Abstract
Objectives
Panaxadiol (PD) is a purified sapogenin of ginseng saponins that exhibits anticancer activity. Irinotecan (IRN) is a second line anticancer drug, but clinical treatment with IRN is limited due to side effects. In this study, we investigated the possible synergistic anticancer effects of PD and IRN on human colorectal cancer cells and explored the potential role of apoptosis in the synergistic activities.
Key findings
The combination of PD and IRN significantly enhanced antiproliferative effects in HCT-116 cells (P < 0.05). Cell cycle analysis demonstrated that combining IRN treatment with PD significantly increased the G1-phase fractions of cells, compared with IRN treatment alone. In apoptotic assays, the combination of PD and IRN significantly increased the percentage of apoptotic cells compared with IRN alone (P < 0.01). Increased caspase 3 and caspase 9 activities were observed after treating with PD and IRN. The synergistic apoptotic effects were also supported by docking analysis, which demonstrated that PD and IRN bound two different chains of the caspase 3 protein.
Conclusions
Data from this study suggested that caspase 3- and caspase 9-mediated apoptosis may play an important role in the PD enhanced antiproliferative effects of IRN on human colorectal cancer cells.
Keywords: Irinotecan, panaxadiol, apoptosis, cell cycle, human colorectal cancer
Introduction
Colorectal cancer is the third most common malignancy in the United States and the second most frequent cause of cancer-related death.[1-2] Although curative surgical resection is possible in 70–80% of patients after diagnosis, the role of chemotherapeutic regimens is limited due to side effects and toxicity. In addition, almost half of patients will die from recurrence of cancer.[3] Previous studies have shown that chemotherapy toxicity could be reduced by chemoadjuvant compounds that potentiate the tumoricidal effects of lower doses.[4-7] Cancer treatment with botanicals such as American ginseng has received increasing attention in recent years.[8-12] Natural products have also been a valuable source of new candidate compounds for therapy.[13-14] Panaxadiol (PD), a pseudoaglycone of a diol-type triterpenoid with a dammarane skeleton (Fig. 1), is an active anticancer compound in steamed American ginseng.[15]
Fig. 1.
Chemical structures of panaxadiol (PD) and irinotecan (IRN).
Irinotecan (IRN) is one of the most common second-line chemotherapeutic agents used for colorectal cancer to control symptoms, maintain or improve quality of life, and prolong survival.[16] The potential risks of treatment-related mortality and morbidity, which consist mainly of neutropenia, nausea, vomiting, diarrhea, and asthenia, must be considered when treating with IRN.[17] Thus, IRN is not usually considered a first-line medicine and is more frequently used in combination with 5-FU as a therapy for metastatic colorectal cancer.[16,18]
In this study, we investigated the combination effects of PD and IRN on two human colorectal cancer cell lines, HCT-116 and SW-480. PD significantly increased the antiproliferative effects of IRN. Furthermore, we observed the combined effect on cell apoptosis, cell cycle and caspase assays to elucidate the possible mechanism of PD and IRN combination effects on colorectal cancer cells. Docking was used to simulate the intermolecular interactions between these compounds and the Caspase-3 protein. This study is the first to investigate the combinatory anticancer effects of IRN and natural compounds from ginseng as an effective chemo-adjuvant for colorectal cancer treatment.
Materials and Methods
Reagents and materials
All cell culture plasticware was obtained from Falcon Labware (Franklin Lakes, NJ) and Techno Plastic Products (Trasadingen, Switzerland). Trypsin, McCoy's 5A and Leibovitz's L-15 media, and phosphate buffered saline were obtained from Mediatech, Inc. (Herndon, VA). Benzylpenicillin (penicillin G)/streptomycin and IRN was obtained from Sigma-Aldrich (St. Louis, MO). An MTS assay kit, CellTiter 96 Aqueous One Solution Cell Proliferation Assay, was obtained from Promega (Madison, WI). An annexin V-FITC apoptosis detection kit was obtained from BD Biosciences (Rockville, MD). PI/RNase staining buffer was obtained from BD Biosciences Pharmingen (San Diego, CA). Cell Lysis Buffer was obtained from Cell Signaling Technology, Inc. (Boston, MA). Caspase 3, 8 and 9 kits (with buffer and synthetic substrates DTT, DEVD-rNA, LEHD-rNA, IETD-rNA) were obtained from BioVison (Mountain View, CA). PD was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
Cell culture
The human colorectal cancer cell lines HCT-116 and SW-480 were obtained from American Type Culture Collection (ATCC). The young adult mouse colon (YAMC) cell line is a conditionally immortalized marina colon epithelial cell line isolated from the H-2Kb–tsA58 mouse (Immortomouse, Charles River Laboratories Chicago, IL, USA). Cells were routinely grown in McCoy's 5A medium (for HCT-116) or Leibovitz's L-15 medium (for SW-480) or RPM 1640 medium (for YAMC) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin (50 units/ml). For YAMC cells 5 units/ml murine IFN-γ, 100 units/ml penicillin and streptomycin, 5 μg/ml insulin, 5 μg/ml transferring were added. Cells were maintained in a tissue culture flask and kept in a humidified incubator (5% CO2 in air at 37°C for HCT-116 and SW-480, 33°C for YAMC). The medium was changed every 2-3 days. When the cells reached 70%-80% confluence, they were trypsinized, harvested, and seeded into a new tissue culture flask.
Cell proliferation assay
The effect of PD and irinotecan on the proliferation of HCT-116 and SW-480 cell lines was determined using a modified trichrome stain (MTS) assay. Cancer cells were plated into a 96-well plate at a density of 1×104 cells/well. After seeding for 24 h, the cells were treated with PD, irinotecan or both at various concentrations. All experiments were performed in triplicate. At the end of the sample exposure period, either 48 h or 72 h, the medium of each well was discarded and 100 μl of fresh medium and 20 μl of CellTiter 96 aqueous solution were added. The plate was returned to the incubator where it remained for 1-4 h in a humidified atmosphere at 37°C. Then 60 μl of medium from each well was transferred to an ELISA 96-well plate, and the absorbance of the formazan product was measured at 490 nm. The blank control was recorded by measuring the absorbance at 490 nm with wells containing medium mixed with CellTiter 96 aqueous solution but no cells. Results were expressed as a percentage of control (vehicle set at 100%).
Cell cycle analysis
The cell cycle profile was assayed by flow cytometry after staining with PI/RNase, and the assay data from PD and IRN were compared. HCT-116 cells were seeded in 24-well tissue culture plates. On the second day, the medium was changed, and cells were treated with PD, irinotecan or both at different concentrations (PD 10 μM, IRN 1, 3, 5, 10, 20 and 30 μM). Cells were incubated for 48 h before harvesting. The cells were fixed gently with 80% ethanol before being placed in a freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 30 μl of phosphate buffered saline (PBS) containing 40 μg/ml propidium iodide and 0.1 mg/ml RNase. Cells were incubated in a dark room for 20 min at room temperature before cell cycle analysis with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and the FlowJo software (Ashland, OR). For each measurement, at least 10,000 cells were counted.
Apoptotic analysis
HCT-116 cells were seeded in 24-well tissue culture plates. After 24 h, the medium was changed and PD, IRN or both drugs were added. After treatment for 48 h, cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then culture medium containing 10% FBS (and floating cells) was added to inactivate trypsin. When gentle pipetting was completed, the cells were centrifuged for 5 min at 1500 g. The supernatant was removed and cells were stained with annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) according to the manufacturer's instructions. Untreated cells were used as control for double staining. Immediately after staining the cells were analyzed by a FACScan flow cytometer. For each measurement, at least 20,000 cells were counted.
Caspase Assay
HCT-116 cells were seeded in 6-well tissue culture plates. After 24 h, the medium was changed and PD, IRN or both drugs were added. After treatment for 6, 12 and 24 h, the medium was removed. 100 μl Cell Lysis Buffer was added and cells were incubated on ice for 10 minutes. Cells were harvested and added with Lysis Buffer to a 1.5 ml tube, then centrifuged for 5 minutes at 1500 g. Protein concentration was assayed with a Bio-Rad Protein Assay kit. Into each well of a 96 well plate, 50 μg protein was diluted by adding Cell Lysis Buffer to make a volume of 100 μl. 50 μl 2X reaction Buffer (containing 10 mM Dithiothreitol, DTT) was added to each well. 5 μl 4 mM DEVD-ρNA (LEHD-ρNA for Caspase 9, IETD ρNA for Caspase 8) substrate (200 μM final concentration) was added, and the plates were incubated at 37°C for 24 hours. The sample plate was read at 400 or 405 nm with a microtiter plate reader.
Docking analysis
The caspase 3 protein (Caspase-X, PDB code: 3KJF) was selected for docking analysis. The Surflex-Dock program was used to determine the binding sites of PD and IRN to caspase 3. The binding affinities of these compounds to different binding sites were also determined, along with the locations of the hydrogen bonds formed.
Statistical analysis
The data are presented as mean ± standard error (SE). A one-way ANOVA and the Student's t-test were used to determine whether the results had statistical significance. The level of statistical significance was set at P<0.05.
Results
Antiproliferative effects of PD and IRN on two human colon cancer cell lines
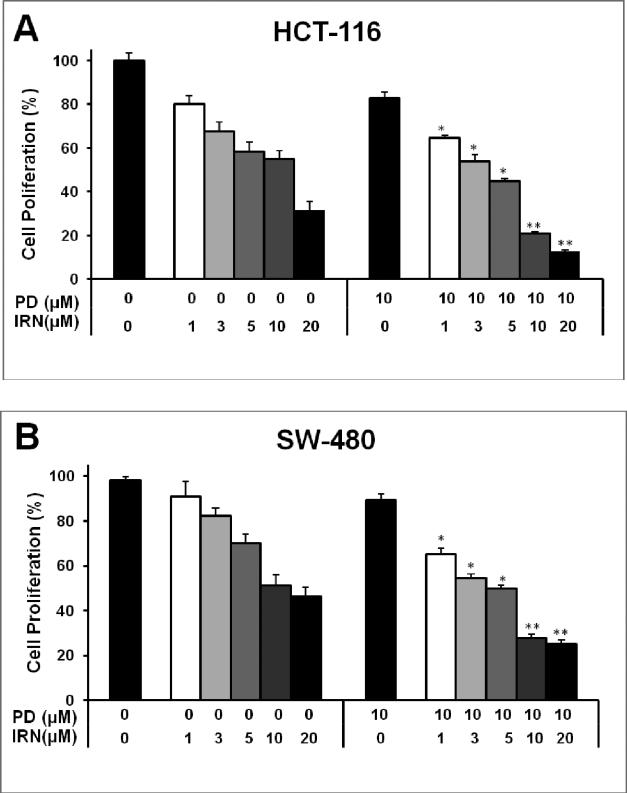
After treatment with PD, IRN or both drugs for 48 h, the proliferation of HCT-116 and SW-480 cells was slightly suppressed dose dependently in each case. Concentrations of irinotecan at 1, 3, 5, 10, 20 μM and/or PD at 10 μM (Fig. 2) were tested. PD exerted about a 19.1% and 10.7% antiproliferative effect at 10 μM on HCT-116 and SW-480 cells, respectively. The half-maximal inhibitory concentration (IC50) of IRN on HCT-116 was at a concentration of 12.1 μM; however, after the addition of PD, the IC50 dropped to a concentration of 4.4 μM. In SW-480 cells, the IC50 of IRN was at 11.8 μM, and after the addition of PD the IC50 dropped to 5.0 μM.
Fig. 2.
Antiproliferative effects of irinotecan and panaxadiol on human colorectal cancer cells. HCT-116 (a) and SW-480 (b) cells were treated with panaxadiol (10 μM) and/or irinotecan (1, 3, 5, 10 or 20 μM) for 48 hours. Data are presented as mean ± SE. *P < 0.05, **P < 0.01, compared to corresponding groups of IRN only.
In addition, we selected the young adult mouse colonic epithelium (YAMC) cell line to evaluate the effect of PD and IRN on normal intestinal epithelial cells. We observed that at the concentration of PD 10 μM combined with IRN 20 μM, the proliferation was 83.5% in YAMC cells. However, the proliferation was only 12.7% in HCT-116 cells. Thus, compared to the significant antiproliferative effect of PD and IRN on colorectal cancer cells, YAMC cells were not obviously affected.
Effects of PD and IRN on cell cycle distribution in HCT-116 cells
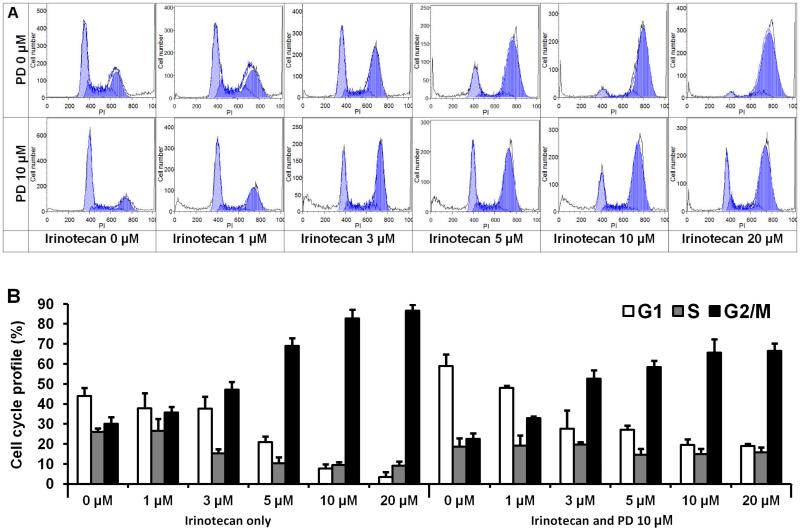
After staining with PI, the assay data from cells treated with PD, IRN, and both drugs at various concentrations were compared. As shown in Fig. 3, compared with control (44.0% in G1 phase, 26.0% in S phase, and 30.0% in G2/M phase), treatment with IRN gradually changed the cell cycle profile of HCT-116 cells. After treatment with IRN at 20 μM, the distribution was 3.4% in G1 phase, 9.2% in S phase, and 87.4% in G2/M phase. At a PD concentration of 10 μM, the fractions of cells were 58.9% (G1 phase), 18.7% (S phase) and 22.4% (G2/M phase). These data show that the G1 phase was slightly arrested. After treatment with 20 μM of IRN plus PD at 10 μM, the cell cycle profile was 19.3% (G1 phase), 16.0% (S phase) and 64.6% (G2/M phase). Notably, IRN arrested the G2/M phase significantly, while combining IRN with PD increased the G1 phase and decreased the G2/M phase, when compared to IRN treatment alone. These data suggest that PD induces cell cycle arrest at the G1 phase, and IRN induces arrest at the G2/M phase. The combination of both increases G1 and G2/M phase arrest, different than that induced by IRN or PD alone.
Fig. 3.
Effects of irinotecan and panaxadiol on HCT-116 cell cycle. HCT-116 cells were treated with panaxadiol (10 μM) and/or irinotecan (1, 3, 5, 10 or 20 μM) for 48 h. Cell cycle profile was determined using flow cytometry after staining with PI/RNase. (a) Representative histograms of cell cycle distribution. (b) Percentage of each cell cycle phase with various treatments or with control. Data are presented as the mean ± SE of triplicate experiments.
Apoptotic effects of PD and IRN on HCT-116 cells
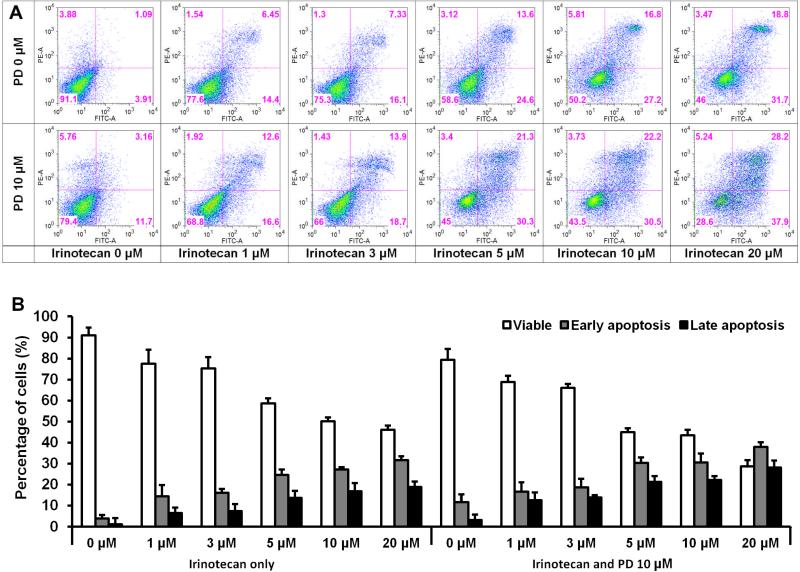
To further characterize the potential mechanisms of the anticancer effect of these two medicines, we performed an apoptotic assay by flow cytometry after staining with annexin V and PI. Compared with the control, it was shown that PD slightly induced apoptosis in HCT-116 cells. At concentrations of 1-20 μM, IRN gradually induced apoptosis. However, the combination with PD was more potent in inducing apoptosis than IRN alone. After treatment for 48 h, the percentage of apoptotic cells induced by PD at 10 μM was 14.8%. The percentage of apoptotic cells induced by IRN at concentrations of 1-20 μM increased from 20.9% to 50.5%. After combination with PD, the percentage range was 29.2% to 66.1%, compared to control at 5.0% (Fig. 4). These results suggested that the antiproliferative effect of IRN plus PD could be mediated by the induction of apoptosis.
Fig. 4.
Effects of irinotecan and panaxadiol on HCT-116 cell apoptosis. HCT-116 cells were treated with panaxadiol (10 μM) and/or IRN (1, 3, 5, 10 or 20 μM) for 48 h. Apoptosis was quantified using flow cytometry after staining with annexin V/PI. (a) Representative scatter plots of PI (y-axis) vs annexin V (x-axis). (b) Percentage of viable, early apoptotic and late apoptotic cells. Data are presented as the mean ± SE of triplicate experiments.
Caspase Assay
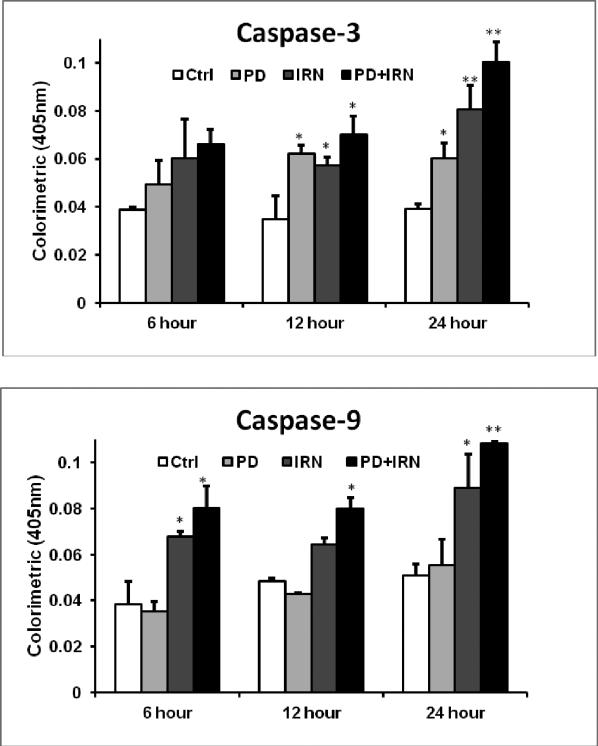
Caspase 3 and caspase 9 are two key proteins of the caspase family of proteases, which are highly conserved in multicellular organisms and function as central regulators of apoptosis. They have been identified as playing a key role in the progression of apoptosis.[19-21] To further characterize the potential mechanism of the anticancer effect of IRN and PD, we performed a Caspase 3/8/9 assay. Compared with the control, we tested the increase in caspase 3 and 9 activities in HCT-116 cells in response to PD at 10 μM, IRN at 10 μM, and the combination of the two. For Caspase-8, the activity was stable with PD, IRN or a combination, and only increased by 2.2% with combination at 24 hours. As shown in Fig. 5, at 6 hours the activity of caspase 3 was increased about 26.8% with PD only, 55.2% with IRN only, 70.7% with the combination; at 12 hours the activity was increased about 79.3% with PD only, 64.9% with IRN only and 102.4% with combination; and after 24 hours the activity was increased about 53.6% with PD only, 105.9% with IRN only, and about 155.7% with the combination. With caspase 9, there was no obvious activity change with PD treatment only, after 6 hours there was about a 76.9% increase with IRN only, and a 109.6% increase with the combination of PD and IRN, at 12 hours, there was about a 33.1% increase with IRN only, and 65.1% with combination; and after 24 hours, there was about a 74.7% increase with IRN only and a 113.1% increase with combination.
Fig. 5.
Effect of PD and IRN on Caspase 3/9 activity in HCT-116 cells. HCT-116 cells were treated with panaxadiol (PD; 10 μM) and/or irinotecan (IRN; 10 μM) for 6, 10 or 24 h. Caspase activities were measured by caspase 3, 9/CPP 32 Colorimetric Assay kits. Data are presented as mean ± SE of mean. *P < 0.05, **P < 0.01, versus corresponding control groups.
Molecular docking of PD and IRN to the caspase 3 protein
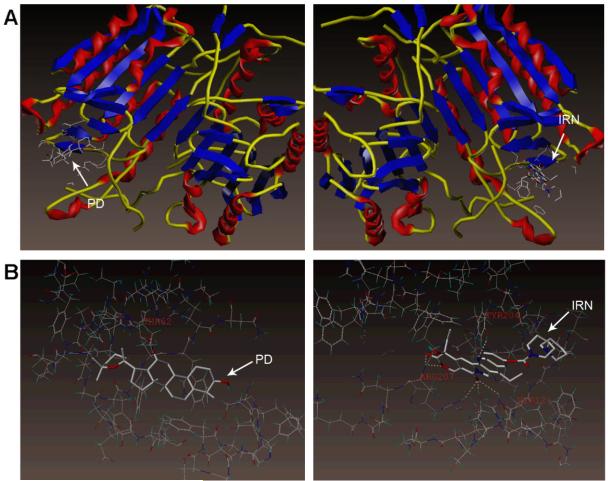
It has been reported that caspase 3 plays a key role in apoptosis and influences the progression of the cell death program. In our study, the combination of PD and IRN displayed a significant pro-apoptotic effect. We selected caspase 3 as our target protein for docking analysis. Fig. 5 shows the interactions between PD, IRN, and caspase 3, and that PD and IRN bind to different binding sites of caspase 3. It can be seen that IRN lies in the left chain (Chain A) of the protein while PD lies in the right chain (Chain B). The docking results (Fig. 6) showed that IRN formed six hydrogen bonds with Arg 207, Tyr 204 and His 121, while PD formed a hydrogen bond with Tyr 62. These results are in agreement with the results of our caspase assay.
Fig. 6.
Docking simulation of irinotecan and panaxadiol on the caspase-3 protein. Panaxadiol (PD) and irinotecan (IRN) bind to distinct binding sites on the protein. A surface and ribbon view is shown above (a), while an insight ligand-site model is shown below (b).
Discussion
Ginseng, including Asian ginseng and American ginseng, occupies a prominent position on the list of the best-selling natural products in the world. Ginseng has been reported to have a wide range of pharmacological effects, including anticancer activity.[22-24] Compared to unsteamed white ginseng, steamed or red ginseng has significantly increased anticancer activity.[25-27]
Using two commonly used colorectal cancer cell lines, HCT-116 and SW-480, we evaluated the interaction of PD and IRN in the inhibition of cancer cell activity. After co-treatment with 10 μM PD plus 1, 3, 5, 10 or 20 μM IRN for 48 h, the IC50 of HCT-116 was determined to be 4.4 μM, much lower than that of IRN alone (12.1 μM); in SW-480 cells the IC50 dropped from 11.8 μM to 5.0 μM. This suggests that PD significantly boosts the antiproliferative effect of IRN on HCT-116 and SW-480 cells and may reduce the dose of IRN needed to achieve the desired effects.
To explore the synergistic antiproliferative effects of PD and IRN, we evaluated the effects of the two compounds on cancer cell cycle regulation. Using the HCT-116 cell line, the cell cycle profile was assayed by flow cytometry after staining with PI. If the DNA is damaged, the cell cycle is halted at the transition from G1 to S phase, or at the transition from G2 to M phase.[28] We found that PD and IRN significantly increased the fractions of cells in G1 and G2/M phases, but decreased the percentage in S phase, suggesting that the combination of PD and IRN damaged cell DNA and inhibited cancer cell growth.
To further characterize the potential mechanism of the anticancer effects of PD plus IRN, we performed an apoptotic assay using flow cytometry. Results showed that the percentage of apoptotic cells induced by the combination was increased compared to that induced by PD or IRN only. This suggests a potential synergistic interaction of the two compounds in the induction of apoptosis.
Apoptosis is a highly regulatory process of programmed cell death, in which the caspase protease family is considered to be a key factor.[21,29] Many reports show that caspase 9 is the initiator associated with intrinsic pathway of apoptosis. Once activated, caspase 9 cleaves and activates a variety of downstream effectors such as caspase 3 etc., which target main regulatory and structural proteins for proteolysis to promote apoptosis.[30] To confirm whether the apoptotic effects induced by IRN plus PD were mediated by the caspase family, we performed a caspase colorimetric assay. Caspase 3, 8, and 9 activities were tested-in HCT-116 cells after exposure to PD, IRN, or the combination treatment at 6, 12 and 24 hours. The results indicated that the activities of caspase 3 and caspase 9 were obviously increased in a time dependent manner (Fig. 5); however, caspase 8 did not show a significant change. Meanwhile, in a docking simulation, PD and IRN got a higher score in the interaction with caspase 3 or caspase 9 than caspase 8. Therefore, we hypothesized that IRN combined with PD treatment might induce HCT-116 cell apoptosis mainly by up-regulating the activities of caspase 3 and caspase 9.
Caspase 3 plays a key role in the progression of the cell death program. In our docking analysis, PD and IRN were found to bind to two different sites on caspase 3 (Fig. 6). IRN showed significant binding affinity with caspase 3. The binding of both compounds to caspase 3 further provides a synergistic mechanism for their pro-apoptotic effects.
Conclusions
In summary, a synergistic anticancer effect of PD and IRN was observed in human colorectal cancer cells. This is the first time that the antiproliferative activity of a ginseng product was evaluated in combination with a semisynthetic camptothecin derivative and second-line antitumor medicine. This observed synergistic effect might be mediated through the induction of apoptosis. Our study also suggests that the anticancer activity of IRN may be enhanced by other natural products. Combined treatment with natural products could significantly reduce the dose of irinotecan needed to achieve the desired treatment effect, and decrease the rate of side effects. This is an important preliminary step in the development of an effective chemoadjuvant for colorectal cancer treatment.
Acknowledgments
This work was supported in part by the NIH/NCCAM grants P01 AT004418 and K01 AT005362.
Footnotes
Conflict of interest
The Author(s) declare(s) that they have no conflicts of interest to disclose.
References
- 1.Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Hawk ET, Levin B. Colorectal cancer prevention. J Clin Oncol. 2005;23:378–391. doi: 10.1200/JCO.2005.08.097. doi: 10.1200/JCO.2005.08.097. [DOI] [PubMed] [Google Scholar]
- 3.Yao Y, et al. Combined chemotherapy of hydroxycampothecin with oxaliplatin as an adjuvant treatment for human colorectal cancer. Tohoku J Exp Med. 2008;215:267–278. doi: 10.1620/tjem.215.267. doi: 10.1620/tjem/215.267. [DOI] [PubMed] [Google Scholar]
- 4.Patel BB, et al. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in mediating growth inhibition of colon cancer cells by modulating EGFR and IGF-1R. Int J Cancer. 2008;122:267–273. doi: 10.1002/ijc.23097. doi: 10.1002/ijc.23097. [DOI] [PubMed] [Google Scholar]
- 5.Politano S, et al. Second-line chemotherapy use in metastatic colon cancer varies by disease responsiveness. Clin Colorectal Cancer. 2008;7:55–59. doi: 10.3816/CCC.2008.n.008. doi: 10.3816/CCC.2008.n.008. [DOI] [PubMed] [Google Scholar]
- 6.Sheikh HY, et al. Alternating irinotecan with oxaliplatin combined with UFT plus leucovorin (SCOUT) in metastatic colorectal cancer. Br J Cancer. 2008;99:577–583. doi: 10.1038/sj.bjc.6604499. doi: 10.1038/sj.bjc.6604499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XL, et al. American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol Rep. 2009;22:943–952. doi: 10.3892/or_00000521. [PubMed] [Google Scholar]
- 8.Duda RB, et al. American ginseng and breast cancer therapeutic agents synergistically inhibit MCF-7 breast cancer cell growth. J Surg Oncol. 1999;72:230–239. doi: 10.1002/(sici)1096-9098(199912)72:4<230::aid-jso9>3.0.co;2-2. PMID: 10589039. [DOI] [PubMed] [Google Scholar]
- 9.King ML, Murphy LL. American ginseng (Panax quinquefolius L.) extract alters mitogen-activated protein kinase cell signaling and inhibits proliferation of MCF-7 cells. J Exp Ther Oncol. 2007;6:147–155. PMID: 17407973. [PubMed] [Google Scholar]
- 10.Luo X, et al. Characterization of gene expression regulated by American ginseng and ginsenoside Rg3 in human colorectal cancer cells. Int J Oncol. 2008;32:975–983. PMID: 18425323. [PMC free article] [PubMed] [Google Scholar]
- 11.Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. PMID: 19051332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qi LW, et al. Isolation and analysis of ginseng: advances and challenges. Nat Prod Rep. 2011;28:467–495. doi: 10.1039/c0np00057d. doi: 10.1039/c0np00057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gullett NP, et al. Cancer prevention with natural compounds. Semin Oncol. 2010;37:258–281. doi: 10.1053/j.seminoncol.2010.06.014. doi: S0093-7754(10)00093-X. [DOI] [PubMed] [Google Scholar]
- 14.Cragg GM, et al. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- 15.Qi LW, et al. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. doi: S0006-2952(10)00457-0. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham D, et al. Optimizing the use of irinotecan in colorectal cancer. Oncologist. 2001;6(Suppl 4):17–23. doi: 10.1634/theoncologist.6-suppl_4-17. PMID: 11585970. [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, et al. Optimisation of irinotecan dose in the treatment of patients with metastatic colorectal cancer after 5-FU failure: results from a multinational, randomised phase II study. Br J Cancer. 2005;92:1055–1062. doi: 10.1038/sj.bjc.6602462. doi: 10.1038/sj.bjc.6602462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanhoefer U, et al. Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol. 2001;19:1501–1518. doi: 10.1200/JCO.2001.19.5.1501. PMID: 11230497. [DOI] [PubMed] [Google Scholar]
- 19.Nicholson DW, Thornberry NA. Apoptosis. Life and death decisions. Science. 2003;299:214–215. doi: 10.1126/science.1081274. doi: 10.1126/science.1081274. [DOI] [PubMed] [Google Scholar]
- 20.Creagh EM, Martin SJ. Caspases: cellular demolition experts. Biochem Soc Trans. 2001;29:696–702. doi: 10.1042/0300-5127:0290696. PMID: 11709057. [DOI] [PubMed] [Google Scholar]
- 21.D'Amelio M, et al. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17:1104–1114. doi: 10.1038/cdd.2009.180. PMID: 19960023. [DOI] [PubMed] [Google Scholar]
- 22.Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29:1109–1118. doi: 10.1111/j.1745-7254.2008.00869.x. doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, et al. Long-term effects of Panax ginseng on disposition of fexofenadine in rats in vivo. Am J Chin Med. 2009;37:657–667. doi: 10.1142/S0192415X09007144. doi: 10.1142/S0192415X09007144. [DOI] [PubMed] [Google Scholar]
- 24.Jang HI, Shin HM. Wild Panax ginseng (Panax ginseng C.A. Meyer) protects against methotrexate-induced cell regression by enhancing the immune response in RAW 264.7 macrophages. Am J Chin Med. 2010;38:949–960. doi: 10.1142/S0192415X10008378. doi: 10.1142/S0192415X10008378. [DOI] [PubMed] [Google Scholar]
- 25.Wang CZ, et al. Steamed American ginseng berry: ginsenoside analyses and anticancer activities. J Agric Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- 26.Wang CZ, et al. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–674. doi: 10.1055/s-2007-981524. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–274. PMID: 15387718. [PubMed] [Google Scholar]
- 28.Hartwell LH, Weinert TA. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989;246:629–634. doi: 10.1126/science.2683079. PMID: 2683079. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RC, et al. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. doi: 10/1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 30.Allan LA, Clarke PR. Apoptosis and autophagy: Regulation of caspase-9 by phosphorylation. FEBS J. 2009;276:6063–6073. doi: 10.1111/j.1742-4658.2009.07330.x. PMID: 19788417. [DOI] [PubMed] [Google Scholar]