Abstract
Intestinal microbiota contributes to diverse mammalian processes including the metabolic function of drugs. It is a potential new territory for drug targeting, especially for dietary herbal products. Because most of herbal drugs are orally administered, the chemical profile and corresponding bioactivities of herbal medicines may be altered by intestinal microbiota. Ginseng is one of the most commonly used herb and it is always an attractive natural product to understand. In this review, after briefly introduce the interactions of herbal products and gut microbiota, we discussed the microbiota-mediated metabolism of ginsenosides in ginseng and red ginseng. In particular, the major metabolite Compound K and its pharmacological advances are commented including anticancer, antidiabetic and antiinflammatory effects. In summary, the intestinal microbiota may play an important role in mediating the metabolism and enhancement of bioactivity of herbal medicines.
Keywords: Intestinal microbiota, Ginseng, Red ginseng, Ginsenosides, Compound K
Introduction
The human intestine is densely populated with microorganisms, and is a site where they exert strong influences on human biology as well as drugs’ fate (Kullberg, 2008). The entire system of the human intestinal microbiota can be pictured as a ‘microbial organ’, which are closely associated with diverse processes including the metabolic function of drugs (Palmer et al., 2007; Fishbein et al., 2008; Jia et al., 2008; Kullberg, 2008). Gut microbiota might be a potential new territory for drug targeting, especially for dietary herbal products (Shin et al., 2006; Li et al., 2009).
Herbal drugs have become the basis of traditional medicines for thousands of years (Xutian et al., 2009), and contribute to be considered valuable materials in medicines (Li et al., 2008; Zhao et al., 2009). Most of herbal drugs are orally administered (Gim et al., 2009; Chen et al., 2010; Lin et al., 2010). Active components of these herbal drugs are inevitably in contact with intestinal microflora. Some are transformed by the intestinal bacteria before being absorbed from the gastrointestinal tract. In some cases, gut bacterial drug metabolism is associated with bioactivity enhancement and/or toxicity diminishment of the metabolite compared with its parent compound (Shin et al., 2006; Li et al., 2009).
Ginseng is one of the world’s most widely used medicinal plants and it is one of the best selling natural products nowadays (Jang and Shin, 2010; Qi et al., 2011a). The major bioactive constituents in ginseng are believe as ginsenoside, a group of triterpene glycosides (Wang et al., 2009; Zuo et al., 2009; Taira et al., 2010). Due to its medicinal effects, ginseng has always been an attractive natural product to study (Zhang et al., 2009; Qi et al., 2011b). Like many other herbal medicines, ginseng is always taken orally. In this form its bioavailability is low because of incomplete absorption (Qi et al., 2010). To date, the biotransformation of ginsenosides to their metabolites by intestinal bacteria has been reported. Some of the metabolites, such as Compound K, have shown various bioactivities.
In this article, we review the role of gut microbiota in mediating the metabolism and enhanced bioactivity of herbal medicines. We first briefly introduce the interactions of herbal products and intestinal microbiota. Taking ginseng as an example, we then discuss the microbiota-mediated metabolism of ginseng and red ginseng. In particular, the major metabolite Compound K and its pharmacological advances are summarized.
Intestinal Microbiota and its Interaction with Herbal Products
Human biological system could be viewed as ‘superorganisms’ involving an indispensable internal ecosystem of the intestinal microbiota. With the development of global system biology, intestinal microbiota has become a hot topic in life sciences (Gill et al., 2006). Human gut microbiota is believed to consist of well over 500 species representing 5 kingdoms of life and 10 trillion microbial cells. They can be roughly divided as beneficial and pathogenic groups (Versalovic and Relman, 2006; Holmes et al., 2008). The human microbiome contains over 100 times more genes than as does the human genome. Microbial communities in vivo include many different bacterial species that are in dynamic, intimate association with each other and with the human host. Accumulating evidence indicates that the intestinal microbiota is a key determiner in energy metabolism and immune function of the host, and has a crucial role in the development of numerous diseases including obesity, diabetes, and even cancers (Ruseler-van Embden et al., 1994; Mazmanian et al., 2008).
Diet is among the most important modifiable determinants of human health. Herbal products are popular dietary supplements since they are natural and therefore safe. An interactive relationship is present between gut microflora and herbal medicines, involving the two important aspects: gut microflora-targeted modulation by herbs and gut microflora-mediated drug metabolism. A large portion of herbs products is water-soluble compositions such as polysaccharides, saponins and inorganic, as well as water-insoluble inorganic materials or polymers. A lot of herbal medicines have been reported as anti-microbial or immunomodulating agents from long-term clinical observations (Schachter, 2008; Wu, 2009; Kano et al., 2010). We believe a large portion of the extraction from herbal products and dietary supplements act as prebiotics or bifidogenic factors by modulating the balance of human gut microbe. From this point, intestinal microbiota might a potential new territory for drug targeting, especially for dietary herbal products. However, limited knowledge has been discovered about the effects of herbs on intestinal microbiota and the molecular details of host–flora interactions are lacking. This subject is interesting but out of the discussion of this review.
Currently, a big challenge for touching herbs is the unavailability of enough quantity and quality of purified single compounds. Therefore, quantitative structure-activity relationship and quantitative metabolism-activity relationship have been summarized, in particular being compared under the same conditions. Many hurdles remain to be overcome in the future for access to subjects as complex as intestinal microbiota and herbal constituents. For example, better separation and detection systems are required for monitoring complex-system herbs and microbiota, and more powerful computational and statistical tools are indispensable for dealing with large networked data sets.
On the other hand, intestinal microbiota metabolism and biotransformation can also modulate the health effects of dietary herbs by altering their absorption and bioavailability. A diverse and numerous microbiota secrete a various array of enzymes giving them substantial metabolic potential which can have major implications for drug stability. Dietary herbs generally consist of hundreds of constituents. A number of them have been shown to be substrates for these bacterial enzymes. However, the capacity of the intestine for metabolism of drug candidates, and the importance of the intestinal microbiota in influencing the disposition, fate, and toxicity of drugs in the host are often overlooked. The major concern with bacterial drug degradation is the behavior of the metabolite.
Many researchers and pharmaceutical industries tend to approach herbal medicines in a characteristically Western way: isolate active ingredients and test their pharmacokinetics and metabolism one at a time. Actually, the metabolism of herbal medicines is far more complex than that of single compounds. The concentration varies in a large range, component-component metabolic competition exists and parent compounds are unpredictable. Based on in vitro and in vivo bioactivity evaluation of selected herbal medicines, the influence of intestinal microbiota has been characterized, and some positive perspective on enhancement of bioactivity of botanical metabolites compare to their parent compounds was observed (Li et al., 2009; Shimada et al., 2010).
Ginseng and its Metabolism by Gut Microbiota
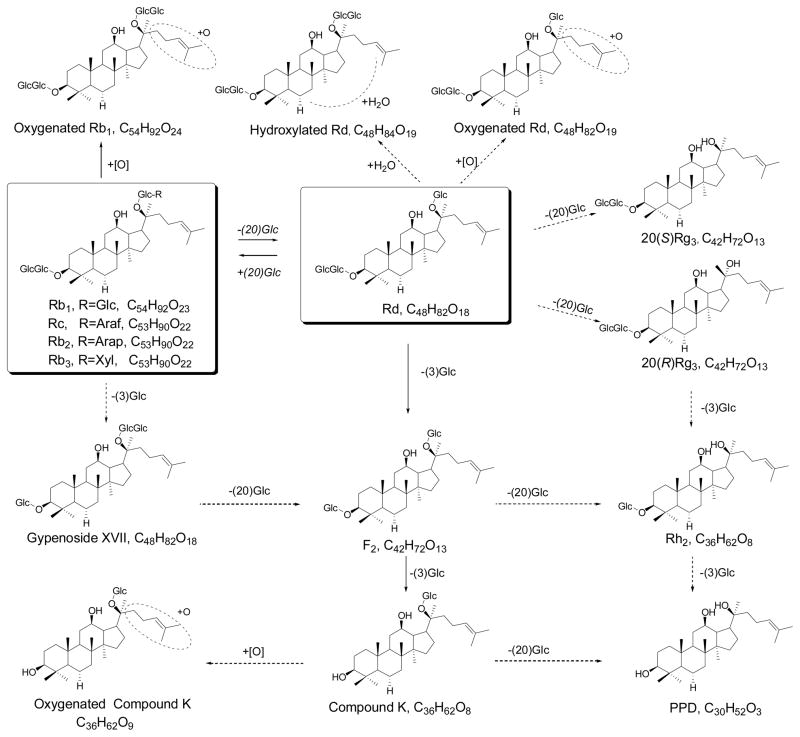
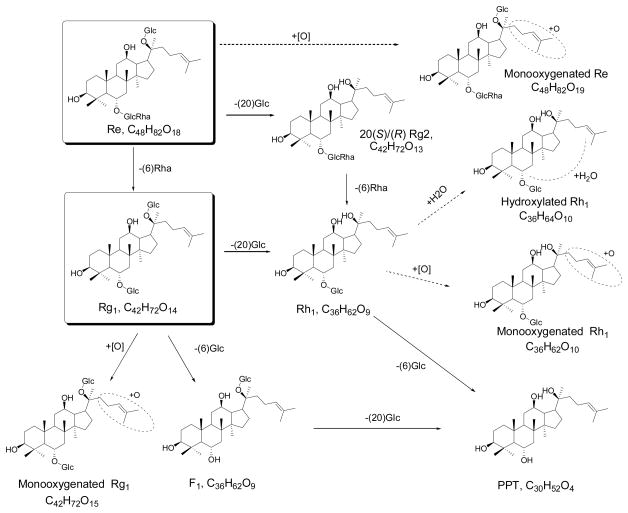
After oral administration, ginseng is metabolized extensively by intestinal bacteria (Hasegawa et al., 1996; Hasegawa, 2004; Lee et al., 2009a; Liu et al., 2009). The conversions of ginsenosides in the gastro-intestinal tract have been largely studied using in vitro and in vivo (Kong et al., 2009; Ruan et al., 2010). The metabolic pathways of protopanaxadiol (PPD)-type and protopanaxatriol (PPT)-type ginsenosides were summarized in Fig. 1 and Fig. 2, respectively. The most popular metabolic pathway is deglycosylation reactions by intestinal bacteria via stepwise cleavage of the sugar moieties (Tawab et al., 2003; Hasegawa, 2004; Liu et al., 2009). In the PPD group, Rb1, Rc, Rb2, Rb3, and Rd are major metabolized to Compound K (Qiang et al., 2006; Yang et al., 2007), demonstrating a preferable selection at C-3 position by intestinal microbiota. In the PPT group, Rg1 and Re are converted to Rh1 and F1 (Wang et al., 2000; Tawab et al., 2003; Yang et al., 2009b). Interestingly, Compound K, an intestinal bacterial metabolite of PPD-type saponins, still contributes to low bioavailability. This possibly resulted from biliary excretion and hepatic metabolism via esterification with fatty acids (Lee et al., 2006; Paek et al., 2006).
Fig. 1.
Metabolic pathway of protopanaxadiol ginsenosides in ginseng by intestinal microbiota. “→” denotes major pathways; “⇢” denotes additional pathways.
Fig. 2.
Metabolic pathway of protopanaxatriol ginsenosides from ginseng by intestinal microbiota. “→” denotes major pathways; “⇢” denotes additional pathways.
As shown in Fig. 1 and 2, oxygenation by intestinal enzymes was observed to be another major metabolic pathway of ginsenosides, in particular for test of single compound (Lai et al., 2009; Yang et al., 2009a). Oxygenation generally occurred on the top-right aliphatic chain (Oian et al., 2005). The gastric acid-mediated hydration reaction was another potential metabolic pathway of ginsenosides.
Tawab reported that after oral administration of Ginsana G115 capsules (4% ginsenosides) to human volunteers, Compound K, F1 and Rh1 or Rg1 were detected to be major metabolites reaching the systemic circulation (Tawab et al., 2003). Recently, the pharmacokinetics of oral administration of ginseng on 32 male subjects was observed. The compound K was absorbed into the circulation 24 hr after intake. There was a correlation between the compound K transforming activity of ginsenoside-Rb1 and the compound K transforming activity of ginseng extract by intestinal microflora. The biotransformation activity from ginsenoside-Rb1 to Compound K was significantly different among individuals (Lee et al., 2009b). Our ongoing studies in human volunteers observed that ginsenoside Rb1 and Compound K reached the systemic circulation after oral administration of American ginseng (unpublished data). This might lead to different effects of ginseng on individuals.
The following aspects should be highlighted for metabolism and pharmacokinetic study of ginseng: (1) Because of competitive absorption and metabolism, administration of a single ginsenoside or of a ginseng extract may lead to different results. (2) The metabolic profiles of ginsenosides might be different between in vitro and in vivo results due to higher exposure of compound concentration in vitro. Also, the bacterial ginsenoside-hydrolyzing effects are known to be different between humans and experimental mice, as well as for different kind of bacteria. (3) The population of the intestinal bacteria is variable, depending on the conditions of the host, including diet, health, and even stress.
Metabolism of Red Ginseng and its Ginsenosides
Red ginseng is prepared by a steaming process on white ginseng. Red ginseng has long been used in clinic as a single herb or a component of prescription especially in Asian countries. In recent years, we noted that increasing reports have shown various pharmacological effects of red ginseng and its constituents, such as anti-inflammatory, anti-oxidative and anticancer effects (Hong and Lyu, 2011; Jung et al., 2011; Lee et al., 2011). In some cases, the bioactivity of red ginseng was compared with white ginseng, and it seemed the red one showed better potential than the white one (Wang et al., 2007; Sun et al., 2011). The chemical profile differs considerably between white and red ginseng, and has been comprehensively reviewed (Wang and Yuan, 2008; Yuan et al., 2010; Sun et al., 2011). During steaming or heating, the polar ginseng saponins decreased, and less polar ginseng saponins increased.
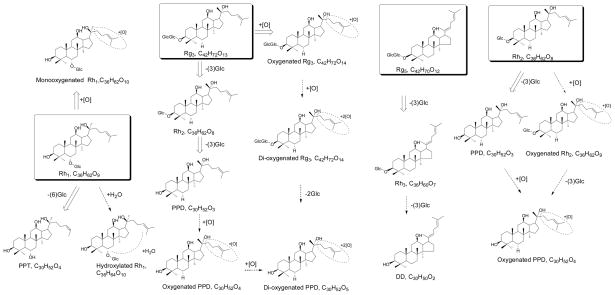
We have known that gastro-intestinal tract plays an important role in determining the metabolic fate of ginsenosides and mediating the bioactivity of white ginseng. We are curious about the metabolic fingerprinting of red ginseng. Because of the great difference in chemical compositions, red ginseng should have difference metabolites. Although the metabolic profiles of red ginseng have been seldom investigated, the individual ginsenosides have been investigated (Qian et al., 2005a; Qian et al., 2005b; Lai et al., 2009). Fig. 3 summarized the major metabolic pathways and metabolites of ginsenosides (Rh1, Rg3, Rg5 and Rh2) in red ginseng. Because of unavailability of purified compounds, some major ginsenosides like Rk1, Rk3 and Rh4 in red ginseng have not been investigated. Similar to the metabolic behavior of those ginsenosides in white ginseng, deglucosylation is the most common pathways for red ginseng ginsenosides in gastrointestinal tract. Oxygenation and hydration reaction were also observed. Several pairs of stereoisomers and positional isomers exist in red ginseng (Yang et al., 2007). Few reports have investigated the metabolic difference between isomers. In some publications, geometric isomers were mistaken from each other.(Cai et al., 2003; Qian et al., 2005c; Xie et al., 2005; Zhao et al., 2010)
Fig. 3.
Metabolic pathway of ginsenosides in red ginseng by intestinal microbiota. “⇨” denotes major pathways; “⇢” denotes additional pathways.
Pharmacological Advances of Compound K
Compound K (20-O-D-glucopyranosyl-20(S)-protopanaxadiol), also known as IH-901, does not occur naturally in ginseng. It is a major metabolite of protopanaxadiol-type ginsenoside formed by intestinal bacteria via the stepwise cleavage of sugar moieties at C-3 position. After oral administration of ginseng extract in animals and human volunteers, Compound K is a main saponin that reaches the systemic circulation in the body. Several reports have shown that various pharmacologic actions of ginseng, including anticancer, antidiabetes, and antiinflammation were mediated at least in the part by this compound.
Anticancer
Compound K has been reported to have potential antitumor effects, stronger than its parent compounds ginsenosides Rb1 and Rd. Several molecular mechanisms exist and collectively converge on various signaling pathways. These pathways include the regulation of the cell cycle, induction of apoptosis, inhibition of angiogenesis, prohibition of invasion, and reduction of inflammatory response. Compound K induced apoptosis in several tumor cell lines by regulating various signaling pathway such as activation of caspase-8 (Cho et al., 2009) and AMP-activated protein kinase (AMPK) (Yoon et al., 2007), suppression of nuclear factor-kappa B (NF-κB) pathways (Choo et al., 2008) and Janus activated kinase 1 (JAK1)-signal transducer and activator of transcription 3 (STAT3) signaling (Park et al., 2011). Also, Compound K suppressed matrix metalloproteinase-9 (MMP-9) expression through inhibition of activator protein-1 (AP-1) and mitogen-activated protein kinase (MAPK) signaling pathways in human astroglioma cells, showing therapeutic potential for controlling the growth and invasiveness of brain tumors (Jung et al., 2006). A recent study showed that CK inhibited basic fibroblast growth factor (bFGF)-induced angiogenesis via regulation of p38 MAPK and AKT in human umbilical vein endothelial cells (Jeong et al., 2010). Though the protective influence of Compound K against cancer has been shown in preclinical studies, this effect needs to be investigated by more scientific clinical trials.
Antidiabetic Effects
Accumulating in vivo evidence suggests that Compound K possesses antidiabetic activity(Han et al., 2007). This compound showed beneficial effects on glucose and lipid metabolisms. Involved mechanisms are activation of peroxisome proliferator-activated receptor γ, increase of GLUT expression, and enhancement of PKA-dependent pathways (Han et al., 2007). Selective phosphatidylinositol-3 kinase inhibitor attenuated the Compound K-mediated effects of glucose uptake, suggesting a key role of phosphatidylinositol-3 kinase pathway (Huang et al., 2010). Yong et al. found that IH-901 treatment ameliorated an insulin resistance through suppressions of endogenous glucose production and lipogenesis in the liver, and activated phosphorylation of AMPK in the HIT-T15 cells (Yoon et al., 2007). Besides, Compound K shows protective effect against beta-cell death, as might contribute to the previously reported anti-diabetic actions of ginseng by anti-apoptotic (Kim et al., 2010). However, literature evidence of antidiabetic potential of ginsenoside Rb1 was not found, while Rb1 is a parent compound of Compound K. Collectively, compare its parent compound, Compound K might be a promising therapeutic agent improving altered glucose and lipid metabolisms revealed in type 2 diabetes mellitus patients.
Other Effects
The antiinflammatory activities of Compound K have been investigated by both in vitro and in vivo models (Choi et al., 2007). Tested cell lines include macrophages RAW 264.7 cells (Cuong et al., 2009), astroglial cells (Park et al., 2009), and mononuclear phagocytes (Yang et al., 2008). Key targets were suppressed by Compound K such as NF-κB activation, pro-inflammatory cytokine, mitogen-activated protein kinase, reactive oxygen species, and mitogen-activated protein kinase (Cuong et al., 2009).
Compound K showed more potent hepatoprotective effects then ginsenoside Rb1 on t-BHP-induced hepatotoxicified liver injury (Lee et al., 2005). Because of its immunomodulatory effects, Compound K played a therapeutic role in the treatment of lethal sepsis through the modulation of Toll-like receptor 4-associated signaling via glucocorticoid receptor binding (Yang et al., 2008). In addition, Compound K can inhibit expression of interferon-γ, thus improving contact dermatitis or psoriasis (Shin et al., 2005).
Summary and Perspectives
Intestinal microbiota can induce comprehensive metabolism of constituents in herbs and might thus increase their bioactivity. Take ginseng as a case, the bioavailability of most original ginsenosides is low, their plasma concentrations may be insufficient to reach to the effects observed under in vitro experimental conditions. More in vivo preclinical models are essential for evaluation of the clinical effectiveness of the ginseng. Although great progress have been made during the last decades, quality control and batch-to-batch consistency are still big challenges for the development of herbal products. Cultivation conditions can change ginsenoside fingerprinting, thus altering ginseng metabolic biofingerprinting.
Growing evidences have been discovering important functions of the intestinal microbiota in human disease-health and provides new vision for the drug-microbiota interactions. With the emerging and development of system biology, we believe more interesting results will be observed in the future. Further well-designed and systematic studies about influence of dietary herbs such as ginseng on intestinal microbiota will open new targets for herb medicines.
Acknowledgments
This work was supported in part by the National Science Foundation of China (No. 81001618), the National Key Technologies R&D Program of China (No. 2008BAI51B01), Program for Changjiang Scholars and Innovative Research Teams in Universities (No. IRT0868), and NIH grants P01 AT004418 and K01 AT005362.
References
- Cai ZW, Qian TX, Wong RNS, Jiang ZH. Liquid chromatography-electrospray ionization mass spectrometry for metabolism and pharmacokinetic studies of ginsenoside Rg3. Anal Chim Acta. 2003;492:283–293. [Google Scholar]
- Chen S, Flower A, Ritchie A, Liu J, Molassiotis A, Yu H, Lewith G. Oral Chinese herbal medicine (CHM) as an adjuvant treatment during chemotherapy for non-small cell lung cancer: A systematic review. Lung Cancer. 2010;68:137–145. doi: 10.1016/j.lungcan.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Cho SH, Chung KS, Choi JH, Kim DH, Lee KT. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer. 2009;9:449. doi: 10.1186/1471-2407-9-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kim M, Ryu J, Choi C. Ginsenosides compound K and Rh(2) inhibit tumor necrosis factor-alpha-induced activation of the NF-kappaB and JNK pathways in human astroglial cells. Neurosci Lett. 2007;421:37–41. doi: 10.1016/j.neulet.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Choo MK, Sakurai H, Kim DH, Saiki I. A ginseng saponin metabolite suppresses tumor necrosis factor-alpha-promoted metastasis by suppressing nuclear factor-kappaB signaling in murine colon cancer cells. Oncol Rep. 2008;19:595–600. [PubMed] [Google Scholar]
- Cuong TT, Yang CS, Yuk JM, Lee HM, Ko SR, Cho BG, Jo EK. Glucocorticoid receptor agonist compound K regulates Dectin-1-dependent inflammatory signaling through inhibition of reactive oxygen species. Life Sci. 2009;85:625–633. doi: 10.1016/j.lfs.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Fishbein T, Novitskiy G, Mishra L, Matsumoto C, Kaufman S, Goyal S, Shetty K, Johnson L, Lu A, Wang A, Hu F, Kallakury B, Lough D, Zasloff M. NOD2-expressing bone marrow-derived cells appear to regulate epithelial innate immunity of the transplanted human small intestine. Gut. 2008;57:323–330. doi: 10.1136/gut.2007.133322. [DOI] [PubMed] [Google Scholar]
- Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gim GT, Kim HM, Kim J, Whang WW, Cho SH. Antioxidant effect of tianwang buxin pills a traditional chinese medicine formula: double-blind, randomized controlled trial. Am J Chin Med. 2009;37:227–239. doi: 10.1142/S0192415X09006795. [DOI] [PubMed] [Google Scholar]
- Han GC, Ko SK, Sung JH, Chung SH. Compound K enhances insulin secretion with beneficial metabolic effects in db/db mice. J Agric Food Chem. 2007;55:10641–10648. doi: 10.1021/jf0722598. [DOI] [PubMed] [Google Scholar]
- Hasegawa H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Sung JH, Matsumiya S, Uchiyama M. Main Ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- Holmes E, Loo RL, Stamler J, Bictash M, Yap IK, Chan Q, Ebbels T, De Iorio M, Brown IJ, Veselkov KA, Daviglus ML, Kesteloot H, Ueshima H, Zhao L, Nicholson JK, Elliott P. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453:396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CE, Lyu SY. Anti-inflammatory and Anti-oxidative Effects of Korean Red Ginseng Extract in Human Keratinocytes. Immune Netw. 2011;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Lin CY, Huang SF, Lin HC, Chang WL, Chang TC. Effect and mechanism of ginsenosides CK and Rg1 on stimulation of glucose uptake in 3T3-L1 adipocytes. J Agric Food Chem. 2010;58:6039–6047. doi: 10.1021/jf9034755. [DOI] [PubMed] [Google Scholar]
- Jang HI, Shin HM. Wild Panax ginseng (Panax ginseng C.A Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am J Chin Med. 2010;38:949–960. doi: 10.1142/S0192415X10008378. [DOI] [PubMed] [Google Scholar]
- Jeong A, Lee HJ, Jeong SJ, Lee EO, Bae H, Kim SH. Compound K inhibits basic fibroblast growth factor-induced angiogenesis via regulation of p38 mitogen activated protein kinase and AKT in human umbilical vein endothelial cells. Biol Pharm Bull. 2010;33:945–950. doi: 10.1248/bpb.33.945. [DOI] [PubMed] [Google Scholar]
- Jia W, Li H, Zhao L, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- Jung JW, Kang HR, Ji GE, Park MS, Song WJ, Kim MH, Kwon JW, Kim TW, Park HW, Cho SH, Min KU. Therapeutic effects of fermented red ginseng in allergic rhinitis: a randomized, double-blind, placebo-controlled study. Allergy Asthma Immunol Res. 2011;3:103–110. doi: 10.4168/aair.2011.3.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Woo MS, Kim SY, Kim WK, Hyun JW, Kim EJ, Kim DH, Kim HS. Ginseng saponin metabolite suppresses phorbol ester-induced matrix metalloproteinase-9 expression through inhibition of activator protein-1 and mitogen-activated protein kinase signaling pathways in human astroglioma cells. Int J Cancer. 2006;118:490–497. doi: 10.1002/ijc.21356. [DOI] [PubMed] [Google Scholar]
- Kano T, Shimizu M, Kanda T, Hijikata Y. Sairei-to therapy on alloimmune recurrent spontaneous abortions and alloimmune-, autoimmune complicated recurrent spontaneous abortions. Am J Chin Med. 2010;38:705–712. doi: 10.1142/S0192415X10008172. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim DH, Kim HY. Compound K protects MIN6N8 pancreatic beta-cells against palmitate-induced apoptosis through modulating SAPK/JNK activation. Cell Biol Int. 2010;34:75–80. doi: 10.1042/CBI20090020. [DOI] [PubMed] [Google Scholar]
- Kong H, Wang M, Venema K, Maathuis A, van der Heijden R, van der Greef J, Xu G, Hankemeier T. Bioconversion of red ginseng saponins in the gastro-intestinal tract in vitro model studied by high-performance liquid chromatography-high resolution Fourier transform ion cyclotron resonance mass spectrometry. J Chromatogr A. 2009;1216:2195–2203. doi: 10.1016/j.chroma.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Kullberg MC. Immunology: soothing intestinal sugars. Nature. 2008;453:602–604. doi: 10.1038/453602a. [DOI] [PubMed] [Google Scholar]
- Lai L, Hao H, Liu Y, Zheng C, Wang Q, Wang G, Chen X. Characterization of pharmacokinetic profiles and metabolic pathways of 20(S)-ginsenoside Rh1 in vivo and in vitro. Planta Med. 2009;75:797–802. doi: 10.1055/s-0029-1185400. [DOI] [PubMed] [Google Scholar]
- Lee HU, Bae EA, Han MJ, Kim NJ, Kim DH. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005;25:1069–1073. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee E, Kim D, Lee J, Yoo J, Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009a;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Lee J, Lee E, Kim D, Yoo J, Koh B. Studies on absorption, distribution and metabolism of ginseng in humans after oral administration. J Ethnopharmacol. 2009b;122:143–148. doi: 10.1016/j.jep.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Lee JS, Choi HS, Kang SW, Chung JH, Park HK, Ban JY, Kwon OY, Hong HP, Ko YG. Therapeutic effect of Korean red ginseng on inflammatory cytokines in rats with focal cerebral ischemia/reperfusion injury. Am J Chin Med. 2011;39:83–94. doi: 10.1142/S0192415X1100866X. [DOI] [PubMed] [Google Scholar]
- Lee PS, Song TW, Sung JH, Moon DC, Song S, Chung YB. Pharmacokinetic characteristics and hepatic distribution of IH-901, a novel intestinal metabolite of ginseng saponin, in rats. Planta Med. 2006;72:204–210. doi: 10.1055/s-2005-916201. [DOI] [PubMed] [Google Scholar]
- Li H, Zhou M, Zhao A, Jia W. Traditional Chinese medicine: balancing the gut ecosystem. Phytother Res. 2009;23:1332–1335. doi: 10.1002/ptr.2590. [DOI] [PubMed] [Google Scholar]
- Li P, Qi LW, Liu EH, Zhou JL, Wen XD. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trends Anal Chem. 2008;27:12. [Google Scholar]
- Lin YC, Chen HW, Kuo YC, Chang YF, Lee YJ, Hwang JJ. Therapeutic efficacy evaluation of curcumin on human oral squamous cell carcinoma xenograft using multimodalities of molecular imaging. Am J Chin Med. 2010;38:343–358. doi: 10.1142/S0192415X10007890. [DOI] [PubMed] [Google Scholar]
- Liu HF, Yang JL, Du FF, Gao XM, Ma XT, Huang YH, Xu F, Niu W, Wang FQ, Mao Y, Sun Y, Lu T, Liu CX, Zhang BL, Li C. Absorption and Disposition of Ginsenosides after Oral Administration of Panax notoginseng Extract to Rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Oian TX, Cai ZW, Wong RNS, Jiang ZH. Liquid chromatography mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005;19:3549–3554. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- Paek IP, Moon Y, Kim J, Ji HY, Kim SA, Sohn DH, Kim JB, Lee HS. Pharmacokinetics of a ginseng saponin metabolite compound K in rats. Biopharm Drug Dispos. 2006;27:39–45. doi: 10.1002/bdd.481. [DOI] [PubMed] [Google Scholar]
- Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Park EM, Kim DH, Jung K, Jung JS, Lee EJ, Hyun JW, Kang JL, Kim HS. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209:40–49. doi: 10.1016/j.jneuroim.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Park S, Lee HJ, Jeong SJ, Song HS, Kim M, Lee EO, Kim DH, Ahn KS, Kim SH. Inhibition of JAK1/STAT3 signaling mediates compound K-induced apoptosis in human multiple myeloma U266 cells. Food Chem Toxicol. 2011 doi: 10.1016/j.fct.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry. 2011a;72:689–699. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011b;28:467–495. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian T, Cai Z, Wong RN, Jiang ZH. Liquid chromatography/mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun Mass Spectrom. 2005a;19:3549–3554. doi: 10.1002/rcm.2232. [DOI] [PubMed] [Google Scholar]
- Qian T, Cai Z, Wong RN, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J Chromatogr B Analyt Technol Biomed Life Sci. 2005b;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Qian TX, Cai ZW, Wong RNS, Mak NK, Jiang ZH. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg(3) J Chromatogr B Analyt Technol Biomed Life Sci. 2005c;816:223–232. doi: 10.1016/j.jchromb.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Qiang TX, Jiang ZH, Cai ZW. High-performance liquid chromatography coupled with tandem mass spectrometry applied for metabolic study of ginsenoside Rb, on rat. Anal Biochem. 2006;352:87–96. doi: 10.1016/j.ab.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Ruan JQ, Leong WI, Yan R, Wang YT. Characterization of Metabolism and in Vitro Permeability Study of Notoginsenoside R1 from Radix Notoginseng. J Agric Food Chem. 2010;58:5770–5776. doi: 10.1021/jf1005885. [DOI] [PubMed] [Google Scholar]
- Ruseler-van Embden JG, Schouten WR, van Lieshout LM. Pouchitis: result of microbial imbalance? Gut. 1994;35:658–664. doi: 10.1136/gut.35.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter SC. Complementary and alternative medical therapies. Curr Opin Neurol. 2008;21:184–189. doi: 10.1097/WCO.0b013e3282f47918. [DOI] [PubMed] [Google Scholar]
- Shimada T, Kondoh M, Motonaga C, Kitamura Y, Cheng L, Shi H, Enomoto T, Tsuruta D, Ishii M, Kobayashi H. Enhancement of anti-allergic effects mediated by the Kampo medicine Shoseiryuto (Xiao-Qing-Long-Tang in Chinese) with lysed Enterococcus faecalis FK-23 in mice. Asian Pac J Allergy Immunol. 2010;28:59–66. [PubMed] [Google Scholar]
- Shin JE, Bae EA, Lee YC, Ma JY, Kim DH. Estrogenic effect of main components kakkalide and tectoridin of Puerariae Flos and their metabolites. Biol Pharm Bull. 2006;29:1202–1206. doi: 10.1248/bpb.29.1202. [DOI] [PubMed] [Google Scholar]
- Shin YW, Bae EA, Kim SS, Lee YC, Kim DH. Effect of ginsenoside Rb1 and compound K in chronic oxazolone-induced mouse dermatitis. Int Immunopharmacol. 2005;5:1183–1191. doi: 10.1016/j.intimp.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Sun S, Qi LW, Du GJ, Mehendale SR, Wang CZ, Yuan CS. Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011;125:1299–1305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira S, Ikeda R, Yokota N, Osaka I, Sakamoto M, Kato M, Sahashi Y. Mass spectrometric imaging of ginsenosides localization in Panax ginseng root. Am J Chin Med. 2010;38:485–493. doi: 10.1142/S0192415X10008007. [DOI] [PubMed] [Google Scholar]
- Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Dispos. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- Versalovic J, Relman D. How bacterial communities expand functional repertoires. PLoS Biol. 2006;4:e430. doi: 10.1371/journal.pbio.0040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Ni M, Sun S, Li XL, He H, Mehendale SR, Yuan CS. Detection of adulteration of notoginseng root extract with other panax species by quantitative HPLC coupled with PCA. J Agric Food Chem. 2009;57:2363–2367. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang BX, Liu TH, Minami M, Nagata T, Ikejima T. Metabolism of ginsenoside Rg(1) by intestinal bacteria II. Immunological activity of ginsenoside Rg(1) and Rh-1. Acta Pharmacol Sin. 2000;21:792–796. [PubMed] [Google Scholar]
- Wu Y. Collateral theory and vascular lesion treatment. Am J Chin Med. 2009;37:241–252. doi: 10.1142/S0192415X09006801. [DOI] [PubMed] [Google Scholar]
- Xie HT, Wang GJ, Sun JG, Tucker I, Zhao XC, Xie YY, Li H, Jiang XL, Wang R, Xu MJ, Wang W. High performance liquid chromatographic-mass spectrometric determination of ginsenoside Rg3 and its metabolites in rat plasma using solid-phase extraction for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;818:167–173. doi: 10.1016/j.jchromb.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Xutian S, Zhang J, Louise W. New exploration and understanding of traditional Chinese medicine. Am J Chin Med. 2009;37:411–426. doi: 10.1142/S0192415X09006941. [DOI] [PubMed] [Google Scholar]
- Yang CS, Ko SR, Cho BG, Shin DM, Yuk JM, Li S, Kim JM, Evans RM, Jung JS, Song DK, Jo EK. The ginsenoside metabolite compound K, a novel agonist of glucocorticoid receptor, induces tolerance to endotoxin-induced lethal shock. J Cell Mol Med. 2008;12:1739–1753. doi: 10.1111/j.1582-4934.2007.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Deng YH, Xu SJ, Zeng X. In vivo pharmacokinetic and metabolism studies of ginsenoside Rd. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;854:77–84. doi: 10.1016/j.jchromb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Yang L, Xu S, Liu C, Su Z. In vivo metabolism study of ginsenoside Re in rat using high-performance liquid chromatography coupled with tandem mass spectrometry. Anal Bioanal Chem. 2009a;395:1441–1451. doi: 10.1007/s00216-009-3121-1. [DOI] [PubMed] [Google Scholar]
- Yang L, Xu SJ, Liu CJ, Su ZJ. In vivo metabolism study of ginsenoside Re in rat using high-performance liquid chromatography coupled with tandem mass spectrometry. Anal Bioanal Chem. 2009b;395:1441–1451. doi: 10.1007/s00216-009-3121-1. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Han EJ, Sung JH, Chung SH. Anti-diabetic effects of compound K versus metformin versus compound K-metformin combination therapy in diabetic db/db mice. Biol Pharm Bull. 2007;30:2196–2200. doi: 10.1248/bpb.30.2196. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Wang CZ, Wicks SM, Qi LW. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Jie J, Zhou Y, Cao Z, Li W. Long-term effects of Panax ginseng on disposition of fexofenadine in rats in vivo. Am J Chin Med. 2009;37:657–667. doi: 10.1142/S0192415X09007144. [DOI] [PubMed] [Google Scholar]
- Zhao H, Wan X, Chen JX. A mini review of traditional Chinese medicine for the treatment of depression in China. Am J Chin Med. 2009;37:207–213. doi: 10.1142/S0192415X09006771. [DOI] [PubMed] [Google Scholar]
- Zhao QA, Zheng X, Jiang J, Zhou H, Hu P. Determination of ginsenoside Rg3 in human plasma and urine by high performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2266–2273. doi: 10.1016/j.jchromb.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Zuo G, Guan T, Chen D, Li C, Jiang R, Luo C, Hu X, Wang Y, Wang J. Total saponins of Panax ginseng induces K562 cell differentiation by promoting internalization of the erythropoietin receptor. Am J Chin Med. 2009;37:747–757. doi: 10.1142/S0192415X09007211. [DOI] [PubMed] [Google Scholar]