Abstract
Hydrolysis of surfactant phospholipids (PL) by secretory phospholipases A2 (sPLA2) contributes to surfactant damage in inflammatory airway diseases such as acute lung injury/acute respiratory distress syndrome. We and others have reported that each sPLA2 exhibits specificity in hydrolyzing different PLs in pulmonary surfactant and that the presence of hydrophilic surfactant protein A (SP-A) alters sPLA2-mediated hydrolysis. This report tests the hypothesis that hydrophobic SP-B also inhibits sPLA2-mediated surfactant hydrolysis. Three surfactant preparations were used containing varied amounts of SP-B and radiolabeled tracers of phosphatidylcholine (PC) or phosphatidylglycerol (PG): 1) washed ovine surfactant (OS) (pre- and postorganic extraction) compared with Survanta (protein poor), 2) Survanta supplemented with purified bovine SP-B (1–5%, wt/wt), and 3) a mixture of dipalmitoylphosphatidylcholine (DPPC), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG) (DPPC:POPC:POPG, 40:40:20) prepared as vesicles and monomolecular films in the presence or absence of SP-B. Hydrolysis of PG and PC by Group IB sPLA2 (PLA2G1A) was significantly lower in the extracted OS, which contains SP-B, compared with Survanta (P = 0.005), which is SP-B poor. Hydrolysis of PG and PC in nonextracted OS, which contains all SPs, was lower than both Survanta and extracted OS. When Survanta was supplemented with 1% SP-B, PG and PC hydrolysis by PLA2G1B was significantly lower (P < 0.001) than in Survanta alone. When supplemented into pure lipid vesicles and monomolecular films composed of PG and PC mixtures, SP-B also inhibited hydrolysis by both PLA2G1B and Group IIA sPLA2 (PLA2G2A). In films, PLA2G1B hydrolyzed surfactant PL monolayers at surface pressures ≤30 mN/m (P < 0.01), and SP-B lowered the surface pressure range at which hydrolysis can occur. These results suggest the hydrophobic SP, SP-B, protects alveolar surfactant PL from hydrolysis mediated by multiple sPLA2 in both vesicles (alveolar subphase) and monomolecular films (air-liquid interface).
Keywords: acute lung injury, pulmonary surfactant, surface tension, surfactant proteins
surfactant damage and dysfunction are important components of inflammatory diseases of the airways (15, 22, 27, 38). Pulmonary surfactant is a complex mixture of phospholipids (PL) (80–90%), neutral lipids (5–10%), and proteins (10%), which maintain airway patency by lowering alveolar surface tension and thus reduce the work of breathing (54). Phosphatidylcholine (PC) and phosphatidylglycerol (PG) make up 90% of the total PL, comprising ∼80% and 10% of human surfactant PLs, respectively. The principal nonlipid surfactant components are proteins. Surfactant protein A (SP-A) is a large, hydrophilic member of the collectin family of proteins and recognizes a variety of microbial ligands (16, 31). As such, SP-A serves a principal role in innate immunity and contributes to the surface activity and structural stability of surfactant (49, 50). SP-B and SP-C, two smaller, highly conserved, hydrophobic proteins, appear intimately associated with maintenance of the dynamic changes in the physical state of surfactant PLs (16, 24) and facilitate the absorption and spreading of PL to form the surface film with each breath cycle (34, 40). SP-B interactions with PG, the most abundant anionic surfactant PL, are particularly important in the maintenance of film function (7, 34). Deficiency of SP-B in humans with congenital SP-B mutations or in knockout mouse models is lethal, and low levels of SP-B correlate with risk of development of acute respiratory distress syndrome (ARDS) in humans (8, 14, 36).
Our laboratory and others have reported on the role of secretory phospholipases (i.e., sPLA2) in inflammation-mediated surfactant dysfunction in both ARDS and asthma (2, 44). The sPLA2s are a large family of phospholipases that hydrolyze PLs at the sn-2-acyl position, generating lysophospholipids and free fatty acids (26). These enzymes have a wide but differential tissue distribution including airway epithelium, alveolar macrophages, and emigrating leukocytes such as neutrophils (5, 13, 33, 45). Thus stimulation of these cells in the inflamed lung can lead to increased extracellular sPLA2 levels and subsequent hydrolysis of pulmonary surfactant, which would cause surfactant dysfunction.
During respiratory cycles, pulmonary surfactant exists in interrelated physical states including monomolecular films, collapsed subphases, and vesicles in the epithelial lining fluid between the film and the airway epithelial cell apical surface (18). All of these surfactant pools are possible targets of sPLA2-mediated damage. In vivo, the degree of surfactant damage caused directly by sPLA2 is dependent on the extent and nature of hydrolysis. This hydrolysis appears partially dependent on the presence of SPs. For example, the hydrophilic SP, SP-A, has been shown to inhibit sPLA2-mediated surfactant hydrolysis (6), but little is known regarding SP-B effects on sPLA2. SPs may be depleted in acute lung injury (ALI) and ARDS (14, 43). Such depletion may alter the balance of sPLA2-mediated hydrolytic damage to surfactant in patients with ALI/ARDS.
In this study, we hypothesized that the hydrophobic SP, SP-B, will also inhibit the sPLA2-mediated hydrolysis of surfactant PLs in models of both surfactant aggregates and monomolecular films.
MATERIALS AND METHODS
Surfactants
Survanta (Ross Laboratories, St. Louis, MO) was donated (R. Dillard, Dept. of Neonatology, Wake Forest University Health Science). Ovine surfactant (OS) was obtained by whole lung saline lavage from female sheep (∼50 kg) immediately after euthanasia in a protocol approved by the Institutional Animal Care and Use Committee. Native OS was prepared by centrifugation at 40,000 g for 1 h and washed twice with normal saline, aliquoted, and stored at −70°C (21). Organic lipid extraction was performed on aliquots of the OS (4), providing a preparation of surfactant PLs containing endogenous hydrophobic SPs. Total protein content of animal surfactant preparations (pellets after ultracentrifugation) were measured using the BCA protein assay (Pierce, Rockford, IL) and reported as mg/mg of PLs. Levels of specific SPs, such as SP-A and SP-B, were not measured in these preparations.
PLs
Dipalmitoylphosphatidylcholine (DPPC), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG) were purchased from Avanti Polar Lipids (Alabaster, AL). For all experiments these lipids were mixed from chloroform stocks in a ratio of 40:40:20 (DPPC:POPC:POPG) as a model of pulmonary surfactant lipid composition. 1-Palmitoyl-[2-palmitoyl-9-10-3H ]-phosphatidylcholine ([3H]-DPPC) was purchased from American Radiolabeled Chemicals (St. Louis, MO). Radiolabeled [3H]-glycerol-PG was prepared by incorporation of [3H]-glycerol (Perkin Elmer, Shelton, CT) by Escherichia coli (E. coli) and purification of the radiolabeled PG by lipid extraction and TLC separation (4, 10). All PLs (with and without addition of SP-B) were mixed in CHCl3:MeOH (90:10) before either drying and suspension in an aqueous buffer (vesicle experiments) or direct application onto an aqueous subphase (monolayer experiments). The resulting vesicles (composed of only PLs) were examined by dynamic light scatter (Malvern Instruments, Westborough, MA) and comprised a population with mean diameter of 140 nm (range of 50–500 nm, not shown).
SPs
The hydrophobic SP, SP-B, was purified from bovine lung surfactant extract (BLES) using column chromatography and identified with SDS-PAGE and amino acid sequencing (41, 52). The hydrophilic SP-A was purified from lung lavage obtained from humans with alveolar proteinosis per the methods of Haagsman et al. (17). Oxidized SP-B was prepared by treatment of BLES with hypochlorous acid before purification of the SP-B as described via LH-20 chromatography (30, 42). Protein levels for purified SP-B and oxidized SP-B were quantified using a modified Lowry procedure (29) containing optimal SDS (30, 53); BLES oxidation did not impact protein quantification. All SP-A and SP-B preparations were provided by Drs. F. Possmayer and R. Veldhuizen.
Mixing of Labeled PLs and SPs
The hydrophobic protein SP-B was mixed with extracted Survanta or pure PL (DPPC:POPC:POPG, 40:40:20) containing trace (<10% of total target PL) labels of [3H]-PG or [3H]-DPPC and dried under a nitrogen gas stream. A solution containing 5 mM Tris pH 7.4 with 0.9% saline and 5 mM calcium chloride was added to the dried extracts (1 mg/ml) and vesicles were formed by bath sonication at 40°C for 20 min. Using analysis with dynamic latter scatter, mixing of SP-B with pure PL vesicles led to significant increase in PL vesicle diameter (range of 400–1,300 nm diameter vesicles, not shown), compared with PL alone. These results are very similar to those reported for a synthetic SP-B mimic (mini-B) (51). Mixing of oxidized SP-B to PL produced a complex mixture of vesicles with a predominant population of larger vesicle size (1,500–1,900 nm diameter) with two additional vesicle populations at 165 nm and >5,000 nm diameters (not shown).
Phospholipases
Bovine Group IB sPLA2 (PLA2G1B) was purchased from Sigma Chemical (St. Louis, MO) with enzyme amounts reported in U/ml using hydrolysis of soy PC as a standard substrate. Group IIA sPLA2 (PLA2G2A) was cloned as described (21, 45) and expressed by transient transfection in Cos 1 cells (ATCC, Manassas, VA). Conditioned tissue culture media was fractionated over heparin Sepharose (4 × 5 ml HiTrap Heparin HP; GE Healthcare Biosciences, Uppsala, Sweden) by elution with a gradient of 0.250–2 M NaCl in 50 mM Tris buffer, pH 7.5. The active fractions were identified by hydrolysis of radiolabeled E. coli membranes as described (21), pooled, dialyzed against 5 mM Tris-buffered saline, pH 7.5, and concentrated over an Amicon YM3 stirred cell membrane (Millipore, Billerica, MA). The enzyme was further fractionated over a C4 column (Phenomenex) with elution on a 5–60% acetonitrile gradient with 0.1% trifluoroacetic acid. Active fractions were pooled, dialyzed against 5 mM Tris saline pH 7.5, and concentrated again over a YM3 membrane. sPLA2 have different affinities for PL substrate head groups (23, 47), and PLA2G2A does not favor hydrolysis of pure PC vesicles used to define units of activity for the commercially available PLA2G1B; therefore PLA2G2A enzyme activity was measured and defined against E. coli-containing radiolabeled oleic acid predominantly in the PG fraction (23). Thus, one E. coli unit equals 2,500 pmol E. coli PL hydrolyzed in 1 h (23) by fractionated PLA2G2A containing ∼570 pg of PLA2G2A as measured by enzyme immunoassay (Cayman Chemical, Ann Arbor, MI).
Hydrolysis of OS, Survanta, and PL Vesicles
For the majority of experiments, the surfactants in 5 mM Tris-buffered saline pH 7.4 with 5 mM CaCl2 were incubated with Group IB sPLA2, (PLA2G1B, bovine group IB sPLA2; Sigma Chemical) in concentrations ranging from 0.1–20 U/ml, for 2 h at 37°C. Experiments using PLA2G2A were performed using 450 E. coli U/ml and gave comparable hydrolysis when compared with 2 U/ml of PLA2G1B. After hydrolysis, samples underwent organic lipid extraction (4) and then separation of intact PLs from either [3H]-lyso-PG or [3H]-oleate using selective TLC systems (23). Fractions were scraped and analyzed for hydrolysis using the percentage of total radioactivity recovered in the lyso-PG (PG hydrolysis) and free fatty acid (PC hydrolysis) fractions.
Hydrolysis of Monomolecular Films
PL mixtures were loaded directly onto an aqueous subphase (5 mM Tris-buffered saline pH 7.4 with 5 mM CaCl2) in a Langmuir Blodgett trough with a platinum Wilhelmy plate (KSV, Helsinki, Finland). Experiments were conducted in two separate troughs: 1) a 17-mm radius trough with a subphase injection port and magnetic stirring bar for experiments on PL films before compression, and 2) a 150 mm × 35 mm isometric trough for hydrolysis experiments after compression and reexpansion of the PL film. Both systems were loaded to a target pressure of 15–30 mN/m, and 10 min was allowed for solvent evaporation. In the isometric trough, the film was compressed using a movable barrier until the collapse pressure of 55–60 mN/m was reached, and 5 min was allowed for reequilibration of the film. The barrier was then moved back to decrease the pressure to 40 mN/m. After stable baselines were recorded for 20 min, the enzyme was injected under the film. The subphase contained 0.9 mM β-cyclodextrin, which binds free fatty acids and lysophospholipids from the air liquid interface posthydrolysis. All monolayer studies were performed at room temperature. Rates of hydrolysis were measured using the rate of change in surface pressure, minus the baseline rate (mN/m per min), after injection of group IB sPLA2 into the subphase buffer.
Statistical Analysis
Results of hydrolysis and SP-B inhibition of hydrolysis were evaluated with Student's paired t-tests with a minimum of three repetitions completed for each experimental condition. Data shown are means ± SD.
RESULTS
Comparison of Surfactant Preparations With Varied Protein Content
Initially, three models of surfactant, each with different levels of endogenous SPs, were used to test the hypothesis that hydrophobic SP-B could modulate sPLA2-mediated hydrolysis of surfactant PLs.
Endogenous SP-B in animal surfactants.
In the first model, OS was harvested, washed repeatedly, and used intact (native) or after extraction with organic solvents, which depletes the surfactant PL of the hydrophilic protein, SP-A. Native and extracted OS were then compared with Survanta, a commercial preparation of extracted bovine surfactant, which contains no hydrophilic SPs, some hydrophobic SP-C, but very low levels of SP-B (32, 37).
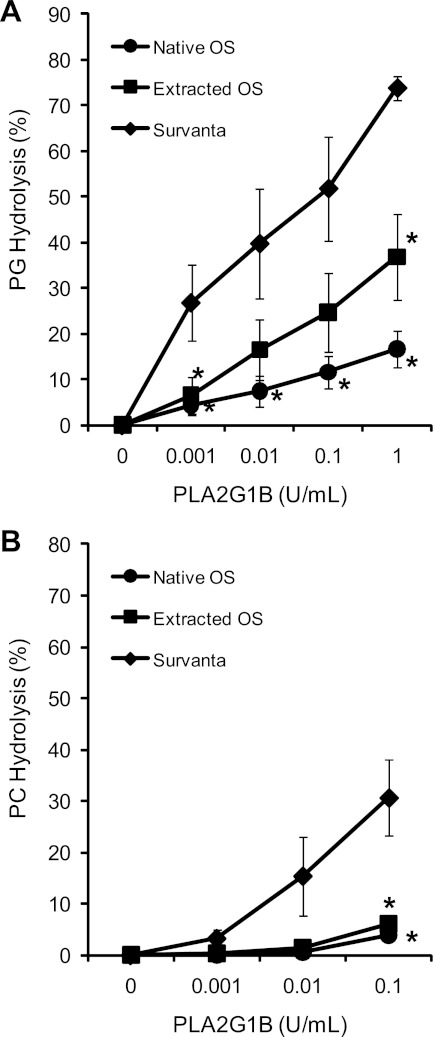
PG hydrolysis by PLA2G1B increased with enzyme concentration in each surfactant preparation. However, PG hydrolysis was highest in the Survanta relative to both the native and extracted OS (Fig. 1A, P < 0.05). Survanta contained the lowest levels of protein in the surfactant pellets (0.02 mg protein/mg PL), with extracted OS containing 0.04 mg protein/mg PL and native OS containing the highest at 0.17 mg protein/mg PL, including both hydrophilic and hydrophobic SPs. Hydrolysis of PC-labeled surfactants in Survanta vs. extracted and native OS produced results similar to PG (Fig. 1B), with both native and extracted OS showing significantly lower hydrolysis than Survanta. Of note, the lower percentage of hydrolysis in this fixed time point experiment is significantly influenced by the approximately fourfold higher PC content of the surfactants relative to PG.
Fig. 1.
Group IB secretory phospholipases A2 (sPLA2) (PLA2G1B) hydrolysis of phosphatidylglycerol (PG) and phosphatidylcholine (PC) in ovine surfactants (OS) and Survanta. PG hydrolysis (A) and PC hydrolysis (B) were measured in 3 surfactant preparations of varying protein content. PLA2G1B enzyme hydrolysis of surfactant phospholipids (PL) was lower in native sheep surfactant, which contained 0.17 mg protein/mg PL, compared with organically extracted sheep surfactant with 0.04 mg protein/ml PL, and to Survanta with 0.02 mg protein/ml of PL. *P < 0.05 compared with Survanta at the same sPLA2 concentration.
Supplementation of animal surfactant with SP-B.
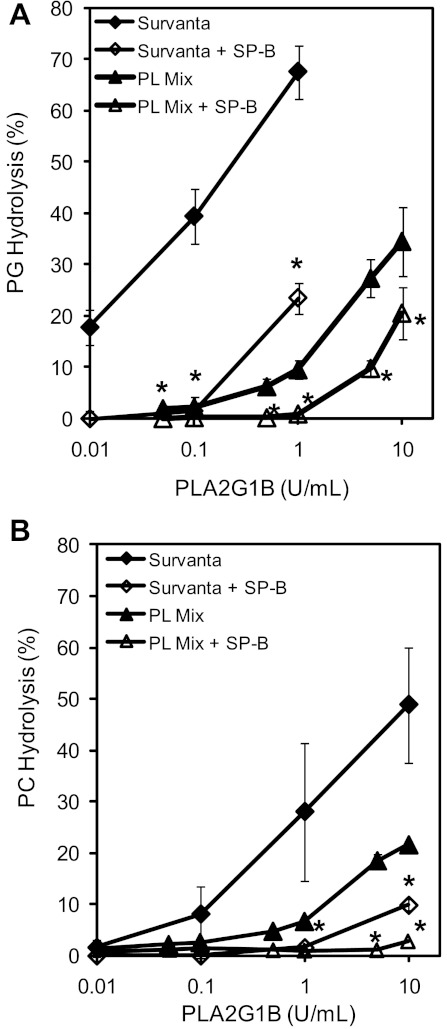
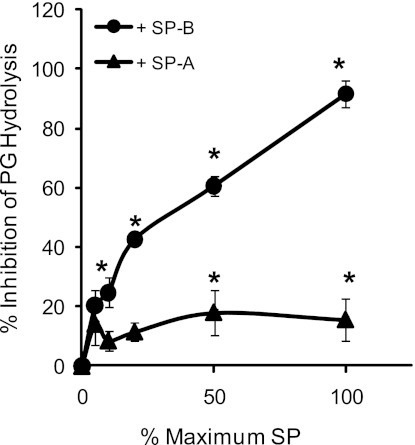
To study more directly the effect of SP-B on sPLA2 hydrolysis of surfactant PLs, SP-B was reconstituted into Survanta. PG hydrolysis by all concentrations of sPLA2 tested was significantly decreased in Survanta supplemented with 1% SP-B compared with pure Survanta alone (23.5 ± 4.3% hydrolysis vs. 67.5 ± 7.4% at 1 U/ml sPLA2, P < 0.001, Fig. 2A). Similarly, hydrolysis of PC-labeled surfactant was significantly inhibited by the addition of 1% SP-B at all but the lowest PLA2G1B concentrations tested (Fig. 2B). Inhibition of sPLA2-mediated PG hydrolysis by SP-B is concentration dependent, increasing over the range of 0.001–0.01 mg SP-B protein/mg PL (Fig. 3). Of note, the inhibition seen with 1% SP-B appears greater than that seen for 10% SP-A (Fig. 3), which are concentrations that are believed to be at or near concentrations in human alveolar surfactant.
Fig. 2.
Surfactant protein-B (SP-B) inhibits Group IB sPLA2 hydrolysis of Survanta and pure PL vesicles. Group IB sPLA2 hydrolysis of PG (A) and PC (B) were measured in Survanta vs. Survanta supplemented with 1% SP-B, and in PL vesicles (PL mix) containing dipalmitoylphosphatidylcholine (DPPC), 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC), and 1-palmitoyl-2-oleoyl-phosphatidylglycerol (POPG) (DPPC:POPC:POPG, 40:40:20) labeled with either [H3]-PG (A) or [H3]-PC (B) and incubated with Group IB sPLA2 (0.1–10 U/ml) at 37°C for 2 h. Hydrolysis of both surfactant substrates decreased in surfactant supplemented with SP-B. Data represent the means ± SD of ≥3 experiments. *P < 0.01 compared with surfactants without SP-B.
Fig. 3.
SP-B inhibits sPLA2 in a concentration-dependent manner. Hydrolysis of PG in Survanta (1 mg PL/ml) by Group IB sPLA2 (0.02 U/ml) demonstrated a concentration-dependent inhibition by SP-B (0–0.01 mg/mg PL) and significant inhibition by SP-A (0–0.1 mg/mg PL). *P < 0.05 compared with control without added SP-B or SP-A.
SP-B supplementation of pure PL mixtures.
As Survanta is a complex mixture of PLs, free fatty acids, and some residual SPs, the hydrolysis experiments were repeated with sonicated vesicles of DPPC:POPC:POPG (40:40:20 mol ratios) that were trace labeled with either PC or PG, and incubated with PLA2G1B. Similar to the results using labeled Survanta, hydrolysis of PG (Fig. 2A) and PC (Fig. 2B) were significantly inhibited by the presence of 1% SP-B at all sPLA2 concentrations examined. Thus, in all models of surfactant vesicles, including three different surfactant preparations, SP-B inhibited hydrolysis of PG and PC by PLA2G1B.
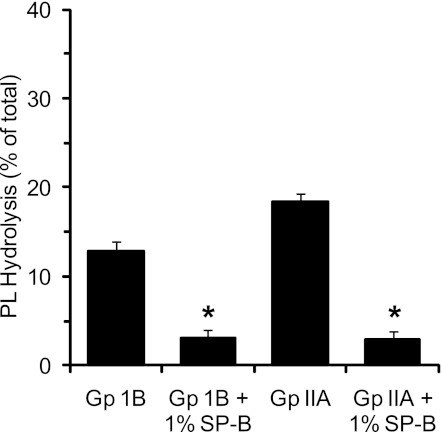
To demonstrate that inhibition of hydrolysis by SP-B was not unique to PLA2G1B, we investigated the effects of SP-B on a second sPLA2, PLA2G2A. We and others have identified PLA2G2A to be elevated in the bronchoalveolar lavage fluid of ALI/ARDS subjects (25, 44). We compared the impact of 1% SP-B on PLA2G1B to PLA2G2A at enzyme levels that gave comparable hydrolysis of the DPPC:POPC:POPG PL vesicle mixture, repeating the design of experiments shown in Fig. 2. Hydrolysis of PG in the vesicles by either enzyme was significantly inhibited by addition of 1% SP-B (Fig. 4).
Fig. 4.
SP-B inhibits PLA2G2A hydrolysis of pure PL vesicles. PL vesicles (DPPC:POPC:POPG, 40:40:20) were labeled with [H3]-PG as in Fig. 2 and incubated with either Group (Gp) IB sPLA2 (2 U/ml) or Group IIA (450 Escherichia coli U/ml) at 37°C for 2 h. Experiments were conducted in the presence and absence of 1% SP-B. Data represent the means ± SD of 4 experiments. *P < 0.05 compared with PL without SP-B.
Effect of SP-B on Hydrolysis of PL in Monomolecular Films
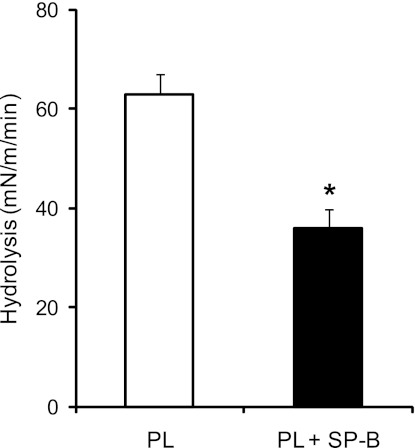
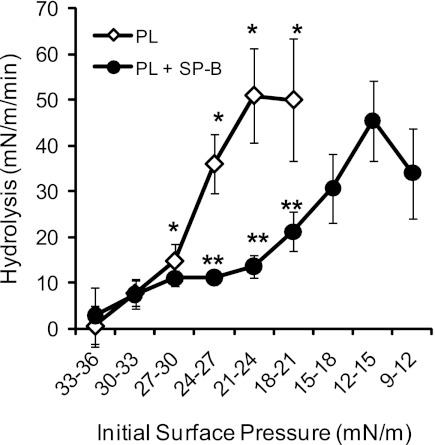
In the lung, surfactant forms an expandable film at the alveolar air liquid interface, which provides a different biophysical form for sPLA2 hydrolysis relative to the aggregates examined above. We used a Langmuir Blodgett trough with a Wilhelmy balance to measure sPLA2 hydrolysis of surfactant films in vitro. Hydrolysis is shown as a change in surface pressure resulting from desorption of the reaction products, free fatty acids, and lysophospholipids from the film and binding to the β-cyclodextrin in the aqueous subphase. Surface pressure impacts the density of PL packing and regulates penetration of the phospholipase into the film. Initial experiments revealed that sPLA2 hydrolyzed the surfactant PL monolayers at a surface pressure at or below 30 mN/m (not shown). With the initial surface pressure of the film set to 17.5 ± 1.5 mN/m, hydrolysis of the monomolecular film by PLA2G1B was significantly reduced in the presence of SP-B, as shown in Fig. 5 (0.62 ± 0.10 mN/m per min vs. 0.36 ± 0.11 mN/m per min, P < 0.001). These data show that SP-B protects a static film that has not yet gone through a cycle of collapse and reexpansion. Because this cycle is ongoing in the breathing lung as components of the surfactant are expelled from the films and new components are included, we did experiments to determine whether SP-B would inhibit sPLA2 in a film first compressed to its collapse pressure and then reexpanded to a lower surface pressure. Figure 6 shows the action of sPLA2 on such films. Hydrolysis of PL is first seen at pressures in the 24–27 mN/m range, increasing to maximum at around 21–24 mN/m. In the presence of SP-B, no hydrolysis was seen until the pressure decreased to 15–18 mN/m. The apparent maximal rates of change in surface pressure with and without SP-B were roughly equal (∼0.5–0.6 mN/m per min). However, it must be emphasized that the pressure changes measured in these experiments are used as surrogate measures for PL hydrolysis but are not quantitatively equivalent to enzymatic rates. More specifically, the surface PL concentration (density) of the film at the lower pressure (12–15 mN/m) when maximum pressure reduction is seen in the experiments with SP-B is roughly 65% that of the PL density (0.99 × 1012 mol/cm2 with SP-B vs. 1.55 × 1012 without SP-B) at the higher pressure (21–24 mN/m) when maximal hydrolysis is achieved in the absence of SP-B. These results suggest that the inhibitory effects of SP-B in compressed and spread films may be mediated through reduction of the surface pressure threshold at which sPLA2 can bind to and/or hydrolyze its PL substrates.
Fig. 5.
SP-B inhibits sPLA2 hydrolysis of PL monomolecular films. Monolayers of PLs with DPPC:POPC:POPG (40:40:20) with and without SP-B (5% m/m) were formed in a Langmuir Wilhelmy trough at concentrations sufficient to achieve an initial surface pressure of 17–20 mN/m. No compression of the PL film was performed before introduction of sPLA2. Group IB sPLA2 (0.07 U/ml) was injected into the subphase, and surface pressure was continuously measured. The data are the means ± SD of at least 3 experiments with and without SP-B. *P < 0.005.
Fig. 6.
SP-B inhibition of monomolecular film hydrolysis is surface pressure dependent. Hydrolysis of monomolecular film PL by Group IB sPLA2 was performed as outlined in Fig. 5 in the presence and absence of SP-B (5%). Enough PL was added onto the film to achieve the desired initial surface pressure (10–50 mN/m). Hydrolysis was measured as the rate of change in surface pressures (mN/m per min) across the specified range of initial surface pressure (mN/m). No PL hydrolysis, seen as a significant rate of change in surface pressure, was seen after addition of sPLA2 at initial surface pressures >30 mN/m. At pressures below 30 mN/m, SP-B inhibited the sPLA2-mediated hydrolysis of surfactant PL, in part by shifting required hydrolysis to lower surface pressures. *P < 0.05 for PL rates vs. those at 30–33 mN/m initial pressure, **P < 0.05 for SP-B inhibition of sPLA2 hydrolysis of PL at same initial pressures.
Effect of Oxidized SP-B on Its Inhibition of sPLA2 Hydrolysis of Surfactant
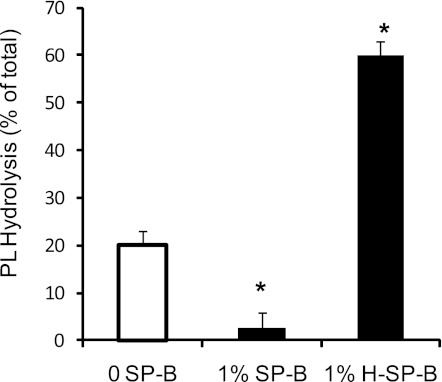
SPs can be oxidized by reduced oxygen species during ALI/ARDS, and it has been demonstrated that, when oxidized, SP-B loses its surface tension-lowering activity (30). Using SP-B oxidized by hypochlorous acid, we examined the impact of oxidation on the ability of SP-B to inhibit sPLA2-mediated hydrolysis. Using a concentration of PLA2G1B that yields moderate hydrolysis of surfactant vesicles, we discovered that equal concentrations of oxidized SP-B (H-SP-B) did not inhibit hydrolysis (Fig. 7). In fact, hydrolysis of surfactant vesicles was enhanced in the presence of H-SP-B, an unexpected but consistently observed and significant result (P < 0.05).
Fig. 7.
Oxidized SP-B does not inhibit sPLA2 hydrolysis of surfactant. Surfactant PL hydrolysis was measured for vesicles using 10 U of Group IB sPLA2 and the PL mix described in as in Fig. 2. Shown are 3 conditions, without SP-B (0 SP-B), with 1% SP-B, and with 1% oxidized SP-B (1%H-SP-B). *P < 0.02 vs. enzyme alone.
DISCUSSION
To examine the potential impact of the essential hydrophobic SP, SP-B, on hydrolysis of pulmonary surfactant by sPLA2, three distinct surfactant preparations were examined: 1) animal-derived surfactants with varying levels of SPs, 2) SP-B supplementation of a modified bovine surfactant with minimal endogenous SPs, and 3) SP-B supplementation of synthetic lipid mixtures containing PC and PG. PLs in the form of lipid vesicles (or aggregates) were studied in all three surfactant preparations, and PLs in films at an air-liquid interface were studied using synthetic lipid mixtures supplemented with SP-B. The cumulative results from these models revealed highly consistent results that indicate susceptibility to PL hydrolysis is inversely correlated with SP-B levels. These findings suggest that SP-B plays an important role in protection of alveolar surfactant from sPLA2 hydrolysis including PLs found at the air-liquid interface and in the subphase of the alveolar lining fluid.
The overall consistency of these results with lipids in both vesicular and monolayer structural conformations is an important observation. These findings indicate that, once secreted, sPLA2 within the alveolar space is capable of hydrolyzing pulmonary surfactant PLs within both the aqueous subphase and at the alveolar air-liquid interface and that SP-B provides an important protective effect, when present. The studies examining PLs in a monolayer conformation (Figs. 5 and 6) also highlight the critical role that surface pressure plays in determining susceptibility of the PL to hydrolysis. Hydrolysis of PL monolayers by Group IB sPLA2 did not occur at or slightly above a surface pressure of 30 mN/m. Although this represents a relatively low surface pressure, it is important to note that there is a broad range of surface pressures during the dynamics of the respiratory cycle with the lowest surface pressures sustained at the peak of inspiration. This range of surface pressures increases dramatically with even lower surface pressures (higher surface tensions), achieved as a result of surfactant damage driven by the acute inflammation characteristic of ALI/ARDS. Our results would suggest that the concomitant depletion of SP-B seen in ALI/ARDS (27) only serves to further enhance the vulnerability of surfactant PLs to sPLA2-mediated hydrolysis by altering the surface pressure threshold at which hydrolysis can be initiated.
The importance of SP-B protection is even further emphasized by the data highlighted in Fig. 7 in which alterations in SP-B through oxidation lead to loss of its ability to inhibit sPLA2 hydrolysis of vesicle PL and apparently enhance hydrolysis. Although oxidized SP-B alone may not account for all increased hydrolysis seen in these experiments (we cannot exclude the presence of oxidized PLs), these results highlight the requirement that SP-B be intact and fully functional to achieve its protective effects (30). When measured in clinical samples, SP-B levels are not assessed for viability and cannot be used to differentiate between intact SP-B and abnormal SP-B that has been altered through oxidation or via additional inflammatory mediators (e.g., serum proteases). Therefore, levels of intact SP-B in ALI/ARDS may be even lower than currently assumed and, if damaged, may be more potentially detrimental by increasing susceptibility to hydrolysis. In addition, mutations of SP-B have been demonstrated to be lethal in both knockout mouse models and human newborns (11, 12, 19, 20, 28). On the basis of our results, it is interesting to speculate whether these mutations might increase susceptibility of the endogenous surfactant to sPLA2-mediated damage and whether this mechanism may be an important contributor to the ensuing progressive respiratory failure and death.
Our results also provide insight into the relative protective effect of SP-B vs. the effect of the other SPs. As mentioned previously, studies have demonstrated the protective effects of SP-A against sPLA2-mediated hydrolysis. Our results in Fig. 1 demonstrate that the combination of hydrophobic SPs (SP-B and SP-C) in extracted OS provide significant protection for both PG and PC hydrolysis in the absence of hydrophilic SPs even at low enzyme concentrations. When present in the complete OS, hydrophilic proteins provided significant additional protection beyond that of the hydrophobic proteins only at higher enzyme concentrations. Furthermore, the results of bovine SP-B and human SP-A (from patients with alveolar proteinosis) supplementation to Survanta (Fig. 3) demonstrate that both provide significant protection against PG hydrolysis. Whether SP-A harvested from animal sources differs from that of humans with pulmonary alveolar proteinosis is unknown (2). The relative protective effects of SP-B vs. SP-C or the potential for additive/synergistic effects between SP-B and SP-C is not addressed in these studies. The data provided in Fig. 1, A and B, suggest potential additive/synergistic effects between the hydrophobic and hydrophilic SPs, but further studies are needed to fully characterize those potential interactions.
The protective effect of SP-B toward the individual PL subclasses within pulmonary surfactant appears to be nonspecific with protection toward PC and PG in all of the tested surfactant preparations. Although it is tempting to compare the relative impact of SP-B on PC vs. PG hydrolysis in these experiments, the studies were not designed to measure direct enzyme kinetics, as hydrolysis was measured at a single fixed time interval after introduction of the enzyme. Also, the predominance of PC (4–8-fold) in each of our surfactant preparations must also be taken into account when interpreting data that reports percentage of hydrolysis of the labeled substrate. It is important to note that the protective effect of SP-B is not limited to PG, despite the presumed important interaction between SP-B and PG in achieving optimal surface tension-lowering activity.
As we have previously demonstrated, PLA2GIB possesses the ability to hydrolyze all three of the major PL subclasses within pulmonary surfactant (PC, PG, and PE) (23). For this reason, PLA2GIB was selected for these initial studies, which aimed to assess how general were the effects of SP-B reduction on hydrolysis by sPLA2. Although PLA2GIB is a human pancreatic sPLA2 that may account for increased sPLA2 activity in ALI/ARDS cases associated with pancreatitis, it does not likely account for the majority of increased sPLA2 activity in either the bronchoalveolar lavage fluid or serum of the majority of ALI/ARDS cases not associated with pancreatitis (35). PLA2G1B was cloned from lung tissue (46) and was reported to increase in both stimulated Type II cells maintained in tissue culture (3) and in murine epithelial cells in response to Pseudomonas aeruginosa (1). However, we and others have demonstrated that PLA2G2A is increased in both serum and lavage fluid in ALI/ARDS and also strongly correlates with PG hydrolysis activity in the bronchoalveolar lavage fluid (see Ref. 44). The present studies demonstrated that SP-B inhibited PLA2G2A as well as PLA2G1B in the pure PL vesicle model at concentrations with equal potency to hydrolyze PG. Inhibition of hydrolysis by SP-B appears to apply to sPLA2 other than PLA2G1B.
Naturally, these results raise questions regarding the potential mechanisms by which SP-B limits sPLA2-mediated hydrolysis of surfactant PLs. The answers to those questions are potentially multiple and may include interactions between all three reaction components: the sPLA2, the PL substrate, and SP-B. After consideration of these potential interactions, we speculate that the two most probable mechanisms are a direct effect of SP-B on the sPLA2 and the effect of SP-B on the physical structure of the lipid making it a less suitable substrate. Although the results reported do not provide evidence to support final conclusions, we believe they provide important preliminary insights toward addressing these questions.
The first mechanism, a direct interaction of SP-B with sPLA2 that blocks activity, such as seen with the interaction of water-soluble SP-A and a different class of sPLA2 (2, 6), appears remote. This conclusion is based on our finding that monomolecular films can be hydrolyzed in the presence of SP-B at a rate comparable to that seen in the absence of SP-B (Fig. 6). The effect of SP-B in this case is to shift hydrolysis to a lower surface pressure.
The second possible mechanism is supported by a number of studies on the influence of SP-B on the physical state of the lipid substrate. Intact SP-B has been shown to cause fusion of PL vesicles producing a heterogeneous population of larger particles. We used dynamic light scattering to determine the effect of SP-B (1% of mass) on PL vesicles as used in the experiments in Figs. 2 and 7. Addition of SP-B caused changes in substrate vesicle diameter, which averaged 140 nm for PL alone (range of 40–500 nm), increasing to a range of 400–1,300 nm for PL plus SP-B (not shown). This increase in size and heterogeneity is consistent with published results (9). This action of SP-B is thought to be part of its physiological function in the formation and maintenance of surfactant. Another action of SP-B is to cause a shift in the PL organization within the vesicle. Using the NH3-terminal fragment, 1–25, of SP-B, Farver et al. (9) showed that DPPC/POPG vesicles were composed of both liquid crystal structures and an isotropic component, usually associated with micelle, as determined by nuclear magnetic resonance spectroscopy. On the basis of the size of these particles, however, a micellar structure was ruled out. Similar features were also reported for a COOH-terminal fragment of SP-B. On the basis of the shift in size seen by us and by Farver et al. (9), it is reasonable to assume that the substrate particles used in this study are composed of a mixture of phases. The shift in the relative amounts of these phases produced by SP-B may lead to the inhibition we report here.
The effect of SP-B on hydrolysis of monolayer films is somewhat more complex because we observed a shift in the pressure dependence rather than an inhibition. In part this can be explained by the study of Pattus et al. (39). Using a radiolabeled pancreatic PLA2, they found that the pressure-dependent association/disassociation of the enzyme from the film controlled activity. From that, we suggest that SP-B is producing a change in the structure of the film that controls PLA2 binding to the film. This could be mediated through phase changes in film structure such as that noted by Takamoto et al. (48). Monomolecular films made of PC and PG have multiple phases that include a gas phase at the lowest pressures, liquid expanded and liquid condensed phases at moderate pressures, and a solid condensed film at high pressures. They demonstrated that SP-B (1–25) partitioned into the PG-rich liquid-expanded phase that remained attached to the film rather than being squeezed out. Because sPLA2 preferentially hydrolyzes PG, this associated PG-rich region could be a target for the PLA2 at low pressures occurring after the reexpansion of the film, as done in our experiments. This speculation demonstrates the complexity of determining the effect of SP-B on hydrolysis of monomolecular films. However, our results thus far point the way for future work on establishing the action of PLA2s on PLs in different physical states that are regulated by SP-B in the lung.
That oxidation of SP-B produced a stimulatory effect on hydrolysis was unexpected. While not pursued in depth, light scattering data of the native vs. oxidized forms of SP-B show that the oxidized form contained the largest and most complex populations of vesicles (three populations with means of 165 nm, 1,952 nm, and >5,000 nm diameters). Thus addition of SP-B or oxidized SP-B caused different effects on the particle sizes and most likely the physical organization of the components. We believe that this indirect evidence further supports our present interpretation that the inhibitory effect of SP-B is mediated through its interaction with the PL.
In summary, our studies utilizing a variety of SPs consistently demonstrate that SP-B, an essential hydrophobic SP, provides protection from sPLA2-mediated hydrolysis. This protection appears to be important for both major PL subclasses in pulmonary surfactant (PC and PG) and for surfactant PLs in both vesicle and monolayer forms, which are present, respectively, in the alveolar lining fluid and at the alveolar air-liquid interface. Protection by SP-B appears to be as important, if not more important, than the protection provided by the most abundant hydrophilic SP, SP-A. Further studies are needed to fully define the mechanism of SP-B inhibition, synergy between SP-B and other SPs, and the spectrum of SP-B protection against multiple PLA2 subclasses.
GRANTS
This work was supported by funds from NIH RO1 HL085248 (R. Hite) and the Canadian Institutes of Health Research (R. Veldhuizen).
DISCLOSURES
Dr. Hite serves as a principal investigator for the Wake Forest Clinical Center of the NIH-sponsored ARDS Network and as a consultant (DSMB Chair) on clinical trials sponsored by Cumberland Pharmaceuticals (Nashville, TN) and the FDA/Emory University (Atlanta, GA). Dr. Hite holds shares (∼150, current worth less than $300) of Discovery Laboratories (Doylestown, PA), which were received as payment for previously completed work as a consultant with that company. All other authors have no conflicts of interest, financial or otherwise, to declare.
ACKNOWLEDGMENTS
We are grateful to J. Figueroa and J. Rose, Dept. of Obstetrics and Gynecology at WFUHS for the donation of sheep surfactant and Dr. Robert Dillard, Dept. of Pediatrics/Neonatology at WFUHS for providing Survanta samples. We are also thankful to Roy Hantgan, Dept. of Biochemistry at WFUHS for assistance with dynamic light scatter measurements.
REFERENCES
- 1. Agassandian M, Miakotina OL, Andrews M, Mathur SN, Mallampalli RK. Pseudomonas aeruginosa and sPLA2 IB stimulate ABCA1-mediated phospholipid efflux via ERK-activation of PPARalpha-RXR. Biochem J 403: 409– 420, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arbibe L, Koumanov K, Vial D, Rougeot C, Faure G, Havet N, Longacre S, Vargaftig BB, Bereziat G, Voelker DR, Wolf C, Touqui L. Generation of lyso-phospholipids from surfactant in acute lung injury is mediated by type-II phospholipase A2 and inhibited by a direct surfactant protein A-phospholipase A2 protein interaction. J Clin Invest 102: 1152– 1160, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, Matthay MA. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol 299: L36– L50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911– 917, 1959 [DOI] [PubMed] [Google Scholar]
- 5. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50 Suppl: S237– S242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chabot S, Koumanov K, Lambeau G, Gelb MH, Balloy V, Chignard M, Whitsett JA, Touqui L. Inhibitory effects of surfactant protein A on surfactant phospholipid hydrolysis by secreted phospholipases A2. J Immunol 171: 995– 1000, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Chang R, Nir S, Poulain FR. Analysis of binding and membrane destabilization of phospholipid membranes by surfactant apoprotein B. Biochim Biophys Acta 1371: 254– 264, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA. Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA 92: 7794– 7798, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farver RS, Mills FD, Antharam VC, Chebukati JN, Fanucci GE, Long JR. Lipid polymorphism induced by surfactant peptide SP-B(1–25). Biophys J 99: 1773– 1782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fine JB, Sprecher H. Unidimensional thin layer chromatography of phospholipids on boric acid-impregnated plates. J Lipid Res 23: 660– 663, 1982 [PubMed] [Google Scholar]
- 11. Floros J, Thomas NJ, Liu W, Papagaroufalis C, Xanthou M, Pereira S, Fan R, Guo X, Diangelo S, Pavlovic J. Family-based association tests suggest linkage between surfactant protein B (SP-B) (and flanking region) and respiratory distress syndrome (RDS): SP-B haplotypes and alleles from SP-B-linked loci are risk factors for RDS. Pediatr Res 59: 616– 621, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Gong MN, Wei Z, Xu LL, Miller DP, Thompson BT, Christiani DC. Polymorphism in the surfactant protein-B gene, gender, and the risk of direct pulmonary injury and ARDS. Chest 125: 203– 211, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Granata F, Nardicchi V, Loffredo S, Frattini A, Ilaria SR, Agostini C, Triggiani M. Secreted phospholipases A(2): A proinflammatory connection between macrophages and mast cells in the human lung. Immunobiology 214: 811– 821, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, Nagae H, Hudson LD, Martin TR. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 160: 1843– 1850, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Griese M. Pulmonary surfactant in health and human lung diseases: state of the art. Eur Respir J 13: 1455– 1476, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Haagsman HP. Structural and functional aspects of the collectin SP-A. Immunobiology 205: 476– 489, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ. The major lung surfactant protein, SP 28–36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem 262: 13877– 13880, 1987 [PubMed] [Google Scholar]
- 18. Hallman M. Lung surfactant, respiratory failure, genes. N Engl J Med 350: 1278– 1280, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Hallman M, Haataja R. Surfactant protein polymorphisms and neonatal lung disease. Semin Perinatol 30: 350– 361, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hamvas A, Nogee LM, Wegner DJ, Depass K, Christodoulou J, Bennetts B, McQuade LR, Gray PH, Deterding RR, Carroll TR, Kammesheidt A, Kasch LM, Kulkarni S, Cole FS. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily a, member 3 genes. J Pediatr 155: 854– 859, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hite RD, Seeds MC, Bowton DL, Grier BL, Safta AM, Balkrishnan R, Waite BM, Bass DA. Surfactant phospholipid changes after antigen challenge: a role for phosphatidylglycerol in dysfunction. Am J Physiol Lung Cell Mol Physiol 288: L610– L617, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Hite RD, Seeds MC, Grier BL, Morris PE, Goodman RB, Schoenfeld DA. The impact of steroids on surfactant in persistent ARDS (ARDSnet LaSRS Trial). Proc Am Thorac Soc 3: A641, 2006 [Google Scholar]
- 23. Hite RD, Seeds MC, Safta AM, Jacinto RB, Gyves JI, Bass DA, Waite BM. Lysophospholipid generation and phosphatidylglycerol depletion in phospholipase A2-mediated surfactant dysfunction. Am J Physiol Lung Cell Mol Physiol 288: L618– L624, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Inchley K, Cockshutt A, Veldhuizen R, Possmayer F. Dissociation of surfactant protein B from canine surfactant large aggregates during formation of small surfactant aggregates by in vitro surface area cycling. Biochim Biophys Acta 1440: 49– 58, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Kim DK, Fukuda T, Thompson BT, Cockrill B, Hales C, Bonventre JV. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 269: L109– L118, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem 77: 495– 520, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Lewis JF, Veldhuizen R. The role of exogenous surfactant in the treatment of acute lung injury. Annu Rev Physiol 65: 613– 642, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Lin Z, Thomas NJ, Wang Y, Guo X, Seifart C, Shakoor H, Floros J. Deletions within a CA-repeat-rich region of intron 4 of the human SP-B gene affect mRNA splicing. Biochem J 389: 403– 412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lowry OH, Rosebrough MJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 193: 265– 275, 1951 [PubMed] [Google Scholar]
- 30. Manzanares D, Rodriguez-Capote K, Liu S, Haines T, Ramos Y, Zhao L, Doherty-Kirby A, Lajoie G, Possmayer F. Modification of tryptophan and methionine residues is implicated in the oxidative inactivation of surfactant protein B. Biochemistry 46: 5604– 5615, 2007 [DOI] [PubMed] [Google Scholar]
- 31. McCormack FX, Whitsett JA. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J Clin Invest 109: 707– 712, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mizuno K, Ikegami M, Chen CM, Ueda T, Jobe AH. Surfactant protein-B supplementation improves in vivo function of a modified natural surfactant. Pediatr Res 37: 271– 276, 1995 [DOI] [PubMed] [Google Scholar]
- 33. Murakami M, Taketomi Y, Sato H, Yamamoto K. Secreted phospholipase A2 revisited. J Biochem 150: 233– 255, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Nag K, Munro JG, Inchley K, Schurch S, Petersen NO, Possmayer F. SP-B refining of pulmonary surfactant phospholipid films. Am J Physiol Lung Cell Mol Physiol 277: L1179– L1189, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Nakos G, Kitsiouli E, Hatzidaki E, Koulouras V, Touqui L, Lekka ME. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit Care Med 33: 772– 779, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Nogee LM, Garnier G, Dietz HC, Singer L, Murphy AM, deMello DE, Colten HR. A mutation in the surfactant protein B gene responsible for fatal neonatal respiratory disease in multiple kindreds. J Clin Invest 93: 1860– 1863, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Notter RH, Wang Z, Egan EA, Holm BA. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids 114: 21– 34, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Panda AK, Nag K, Harbottle RR, Rodriguez-Capote K, Veldhuizen RA, Petersen NO, Possmayer F. Effect of acute lung injury on structure and function of pulmonary surfactant films. Am J Respir Cell Mol Biol 30: 641– 650, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Pattus F, Slotboom AJ, De Haas GH. Regulation of phospholipase A2 activity by the lipid-water interface: a monolayer approach. Biochemistry 18: 2691– 2697, 1979 [DOI] [PubMed] [Google Scholar]
- 40. Possmayer F. The role of surfactant-associated proteins. Am Rev Respir Dis 142: 749– 752, 1990 [DOI] [PubMed] [Google Scholar]
- 41. Qanbar R, Cheng S, Possmayer F, Schurch S. Role of the palmitoylation of surfactant-associated protein C in surfactant film formation and stability. Am J Physiol Lung Cell Mol Physiol 271: L572– L580, 1996 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez-Capote K, Manzanares D, Haines T, Possmayer F. Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys J 90: 2808– 2821, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmidt R, Markart P, Ruppert C, Wygrecka M, Kuchenbuch T, Walmrath D, Seeger W, Guenther A. Time-dependent changes in pulmonary surfactant function and composition in acute respiratory distress syndrome due to pneumonia or aspiration. Respir Res 8: 55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seeds MC, Grier BL, Suckling BN, Safta AM, Long DL, Waite BM, Morris PE, Hite RD. Secretory phospholipase A2 mediated depletion of phosphatidylglycerol in early Acute Respiratory Distress Syndrome. Am J Med Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seeds MC, Jones KA, Duncan HR, Willingham MC, Borgerink HM, Woodruff RD, Bowton DL, Bass DA. Cell-specific expression of group X and group V secretory phospholipases A(2) in human lung airway epithelial cells. Am J Respir Cell Mol Biol 23: 37– 44, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Seilhamer JJ, Randall TL, Yamanaka M, Johnson LK. Pancreatic phospholipase A2: Isolation of the human gene and cDNAs from porcine pancreas and human lung. DNA (NY) 5: 519– 527, 1986 [DOI] [PubMed] [Google Scholar]
- 47. Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J Biol Chem 277: 48535– 48549, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Takamoto DY, Lipp MM, von Nahmen A, Lee KY, Waring AJ, Zasadzinski JA. Interaction of lung surfactant proteins with anionic phospholipids. Biophys J 81: 153– 169, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van Iwaarden F, Welmers B, Verhoef J, Haagsman HP, van Golde LMG. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol 2: 91– 98, 1990 [DOI] [PubMed] [Google Scholar]
- 50. van Iwaarden JF, Van Strijp JAG, Visser H, Haagsman HP, Verhoef J, van Golde LMG. Binding of surfactant protein A (SP-A) to herpes simplex virus type 1-infected cells is mediated by the carbohydrate moiety of SP-A. J Biol Chem 267: 25039– 25043, 1992 [PubMed] [Google Scholar]
- 51. Waring AJ, Walther FJ, Gordon LM, Hernandez-Juviel JM, Hong T, Sherman MA, Alonso C, Alig T, Braun A, Bacon D, Zasadzinski JA. The role of charged amphipathic helices in the structure and function of surfactant protein B. J Pept Res 66: 364– 374, 2005 [DOI] [PubMed] [Google Scholar]
- 52. Yu SH, Possmayer F. Comparative studies on the biophysical activities of the low-molecular-weight hydrophobic proteins purified from bovine pulmonary surfactant. Biochim Biophys Acta 961: 337– 350, 1988 [DOI] [PubMed] [Google Scholar]
- 53. Yu SH, Wallace D, Bhavnani B, Enhorning G, Harding PG, Possmayer F. Effect of reconstituted pulmonary surfactant containing the 6000-Dalton hydrophobic protein on lung compliance of prematurely delivered rabbit fetuses. Pediatr Res 23: 23– 30, 1988 [DOI] [PubMed] [Google Scholar]
- 54. Zuo YY, Veldhuizen RA, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant—inhibition, enhancement and evaluation. Biochim Biophys Acta 1778: 1947– 1977, 2008 [DOI] [PubMed] [Google Scholar]