cardiovascular risk among HIV patients: a cross-sectional study. PLoS One. 2011;6:e25032. doi: 10.1371/journal.pone.0025032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddler SA, Li X, Otvos J, et al. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2008;48:281–8. doi: 10.1097/QAI.0b013e31817bbbf0. [DOI] [PubMed] [Google Scholar]
- 43.Hadigan C, Borgonha S, Rabe J, Young V, Grinspoon S. Increased rates of lipolysis among human immunodeficiency virus-infected men receiving highly active antiretroviral therapy. Metabolism. 2002;51:1143–7. doi: 10.1053/meta.2002.34704. [DOI] [PubMed] [Google Scholar]
- 44.Reeds DN, Mittendorfer B, Patterson BW, Powderly WG, Yarasheski KE, Klein S. Alterations in lipid kinetics in men with HIV-dyslipidemia. Am J Physiol Endocrinol Metab. 2003;285:E490–7. doi: 10.1152/ajpendo.00118.2003. [DOI] [PubMed] [Google Scholar]
- 45.Hellerstein MK, Grunfeld C, Wu K, et al. Increased de novo hepatic lipogenesis in human immunodeficiency virus infection. J Clin Endocrinol Metab. 1993;76:559–65. doi: 10.1210/jcem.76.3.8445011. [DOI] [PubMed] [Google Scholar]
- 46.van Wijk JP, Cabezas MC, de Koning EJ, Rabelink TJ, van der Geest R, Hoepelman IM. In vivo evidence of impaired peripheral fatty acid trapping in patients with human immunodeficiency virus-associated lipodystrophy. J Clin Endocrinol Metab. 2005;90:3575–82. doi: 10.1210/jc.2004-2343. [DOI] [PubMed] [Google Scholar]
- 47.Joy T, Keough HM, Hadigan C, et al. Dietary fat intake and relationship to serum lipid levels among HIV-infected subjects with metabolic abnormalities in the era of HAART. AIDS. 2007;21:1591–600. doi: 10.1097/QAD.0b013e32823644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones SP, Waitt C, Sutton R, Back DJ, Pirmohamed M. Effect of atazanavir and ritonavir on the differentiation and adipokine secretion of human subcutaneous and omental preadipocytes. AIDS. 2008;22:1293–8. doi: 10.1097/QAD.0b013e3283021a4f. [DOI] [PubMed] [Google Scholar]
- 49.Adler-Wailes DC, Liu H, Ahmad F, et al. Effects of the human immunodeficiency virus-protease inhibitor, ritonavir, on basal and catecholamine-stimulated lipolysis. J Clin Endocrinol Metab. 2005;90:3251–61. doi: 10.1210/jc.2004-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richmond SR, Carper MJ, Lei X, Zhang S, Yarasheski KE, Ramanadham S. HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters. Biochim Biophys Acta. 2010;1801:559–66. doi: 10.1016/j.bbalip.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.