Abstract
Carcinoembryonic cell adhesion molecule 6 (CEACAM6) is a glycosylated, glycophosphatidylinositol-anchored protein expressed in epithelial cells of various primate tissues. It binds gram-negative bacteria and is overexpressed in human cancers. CEACAM6 is associated with lamellar bodies of cultured type II cells of human fetal lung and protects surfactant function in vitro. In this study, we characterized CEACAM6 expression in vivo in human lung. CEACAM6 was present in lung lavage of premature infants at birth and increased progressively in intubated infants with lung disease. Of surfactant-associated CEACAM6, ∼80% was the fully glycosylated, 90-kDa form that contains the glycophosphatidylinositol anchor, and the concentration (3.9% of phospholipid for adult lung) was comparable to that for surfactant proteins (SP)-A/B/C. We examined the affinity of CEACAM6 by purification of surfactant on density gradient centrifugation; concentrations of CEACAM6 and SP-B per phospholipid were unchanged, whereas levels of total protein and SP-A decreased by 60%. CEACAM6 mRNA content decreased progressively from upper trachea to peripheral fetal lung, whereas protein levels were similar in all regions of adult lung, suggesting proximal-to-distal developmental expression in lung epithelium. In adult lung, most type I cells and ∼50% of type II cells were immunopositive. We conclude that CEACAM6 is expressed by alveolar and airway epithelial cells of human lung and is secreted into lung-lining fluid, where fully glycosylated protein binds to surfactant. Production appears to be upregulated during neonatal lung disease, perhaps related to roles of CEACAM6 in surfactant function, cell proliferation, and innate immune defense.
Keywords: alveolar type II cells, lung lining fluid, surfactant, airway epithelium
carcinoembryonic cell adhesion molecule 6 (CEACAM6) (also called NCA, NCA-50/90, and CD66c) is a member of the carcinoembryonic antigen (CEA) gene family, consisting of 29 related genes. CEA proteins function as intercellular homophilic and heterophilic adhesion molecules and have signaling properties (18). CEACAM6 contains a glycophosphatidylinositol (GPI) membrane anchor (23) and is targeted to lipid rafts in apical membranes of polarized epithelial cells (26). CEACAM6 binds a variety of gram-negative bacteria and mediates internalization and phagocytosis, participating in innate immune defense in the intestine (13). The CEACAM6 gene is not present in rodents, and its emergence in primates may represent pathogen-host coevolution, providing different protein structures with selective bacterial binding properties.
Expression of CEACAM6 and closely related CEACAM5 is deregulated and overexpressed in cancers of colorectal epithelium, with surface levels inversely correlated with both the degree of colonocyte differentiation (21) and positive clinical outcome (22). The two CEACAMs are also expressed in a high proportion of tumor cell lines derived from breast, ovary, pancreas, prostate, and lung (11). It has been proposed that CEACAM5/6 overproduction has a causative role in tumorgenesis, acting via an imbalance of cell surface adhesion molecules that disrupts differentiation, inhibits apoptosis, and promotes both tumor formation and metastases (21, 28). Overexpression of CEACAM6 also occurs in Crohn's disease, where it promotes uptake and colonization of small intestine epithelium by adherent, invasive E. coli (6).
Two earlier studies identified CEACAM6 immunoreactivity in normal adult lung, with localization to both alveolar and airway epithelium (30, 32). In a previous study with human fetal lung, our laboratory identified CEACAM6 as one of the genes upregulated during hormone-induced differentiation of lung type II cells in vitro (33). An important function of mature type II cells is production of pulmonary surfactant, a phospholipid (PL)-protein mixture that is essential for normal respiration by virtue of its ability to reduce surface tension and prevent collapse of air spaces. We also found that CEACAM6 is a target protein of thyroid transcription factor-1 (product of Nkx2.1), which is required for differentiation of type II cells and expression of surfactant-associated proteins (SP) (25). In additional recent studies with cultured fetal type II cells, we presented evidence that CEACAM6 is associated with surfactant-containing lamellar bodies, is found in lung fluid of infants, and protects surfactant from inhibition by extraneous proteins in vitro (24). Based on these observations, we hypothesized that CEACAM6 in human lung is a SP.
In this study we describe the ontogeny of CEACAM6 in lung fluid and expression in epithelial cells from premature infants and adults. We determined the content of different isoforms and distribution of the protein between surfactant and supernatant fractions of lung fluid. Our findings indicate that 90-kDa CEACAM6 is tightly associated with surfactant at a concentration comparable to that for SPs-A/B/C.
MATERIALS AND METHODS
Patient population.
We studied repository samples of tracheal aspirate fluid (TAF) from three cohorts of premature ventilated infants. In one cohort, serial aspirate samples (n = 42) were taken from eight inborn infants <30-wk gestation with bronchopulmonary dysplasia who were intubated from birth through 8–14 wk. The other cohorts consisted of infants who were 24–31 wk gestation and <1,250 g birth weight. These infants were intubated at birth for stabilization and administration of replacement surfactant to ameliorate respiratory distress syndrome. The day 1 (d-1) group of 8 infants had TAFs collected at 9–24 h of life, and the other cohort of 29 infants with developing bronchopulmonary dysplasia had samples taken at 14 days (d-14). An additional 21 TAF samples were obtained from 10 infants of <2 mo of age who were intubated for conditions other than acute respiratory failure; these included congenital anomalies (congenital cystic adenomatoid malformation, congenital diaphragmatic hernia, tracheoesophageal fistula, Pierre Robin syndrome, gastroscisis, and jejunal atresia) and other conditions (persistent pulmonary hypertension of newborn, respiratory syncytial virus pneumonia, and seizures). TAFs were obtained under an Internal Review Board-approved protocol. Pathological specimens of human fetal lung from abortuses of 12- to 22-wk gestation were obtained from Advanced Bioscience Resources (Alameda, CA) and/or the Birth Defects Laboratory of the University of Washington (Seattle, WA). Adult bronchoalveolar lavage (n = 7) and tissue (n = 6) were obtained from donated adult human lungs, which were not used for transplantation, from the Northern California Transplant Donor Network.
Cell isolation.
Epithelial cells from trachea, main stem bronchus, and proximal airways of adult lung were obtained by careful scraping of the inner lumen with a cell scraper and rinsing the cells into a centrifuge tube with phosphate-buffered saline (PBS), pH 7.4. The cells were spun at 500 g for 5 min, the supernatant removed, and protease inhibitors were applied to the cell pellet before freezing at −80°C until use. Type II cells were isolated from donor lungs at 93% purity, as described (2). In some experiments, epithelial cells of adult peripheral lung were isolated by elastase treatment and FACS sorted, as previously described (17). Cells were initially treated with human IgG (50 μg/ml; Sigma) to block nonspecific binding of mouse monoclonal primary antibodies against the type II cell-specific HTII-280 protein. Subsequently, cells were exposed to tissue culture supernatant containing HTII-280 antibody (1:20 dilution) for 5 min and centrifuged at 300 g for 12 min. Alexa 488 anti-mouse IgM (Invitrogen, Carlsbad, CA) and anti-HTI-56 (14) (directly labeled with Alexa 610-RPE using Zenon technology, Invitrogen) were then added, and the cells were sorted on a Aria FACS instrument (BD Biosciences, San Jose, CA). FACS-sorted cells were deposited on glass slides using Cytospin (Shandon, Astmoor, UK), and both cell preparations were >99% pure by immunostaining for HTI-56 and HTII-280.
Processing of TAF samples.
After centrifugation at 500 g for 5 min to remove cells, TAF supernatant was removed, stored at −80°C in the presence of protease inhibitors, and subsequently centrifuged at 27,000 g for 60 min to isolate large-aggregate surfactant pellet and supernatant fractions. Freezing of lavage fluid, which occurred for all TAF samples, may affect separation of large-aggregate surfactant from other forms; this could not be examined in the repository specimens. Large-aggregate pellet was resuspended in surfactant buffer (10 mM Tris, 154 mM NaCl, 1.5 mM CaCl2, pH 7.4), and an aliquot was extracted (10) and assayed for PL content (7). Total protein was measured on both fractions using QuantiPro BCA assay system (Sigma, St. Louis, MO).
CEACAM6 immunodot assay.
CEACAM6 was quantified using a modification of an immunodot assay previously developed for measurement of SP-B (4). Recombinant CEACAM6 (rCEACAM6, R & D Systems, Minneapolis, MN) was used as standard for quantitative assay of CEACAM6 levels in samples. rCEACAM6 was diluted to 1 ng/μl in 125 mM Tris·HCl, 100 mM DTT, 0.1% MEGA-8 (Calbiochem, La Jolla, CA), pH 6.8, and heated at 95°C for 10 min; using a V-bottom 96-well plate, 30 ng of the treated standard in a total volume of 500 μl were serially diluted by 25% (375 μl into 125-μl PBS eight times). rCEACAM6 must be denatured in order for it to be immunoreactive using the anti-human CEACAM6 antibody mentioned below. TAF supernatant and pellet samples (6.65 and 1.33 μg total protein, respectively) were added to PBS in a total volume of 500 μl and serially diluted as for the standard. One hundred microliters of each dilution were applied to 0.2-μm nitrocellulose membrane (Whatman, Dassel, Germany) in a 96-well, dot-blot manifold under negative pressure. All blots were run with two internal control samples of known CEACM6 concentration to evaluate reproducibility of the assay. After being blocked with 5% nonfat milk solution for 60 min, the blot was incubated with mouse anti-human CEACAM6, clone 9A6 (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:1,000 dilution. After washing, the blot was incubated with secondary antibody conjugated to horseradish peroxidase, washed again, and developed with ECL Plus (GE Healthcare, Piscataway, NJ), according to manufacturer's instructions. Blots were exposed to X-ray film and then imaged on a Storm 840 phosphoimager equipped with a blue fluorescence/chemifluorescence detector and quantified using Imagequant software (GE Healthcare, Piscataway, NJ). Values for each sample that fell on the linear portion of the standard curve were averaged and expressed as percentage of protein (supernatant and pellet fractions) and/or as percent of PL (pellet). The assay has a fivefold linear range with an intra- and interassay variability of 5 and 7%, respectively. Adult tracheal, bronchial, and proximal airways cells and peripheral lung tissue were assayed in a similar fashion. Before development of the immunodot assay, the specificity of the antibody was confirmed by Western blot. SP-B was assayed in a immunodot assay using a polyclonal anti-SP-B antibody (Millipore, Billerica, MA), as previously reported (4).
Western blot.
Analysis of CEACAM6 and SP-A was performed by standard methods. Samples were denatured in Laemmli buffer containing 100 mM DTT and run on 4–15% Tris·HCl gradient gel. The proteins were then transferred to 0.2-μm nitrocellulose. The membrane was washed and processed for CEACAM6 detection, as above. In some instances, SP-A was simultaneously detected with CEACAM6 by Western blot. In that case, blots were incubated with both mouse anti-human CEACAM6 and rabbit anti-sheep SP-A antibody (1:5,000, a gift of Sam Hawgood), followed by simultaneous incubation with anti-mouse and anti-rabbit horseradish peroxidase antibodies. In some cases, blots were scanned, and optical densities of the bands were quantified using Image Pro Plus (MediaCybernetics, Bethesda, MD). Alternatively, infrared-detectable secondary antibody (goat anti-mouse IgG Alexa-680 tagged, Invitrogen, Carlsbad, CA) was used, and the Odyssey Imaging system (Licor, Lincoln, NE) was used for detection and quantification.
RT-PCR.
Total RNA was prepared from lung cells and bronchial samples with RNA STAT-60 (Tel-Test, Friendswood, TX) and treated with RQ1 RNase-free DNase. Integrity and purity were determined with an Agilent 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA). Real-time PCR reactions were performed in the Real-Time PCR Facility of the Children's Hospital of Philadelphia Research Institute, as previously described (25). CEACAM6, epithelial cell adhesion molecule, SCGB1A1 and 18S primers and probes were purchased from Applied Biosystems (Carlsbad, CA). The CEACAM6 (Hs00366002_m1) probe sequence was CAGGAAGACTGGCAGATTGGACCAG, which spans exons 5 and 6. Semiquantitative RT-PCR was performed on RNA that was purified from FACS-sorted type I and type II cell preparations, as well as tissue from the same adult lung specimen, using Qiagen RNeasy isolation kit with DNase digestion (Valencia, CA). RNA was reverse transcribed using Ambion RETROscript kits (Applied Biosystems/Ambion, Austin, TX). PCR and agarose gel electrophoresis was performed on 70 ng of cDNA from each sample, plus a no cDNA control (21, 25, 29 cycles) using an annealing temperature of 55°C in a PT-200 Peltier Thermal Cycler (MJ Research, Waltham, MA). Primer sequences used are specific for human CEACAM6 (12) with an expected product of 353 bp: 5′ primer = 5′-TACTCAGCGTCAAAAGGAAC-3′ and 3′ primer = 5′-AGAGACTGTGATCATCGTGA-3′.
Immunofluorescent staining.
Fetal tracheal and bronchial tissues were fixed overnight in 4% paraformaldehyde and then transferred to 4% paraformaldehyde + 30% sucrose solution for cryopreservation overnight at 4°C. The tissue was then sliced into small pieces and embedded in O.C.T. Compound (Tissue-Tek, Torrance, CA) and frozen at −80°C until use. Five-micrometer cryosections were cut using a Microm HM560 cryostat (Walldorf). Sections were permeabilized and stained as previously described (16, 24). All antibodies were applied successively. Briefly, primary antibodies were anti-human CEACAM6 (1:100) and anti-pan cytokeratin (1:100, H-240, Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies, tagged with Alexa 488 or Alexa 546, were used at 1:400 (Invitrogen, Camarillo, CA). Postmortem lung samples in paraffin sections were treated by antigen retrieval (no. H-3300, Vector Laboratories, Burlingame, CA), permeabilized with 0.3% Triton X-100, and immunostained with anti-human CEACAM6 (1:100), secondary antibody goat anti-mouse-Alexa 546 tagged, followed by rabbit anti-sheep SP-B (1:100, Chemicon, Temecula, CA), and then secondary goat anti-rabbit-Alexa 488 tagged (1:400). Sections were imaged using an Olympus IX81 microscope equipped with epifluorescence and Metamorph imaging system (Universal Imaging, West Chester, PA). Cytospins of freshly isolated adult type II cells were fixed in 4% paraformaldehyde over night, washed, and stored at −20°C until use. Cytospins were permeabilized and stained for CEACAM6 (1:100) and HTII 280, a type II cell-specific marker in human lung (1:200) (17). Adult trachea sections were treated in a similar fashion and stained with CEACAM6 and rabbit anti-pan-cytokeratin (1:100, Invitrogen). Secondary antibodies used for detection were anti-mouse IgG Alexa 488 (1:1,000) and anti-mouse IgM Alexa 594-tagged (1:500) antibodies for cytospins. Anti-mouse Alexa 594 (1:500) and anti-rabbit Alexa 488 (1:100) were used for adult trachea sections. Slides were mounted in Prolong containing 4',6-diamidino-2-phenylindole (Invitrogen), and images were obtained using a Leitz Orthoplan 2 microscope (Leica Microsystems, Bannockburn, IL). Each fluorescent image was captured in a separate channel using a Leica DC500 digital camera.
Buoyant density centrifugation.
To estimate the affinity of CEACAM6 for PL, three separate pooled samples of d-14 surfactant pellets (320–400 μg of PL) were centrifuged up through a KBr gradient using a modification of a previously described method (8). Before centrifugation, an aliquot of the pooled surfactant pellets was removed for assay. The remaining sample was placed at the bottom of a Beckman Ultra-Clear Centrifuge tube (no. 344057), and solid KBr was added to equal 16% by volume. The mixture was then overlaid with 2.3 ml of 13% KBr solution, followed by another overlay of 1.5 ml 0.9% NaCl. The samples were centrifuged at 116,000 g for 100 min at 4°C in an Optima LE-80K ultracentrifuge using a SW50.1 rotor (Beckman Coulter, Brea, CA). The lipid layer was then removed from the saline and KBr interface with a 25G needle and syringe, washed with 0.9% NaCl to remove residual KBr, and repelleted at 27,000 g for 60 min at 4°C. The postcentrifugation samples were resuspended in surfactant buffer and assayed for protein and PL content as described above. Samples were diluted to 1.5 μg PL/μl, and equal amounts of PL from pre- and postcentrifugation samples were loaded on a single PAGE gel. The Western blot was probed for CEACAM6 and SP-A, and band intensities were quantitated by densitometry. SP-B was measured in the samples by immunodot assay. The data were initially expressed as content of each protein per PL, and then the postcentrifugation value was expressed as percentage of the precentrifugation value.
RESULTS
In our laboratory's previous publication (24), we reported the presence of CEACAM6 in TAF from intubated premature infants. To further characterize this finding, we now describe CEACAM6 content in lung epithelium and lavage fluid at different postnatal ages, the content of different isoforms, influence of lung injury and infection, and studies of CEACAM6 affinity for surfactant. The studies of CEACAM6 in infants use repository samples of TAF from inborn infants <32 wk gestation, born between 1996 and 2004, with serial collection of TAF samples from birth while intubated. At the time, infants of <1,250-g birth weight were routinely intubated at birth for stabilization and administration of replacement surfactant. The d-1 group of 8 infants had TAF collected at 9–24 h of age and were 26.9 ± 2.1 wk (mean ± SD) gestation, and the 29 intubated infants with samples at d-14 were 25.5 ± 1.4 wk (P < 0.05) gestation; the lower mean gestational age at d-14 reflects the greater occurrence of chronic lung disease requiring continued intubation in younger infants. Within both groups, there was no significant correlation between TAF CEACAM6 content and gestational age. Most infants of this gestational age who are intubated at d-14 will have a diagnosis of bronchopulmonary dysplasia at 36 wk postmenstrual age (5).
Content and surfactant association.
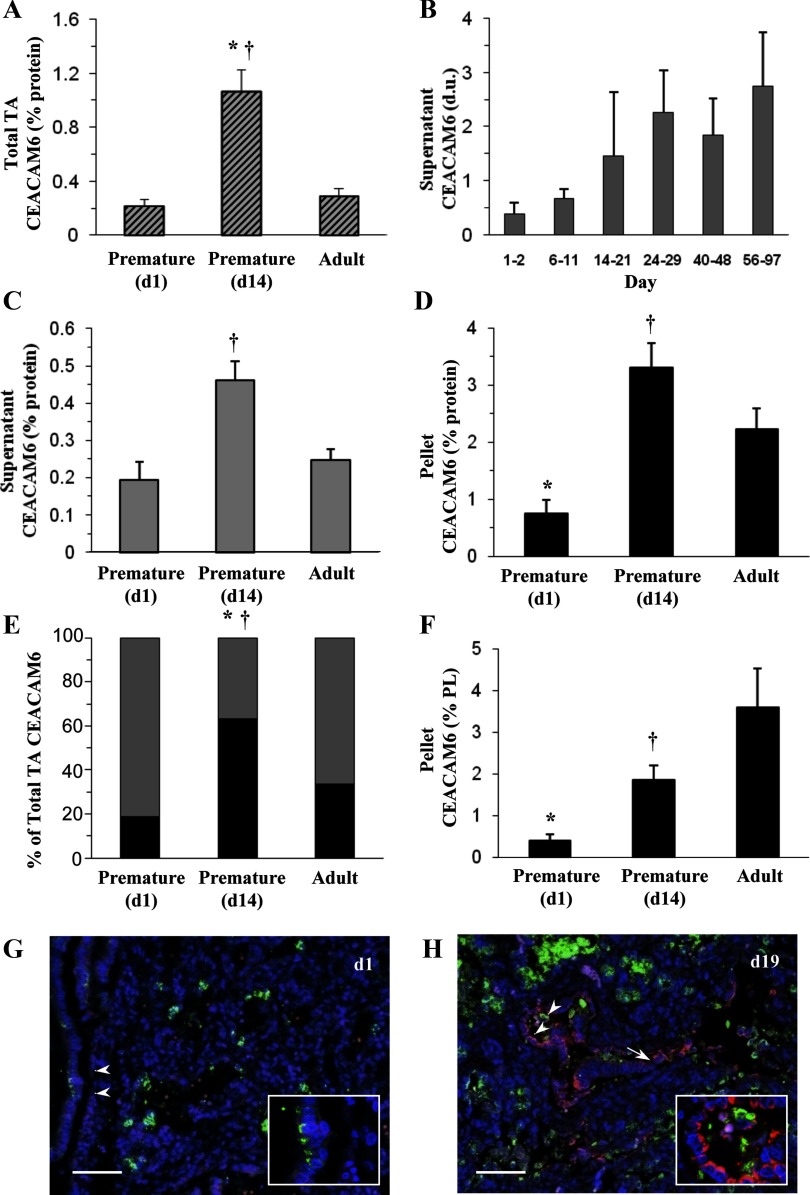
Total CEACAM6 content was determined by immunodot and Western assays of infant TAF and lavage fluid of adult donor lungs obtained postmortem. Expressed per milligrams total protein, CEACAM6 content in TAF of premature infants (Fig. 1A) was ∼0.2% on d-1 and was approximately fivefold greater in the d-14 cohort, exceeding the level in adult (0.29%). The relative levels of CEACAM6 in premature vs. adult lungs, expressed per total protein, are influenced by a higher protein/PL content in adult fluid (data not shown), which likely results from mechanical ventilation and/or the delay in performing the lavage procedure on donor lungs. The postnatal pattern in CEACAM6 was confirmed in eight other intubated premature infants with repeated determinations over 14 wk. There was a progressive increase of approximately sevenfold in CEACAM6 content over this interval (Fig. 1B); by linear regression analysis (not shown) r = 0.58 and P < 0.0001 for 42 samples. CEACAM6 was detected in both TAF supernatant and the large-aggregate surfactant fraction. The relative levels of CEACAM6/total protein in the supernatant (Fig. 1C) and surfactant (Fig. 1D) fractions for the three groups of samples was similar to that for total TAF, with highest levels observed in the d-14 cohort. Figure 1E shows the distribution of CEACAM6 between supernatant and surfactant pellet in the sample sets. Surfactant-associated CEACAM6 represented 60% of the total CEACAM6 for d-14 infants compared with 20 and 33% for d-1 and adult, respectively (P < 0.004). The different supernatant-to-pellet distribution for d-14 may reflect a combination of low surfactant content in newborn premature infants and relative amounts of other proteins that compete for lipid binding.
Fig. 1.
Content of carcinoembryonic cell adhesion molecule 6 (CEACAM6) in lung epithelium lining fluid. A: total immunoreactive CEACAM6 by immunodot assay in fluid of infants and adults expressed as percentage of total protein. There is a significant increase in CEACAM6 concentration between day 1 (d1; n = 8) and day 14 (d14; n = 29) after birth to a level exceeding that of the adult (n = 6). B: postnatal increase in supernatant CEACAM6 in intubated premature infants over ∼12 wk with repeated sampling and Western analysis. Densitometric data [density units (d.u.)] are shown for 42 tracheal aspirate fluid (TAF) samples from 8 infants. Linear regression of all data (not shown) is as follows: r = 0.58 and P < 0.0001. C: CEACAM6 concentration per total protein in TAF supernatant after removal of large-aggregate surfactant by centrifugation. D: CEACAM6 concentration in large-aggregate surfactant pellet. The relative levels of CEACAM6/total protein in the supernatant (C) and the pellet (D) fractions for the 3 groups is similar to that for total TAF (A), with highest levels observed at d14. E: relative distribution of CEACAM6 between supernatant and surfactant fractions for the 3 age groups; a relatively greater distribution to the surfactant pellet was observed at d14. Solid bars, surfactant pellet; shaded bars, supernatant. F: CEACAM6 content in surfactant expressed as percentage of phospholipid (PL); concentration increases from 0.5% in d-1 infants to 3.9% in adult. Values are means ± SE. G and H: immunostaining for CEACAM6 in uninflated paraffin sections of postmortem lung. *P < 0.05 vs. adult. †P < 0.05 vs. d1. G: d1 premature infant. Note low level of surfactant protein (SP)-B staining (green) and very low signal for CEACAM6 (red); 4',6-diamidino-2-phenylindole (DAPI) staining (blue) identifies nuclei. Inset shows higher power view of intracellular SP-B staining. H: d19 premature infant with chronic lung disease. The section contains a terminal respiratory unit (running horizontally) showing apparent epithelial cell hyperplasia, as represented in the alveolus (arrowheads) and in the corresponding higher power inset. SP-B (green) is present as punctate intracellular and aggregated extracellular staining. Apical CEACAM6 staining (red) is observed in some cells of both alveolus and terminal airway (arrow), which do not express SP-B. Images are representative of 4 premature infants at each age and illustrate increased CEACAM6 signal in the older infants, who all had evolving chronic lung disease, consistent with findings in TAF. Bar = 70 μm. TA, tracheal aspirate; PL, phospholipid.
Figure 1F shows the concentration of CEACAM6 in the surfactant pellet expressed as percentage of PL. For premature infants, CEACAM6 was 0.5% on d-1 and 2.2% on d-14 (P < 0.05) compared with an adult level of 3.9%. These values for CEACAM6 are in the range reported for SP-A, SP-B, and SP-C, known SPs (3). The pellet content of CEACAM6 was not statistically different between d-14 and adult surfactant normalized to both total protein (Fig. 1D) and PL (Fig. 1F); the trend toward higher CEACAM6 per PL in adult lung fluid reflects the higher total protein-to-PL ratio in adult fluid. Because CEACAM6 content of isolated surfactant may be influenced by the amount of extracellular surfactant, we calculated surfactant recovery in TAF by normalizing to total protein in the sample. For d-1 and d-14 samples, PL recovery in the pellet was similar (43.4 ± 33.6% and 32.1 ± 35.2%, respectively, nonsignificant). Thus the increase in surfactant-associated CEACAM6 during the first 2 postnatal wk is independent of total recovered surfactant as measured, consistent with increased production and/or secretion of CEACAM6.
A limited number of TAF samples were available from intubated infants <2 mo of age with diagnoses other than chronic lung disease. By Western analysis and densitometry, higher signals (3.4-fold; P < 0.01) were observed for two different TAF samples from two infants with respiratory syncytial virus infection compared with eight infants with noninfectious abnormalities. Based on these preliminary observations, we propose that infectious events in the lung increase production of CEACAM6, perhaps representing a component of the innate immune response to neutralize microorganisms as has been described in intestine (13).
To further examine the postnatal increase in TAF CEACAM6, we performed immunostaining of lung tissue of premature infants. CEACAM6/CEA immunoreactivity has been reported for alveolar epithelium of adult human lung (30, 32), and previously we found that fetal human type II cells in monolayer culture expressed CEACAM6 (24). Figure 1, G and H, illustrates CEACAM6 expression in representative postmortem lung sections of premature newborns at 1 day of age and in the third postnatal week for infants with lung disease, respectively. Some airway and alveolar cells have a weak CEACAM6 signal (red) in d-1 tissue, and there is relatively low expression of SP-B (green), as expected with prematurity. By contrast, more cells show a stronger CEACAM6 signal in the d-19 tissue. Of interest, most hyperplastic alveolar cells of injured d-19 lungs (e.g., Fig. 1H, inset) were immunopositive for CEACAM6, and there was no apparent cellular colocalization with SP-B.
These results establish the presence of CEACAM6 in lung lining fluid shortly after premature birth and increasing concentrations postnatally for intubated infants; 20–60% of extracellular CEACAM6 was associated with surfactant. To further examine CEACAM6 interaction with surfactant, we determined the isoform pattern for the protein.
Surfactant-associated CEACAM6 is primarily 90 kDa.
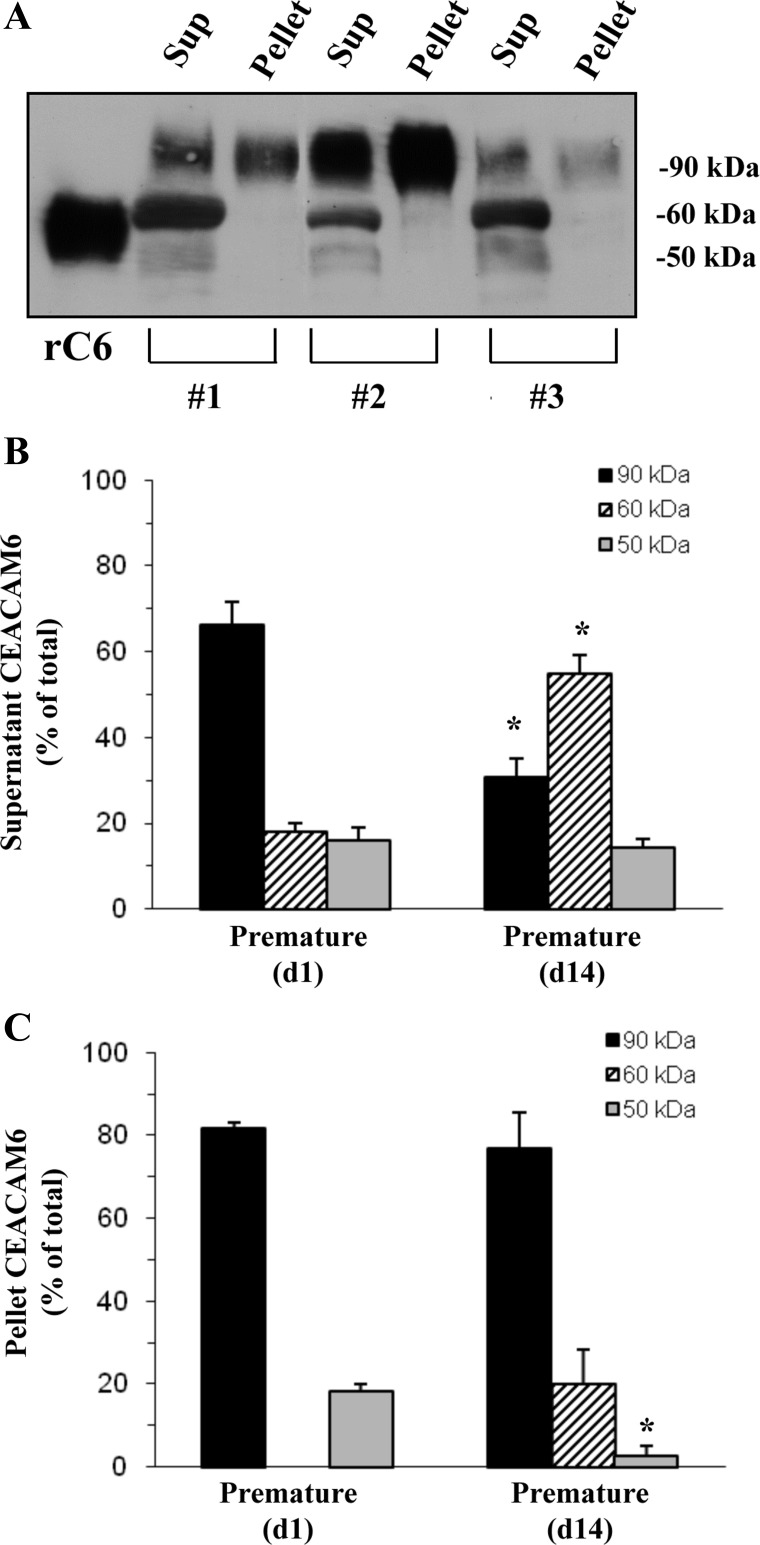
By Western analysis of TAF, we found CEACAM6 immunoreactivity in three bands at 90, 60, and 50 kDa, similar to previous reports for other nonpulmonary tissues (18). The supernatant fraction of TAF contained all three bands, while the surfactant pellet had primarily 90-kDa CEACAM6; rCEACAM6, which was used as a standard, migrated at 55 kDa, presumably reflecting partial glycosylation compared with the in vivo protein (Fig. 2A). The 90-kDa isoform was ∼65% of total CEACAM6 in TAF supernatant on d-1, but represented ∼30% (P = 0.001) at d-14 (Fig. 2B). In the surfactant pellet, the 90-kDa isoform accounted for ∼80% of total immunoreactivity at both postnatal ages (Fig. 2C). In the adult samples, 90-kDa CEACAM6 was ∼70 and 90% of total in supernatant and surfactant pellet fractions, respectively (data not shown), with the remainder of signal appearing at <50 kDa and likely representing degradation products resulting from postmortem proteolysis. We propose that fully glycosylated CEACAM6, which contains the GPI moiety, binds to surfactant with higher affinity than the other, less glycosylated, forms that presumably lack the GPI anchor.
Fig. 2.
Isoforms of CEACAM6 in lung fluid. A: representative Western for three d14 infants. Supernatant (Sup) contains bands at ∼90, 60, and 50 kDa, while surfactant pellets (Pellet) contain primarily 90-kDa CEACAM6. Recombinant CEACAM6 (rC6) is not fully glycosylated and runs at ∼55 kDa. Total protein loaded was 5 μg for supernatant, 1 μg for pellet, and 0.02 μg for rCEACAM6; different protein loading was required to provide comparable signals for scanning densitometry. B: densitometric quantitation of supernatant CEACAM6 distribution between isoforms at d1 and d14. C: densitometric quantitation of pellet CEACAM6 distribution between isoforms. The 90-kDa form was 65–80% of the total CEACAM6 in each comparison, except for supernatant d14 samples (30%). Values are means ± SE for 6 samples at d1 and 9 samples at d14. *P < 0.001 vs. d1.
Affinity of CEACAM6 for surfactant.
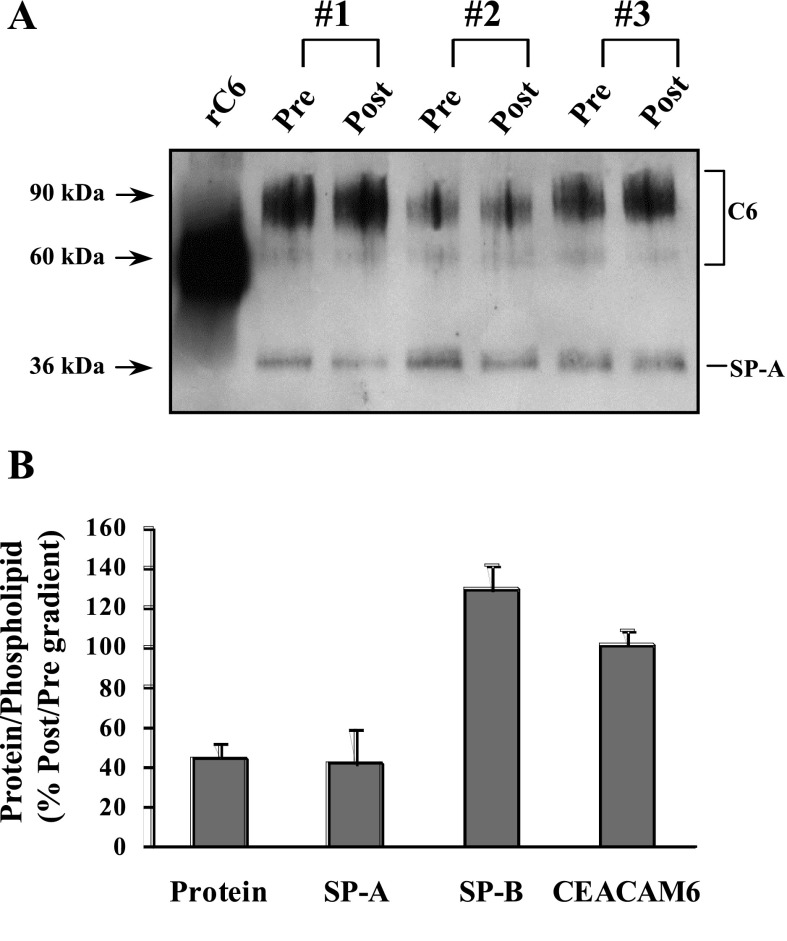
Our previous studies observed immunostaining for CEACAM6 within lamellar bodies of fetal type II cells, colocalizing with SP-B (24), and the current data confirm that CEACAM6 is associated with surfactant isolated from epithelial lining fluid of infants and adults. To examine the relative affinity of CEACAM6 for surfactant, we performed experiments in which surfactant lipids (and associated proteins) are isolated by discontinuous density gradient centrifugation. In this procedure, surfactant pellet is mixed with 16% KBr and overlayered with solutions of 13% KBr and saline. On centrifugation, lipids float to the KBr/saline interface, and nonassociated proteins are pelleted at the bottom of the tube. Figure 3A shows a Western blot for pooled surfactant samples before (Pre) and after (Post) density gradient centrifugation with equal loading of PL. The CEACAM6 signal (top band) is identical before and after centrifugation, indicating equivalent concentrations of the protein in the lipid band. By contrast, the SP-A signal is lower in each postcentrifugation sample. As summarized in Fig. 3B, post- vs. precentrifugation concentrations of CEACAM6 and SP-B (by immunodot assay) per μg PL were ≥100% compared with ∼40% for both total protein and SP-A. These results indicate a relatively high affinity of 90-kDa CEACAM6 for surfactant, apparently greater than for SP-A, and support designation of CEACAM6 as a SP.
Fig. 3.
CEACAM6 (C6) association with surfactant after density gradient flotation. A: Western blot. Three pooled samples of d14 infant surfactant pellets were subjected to KBr gradient centrifugation, and the surfactant band was obtained. Equal fractions (2 μg of PL) of the initial pellet sample (Pre) and postcentrifugation surfactant band (Post) were analyzed by Western blotting for CEACAM6 and SP-A. Pre and Post CEACAM6 bands are similar, whereas the SP-A signal is reduced for each Post sample. B: concentration of proteins in the surfactant band. Content of SP-A and CEACAM6 was determined by densitometry of the Western. SP-B content was measured by immunodot assay, and total protein was determined by the BCA assay. SP-B and CEACAM6 concentrations were identical in the pre- and postgradient surfactant compared with ∼40% of SP-A and total protein after flotation. Values are means ± SE; n = 3. P < 0.01 for Pre vs. Post for protein and SP-A.
Source of TAF CEACAM6.
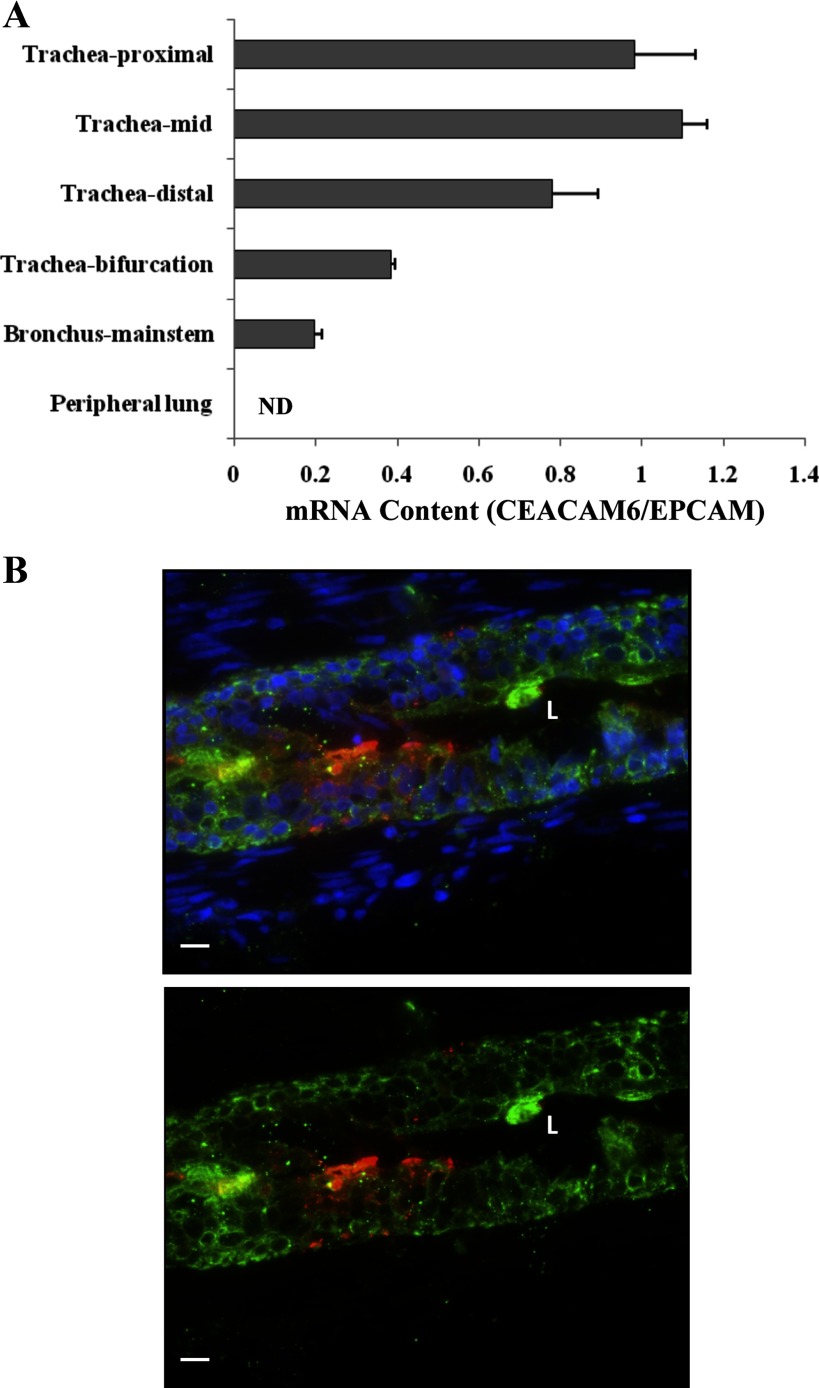
Suctioning to acquire infant TAF occurs just above the bifurcation of main stem bronchi, and the epithelial lining fluid obtained represents secretions of airways plus alveolar and terminal airway fluid that has moved up the airways by surface spreading, as well as ciliary action. Thus CEACAM6 in TAF could arise from peripheral lung spaces, proximal branching airways, main stem bronchi, and/or trachea. To explore the source of CEACAM6 in infant TAF, we examined these regions of human fetal lung. The highest content of CEACAM6 mRNA, as assessed by real-time PCR, occurred in the proximal two-thirds of the trachea, with reduced content in lower regions of the trachea and main stem bronchi; CEACAM6 transcript was not detected in peripheral lung of the fetus (Fig. 4A). Consistent with these results, parallel studies using Western analysis detected 90-kDa CEACAM6 in lysate of main stem bronchi (n = 3), but very low to undetectable levels of signal in peripheral lung tissue (data not shown). The presence of CEACAM6 was confirmed by positive immunostaining of epithelial cells of main stem bronchi (Fig. 4B) and trachea (not shown). The low expression of CEACAM6 in peripheral fetal lung before birth, as also illustrated in Fig. 1G, reflects lack of cellular differentiation at this gestational age. Thus these findings indicate that both airways and terminal air spaces may contribute to CEACAM6 in TAF of premature infants.
Fig. 4.
CEACAM6 expression in airway and lung tissue of human fetal lung. A: mRNA content by quantitative PCR in regions of airway, proximal to distal, and peripheral lung tissue. Data for CEACAM6 are normalized to epithelial cell adhesion molecule (EPCAM), an epithelial cell marker, and demonstrate decreasing CEACAM6 expression in distal air spaces and lung tissue. Normalized to 18S RNA (not shown), there was no consistent change in EPCAM and SCGB1A1 (CC10) expression along the airway. Values are means ± SE for triplicate determinations in an 18-wk gestation lung. Similar results were obtained with a 19-wk gestation lung. B: representative immunostaining for main stem bronchi showing CEACAM6 signal in the epithelium, but not surrounding interstitium. Green, cytokeratin; red, CEACAM6; blue, DAPI (top panel only); L, lumen of airway; bar = 10 μm.
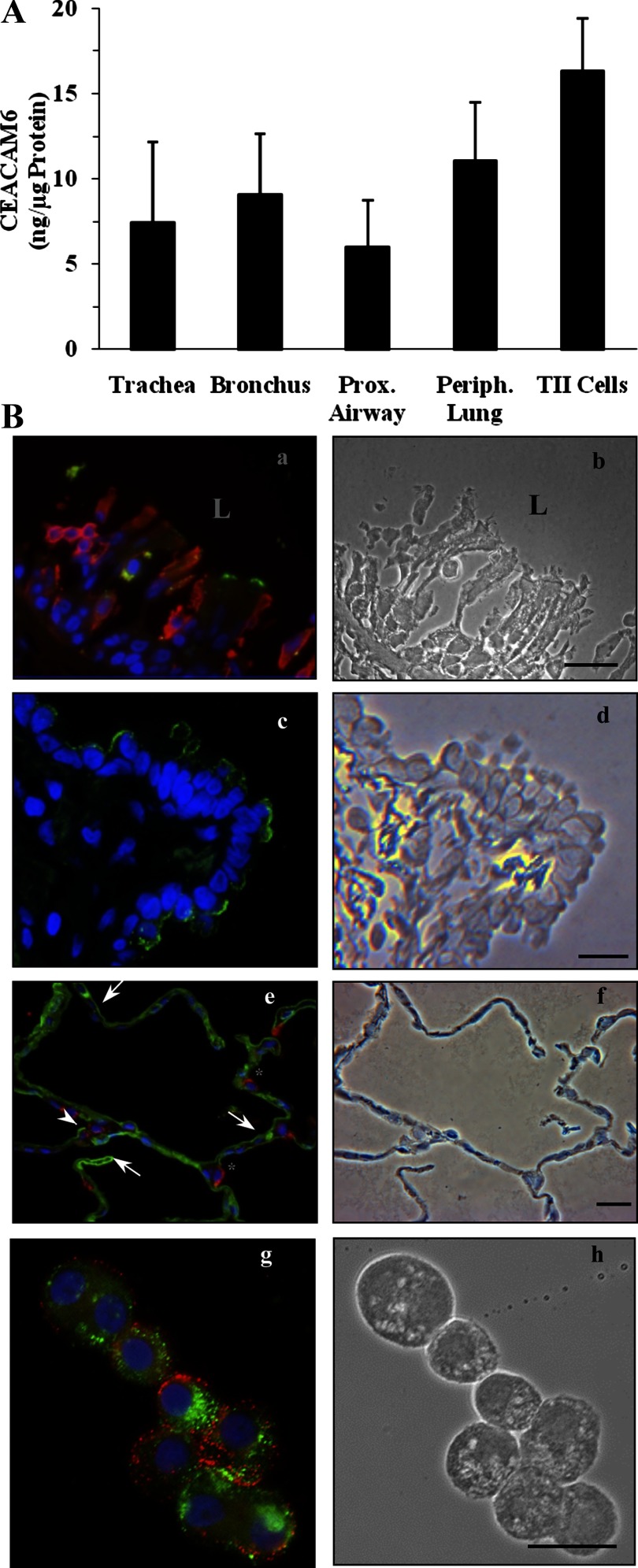
We also examined the distribution of CEACAM6 within adult lung. For these experiments, we measured CEACAM6 in epithelium scraped from airways and in peripheral lung tissue, as well as isolated type II cells. Similar levels of CEACAM6 were found in each of these specimens, as summarized in Fig. 5A. The finding of CEACAM6 in adult type II cells is consistent with our laboratory's previous mRNA data (2). Expression of CEACAM6 in airway is illustrated by the immunostaining images of tracheal epithelium (Fig. 5Ba, green stain) and of distal airway epithelium (Fig. 5Bc, green stain). In the alveolar epithelium (Fig. 5Be), CEACAM6 staining (green) was observed in most type I cells (negative for SP-B, red) and in approximately one-half of the type II cells, as noted by positive signal for SP-B. Isolated type II cells (Fig. 5Bg) also were positive for CEACAM6 (green staining); in this experiment, ∼60% of the cells that were positive for HTII-280, the type II cell marker, were also positive for CEACAM6.
Fig. 5.
CEACAM6 expression in airway epithelium and peripheral tissue of adult human lung. A: CEACAM6 content by immunodot assay in 5 regions, proximal to distal, using scraped epithelial cells from airways, lung tissue from the periphery, and isolated type II (TII) cells. CEACAM6 is detected in all airways and alveolar cells, with no significant differences in concentrations by ANOVA. Values are means ± SE for 3–4 lungs. B: representative immunostaining. a: CEACAM6 signal (green) in some tracheal epithelial cells of a postmortem adult lung, but not in underlying interstitium. Red, cytokeratin; blue, DAPI. b: Phase image of section in a; note disrupted epithelium. L, lumen of airway. c: CEACAM6 signal (green) is detected in many, but not all, distal airway cells; this section was negative for HTII-280 [a type II cell membrane marker (red); blue, DAPI]. d: Phase image of section in c. e: Alveolar epithelium of inflation-fixed adult lung showing CEACAM6 signal (green) in epithelial cells consistent with type I cells (arrows) and in some (arrowhead), but not all, type II cells, as identified by positive SP-B (red) staining. *SP-B-positive cells that are negative for CEACAM6. f: Phase image of section in e. g: Freshly isolated type II cells with CEACAM6 signal (green) and HTII-280 (red) showing colocalization in 6 of the 7 cells in the field. h: Phase-contrast image of cells. Blue, DAPI staining. Bars = 20 μm for all images.
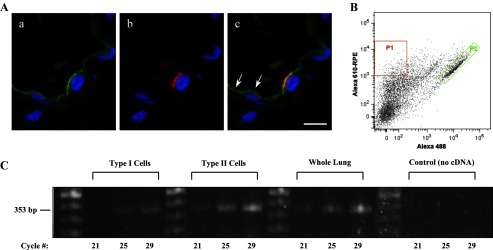
Results of our laboratory's previous studies (2, 24) plus the data of Fig. 5, Be and Bg, establish the expression of CEACAM6 in type II cells of the alveolus. However, the presence of CEACAM6 immunoreactivity throughout most of the alveolar epithelium (Fig. 5Be and Refs. 30, 32), including type I cells, could result from gene expression and/or shedding and membrane insertion of CEACAM6, a known property of GPI proteins (27) and of CEACAM6 in A549 cells (31). We, therefore, further examined this issue as summarized in Fig. 6. By high magnification microscopy (Fig. 6A), immunostaining of CEACAM6 was intense on the apical surface of type II cells (as identified by HTII-280 colocalization, Fig. 6Ab); by contrast, type I cells demonstrated fainter, punctate CEACAM6 staining. To determine whether type I cells synthesize CEACAM6, we isolated highly purified populations of these cells from adult lung by flow cytometry (Fig. 6B). Results of RT-PCR and agarose gel electrophoresis for type I and type II cells, shown in Fig. 6C, indicate signal in both cell types; by real-time PCR the levels of CEACAM6 transcript were similar (type I/type II = 0.98 ± 0.06, n = 3). However, these quantitative results may not accurately reflect relative content of transcript in vivo because CEACAM6 was detected by immunostaining in most type I cells, but only ∼50% of type II cells, and because relative stability of transcript during cell isolations is unknown. Thus the CEACAM6 gene is expressed in both alveolar cell types, although our results do not rule out the possibility that “painting” of the type I cell membrane with alveolar CEACAM6 shed from other cell types also occurs.
Fig. 6.
CEACAM6 expression in adult alveolar epithelial cells. A: representative high-magnification images of immunostaining from cryosections of inflation-fixed peripheral lung (alveolar region). a: CEACAM6 signal (green) of differing intensity is observed in cells of the alveolar epithelium. b: HTII-280 (red) signal in the same field as a, showing one positive type II cell in the field. c: Merge of a and b, with yellow signal showing colocalization of CEACAM6 and HTII-280; CEACAM6 signal also occurs in type I cells on either side of the type II cell (e.g., arrows). Blue, DAPI in all images; n = 2 lungs; bar = 10 μm. B: scattergram of human alveolar type I and type II cells isolated by FACS. Two cell populations were selected: P1 (red), high anti-HTI-56 Alexa 610-RPE-staining type I cells; and P2 (green), high anti-HTII-280 Alexa 488-staining type II cells. C: agarose gel electrophoresis after RT-PCR for CEACAM6 of type I cells and type II cells, isolated by FACS sorting, as well as whole lung tissue from the same lung. CEACAM6-specific primers identified product at the expected size of 353 bp from each RNA preparation, whereas no product was observed in the no cDNA control; cycle numbers = 21, 25, and 29.
DISCUSSION
CEACAM6 is expressed in epithelia of various human tissues, where it is involved in innate defense and control of cell proliferation, but pulmonary expression of CEACAM6 has not been well characterized. Our laboratory recently identified CEACAM6 as one of the genes upregulated during hormone-induced differentiation of lung type II cells in vitro (33) and subsequently confirmed mRNA expression in type II cells isolated from adult lung (2). In the present study, we investigated CEACAM6 expression in lung epithelial cells and lining fluid of infants and adults. The major new findings include expression of CEACAM6 in all levels of airways, as well as both the type I and type II cells of the alveolar epithelium of adult lung, association of the 90-kDa protein with surfactant in vivo, and likely upregulated production during lung injury of premature infants. The distribution and regulated expression support a role for CEACAM6 in maintenance of normal air space function and response to pathogens and injury.
In our laboratory's previous report, CEACAM6 was first detected in peripheral human fetal lung tissue at ∼24 wk gestation, and levels increased throughout the third trimester and after birth (24). This developmental pattern is typical of a number of regulated genes of type II cells, including SPs-A, -B, and -C, and reflects phenotypic changes with cell differentiation (1). Based on the tissue studies, we were surprised that CEACAM6 was detected in TAF of premature infants (gestational age 27 wk) on the first postnatal day (Fig. 1). A likely explanation is the finding with fetal lung (Fig. 4) that CEACAM6 is expressed in epithelium of upper airways at considerably higher levels than in the lung periphery; presumably much of the CEACAM6 in newborn infant TAF originates from airways. The data for CEACAM6 in adult lung (Fig. 5) are consistent with continuing production in airways plus a developmental increase that occurs in a proximal-to-distal pattern. We speculate that lung injury, which, in the case of premature infants with chronic lung disease, includes oxidative stress, inflammation, and infection, stimulates production of CEACAM6 by lung epithelium, resulting in elevated TAF levels postnatally. In preliminary immunostaining studies (Fig. 1H), we observed increased intensity and distribution of CEACAM6 immunoreactivity in the hyperplastic alveolar epithelium that develops in response to injury, whereas, in adult lung, CEACAM6 is expressed in only a subpopulation of type II cells. It is possible that CEACAM6, via its antiapoptotic activity for lung epithelial cells (24), promotes cell proliferation in response to lung injury, while also contributing to innate immune defense, along with other lung proteins, such as SP-A and SP-D. Our laboratory is currently examining CEACAM6 expression in additional sections of injured infant lung and in bleomycin-injured lungs of transgenic mice expressing human CEACAM6 (12).
There is accumulating evidence that CEACAM6 is a SP. Previously, our laboratory found that the protein is localized within lamellar bodies, as well as at the cell surface, of cultured fetal lung type II cells; moreover, CEACAM6 influenced surfactant surface-active properties in vitro, consistent with a physical interaction (24). In the present study, we observed that a fraction of CEACAM6 in TAF sedimented with large-aggregate surfactant on centrifugation. To more rigorously test this interaction, we performed buoyant density centrifugation, which results in surfactant localization at a density interface. The concentration of CEACAM6, normalized to total PL, was the same pre- and postcentrifugation, consistent with a relatively high affinity of CEACAM6 for surfactant. By contrast, the concentration of both SP-A and total protein decreased 60% after centrifugation. After lipid extraction of surfactant with organic solvents, only the lipoproteins SP-B and SP-C remain associated with lipid, indicating that all other proteins, while possessing lipophilic properties, have lower lipid affinity. Other than for SPs and CEACAM6, there is relatively little information regarding specific proteins that associate with surfactant in both normal and disease states. An earlier study using two-dimensional PAGE identified at least 50 different proteins associated with human lung lamellar bodies (15). A recent proteomic analysis of rat lung lamellar bodies identified >500 proteins; while many of these proteins had lipid-related functions, other proteins had antimicrobial and antioxidant functions consistent with the presence of CEACAM6 in human lamellar bodies and extracellular surfactant (29).
CEACAM6 contains a lipophilic 32-amino acid region at the carboxy terminus, as well as a GPI anchor, which is critical for insertion of the protein into lipid rafts of the cell surface membrane (18). Both of these moieties may contribute to binding of CEACAM6 to surfactant lipids. The identity of specific lipids involved in CEACAM6 association with surfactant is not presently known; cholesterol and sphingomyelin of the exoplasmic layer participate in binding of GPI proteins to the cell membrane (19), and these lipids are present at low levels in surfactant. We found that surfactant-associated CEACAM6 is predominantly 90 kDa, the full length and fully glycosylated isoform (Fig. 2). The lower molecular weight isoforms detected on Western blot may represent degradation products or partially glycosylated precursors, as previously observed using an inhibitor (mannosamine) of GPI incorporation (24). These forms were more abundant in d-14 infant TAF than in d-1 or adult TAF. At 2 wk of age, these infants had developing chronic lung disease of sufficient severity to require continuing intubation with mechanical ventilation and supplemental oxygen. The disease process and/or treatment may interfere with glycosylation/GPI anchor incorporation, damage epithelial cells with release of partially glycosylated CEACAM6, or enhance degradation of the 90-kDa protein secondary to increased levels of inflammatory proteases.
CEACAM6 likely enters air spaces by both exocytosis of lamellar bodies from type II cells and via shedding of cell surface-attached protein, as occurs in other tissues (18). In cultured fetal lung cells, at least, some CEACAM6 is found within intracellular lamellar bodies and is surfactant associated in the culture medium (24). Presumably this occurs in vivo, and stimulated surfactant secretion in response to endogenous secretagogues may be one mechanism to enhance alveolar content of CEACAM6 in stressful conditions. However, we propose that most CEACAM6 in airways and alveoli occurs by shedding from epithelial cells throughout the lung, and we speculate that free CEACAM6 in upper airways has primarily an antimicrobial role, whereas CEACAM6 in lower airways also has a surfactant-related function. If this conclusion regarding the source of CEACAM6 is correct, then much of the binding of CEACAM6 to surfactant must occur in the lung lining fluid rather than during surfactant synthesis in type II cells. We have been unable to test this proposal directly due to unavailability of fully glycosylated rCEACAM6; partially glycosylated rCEACAM6 doses not bind to surfactant (data not shown), in contrast to endogenous CEACAM6 (24).
As discussed, CEACAM6 is expressed in epithelia of several tissues and functions in cellular adhesion, binds gram-negative bacteria, and is tumor promoting. Our previous study confirmed antiapoptotic activity of CEACAM6 in cultured human fetal lung cells and provided initial evidence for binding to surfactant and in vitro protection of surface activity in the presence of inhibitory proteins. Based on both in vitro bacterial binding data and studies in nonpulmonary tissues, it is expected that CEACAM6 in lung lining fluid binds gram-negative bacteria as one component of the pulmonary innate immune defense. Of interest, CEACAM6 binds human-specific respiratory organisms, including Haemophilius influenzae and Moraxella catarrhalis (20), and respiratory colonization with these microorganisms in infancy is associated with increased risk for asthma (9). Our present findings on distribution of CEACAM6 suggest the protein may have multiple functions in both airways and alveoli of normal and diseased lungs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-088193 (P. L. Ballard, L. W. Gonzales), HL-024075 (P. L. Ballard), and HL-093026 (J.-W. Lee), and the Foundation of Anesthesia Education and Research (J.-W. Lee).
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.C., N.A.B., L.W.G., J.-W.L., and R.F.G. performed experiments; C.J.C., N.A.B., L.W.G., R.F.G., and P.L.B. analyzed data; C.J.C., N.A.B., L.W.G., R.F.G., and P.L.B. interpreted results of experiments; C.J.C., N.A.B., L.W.G., and R.F.G. prepared figures; C.J.C. drafted manuscript; C.J.C., N.A.B., L.W.G., J.-W.L., R.F.G., and P.L.B. approved final version of manuscript; N.A.B., L.W.G., J.-W.L., R.F.G., and P.L.B. edited and revised manuscript; L.W.G. and P.L.B. conception and design of research.
ACKNOWLEDGMENTS
We thank X. Fang for preparing adult type II cells, and both P. Wang and A.M. Barrette for technical assistance.
REFERENCES
- 1. Ballard PL. The glucocorticoid domain in the lung and mechanisms of action. In: Endocrinology of the Lung: Development and Surfactant Synthesis, edited by Mendelson CR. Totowa, NJ: Humana, 2000, p. 1–44. [Google Scholar]
- 2. Ballard PL, Lee JW, Fang X, Chapin C, Allen L, Segal MR, Fischer H, Illek B, Gonzales LW, Kolla V, Matthay MA. Regulated gene expression in cultured type II cells of adult human lung. Am J Physiol Lung Cell Mol Physiol 299: L36–L50, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ballard PL, Merrill JD, Godinez RI, Godinez MH, Truog WE, Ballard RA. Surfactant protein profile of pulmonary surfactant in premature infants. Am J Respir Crit Care Med 168: 1123–1128, 2003. [DOI] [PubMed] [Google Scholar]
- 4. Ballard PL, Ning Y, Polk D, Ikegami M, Jobe AH. Glucocorticoid regulation of surfactant components in immature lambs. Am J Physiol Lung Cell Mol Physiol 273: L1048–L1057, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, Walsh MC, Durand DJ, Mayock DE, Eichenwald EC, Null DR, Hudak ML, Puri AR, Golombek SG, Courtney SE, Stewart DL, Welty SE, Phibbs RH, Hibbs AM, Luan X, Wadlinger SR, Asselin JM, Coburn CE. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med 355: 343–353, 2006. [DOI] [PubMed] [Google Scholar]
- 6. Barnich N, Carvalho FA, Glasser AL, Darcha C, Jantscheff P, Allez M, Peeters H, Bommelaer G, Desreumaux P, Colombel JF, Darfeuille-Michaud A. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest 117: 1566–1574, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 234: 466–468, 1959. [PubMed] [Google Scholar]
- 8. Bentejac M, Lecerf J, Bugaut M, Delachambre M. Turnover and uptake of double-labelled high-density lipoprotein sphingomyelin in the adult rat. Biochem Biophys Acta 959: 349–360, 1988. [DOI] [PubMed] [Google Scholar]
- 9. Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, Stage M, Pipper CB. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 357: 1487–1495, 2007. [DOI] [PubMed] [Google Scholar]
- 10. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 11. Blumenthal RD, Hansen HJ, Goldenberg DM. Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res 65: 8809–8817, 2005. [DOI] [PubMed] [Google Scholar]
- 12. Chan CH, Stanners CP. Novel mouse model for carcinoembryonic antigen-based therapy. Mol Ther 9: 775–785, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med 185: 1557–1564, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dobbs LG, Gonzalez R, Allen L, Froh D. HTI56, an integral membrane protein specific to human alveolar type I cells. J Histochem Cytochem 47: 129–137, 1999. [DOI] [PubMed] [Google Scholar]
- 15. Froh D, Ballard PL, Williams MC, Gonzales J, Goerke J, Odom MW, Gonzales LW. Lamellar bodies of cultured human fetal lung: content of surfactant protein A (SP-A), surface film formation and structural transformation in vitro. Biochim Biophys Acta 1052: 78–89, 1990. [DOI] [PubMed] [Google Scholar]
- 16. Gonzales LW, Guttentag SH, Wade KC, Postle AD, Ballard PL. Differentiation of human pulmonary type II cells in vitro by glucocorticoid plus cAMP. Am J Physiol Lung Cell Mol Physiol 283: L940–L951, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez RF, Allen L, Gonzales L, Ballard PL, Dobbs LG. HTII-280, a biomarker specific to the apical plasma membrane of human lung alveolar type II cells. J Histochem Cytochem 58: 891–901, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 9: 67–81, 1999. [DOI] [PubMed] [Google Scholar]
- 19. Hanada K, Nishijima M, Akamatsu Y, Pagano RE. Both sphingolipids and cholesterol participate in the detergent insolubility of alkaline phosphatase, a glycosylphosphatidylinositol-anchored protein, in mammalian membranes. J Biol Chem 270: 6254–6260, 1995. [DOI] [PubMed] [Google Scholar]
- 20. Hill DJ, Virji M. A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol Microbiol 48: 117–129, 2003. [DOI] [PubMed] [Google Scholar]
- 21. Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Invest 76: 703–716, 1997. [PubMed] [Google Scholar]
- 22. Jantscheff P, Terracciano L, Lowy A, Glatz-Krieger K, Grunert F, Micheel B, Brummer J, Laffer U, Metzger U, Herrmann R, Rochlitz C. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol 21: 3638–3646, 2003. [DOI] [PubMed] [Google Scholar]
- 23. Kammerer R, Popp T, Singer BB, Schlender J, Zimmermann W. Identification of allelic variants of the bovine immune regulatory molecule CEACAM1 implies a pathogen-driven evolution. Gene 339: 99–109, 2004. [DOI] [PubMed] [Google Scholar]
- 24. Kolla V, Gonzales LW, Bailey NA, Wang P, Angampalli S, Godinez MH, Madesh M, Ballard PL. Carcinoembryonic cell adhesion molecule 6 in human lung: regulated expression of a multifunctional type II cell protein. Am J Physiol Lung Cell Mol Physiol 296: L1019–L1030, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kolla V, Gonzales LW, Gonzales J, Wang P, Angampalli S, Feinstein SI, Ballard PL. Thyroid transcription factor in differentiating type II cells: regulation, isoforms, and target genes. Am J Respir Cell Mol Biol 36: 213–225, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuroki M, Abe H, Imakiirei T, Liao S, Uchida H, Yamauchi Y, Oikawa S, Kuroki M. Identification and comparison of residues critical for cell-adhesion activities of two neutrophil CD66 antigens, CEACAM6 and CEACAM8. J Leukoc Biol 70: 543–550, 2001. [PubMed] [Google Scholar]
- 27. Lauc G, Heffer-Lauc M. Shedding and uptake of gangliosides and glycosylphosphatidylinositol-anchored proteins. Biochim Biophys Acta 1760: 584–602, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Ordonez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res 60: 3419–3424, 2000. [PubMed] [Google Scholar]
- 29. Ridsdale R, Na CL, Xu Y, Greis KD, Weaver T. Comparative proteomic analysis of lung lamellar bodies and lysosome-related organelles. PLos One 6: e16482, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scholzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol 156: 595–605, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singer BB, Scheffrahn I, Kammerer R, Suttorp N, Ergun S, Slevogt H. Deregulation of the CEACAM expression pattern causes undifferentiated cell growth in human lung adenocarcinoma cells. PLos One 5: e8747, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsutsumi Y, Onoda N, Misawa M, Kuroki M, Matsuoka Y. Immunohistochemical demonstration of nonspecific cross-reacting antigen in normal and neoplastic human tissues using a monoclonal antibody. Comparison with carcinoembryonic antigen localization. Acta Pathol Jpn 40: 85–97, 1990. [DOI] [PubMed] [Google Scholar]
- 33. Wade KC, Guttentag SH, Gonzales LW, Maschhoff KL, Gonzales J, Kolla V, Singhal S, Ballard PL. Gene induction during differentiation of human pulmonary type II cells in vitro. Am J Respir Cell Mol Biol 34: 727–737, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]