Abstract
Atherosclerosis preferentially occurs in areas of turbulent flow and low fluid shear stress, whereas laminar flow and high shear stress are atheroprotective. Inflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and IL-1 stimulate expression of endothelial cell (EC) genes that may promote atherosclerosis. TNF-α and IL-1 regulate gene expression in ECs, in part, by stimulating mitogen-activated protein kinases (MAPK), which phosphorylate transcription factors. We hypothesized that steady laminar flow inhibits cytokine-mediated activation of MAPK in EC. To test this hypothesis, we determined the effects of flow (shear stress = 12 dynes/cm2) on TNF-α and IL-1-stimulated activity of three MAPK in human umbilical vein ECs (HUVEC): extracellular signal-regulated kinase (ERK1/2), p38, and c-Jun N-terminal kinase (JNK). Flow alone stimulated ERK1/2 and p38 activity but decreased JNK activity compared with static controls. TNF-α or IL-1 alone activated ERK1/2, p38, and JNK maximally at 15 min in HUVEC. Preexposing HUVEC for 10 min to flow inhibited TNF-α and IL-1 activation of JNK by 46% and 49%, respectively, but had no significant effect on ERK1/2 or p38 activation. Incubation of HUVEC with PD98059, which inhibits flow-mediated ERK1/2 activation, prevented flow from inhibiting cytokine activation of JNK. Phorbol 12-myristate 13-acetate, which strongly activates ERK1/2, also inhibited TNF-α activation of JNK. These findings indicate that fluid shear stress inhibits TNF-α-mediated signaling events in HUVEC via the activation of the ERK1/2 signaling pathway. Inhibition of TNF-α signal transduction represents a mechanism by which steady laminar flow may exert atheroprotective effects on the endothelium.
Keywords: endothelium, protein kinase, signal transduction
Many findings suggest that steady laminar flow in blood vessels activates signal transduction events that lead to expression of atheroprotective genes (1, 2). The nature and magnitude of shear stress plays an important role in long-term maintenance of the structure and function of the blood vessel. In “linear” areas of the vasculature blood flows in ordered laminar patterns in a pulsatile fashion and endothelial cells (ECs) experience pulsatile shear stress with fluctuations in magnitude that yield a mean positive shear stress. At areas of abrupt curvatures in the vasculature, as in the carotid bifurcation, the laminar flow of blood is disrupted and separate flow patterns result (3–8). The significance of these flow patterns is demonstrated by studies that correlate development of atherosclerotic lesions (fatty streaks and small plaques) with areas of the carotid that experience these flow reversals with low time-averaged shear stress (3, 4). Regions of the carotid bifurcation that experience steady nonoscillatory shear stress as the result of laminar blood flow patterns are relatively protected from atherosclerosis. Examples of the atheroprotective nature of steady laminar flow are inhibition of E-selectin expression and suppression of vascular cell adhesion molecule 1 (VCAM-1) induction by cytokines such as IL-1 and tumor necrosis factor α (TNF-α) (5). An example of the importance of the magnitude of shear stress is the markedly greater VCAM-1 expression and monocyte binding to the carotid in areas of low shear stress compared with high shear stress (6).
A useful approach to gain insight into mechanisms by which flow regulates EC cell function has been to study flow-mediated signal transduction events. Our lab, as well as others, has shown that members of the mitogen-activated protein kinase (MAPK) family are activated by steady laminar flow in a time- and force-dependent manner (7–13). Recently, two groups showed that steady laminar flow stimulated c-Jun NH2-terminal kinases (JNK1 and JNK2) to a greater extent than extracellular signal-regulated kinase (ERK)1/2 (12, 14). However, these results are inconsistent with the concept that flow promotes atheroprotective events because JNK activity is stimulated by inflammatory cytokines (15, 16), which are thought to promote atherosclerosis (17). We believe that more appropriate experimental conditions to investigate the effects of flow on JNK activity would be in the presence of cytokine stimulation. Under these conditions, we hypothesized that flow would inhibit signaling events mediated by cytokines such as IL-1 and TNF-α.
In the present study, we show that steady laminar flow, via the activation of the ERK1/2 signaling pathway, inhibits cytokine-mediated activation of JNK. These findings suggest that an important mechanism by which laminar flow may be atheroprotective is via the inhibition of specific downstream signaling events activated by inflammatory cytokines in ECs.
Methods
Cell Culture and Flow Experiments.
ECs were isolated from human umbilical veins (HUVEC) as described (9). Cells at passages 1–3 were grown in RPMI 1640 (GIBCO/BRL) supplemented with 20% FBS (HyClone), heparin (Sigma), and EC growth factor. At least four different HUVEC preparations were used for the experiments shown. Two different devices were used to create fluid shear stress in vitro: the parallel plate chamber and the cone and plate viscometer (18). Cells were grown in 2 × 4-cm slides of tissue culture plastic cut from the bottom of tissue culture dishes coated with gelatin (for the parallel plate chamber) or 60-mm dishes coated with gelatin (for the cone and plate viscometer). Upon reaching 95% confluence, fresh media were added; 2 days later, cells were rinsed free of culture media with Hanks' balanced salt solution containing 130 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 1.0 mM MgCl2, 20 m M Hepes, pH 7.4, with 10 mM glucose and 10% serum added and either maintained in static condition or exposed to flow (fluid shear stress = 12 dynes/cm2) in a parallel plate chamber at 37°C, as described (9). After varying times of exposure to flow, cells were washed gently with ice-cold PBS (composition: 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.3), and cell lysates were obtained by freeze thaw and subsequent sonication.
JNK Assays.
Protein concentration in cell lysates was determined by Bradford protein assay and JNK precipitated by incubating 100 μg of cell lysate with 3 μg of glutathione S-transferase (GST)-c-Jun (1) coupled to glutathione agarose for 4 h at 4°C with constant rotation. Beads were then washed three times with 1 ml of buffer B (12.5 mM 4-morpholinepropanesulfonic acid, pH 7.2/12.5 mM β-glycerophosphate/0.5 mM EGTA/7.5 mM MgCl2/1% Nonidet P-40/1 mM DTT) plus 0.25 M NaCl. JNK activity was assayed by resuspending beads in buffer B plus 10 mM MgCl2, 1 mM MnCl2, and 50 μM [γ-32P]ATP or 100 μM ATP and then incubating for 30 min at 30°C with constant vortexing. The reaction was terminated with SDS sample buffer and boiled for 5 min; proteins were then size-fractionated by 10% SDS/PAGE. Detection of phosphorylated GST-c-jun was performed by autoradiography or by Western blot analysis by using a phosphospecific c-jun antibody (New England Biolabs). The resulting autoradiograms and Western blots were then analyzed by densitometry. In addition, endogenous phospho-c-jun was measured by Western blot (see below) as another means to determine JNK activity.
Western Blot Analysis for Endogenous c-jun Phosphorylation, ERK1/2, and p38 Activation.
Cell lysates were subjected to SDS/PAGE under reducing conditions, and proteins were transferred to nitrocellulose filters (Hybond, Amersham Pharmacia). To ensure quantitative transfer of proteins, the filters were stained with Ponceau S. The membrane was blocked for 2 h at room temperature with a commercial blocking buffer (GIBCO/BRL) or 5% milk in TBS with 0.1% Tween-20. The blots were incubated overnight at 4°C with the primary antibodies (phosphospecific c-jun Ser-63 and phosphospecific c-jun Ser-73, phosphospecific ERK1/2, or p38 antibody (New England Biolabs), followed by incubation for 1–2 h with secondary antibody (horseradish peroxidase-conjugated). Immunoreactive bands were visualized by chemiluminescence (Amersham Pharmacia ECL or Pierce ECL). The resulting autoradiograms were then analyzed by densitometry. All experiments were performed at least three times. Statistical analysis was performed by using EXCEL 5.0 and Student's two-tailed t test.
Results
Flow Decreases Basal JNK Activity and Increases ERK1/2 and p38 Activity in HUVEC.
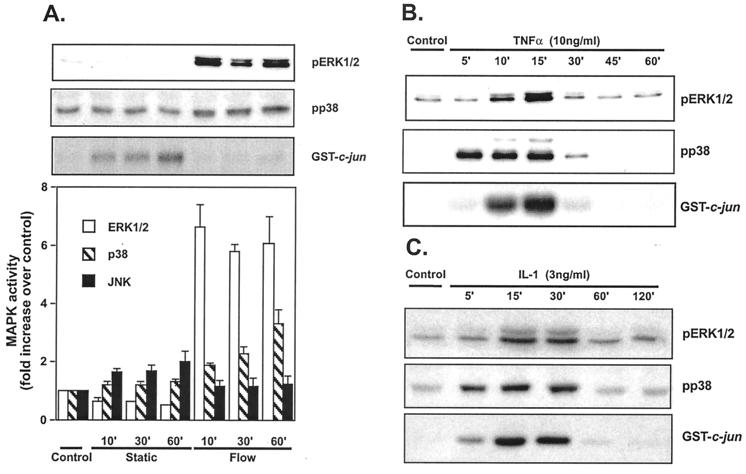
Previous studies found that flow alone stimulated JNK activity in bovine aortic ECs (12, 14). To study the effects of flow on JNK activity in unstimulated HUVEC, cells were exposed to steady laminar flow (shear stress = 12 dynes/cm2) for varying times. As controls, HUVEC on cover slips were washed with flow medium and maintained in static conditions. JNK activity, measured by GST-c-jun phosphorylation, was minimal in confluent HUVEC that remained in growth medium (Control, Fig. 1A Upper). However, after changing the medium, there was a small but significant increase in JNK activity in cells maintained in static culture, which reached a peak at 60 min (2.0 ± 0.5-fold increase, n = 5, P < 0.05; Static, Fig. 1A). This result suggests that agonists present in fresh tissue culture medium, which contains 10% serum, activate JNK to a small extent. Stimulation of HUVEC by flow in fresh tissue culture medium caused no significant change in JNK activity (1.1 ± 0.3-, 1.1 ± 0.4-, and 1.2 ± 0.4-fold increases at 10, 30, and 60 min; Flow, Fig. 1A). However, relative to the static cultures, JNK activity in cultures exposed to flow was significantly inhibited (although to a small extent) by 31%, 35%, and 40% at the indicated times. ERK1/2 and p38 activity were also very low in confluent HUVEC that remained in growth medium (Control, Fig. 1A Upper). After changing the medium, there was no significant change in ERK1/2 and p38 activity in cells maintained in static culture. In response to flow, there was a rapid increase in ERK1/2 activity, which peaked at 10 min (6.6 ± 0.9-fold increase, n = 5, P < 0.05; Flow, Fig. 1A) and was sustained for at least 60 min. In contrast, p38 activity increased much more slowly, with peak at 60 min (2.3 ± 0.5-fold; n = 5, P < 0.05). These results demonstrate that flow, in the absence of hormonal stimulation, significantly activates ERK1/2 and p38 while decreasing JNK activity to a small extent in HUVEC.
Figure 1.
Effect of flow, TNF-α, and IL-1 on ERK1/2, p38, and JNK activities. (A) HUVEC were maintained in static conditions or exposed to flow (shear stress = 12 dynes/cm2) for the indicated times. Cell lysates were prepared and analyzed for ERK1/2 activity by phospho-ERK Western blot, for p38 activity by phospho-p38 Western blot, and for JNK activity by in vitro kinase assay by using GST-jun as a substrate and anti-phospho-c-jun antibodies for detection. Error bars represent SEM (n = 5). HUVEC were stimulated with 10 ng/ml TNF-α (B) or 3 ng/ml IL-1 (C) for the indicated times and analyzed for MAPK activity. Analysis of different experiments was performed by normalizing autoradiographic intensity of control cells in each experiment to an arbitrary value of 1.0. Error bars represent SEM (n = 3).
Cytokines Are Powerful Activators of JNK, ERK1/2, and p38 in HUVEC.
To determine the effects of TNF-α and IL-1 on JNK, ERK1/2, and p38 activity in HUVEC, confluent HUVEC were exposed to 10 ng/ml TNF-α or 3 ng/ml IL-1 under static conditions. TNF-α rapidly increased JNK, ERK1/2, and p38 activity in HUVEC (Fig. 1B) as reported by others (5, 15, 19). JNK activity increased within 5 min, peaked at 15 min (8.5 ± 0.8-fold), and returned to baseline at 60 min. ERK1/2 activation by TNF-α was similar with an increase at 10 min (6.0 ± 0.9-fold), a peak at 15 min (4.0-fold), and a return to baseline at 30 min (Fig. 1B). p38 activity in response to TNF-α was similar to JNK with peak at 15 min (6.1 ± 1.1-fold). Similar results were found with IL-1 (Fig. 1C) with peak activation of all three MAPK at 15–30 min of ≈4-fold.
Flow Inhibits TNF-α Stimulation of JNK Activity but Not ERK1/2 and p38 Activity.
To study the effect of flow on TNF-α and IL-1-mediated JNK, p38, and ERK1/2 activation, we used the following “preconditioning” protocol for all experiments. In brief, cells were exposed to flow (shear stress = 12 dynes/cm2) for 10 min or maintained in medium for 10 min under static conditions. Cells were then kept in static conditions for an additional 15 min, resulting in a total of 25 min, or stimulated with TNF-α (10 ng/ml) or IL-1 (3 ng/ml) for 15 min. There was no difference in the effect of preconditioning when cells were exposed to flow for up to 120 min (not shown).
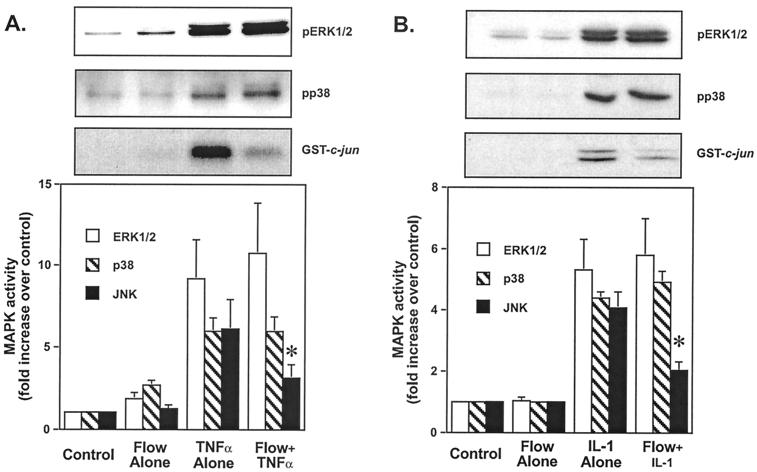
Flow alone caused little or no significant increase in JNK or p38 activity at these short times (1.2 ± 0.2-fold and 1.1 ± 0.3-fold increases, respectively) measured by phosphorylation of GST-c-Jun and phospho-p38 (Fig. 2). Flow stimulated a small (1.9 ± 0.5-fold) increase in ERK1/2 activity measured by phospho-ERK1/2 antibodies as reported (9, 13). The magnitude of the increase in ERK1/2 activity was smaller than shown in Fig. 1A because the cells were placed in static conditions for 15 min after exposure to flow for 10 min. Incubation in the absence of flow results in inactivation of ERK1/2 likely due to action of the MAPK phosphatases. TNF-α alone stimulated a 9.2 ± 2.6-fold increase in ERK1/2, a 5.9 ± 1.0-fold increase in p38, and a 6.1 ± 2.0-fold increase in JNK activity compared with controls maintained continuously under static conditions for 25 min (Fig. 2A). In other experiments with lower concentrations of TNF-α, similar effects on p38, JNK, and ERK1/2 were observed (not shown). Exposure to flow significantly inhibited TNF-α mediated activation of JNK by 46% (3.3 ± 1.0-fold vs. 6.1 ± 2.0-fold increase, P = 0.029, n = 5; *, Fig. 2A). In contrast, flow caused a 17% increase in ERK1/2 activation by TNF-α, which did not differ significantly compared with TNF-α alone (10.8 ± 3.2-fold vs. 9.2 ± 2.6-fold increase, Fig. 2A). There was no significant effect of flow on TNF-α-mediated activation of p38 (Fig. 2A).
Figure 2.
Flow preexposure inhibits TNF-α and IL-1-mediated JNK activation, without effect on ERK1/2 and p38 activation. HUVEC were subjected to the following “preconditioning” protocol: maintained in static conditions for 25 min (Control), exposed to flow for 10 min and then held static for 15 min (Flow Alone), maintained in static conditions for 10 min followed by TNF-α (A) or IL-1 (B) stimulation for 15 min (TNF-α or IL-1 alone), or subjected to flow for 10 min followed by TNF-α (A) or IL-1 (B) stimulation for 15 min (Flow + TNF-α or IL-1). Cell lysates were prepared and analyzed for ERK1/2 activity by phospho-ERK Western blot, for p38 activity by phospho-p38 Western blot, and for JNK activity by in vitro kinase assay by using GST-jun as a substrate and anti-phospho-c-jun antibodies for detection. Densitometric analysis was performed as in Fig. 1. Error bars represent SEM (n = 5).
Flow had similar effects on IL-1 activation of p38, ERK1/2, and JNK. Similar to TNF-α, flow significantly inhibited IL-1 activation of JNK compared with IL-1 alone by 49% (2.0 ± 0.4-fold vs. 4.1 ± 0.6-fold increase, P = 0.04, n = 4; *, Fig. 2B), whereas flow did not significantly alter IL-1 activation of ERK1/2 and p38 compared with IL-1 alone (5.3 ± 1.1-fold vs. 5.8 ± 1.3-fold increases and 4.0 ± 0.3-fold vs. 4.9 ± 0.5-fold, Fig. 2B). In summary, these results demonstrate that flow inhibits TNF-α and IL-1-mediated activation of JNK specifically. The fact that activation of p38 and ERK1/2 by these cytokines was unaffected suggests that the inhibitory effect of flow was not because of alterations in binding of TNF-α or IL-1 to their receptors but was due to inhibition of a shared downstream mediator.
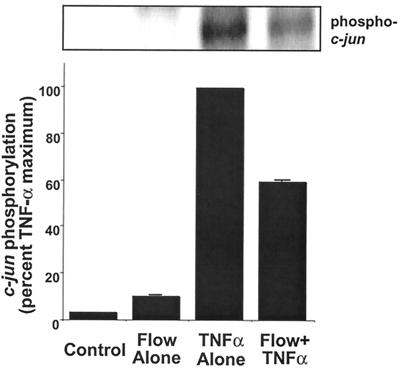
To examine further the effects of flow on TNF-α mediated JNK activation, we measured phosphorylation of endogenous c-jun, the downstream substrate of JNK. TNF-α (10 ng/ml) alone increased c-jun phosphorylation more than 50-fold, making relative quantitation by fold increase difficult (Fig. 3). Thus, results were normalized to TNF-α as a maximal stimulus (100%). In these experiments, flow alone stimulated c-jun phosphorylation to a small extent (11 ± 3% of TNF-α). However, flow significantly inhibited TNF-α-mediated c-jun phosphorylation by 40% (P = 0.003, n = 3, Fig. 3). There was also a significant decrease in nuclear c-jun phosphorylation in cells exposed to TNF-α after preconditioning with flow (not shown).
Figure 3.
Flow preexposure inhibits TNF-α-mediated phosphorylation of c-jun. HUVEC were subjected to the same preconditioning protocol described in Fig. 2. Cell lysates were prepared and analyzed for c-jun phosphorylation by phospho-c-jun Western blot. Densitometric analysis was performed as in Fig. 1. Error bars represent SEM (n = 3).
Flow Inhibition of JNK Activation by TNF-α Depends on Activation of the ERK1/2 Pathway.
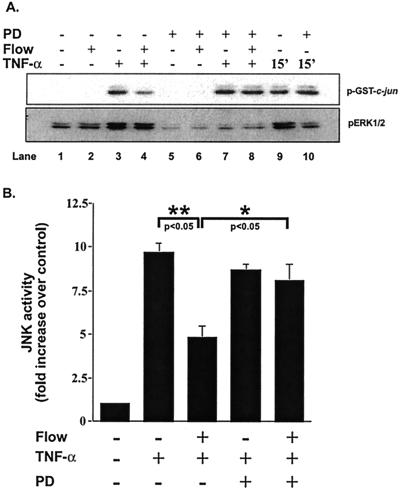
Because crosstalk among MAPK family members has been shown to regulate activity (20–22), we investigated the possibility that ERK1/2 activation might be responsible for the flow inhibition of JNK activation by TNF-α. To inhibit ERK1/2 activation, cells were pretreated for 30 min with 30 μM PD98059, a known MEK1/2 inhibitor. The efficacy and specificity of PD98059 as an inhibitor of ERK1/2 was determined by measuring its effects on flow and TNF-α-mediated activation of ERK1/2 and JNK. PD98059 (30 μM) completely inhibited TNF-α and flow-mediated ERK1/2 activation (pERK1/2 in Fig. 4A, compare lanes 6 and 7 to lanes 2 and 3). However, PD98059 had no inhibitory effect on TNF-α-mediated JNK activation (p-GST-c-jun in Fig. 4A, compare lane 7 to lane 3, and compare bars 2 and 4 in Fig. 4B).
Figure 4.
Effect of PD98059 on ERK1/2 and JNK activation stimulated by flow and TNF-α. HUVEC were pretreated with 30 μM PD98059 for 30 min or vehicle and then subjected to the preconditioning protocol described in Fig. 2. Cells were then stimulated with TNF-α or subjected to flow followed by TNF-α stimulation in the presence or absence of 30 μM PD98059. In lanes 9 and 10, TNF-α was added for only 15 min in the absence (lane 9) or presence (lane 10) of PD98059 to show that ERK1/2 was inhibited. Assay of JNK activity was performed by using GST-c-jun as a substrate and anti-phospho-c-jun antibodies for detection and assay of ERK1/2 activity by phospho-ERK Western blot (A). Densitometry was performed as in Fig. 1. Error bars represent SEM (n = 3).
The effect of PD98059 on flow inhibition of TNF-α-mediated JNK activation was then studied. Preexposure to flow caused 50 ± 5% inhibition of TNF-α-mediated JNK activation (p-GST-c-jun in Fig. 4A, compare lane 3 to lane 4 and compare bars 2 and 3 in Fig. 4B). However, in the presence of PD98059, the flow-mediated inhibition of JNK was completely prevented (p-GST-c-jun in Fig. 4A, compare lane 4 to lane 8, and compare bars 3 and 5 in Fig. 4B). These experiments demonstrate that the inhibition of TNF-α-mediated activation of JNK by flow depends on flow activation of the ERK1/2 signaling pathway.
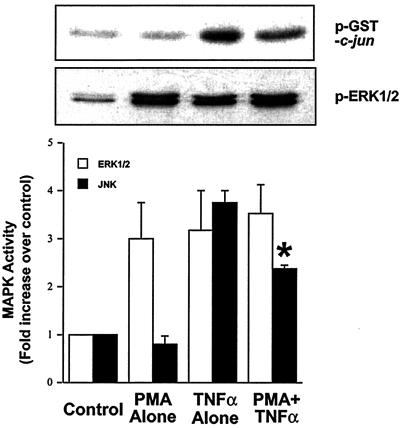
Because flow activation of the ERK1/2 pathway inhibits TNF-α activation of JNK, we investigated whether other agonists of this pathway would be able to inhibit JNK activation by TNF-α. HUVEC were exposed to either vehicle for 25 min, 200 nM phorbol 12-myristate 13-acetate (PMA, a phorbol ester known to activate ERK1/2) for 25 min, vehicle for 10 min followed by TNF-α for 15 min or PMA for 10 min followed by TNF-α for an additional 15 min. PMA, TNF-α, and PMA + TNF-α all increased ERK1/2 activity as measured by phosphoERK1/2 antibodies (Fig. 5). PMA alone did not cause an increase in JNK activity, whereas TNF-α did. Preexposure of HUVEC to PMA for 10 min inhibited TNF-α-mediated activation of JNK (Fig. 5). Furthermore, because TNF-α activates both the ERK1/2 and JNK pathways with a similar time course, we studied the effect of PD98059 on TNF-α activation of JNK. PD98059 blocked TNF-α activation of ERK1/2 and enhanced JNK activity (data not shown). These results further demonstrate that the activation of ERK1/2 inhibits TNF-α-mediated activation of JNK.
Figure 5.
PMA activation of ERK1/2 inhibits TNF-α activation of JNK. HUVEC were treated with either vehicle for 25 min, 200 nM PMA for 25 min, vehicle for 10 min followed by TNF-α for 15 min, or 200 nM PMA for 10 min followed by TNF-α for 15 min. Cell lysates were prepared and analyzed for ERK1/2 activity by phospho-ERK Western blot and for JNK activity by in vitro kinase assay by using GST-jun as a substrate. Densitometric analysis was performed as in Fig. 1. Error bars represent SEM (n = 3).
Discussion
The major finding of this study is that steady laminar fluid shear stress inhibits TNF-α and IL-1-mediated activation of JNK in HUVEC. This inhibition is specific for JNK because TNF-α and IL-1 activation of p38 and ERK1/2 are not inhibited by flow. The mechanism for this inhibition requires the flow-mediated activation of the ERK1/2 signaling pathway. Thus, the present study illustrates an important physiologic example of inhibitory crosstalk among the MAPK. Although previous investigators have shown that flow inhibits TNF-α-mediated gene expression (1, 2), the present study shows selective effects of flow on IL-1 and TNF-α-mediated signal transduction. The effect of flow on JNK activation by TNF-α and IL-1 would be expected to have important consequences for EC function, as JNK is an important regulator of genes whose expression depends on c-Jun phosphorylation and AP-1 activation. Thus, our results provide a mechanism by which flow may alter endothelial gene expression.
Because ECs integrate a variety of hormonal and biomechanical signals, it is logical that members of the MAPK family would be important in endothelial function. We and others have previously shown that flow rapidly activates ERK1/2 and p38 (3–8). More recently, we have observed that big MAP kinase 1 (BMK1) is also activated by flow (9). We have been unable to show significant flow-mediated stimulation of JNK (this study). This suggests important differences in the upstream pathways stimulated by flow that lead to activation of the MAPKs.
The most likely mechanism for flow inhibition of TNF-α and IL-1 would be crosstalk among MAPKs. Several groups, including our lab, have found that one MAPK (e.g., p38 or JNK) may inhibit or oppose the activation of another MAPK (e.g., ERK1/2) (23–25). Based on the effect of PD98059 in the present study, it seems that the ERK1/2 pathway is the most logical candidate mediator for flow-mediated JNK inhibition. The mechanisms responsible for MAPK inhibitory crosstalk of JNK remain unknown. Logical possibilities include effects on upstream signaling molecules of the JNK pathway by MAPK [e.g., apoptosis signal-regulated kinase (ASK) and p21-activated kinase], as well as changes in subcellular localization and stimulation of phosphatases that dephosphorylate and inactivate JNK. Phosphatases that may regulate JNK activity include the MAPK phosphatases, protein phosphatase 2, and Shp2 protein tyrosine phosphatase (10–19, 23, 24). Further studies will be required to determine which JNK phosphatases are activated by flow.
The most likely mechanism for ERK1/2 inhibition of IL-1 and TNF-α JNK activation is via an effect on a shared downstream mediator. Activation of JNK by TNF-α is thought to be mediated by a family of intracellular signaling molecules known as TNF-α receptor-associated factors (TRAFs) (25). Studies from TRAF2 transgenic and knockout mice demonstrated that TRAF2 is essential for activation of JNK in response to TNF-α but not IL-1 (26, 27). In contrast, TRAF6 specifically mediates IL-1-induced JNK activation (20, 21). Thus, it is not likely that flow inhibits JNK via effects on TRAF family members because this would require inhibition of both TRAF2 and TRAF6. Downstream activators of JNK include the MAPK kinase kinases that include MEKK1, TAK1, and ASK1 (22, 28, 29). ASK1 seems to be involved in JNK activation only in response to pro-inflammatory cytokines and stress stimuli (30–32), and a kinase inactive form of ASK1 functions as a dominant negative in cytokine-induced JNK activation (30, 32). Thus, future studies of ASK1 as a possible downstream target for ERK1/2 may provide important insight into flow-mediated regulation of TNF-α signaling.
Recently, PD98059 has been shown to inhibit the MEK5/BMK1 pathway as well as the ERK1/2 pathway (33). Although it is possible that the flow inhibition of JNK may be mediated by BMK1, our data suggest that it is ERK1/2 that is responsible for this inhibition. Our lab has shown that PMA, a phorbol ester that activates ERK1/2, does not activate BMK1 in ECs (data not shown) and vascular smooth muscle cells (34). Our findings about PMA, which activates ERK1/2 and inhibits TNF-α activation of JNK, support our hypothesis that flow inhibition of JNK is via the ERK1/2 pathway.
Our results are consistent with recent findings that flow inhibits TNF-α-mediated events in ECs. Of most relevance is the TNF-α and JNK-dependent stimulation of E-selectin expression in ECs. Min and Pober (35) showed that overexpression of N-terminal truncated c-Jun or catalytically inactive JNK inhibited TNF-α-induced transcription of an E-selectin promoter–reporter gene but not a κB promoter–reporter gene. Tsao et al. (36) found that flow inhibited TNF-α-mediated increases in NF-κB activity, VCAM-1 expression, and endothelial adhesiveness for monocytes. Based on the findings of Tsao et al. (36) and Khan et al. (37), it seems that the inhibitory effect of flow on VCAM-1 expression may be related to increases in NO production. However, preliminary studies suggest that JNK activation may not be regulated by either changes in NO or cyclic nucleotides (data not shown), suggesting that other pathways activated by flow may be important. Further, more detailed studies are required to determine the roles of NO and cyclic nucleotides in this system.
The results of the present study differ from those of Li et al. (38) and Jo et al. (7), who demonstrated JNK activation by steady laminar flow. We found no significant increase in JNK activity in response to flow alone in 8 of 10 experiments. In fact, we observed a small decrease in JNK activity by flow when compared with static cultures that received fresh medium in our experimental protocol. In the occasional experiments that showed increased JNK activity, the magnitude was very small (≈10%) compared with TNF-α. Possible explanations for the differences in the present study and previous studies include the fact that we used HUVEC rather than bovine aortic endothelial cells; our cells were several days postconfluent and growth arrested by contact inhibition, whereas Jo et al.(7) used serum deprivation to cause growth arrest; and we did not study transfected cells. In particular, the population of ECs that are transfected represents only a small minority of cells (transfection efficiency of ECs ≈5–10%) and may represent a population in which JNK is activated by flow. Another potentially important difference between the present study and those of Jo and Li is that the magnitude of ERK1/2 activation is much greater in our studies. Because we find that JNK inhibition seems to require ERK1/2 activation, it is logical that these investigators may have observed JNK activation.
In summary, the present study demonstrates that fluid shear stress inhibits TNF-α and IL-1 activation of JNK but not p38 or ERK1/2. Furthermore, the mechanism for this inhibition is via flow-mediated activation of ERK1/2, suggesting inhibitory crosstalk among the MAPK. Further studies should investigate and determine the mechanisms for the inhibitory crosstalk among MAPK in response to fluid shear stress.
Acknowledgments
This study was supported by grants from the National Institutes of Health (PO1-HL18645) and the American Heart Association (to B.C.B.) and from the Howard Hughes Medical Institute (to J.S.). R.H. is a trainee in the Medical Scientist Training Program funded by National Institutes of Health Grant T32 GM07356.
Abbreviations
- ERK1/2
extracellular signal-regulated kinase
- EC
endothelial cell
- HUVEC
human umbilical vein ECs
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- TNF-α
tumor necrosis factor-α
- PMA
phorbol 12-myristate 13-acetate
- GST
glutathione S-transferase
- BMK1
big MAP kinase 1
- TRAF
TNF-α receptor-associated factor
- ASK
apoptosis signal-regulated kinase
- VCAM-1
vascular cell adhesion molecule 1
References
- 1.Gimbrone M A, Jr, Nagel T, Topper J N. J Clin Invest. 1997;99:1809–1813. doi: 10.1172/JCI119346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traub O, Berk B C. Arterioscler Thromb Vasc Biol. 1998;18:677–685. doi: 10.1161/01.atv.18.5.677. [DOI] [PubMed] [Google Scholar]
- 3.Tseng H, Peterson T E, Berk B C. Circ Res. 1995;77:869–878. doi: 10.1161/01.res.77.5.869. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi M, Berk B C. J Clin Invest. 1996;98:2623–2631. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shyy J Y, Lin M C, Han J, Lu Y, Petrime M, Chien S. Proc Natl Acad Sci USA. 1995;92:8069–8073. doi: 10.1073/pnas.92.17.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishida T, Peterson T E, Kovach N L, Berk B C. Circ Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 7.Jo H, Sipos K, Go Y M, Law R, Rong J, McDonald J M. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- 8.Traub O, Monia B P, Dean N M, Berk B C. J Biol Chem. 1997;272:31251–31257. doi: 10.1074/jbc.272.50.31251. [DOI] [PubMed] [Google Scholar]
- 9.Yan C, Takahashi M, Okuda M, Lee J D, Berk B C. J Biol Chem. 1999;274:143–150. doi: 10.1074/jbc.274.1.143. [DOI] [PubMed] [Google Scholar]
- 10.Haneda M, Sugimoto T, Kikkawa R. Eur J Pharmacol. 1999;365:1–7. doi: 10.1016/s0014-2999(98)00857-7. [DOI] [PubMed] [Google Scholar]
- 11.Duff J L, Monia B P, Berk B C. J Biol Chem. 1995;270:7161–7166. doi: 10.1074/jbc.270.13.7161. [DOI] [PubMed] [Google Scholar]
- 12.Tanoue T, Moriguchi T, Nishida E. J Biol Chem. 1999;274:19949–19956. doi: 10.1074/jbc.274.28.19949. [DOI] [PubMed] [Google Scholar]
- 13.Vinals F, Pouyssegur J. Mol Cell Biol. 1999;19:2763–2772. doi: 10.1128/mcb.19.4.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanada M, Kobayashi T, Ohnishi M, Ikeda S, Wang H, Katsura K, Yanagawa Y, Hiraga A, Kanamaru R, Tamura S. FEBS Lett. 1998;437:172–176. doi: 10.1016/s0014-5793(98)01229-0. [DOI] [PubMed] [Google Scholar]
- 15.Gaits F, Shiozaki K, Russell P. J Biol Chem. 1997;272:17873–17879. doi: 10.1074/jbc.272.28.17873. [DOI] [PubMed] [Google Scholar]
- 16.Shiozaki K, Russell P. EMBO J. 1995;14:492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf C M, Reynolds J E, Morana S J, Eastman A. Exp Cell Res. 1997;230:22–27. doi: 10.1006/excr.1996.3401. [DOI] [PubMed] [Google Scholar]
- 18.Schonthal A H. Semin Cancer Biol. 1995;6:239–248. doi: 10.1006/scbi.1995.0031. [DOI] [PubMed] [Google Scholar]
- 19.Fukunaga K, Noguchi T, Takeda H, Matozaki T, Hayashi Y, Itoh H, Kasuga M. J Biol Chem. 2000;275:5208–5213. doi: 10.1074/jbc.275.7.5208. [DOI] [PubMed] [Google Scholar]
- 20.Baud V, Liu Z G, Bennett B, Suzuki N, Xia Y, Karin M. Genes Dev. 1999;13:1297–1308. doi: 10.1101/gad.13.10.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel D V. Nature (London) 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 22.Derijard B, Hibi M, Wu I, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 23.Feng G S. Exp Cell Res. 1999;253:47–54. doi: 10.1006/excr.1999.4668. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z Q, Lu W, Feng G S. J Biol Chem. 1998;273:4904–4908. doi: 10.1074/jbc.273.9.4904. [DOI] [PubMed] [Google Scholar]
- 25.Arch R H, Gedrich R W, Thompson C B. Genes Dev. 1998;12:2821–2830. doi: 10.1101/gad.12.18.2821. [DOI] [PubMed] [Google Scholar]
- 26.Lee S Y, Reichlin A, Santana A, Sokol K A, Nussenzweig M C, Choi Y. Immunity. 1997;7:703–713. doi: 10.1016/s1074-7613(00)80390-8. [DOI] [PubMed] [Google Scholar]
- 27.Yeh W C, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa J L, Ferrick D, Hum B, Iscove N, et al. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 28.Ichijo H. Oncogene. 1999;18:6087–6093. doi: 10.1038/sj.onc.1203129. [DOI] [PubMed] [Google Scholar]
- 29.Ip Y T, Davis R J. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 30.Chang H Y, Nishitoh H, Yang X, Ichijo H, Baltimore D. Science. 1998;281:1860–1863. doi: 10.1126/science.281.5384.1860. [DOI] [PubMed] [Google Scholar]
- 31.Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 32.Nishitoh H, Saitoh M, Mochida Y, Takeda K, Nakano H, Rothe M, Miyazono K, Ichijo H. Mol Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 33.Kamakura S, Moriguchi T, Nishida E. J Biol Chem. 1999;274:26563–26571. doi: 10.1074/jbc.274.37.26563. [DOI] [PubMed] [Google Scholar]
- 34.Abe J, Kusuhara M, Ulevitch R J, Berk B C, Lee J D. J Biol Chem. 1996;271:16586–16590. doi: 10.1074/jbc.271.28.16586. [DOI] [PubMed] [Google Scholar]
- 35.Min W, Pober J S. J Immunol. 1997;159:3508–3518. [PubMed] [Google Scholar]
- 36.Tsao P S, Buitrago R, Chan J R, Cooke J P. Circulation. 1996;94:1682–1689. doi: 10.1161/01.cir.94.7.1682. [DOI] [PubMed] [Google Scholar]
- 37.Khan B V, Harrison D G, Olbrych M T, Alexander R W, Medford R M. Proc Natl Acad Sci USA. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y S, Shyy J Y, Li S, Lee J, Su B, Karin M, Chien S. Mol Cell Biol. 1996;16:5947–5954. doi: 10.1128/mcb.16.11.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]