Abstract
The neurotransmitter dopamine and its dopamine receptor D2 (D2DR) agonists are known to inhibit vascular permeability factor/vascular endothelial growth factor (VEGF)-mediated angiogenesis and vascular permeability. Lung injury is a clinical syndrome associated with increased microvascular permeability. However, the effects of dopamine on pulmonary edema, a phenomenon critical to the pathophysiology of both acute and chronic lung injuries, have yet to be established. Therefore, we sought to determine the potential therapeutic effects of dopamine in a murine model of lipopolysaccharide (LPS)-induced acute lung injury (ALI). Compared with sham-treated controls, pretreatment with dopamine (50 mg/kg body wt) ameliorated LPS-mediated edema formation and lowered myeloperoxidase activity, a measure of neutrophil infiltration. Moreover, dopamine significantly increased survival rates of LPS-treated mice, from 0–75%. Mechanistically, we found that dopamine acts through the VEGF-VEGFR2 axis to reduce pulmonary edema, as dopamine pretreatment in LPS-treated mice resulted in decreased serum VEGF, VEGFR2 phosphorylation, and endothelial nitric oxide synthase phosphorylation. We used D2DR knockout mice to confirm that dopamine acts through D2DR to block vascular permeability in our lung injury model. As expected, a D2DR agonist failed to reduce pulmonary edema in D2DR−/− mice. Taken together, our results suggest that dopamine acts through D2DR to inhibit pulmonary edema-associated vascular permeability, which is mediated through VEGF-VEGFR2 signaling and conveys protective effects in an ALI model.
Keywords: dopamine receptor D2, vascular endothelial growth factor
acute lung injury (ALI) and its severe form, the acute respiratory distress syndrome (ARDS), are prevalent causes of morbidity and mortality (51). One of the hallmarks of ALI is the accumulation of protein-rich alveolar edema fluid resulting from impaired vascular barrier properties (29, 33, 50, 53). Not only are vascular filtration coefficient and protein permeability increased, injured lungs are also defective in alveolar fluid clearance (35, 52). Although β-receptor agonists such as albuterol and isoproterenol reliably increase alveolar fluid clearance in experimental animals with injured lungs, their use in patients (42) has met limited success (32). Dopamine, a neurotransmitter, acts via two receptor isoforms (dopamine receptor D1 and D2, D1DR and D2DR). D1DR has been shown to increase transepithelial fluid flux through the trafficking of NaKATPase to the basolateral membrane of type II alveolar epithelial cells (5). Moreover, activation of D2DR induces NaKATPase gene expression (17). However, the actions of dopamine on pulmonary vascular barrier properties may not be limited to effects on transepithelial sodium transport. Activation of D2DR has also been implicated in the regulation of vascular endothelial growth factor (VEGF)-induced vascular permeability as well as tumor angiogenesis (2, 9, 46). Many experimental and human studies support the hypothesis that VEGF plays a critical role in shaping the vascular barrier function in ALI (3, 6, 15, 18, 25). In normal human lungs, the VEGF concentration in alveolar lining fluid is greater than that in plasma (43). Epithelial injury leads to decompartmentalization of VEGF, permitting its migration across tight junctions from the alveolar space to the vascular compartment (16, 25, 27, 49), and is therefore thought to play a pathogenic role in noncardiogenic pulmonary edema (26, 38).
Using a mouse ovarian tumor model, our group previously reported that the endothelium expresses the ligand dopamine along with its cognate D2DR, thereby regulating vascular permeability and endothelial cell (EC) barrier integrity (2, 7). Furthermore, we demonstrated that dopamine, through a D2DR-dependent mechanism, causes the downregulation of VEGFR2 phosphorylation (47). We now provide evidence that the same mechanisms operate in murine endotoxin-induced ALI and can be targeted to improve morbidity and mortality in this preclinical model. Sepsis is the most common cause of ALI in humans (50, 51). Administration of the gram-negative bacterial endotoxin lipopolysaccharide (LPS) has been widely used as an animal model of sepsis-related lung injury. LPS is known to cause overexpression of VEGF (11, 12, 22, 54), which in turn upregulates nitric oxide (NO) production through phosphorylation of VEGFR2 and endothelial NO synthase (eNOS) (13, 14, 40). As postulated, our results suggest that dopamine acts through D2DR to inhibit pulmonary edema-associated vascular permeability, which is mediated through VEGF-VEGFR2 signaling, and conveys protective effects in an ALI model.
MATERIALS AND METHODS
Animals
Pathogen-free male Balb/C mice purchased from the National Cancer Institute (Frederick, MD) and D2DR knockout C57Bl/6 mice from Jackson Laboratory (Bar Harbor, ME) were used in LPS-induced ALI. The animals were housed in separate cages in a temperature-controlled room with alternating 12-h:12-h light/dark cycles and were allowed 1 wk to acclimate to their surroundings. The animals were also fed a standard diet.
Reagents
LPS (Salmonella enterica), FITC-albumin, hexadecyl-trimethyl-ammonium bromide, hydrogen peroxide, quinpirole, ectilopride, and O-dianisidine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). The antibodies used were phospho-eNOS (S1177) and total eNOS (BD Biosciences, Franklin Lakes, NJ), VEGFR-2 (SC-504, Santa Cruz Biotechnology, Santa Cruz, CA), and phospho-tyrosine 4G10 clone (Millipore, Billerica, MA).
Dopamine Preparation
A pharmaceutical-grade dopamine solution (40 mg/ml) was purchased from the Mayo Clinic Pharmacy. Dopamine (50 mg/kg body wt), quinpirole (10 mg/kg body wt), and eticlopride (10 mg/kg body wt) doses were selected on the basis of prior studies demonstrating their regulatory effects on vascular permeability (2).
Experimental Procedure
The wild-type animals were divided into six treatment groups as control (sham treated, n = 20), dopamine alone (n = 20), LPS alone (n = 20), dopamine + LPS (n = 20), quinpirole (D2DR specific agonist) + LPS (n = 20), and dopamine + LPS + eticlopride (D2DR antagonist, n = 20). In D2DR knockout study, animals were divided into six treatment groups as control (sham-treated D2−/−, n = 14), LPS alone (D2−/−, n = 14), quinpirole + LPS (+/+, n = 14), quinpirole + LPS (+/−, n = 14), quinpirole + LPS (D2−/−, n = 14), and dopamine + LPS (D2−/−, n = 14). The Mayo Clinic Institutional Animal Care and Use Committee approved all experimental procedures. LPS (400 μg/mouse) was injected intraperitoneally (IP) to induce lung edema (54). The control and treated animals were euthanized by spinal break. One group of animals was treated 30 min before the onset of LPS administration with an IP injection of dopamine (50 mg/kg body wt). One dose of dopamine was given the day of LPS treatment, and an additional dose was given the following day. The same procedure was repeated with quinpirole (10 mg/kg body wt).
Lung Injury Analysis
Lung injury is a broad term that can be applied to conditions ranging from mild interstitial edema without cellularity to massive and fatal destruction of the lung. Here, we used physical and biochemical methods to investigate the effect of dopamine on LPS-induced lung injury. The LPS-challenged animals become very sick and started dying within 2–3 h at this stage. We selected 24 h as the time point in all groups for histological comparison except for bronchoalveolar lavage (BAL) analysis, which was done at 6 h as well as 24 h after LPS challenge.
Lung Water Content
After completion of the animal experiment, mice were euthanized by spinal break. The left lung was harvested for the wet-to-dry weight ratio. The dry weight was determined after incubating the lungs at 80°C for 72 h, and the wet-to-dry weight ratio was calculated (56).
Histology
The right lung was inflated with 10% (vol/vol) formaldehyde embedded in paraffin, and cut into 5-μm-thick sections. Sections were stained with hematoxylin and eosin, and images were taken with a Nikon Eclipse E800 microscope with a ×20 objective. The extent of lung injury was determined by a blinded observer using a semiquantitative score based on congestion, interstitial edema, neutrophil infiltration, and air space hemorrhage, as follows: 0 = no change; 1+ = focal, mild, subtle change, 2+ = multifocal mild changes; 3+ = multifocal prominent changes; and 4+ = extensive prominent changes (1, 48). Histological comparison was done at 24 h in all groups.
Survival
A survival study was carried out separately with initial intervention. Animals (n = 8, each group) were observed daily and survival calculated 158 h after the initial intervention. D2DR knockout animals (n = 8, each group) were monitored up to 32 h.
Biochemical Assays
Pulmonary microvascular permeability.
FITC-labeled albumin, a macromolecular marker, is widely used to evaluate pulmonary microvascular permeability (56). Two hours before euthanasia, FITC-labeled albumin (5 mg/kg body wt) was administered via a tail-vein injection at 6 h (n = 3) and at 24 h (n = 3). Immediately after euthanasia, the lungs were lavaged three times with phosphate-buffered saline (0.5 ml per lavage) and the samples combined. Fluid recovery was roughly 95%. The BAL samples were centrifuged at 3,000 revolution/min for 10 min. FITC fluorescence in the BAL fluid was measured using a fluorescence spectrophotometer with excitation at 484 nm and emission at 510 nm (56).
Myeloperoxidase assay.
Tissue myeloperoxidase (MPO) activity is a quantitative marker for the presence of neutrophils in the lungs (55). MPO activity was measured in tissue homogenates at 24 h after LPS injection in placebo and dopamine treated animals (n = 6). The MPO activity was expressed as absorbance per gram of tissue (39).
VEGF-A Levels and VEGFR-2 Phosphorylation
Serum was collected from the different experimental groups at 24 h after the LPS challenge. Mouse VEGF-164 levels in serum were measured by an ELISA assay (R&D Systems, Minneapolis, MN) with a specific mouse VEGF primary antibody that has no cross reactivity with human VEGF. Phosphorylation of VEGFR2 was assessed by Western blot analysis. Frozen whole-lung tissues were homogenized in 400 μl of RIPA lysis buffer supplemented with protease and phosphatase inhibitors. The homogenates were centrifuged at 14,000 revolution/min for 15 min at 4°C, and the supernatants were collected. VEGFR2 was immunoprecipitated with a VEGFR2 antibody and resolved on a 10% SDS-PAGE. The samples were analyzed for VEGFR2 phosphorylation using the antiphosphotyrosine clone 4G10. A mouse anti-IgG-horseradish peroxidase conjugate was used to detect the band with the enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ). The intensities of the bands were analyzed using Image J from the National Institutes of Health.
Measurement of eNOS
Histology slides were stained for eNOS using a blood vessel staining kit (ECM590, Millipore) per the manufacturer's instructions. Immunohistological staining of slides from all treatment groups was performed with phospho-eNOS (Serine 1177) and total eNOS antibodies (BD Biosciences). A quantitative analysis of immunostained slides was done with Metamorph software (greater than three slides per group).
Statistics
The data in the figures are represented as means and SD. The error bar was based on SD values. Differences between the treated groups vs. the injured group (LPS/saline) were assessed using the unpaired Student's t-tests for unpaired observation, and ANOVA and/or Dunn's test. Statistical significance was assigned when the P value was ≤0.05.
RESULTS
Given the well-established role of dopamine in preventing vascular permeability, we sought to determine the potential therapeutic effects of dopamine in a murine model of ALI. To this end, we challenged mice with LPS to induce pathophysiological features of pulmonary edema such as alveolar flooding and collapse, tissue infiltration with inflammatory cells, and pulmonary hemorrhage.
Dopamine Modulates the Pathophysiological Features of LPS-Induced ALI
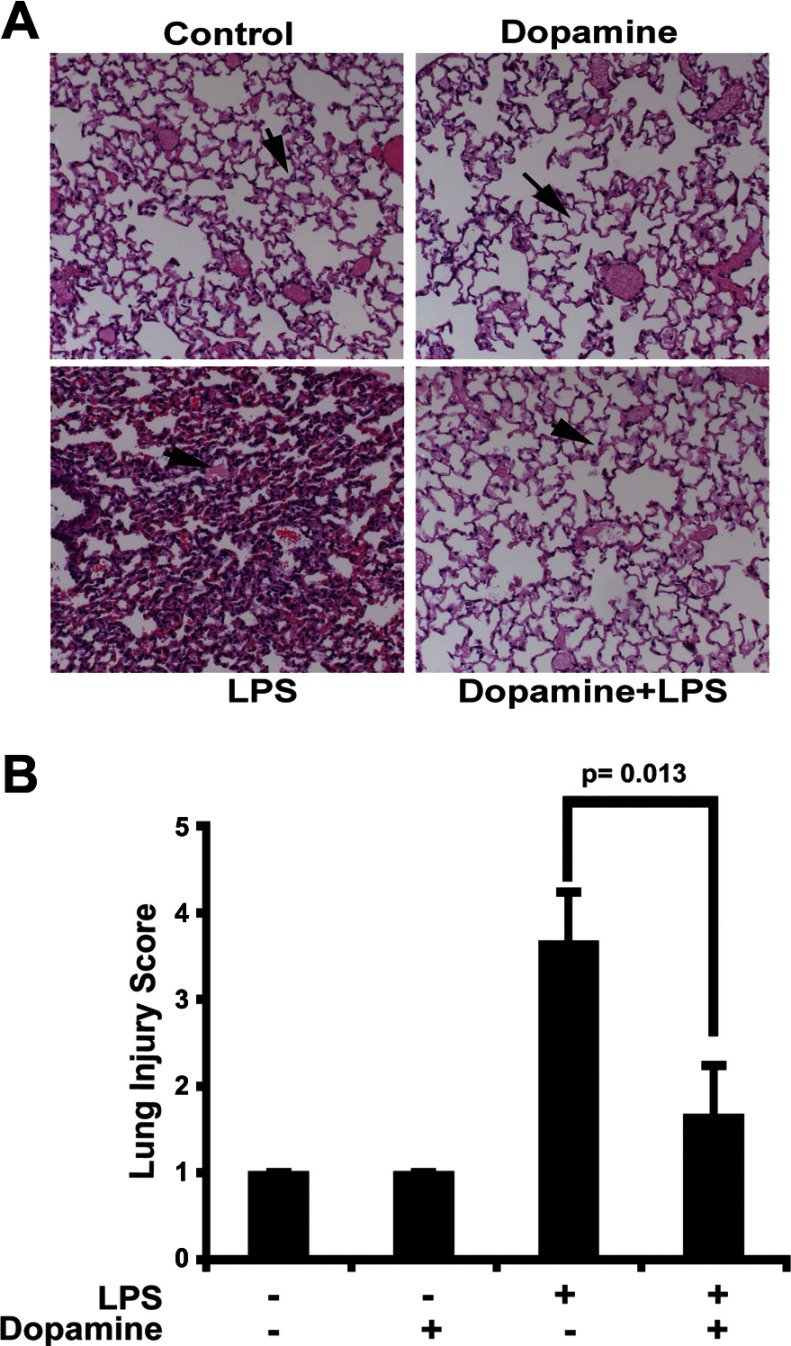
The intraperitoneal administration of LPS was associated with alveolar flooding, airspace collapse, tissue infiltration with inflammatory cells, and perivascular hemorrhage (n = 6, Fig. 1A). These histopathological features of ALI were much less pronounced in animals pretreated with dopamine as reflected in the statistically significant difference in histological lung injury scores (P = 0.013 vs. sham, n = 6, Fig. 1B). These results are consistent with the hypothesis that dopamine affords pulmonary vascular barrier protection against proinflammatory insults.
Fig. 1.
A: histopathology of lung. No signs of pulmonary edema are visible in the sham- and dopamine-treated mice. A significant accumulation of fluid is visible in the alveolar space, with damaged alveolar architecture in lipopolysaccharide (LPS)-treated mice. No signs of pulmonary edema are visible among the stable alveolar architecture of the dopamine + LPS-treated mice (magnification, ×20). The samples were taken at 24 h after the LPS and or dopamine challenge. B: lung injury scoring. We did the extent of lung injury using semiquantitative scoring method (see materials and methods). We observed no significant change in the sham- and dopamine-treated groups, but there was a significant change in LPS- and dopamine + LPS-treated mice. All samples were taken after 24 h after LPS and/or dopamine challenge.
Dopamine Ameliorates the LPS-Induced Increase in Lung Water and Microvascular Protein Permeability
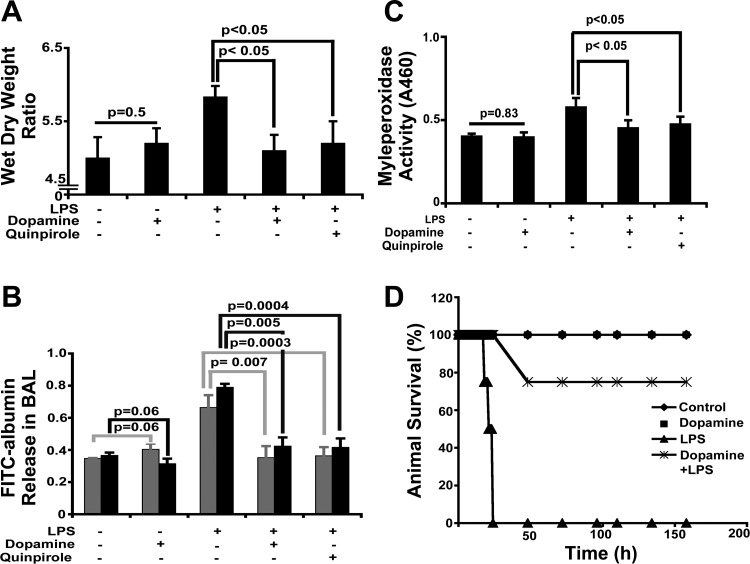
The intraperitoneal administration of LPS was associated with a statistically significant increase in lung water as reflected in the greater wet-to-dry weight ratio compared with sham-treated controls (P < 0.05 vs. sham, n = 6, Fig. 2A). Consistent with the histopathological findings, the pretreatment of LPS challenged mice with dopamine prevented the increase in lung water, resulting in a wet-to-dry weight ratio similar to that measured in sham-treated controls. The administration of the D2DR-specific agonist quinpirole before LPS challenge was equally effective in preventing pulmonary edema, suggesting that the mechanism of dopamine-mediated barrier protection to proinflammatory insults involves D2DR activation.
Fig. 2.
Effects of LPS and dopamine on lungs. The water content of lungs was determined by calculating the wet-to-dry weight (see materials and methods) at 24 h in sham (n = 6)-, dopamine- (n = 6), LPS- (n = 6), dopamine + LPS- (n = 6), and quinpirole + LPS-treated (n = 6) mice (A). The measurement of FITC-albumin release is indicative of vascular leakiness in the bronchioalveolar lavage (BAL) of sham- (n = 3), dopamine- (n = 3), LPS- (n = 3), dopamine + LPS-, and quinpirole + LPS-treated mice at 6 h (gray bar) and 24 h (solid black bar) (B). Pulmonary sequestration of neutrophils was assessed by lung myeloperoxidase (MPO) activity in sham- (n = 6), LPS- (n = 6), dopamine- (n = 6), dopamine + LPS- (n = 6), and quinpirole + LPS- (n = 6) treated mice at 24 h (C). Animal survival was monitored up to 158 h in sham- (n = 8), dopamine- (n = 8), LPS-(n = 8), and LPS + dopamine- (n = 8) treated groups based on animal activity, movement, and physical examination (D).
The effects of dopamine on pulmonary microvascular protein permeability was inferred from FITC-albumin concentrations in BAL fluid obtained at 6 h (P = 0.007 vs. sham, n = 3, Fig. 2B) and 24 h (P < 0.0003 vs. sham, n = 3, Fig. 2B) after an LPS challenge. FITC-albumin concentrations of LPS-treated mice were greater than those of sham-treated controls at both time points. In contrast, pretreatment with dopamine largely prevented the translocation of FITC-albumin into the alveolar space, indicating preservation/restoration of microvascular protein permeability. A similar response was observed in LPS-challenged animals pretreated with quinpirole (P = 0.005 vs. sham, n = 3, Fig. 2B at 6 h and P < 0.0004 vs. sham, n = 3, Fig. 2B at 24 h) in support of the role of D2DR in dopamine-mediated barrier protection.
Dopamine Prevents LPS-Mediated Neutrophil Recruitment
Neutrophil recruitment in response to injury was inferred from MPO activity in lung homogenates of LPS- and placebo-treated mice. As anticipated from the histopathological findings and numerous reports in the literature, LPS induced neutrophil recruitment and therefore raised MPO activity of lung tissue homogenates. Pretreatment with dopamine and quinpirole substantially reduced MPO activity (P < 0.05 vs. sham, n = 6, Fig. 2C) in lungs of LPS-treated mice, indicating that D2DR agonists inhibit neutrophil recruitment to proinflammatory insults.
Dopamine Increases Survival Rates of LPS-Challenged Mice
In preliminary observations, we found that animals became very sick and unable to move around 24 h (n = 8, Fig. 2D) and died around 24–27 h (Fig. 2D), whereas 75% of mice pretreated with dopamine were alive at 158 h (n = 6, Fig. 2D), suggesting that dopamine-mediated hemodynamic effects along with protection of endothelial barrier integrity may be contributing to the increased lifespan in this preclinical ALI model (41).
Dopamine Inhibits Pulmonary Edema by Decreasing Serum VEGF Levels and Phosphorylation of VEGFR2
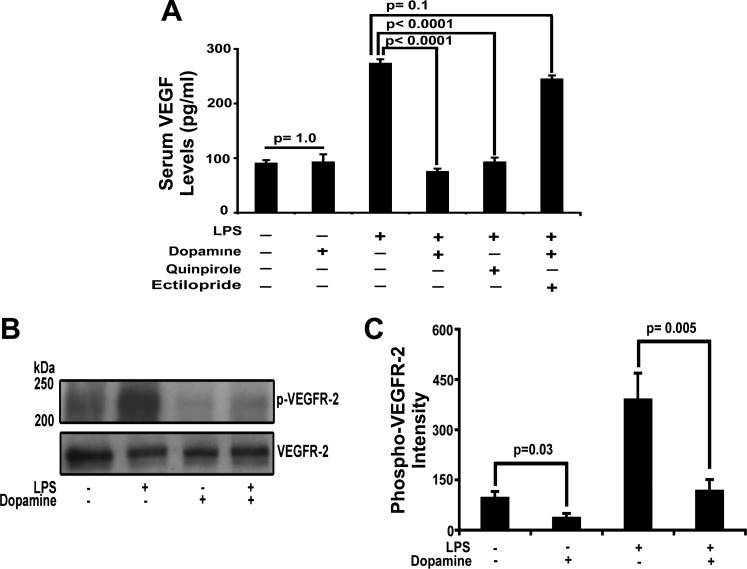
We previously demonstrated in an ovarian tumor model that dopamine acts through D2DRs to induce endocytosis of VEGFR2, preventing VEGF binding and receptor activation, thereby preserving vascular barrier properties. Because serum VEGF levels are increased in noncardiogenic pulmonary edema, we tested whether dopamine altered the lung response to an LPS challenge by a similar mechanism. As postulated, we observed a significant reduction in serum VEGF levels (P < 0.0001 vs. sham, n = 6, Fig. 3A) and VEGFR2 (P = 0.005 vs. sham, n = 6, Fig. 3, B and C) phosphorylation in dopamine-pretreated animals challenged with an LPS-induced lung injury compared with sham-treated groups. Our findings are consistent with, but not definitive for, the effect of dopamine on systemic VEGF levels. Similar results were obtained using the D2DR-specific agonist quinpirole (P < 0.0001 vs. sham, n = 6, Fig. 3A). Eticlopride, (an antagonist of D2DR) abrogated dopamine-mediated decrease in VEGF (P = 0.1 vs. sham, n = 6, Fig. 3A) levels. These results provided a further support that D2DR, but not D1DR, plays a role in preventing pulmonary edema by inhibiting VEGF-VEGFR2 signaling.
Fig. 3.
Effect of dopamine on serum vascular endothelial growth factor (VEGF) levels and VEGFR2 receptor phosphorylation. Serum VEGF levels were assessed with ELISA as well as the phosphorylation of VEGFR2 by Western blotting at 10% SDS-PAGE on lung homogenate in sham- (n = 6), LPS- (n = 6), dopamine- (n = 6), dopamine + LPS- (n = 6), quinpirole + LPS- (n = 6), dopamine + ectilopride (dopamine receptor D2, D2DR, antagonist)+ LPS-treated (n = 6) mice. We observed a significant reduction in serum VEGF level in dopamine- and quinpirole-pretreated mice (n = 6; A). We also assessed VEGFR2 phosphorylation in sham-, LPS-, dopamine-, and dopamine + LPS-pretreated animals (B). Quantitative analysis of VEGFR2 phosphorylation (n = 6) was done with National Institutes of Health imaging software (C). All samples were taken at 24 h after LPS challenge.
Dopamine Inhibits VEGFR2 Signaling
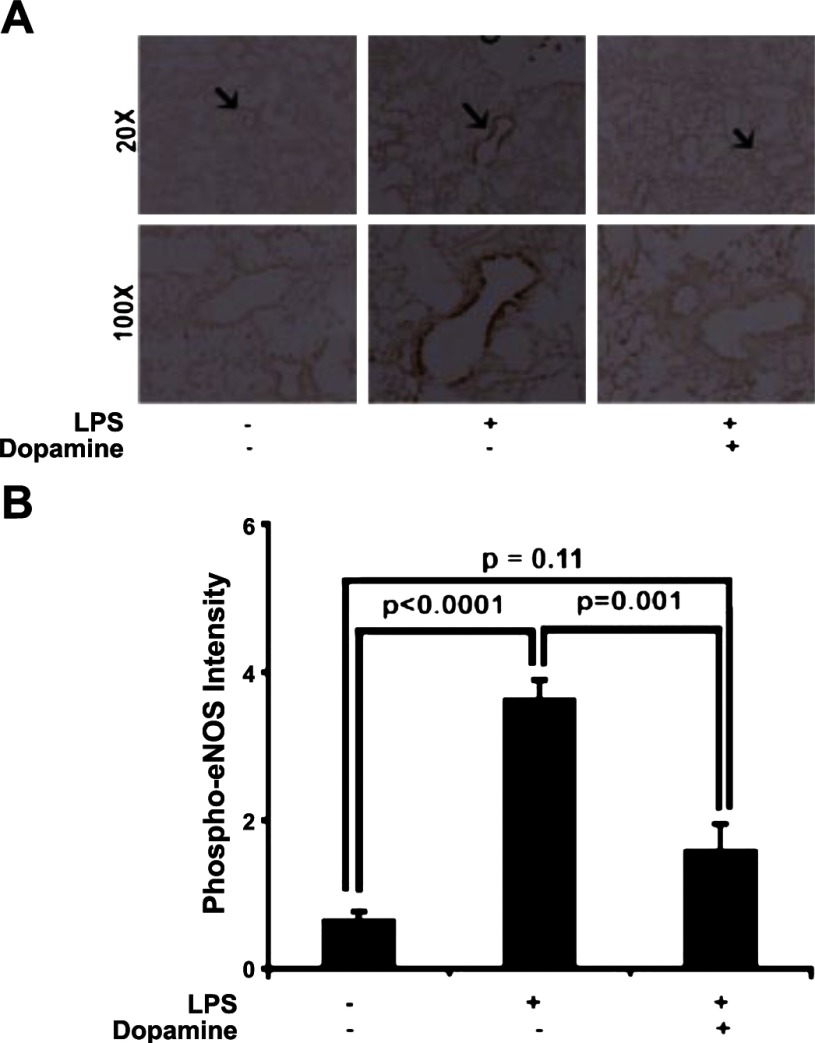
To further demonstrate that VEGFR-2 signaling plays a critical role in sepsis-induced lung injury, we used immunohistochemistry to evaluate eNOS levels, a downstream target of VEGFR-2 signaling. On the basis of our results illustrating effects of dopamine on the VEGF-VEGFR2 signaling axis, we expected decreased levels of phospho-eNOS (10, 30) in dopamine-treated mice challenged with LPS. However, there was no significant change in eNOS phosphorylation observed in sham-treated and dopamine-pretreated LPS animals (P = 0.11, n = 6, Fig. 4A), indicating that the reduction in VEGFR2 phosphorylation influenced eNOS activation (P = 0.001 vs. sham, n = 6, Fig. 4, A and B). Although we did not observe significant change in protein expression levels (P = 0.33 vs. sham, n = 5, Supplemental Fig. S1, A and B; supplemental figures are available online at the American Journal of Physiology Lung Cellular and Molecular Physiology website), we observed a significant reduction in eNOS phosphorylation in these mice, compared with LPS-treated animals.
Fig. 4.
Effect of dopamine and LPS on endothelial nitric oxide synthase (eNOS). Immunostaining of histology slides with phospho-eNOS (Serine 1177) from sham- (n = 6), LPS- (n = 6), and dopamine + LPS-treated (n = 6) groups (black arrows indicate the location of eNOS phosphorylation) (A). Quantitative analysis of immunostained slides with phospho-eNOS was performed with metamorph software (more than three slides per group; B). All samples were taken after 24 h of LPS challenge.
Validation of Postulated Mechanisms in a D2DR Knockout Mouse Model
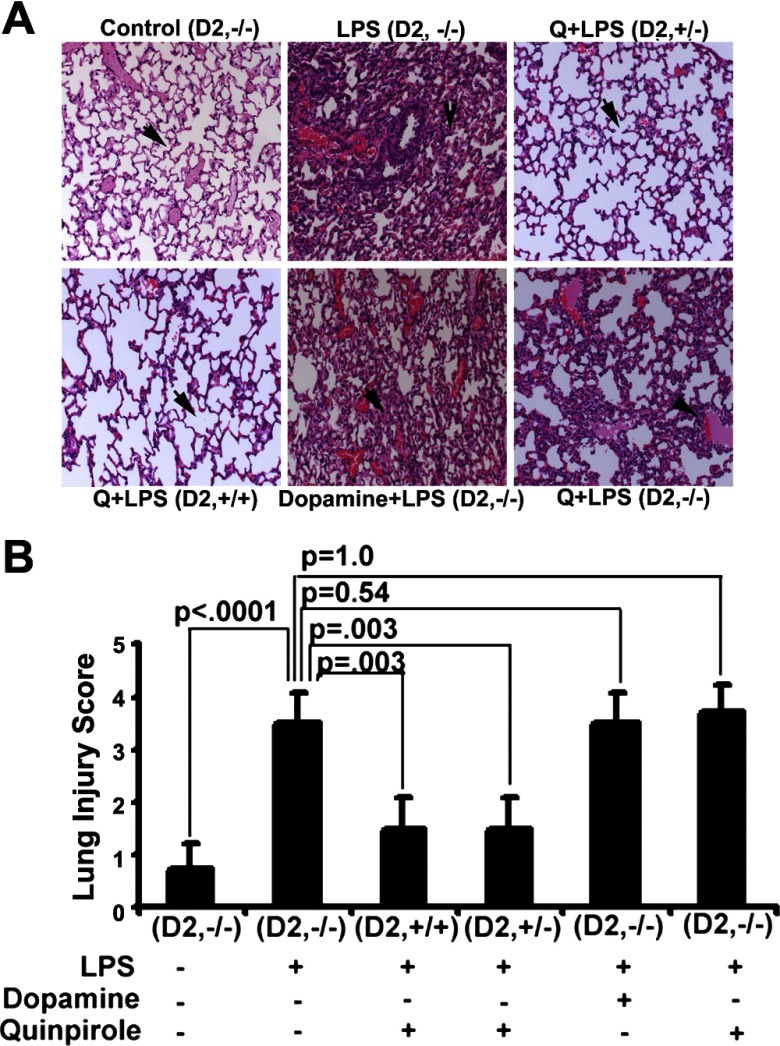
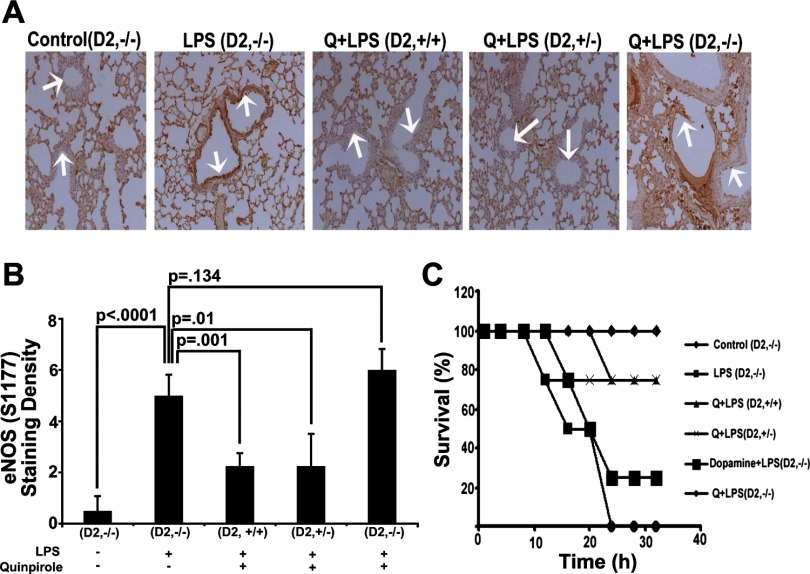
D2DR (−/−), heterozygous (+/−) and wild-type mice (+/+) were challenged with LPS, and effects on histopathology, phospho-eNOS tissue labeling, and survival were examined. Pretreatment with dopamine or the D2DR agonist quinpirole prevented LPS-induced lung injury in wild-type as well as heterozygous D2DR (+/−) mice. These drugs had no lung protective effect in D2DR-null mice. (P = 0.003 vs. sham, n = 6, Fig. 5, A and B). In keeping with these results, the prophylactic administration of dopamine and/or quinpirole was associated with decreased phospho-eNOS labeling in wild-type and heterozygous D2DR (+/−) but failed to ameliorate LPS-induced activation of eNOS in D2DR-null mice (P = 0.54, n = 6, Fig. 6, A and B; P = 1.0, n = 5, Supplemental Fig. S2, A and B). Quinpirole significantly improved the survival rates (n = 8 each group) of LPS-treated wild-type and D2DR (+/−) (n = 6, Fig. 6C) mice but failed to improve the survival of in D2DR (−/−) animals at 32 h (Fig. 6C). Taken together, these results show that dopamine protects against LPS-mediated inflammation and barrier dysfunction via a D2DR activation-dependent mechanism and that a single allele of the D2DR gene appears sufficient to mediate this effect.
Fig. 5.
Effect of D2DR knockout on pulmonary edema. Similar experiments were undertaken in wild-type (+/+, n = 6), heterozygous (+/−, n = 6), and homozygous deleted (−/−, n = 6) D2DR with C57BL/6 mice. No signs of pulmonary edema (black arrow) were visible in the sham- and quinpirole (D2-specific agonist)-treated D2DR wild-type (+/+, n = 6), heterozygous (+/−, n = 6) mice, and quinpirole + LPS-treated mice (n = 6) with stable alveolar architecture. There were high levels of accumulation of fluid in the alveolar space, with damaged alveolar architecture in D2DR homozygous deleted (−/−, n = 6) mice and in LPS-challenged mice. Pretreatment dopamine and or quinpirole did not improve the features of ALI in homozygous deleted group (black arrow; ×20; A). We used a semiquantitative lung injury scoring method as described (see materials and methods) based on previous reports to assess the level of injury in these mice (B).
Fig. 6.
Role of D2DR knockout on activation of eNOS in lung injury model. Immunostaining of histology slides with phospho-eNOS (Serine 1177) from wild-type (+/+, n = 6), heterozygous (+/−, n = 6), homozygous D2DR (−/−, n = 6) deleted mice treated with LPS alone (n = 6) or with quinpirole and LPS (n = 6, A). Quantitative analysis of immunostained slides was performed using metamorph software (more than 3 slides per group, n = 6, B). We also monitored the animal survival profile of each group (n = 8 each group) for 32 h. We observed significant mortality in homozygously (−/−) deleted D2DR mice (n = 0), even on quinpirole pretreatment (n = 0). The quinpirole improved animal survival in heterozygous (+/−, n = 6) and wild-type (+/+, n = 6) animals (C).
DISCUSSION
Our results clearly demonstrate that, in a clinically relevant murine model of sepsis, pretreatment with dopamine and quinpirole significantly attenuates the characteristic features of ALI. Specifically, decrease in the wet-to-dry lung weight ratio, preserved aeration of alveoli as well as intravascular retention of albumin, consistent with barrier protection. These observations are consistent with our premise that the barrier-protective mechanism facilitated by dopamine or quinpirole is mediated by its interaction with VEGF signaling. Our hypothesis is substantiated by the observation that pretreatment with dopamine significantly decreases VEGF level as well as VEGFR2 phosphorylation. However, we do not exclude the possibility of an effect on the delivery of VEGF to alveolar endothelium via recruited monocyte/macrophages and neutrophils, both of which have VEGF in their granules, especially given the observed effect of dopamine on the inhibition of lung parenchymal inflammation. Although our results are indicative of the fact that dopamine mediated barrier protection, in our murine model sepsis is predominantly mediated by VEGF pathway. There are several alternative or complementary mechanisms through which dopamine may influence the formation and clearance of pulmonary edema.
It is a well-established fact that the clinical use of dopamine might significantly increase venous return and cardiac-filling pressure in patients with cardiac dysfunction, sepsis, and respiratory failure (20, 45). We did not measure left atrial or pulmonary venous pressure in our animal model, but we observed a significant reduction in the levels of lung water content, as assessed by weight and histology, in dopamine-treated mice. These results are in accord with the well-established effects of dopamine on alveolar fluid clearance in ALI (31, 34–37). Although we did not measure alveolar fluid clearance independent of lung water or protein permeability, we concluded that a significantly decreased concentration of FITC-albumin in the BAL fluid of dopamine- and LPS-treated mice would not be consistent with a clearance-dominated mechanism of action. In contrast, with capillary fluid egress, alveolar fluid clearance remains the most viable and active energy-dependent process, mediated by coordinated interactions between apical sodium channels and basolateral Na/K ATPase. Both type 1 and type 2 alveolar epithelial cells possess the machinery for transepithelial ion transport, though water simply moves along osmotic gradients (4). Typical alveolar fluid clearance-measurement methods infer water flux from changes in alveolar concentration relative to cell-impermeable molecules such as albumin. Therefore, we anticipated a higher rather than lower FITC-albumin concentration in the BAL fluid of dopamine-treated mice if enhanced clearance had been the dominant mechanism. The preponderance of our data supports the fact that observed dopamine-induced change in albumin extravasation likely arises from the changes in the hydraulic conductance. However, the conclusion that vasoconstriction prevents alveolar edema formation cannot be made on data obtained from albumin extravasation ratio alone.
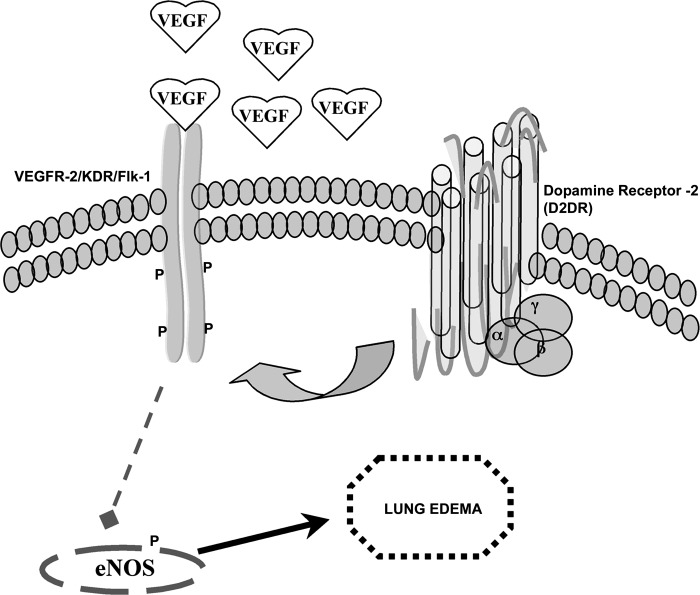
The numerous therapeutic interventions include simvastatin, adenosine triphosphate (ATP), sphingosine 1-phosphate (S1P) and activated protein C (APC), currently (23) being proposed to protect and maintain endothelial barrier integrity in ALI. A previous report from our group clearly enunciated the role of D2DR in protection of endothelial barrier integrity and modulation of vascular permeability (47). The above-mentioned report became the premise for testing the role of a direct modulator of EC barrier function in our murine model of ALI (23). Excessive production of NO has also been implicated in pathophysiology of many diseases including sepsis. Previous reports have suggested that increased phosphorylation of eNOS upon lung injury plays a pivotal role in vascular permeability (8, 19, 21); however, a consensus has not been reached on the mechanism of activation, as the signaling cues correlating VEGF-NOS pathways has not been ascertained until now (24). In this context, a previous report from Knoll and colleagues suggests that indeed VEGFR2 activation upregulates eNOS activation and NO production (28). Another possibility is that there is intracellular cross talk between the D2DR signaling pathway and the phosphorylation of eNOS (44). None of these possibilities can be definitively excluded in the current findings. These observations are in sync with the earlier observations pertaining to dopamine-mediated inhibition of eNOS phosphorylation in vivo and correspond to its role in vascular permeability. Here, we have conclusively demonstrated for the first time that dopamine, acting through its D2 receptors, could significantly attenuate pulmonary edema, suggesting that inexpensive drugs such as dopamine or its specific D2DR agonists could have potential use as an agonist in the treatment of sepsis-induced lung injury (Fig. 7).
Fig. 7.
Proposed model of D2DR role in LPS-induced pulmonary edema. LPS is known to induce overexpression of VEGF in the lung environment, leading to VEGFR2 receptor phosphorylation. The activated VEGFR2 induces the phosphorylation of eNOS and, ultimately, leads to vascular permeability. Activation of D2DR impaired VEGFR2 receptor phosphorylation during sepsis-induced lung injury. This could be the one possible mechanism that inhibits pulmonary edema.
Although there is a large body of literature documenting the pathophysiology and treatment of severe sepsis, there is still an unacceptably high rate of mortality, emphasizing the need to develop novel cost-effective, clinically effective therapeutic strategies. We anticipate that the observations made in the current study will form the rationale for novel cost-effective therapeutic strategies. Further explorative studies are required to investigate the effects of other D2DR agonists and tissue-specific knockdown of D2DR to delineate the role in lung injury. In summary, this study not only reveals a novel link between D2DR and pulmonary edema but also indicates that dopamine and or its D2DR agonists may have a therapeutic value in sepsis-induced lung injury.
Dopamine acts through D2DR to inhibit pulmonary edema-associated vascular permeability mediated through VEGF-VEGFR2 signaling, conveying protective effects in an ALI model. We demonstrated that D2DR plays a critical role in preventing VEGFR2 activation and the subsequent fluid influx associated with lung injury. Our findings highlight the promise of dopamine or D2DR agonists as potential novel therapies for the treatment of pulmonary edema.
GRANTS
This work was partly supported by NIH grants CA78383, HL072178, and HL70567 and Bruce and Martha Atwater Foundation grant to D. Mukhopadhyay.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
REFERENCES
- 1.Baltalarli A, Goksin I, Sirin H, Ortaç R, Onem G, Baltalarli B, Rendex O, Saçar M. Does anti-inflammatory therapy attenuate the lung injury caused by ischemia/reperfusion of the lower extremities in the rabbit? Int J Thoracic Cardiovasc Surg 3: 22, 2000. [Google Scholar]
- 2.Basu S, Nagy JA, Pal S, Vasile E, Eckelhoefer IA, Bliss VS, Manseau EJ, Dasgupta PS, Dvorak HF, Mukhopadhyay D. The neurotransmitter dopamine inhibits angiogenesis induced by vascular permeability factor/vascular endothelial growth factor. Nat Med 7: 569–574, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Becker PM, Alcasabas A, Yu AY, Semenza GL, Bunton TE. Oxygen-independent upregulation of vascular endothelial growth factor and vascular barrier dysfunction during ventilated pulmonary ischemia in isolated ferret lungs. Am J Respir Cell Mol Biol 22: 272–279, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Berthiaume Y, Matthay MA. Alveolar edema fluid clearance and acute lung injury. Respir Physiol Neurobiol 159: 350–359, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertorello AM, Sznajder JI. The dopamine paradox in lung and kidney epithelia: sharing the same target but operating different signaling networks. Am J Respir Cell Mol Biol 33: 432–437, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhandari V, Choo-Wing R, Lee CG, Yusuf K, Nedrelow JH, Ambalavanan N, Malkus H, Homer RJ, Elias JA. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol 39: 420–430, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhattacharya R, Sinha S, Yang SP, Patra C, Dutta S, Wang E, Mukhopadhyay D. The neurotransmitter dopamine modulates vascular permeability in the endothelium. J Mol Signal 3: 14, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA 102: 904–908, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakroborty D, Sarkar C, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Depleted dopamine in gastric cancer tissues: dopamine treatment retards growth of gastric cancer by inhibiting angiogenesis. Clin Cancer Res 10: 4349–4356, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Edirisinghe I, Arunachalam G, Wong C, Yao H, Rahman A, Phipps RP, Jin ZG, Rahman I. Cigarette-smoke-induced oxidative/nitrosative stress impairs VEGF- and fluid-shear-stress-mediated signaling in endothelial cells. Antioxid Redox Signal 12: 1355–1369, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Frey EA, Finlay BB. Lipopolysaccharide induces apoptosis in a bovine endothelial cell line via a soluble CD14 dependent pathway. Microb Pathog 24: 101–109, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Fujita M, Kuwano K, Kunitake R, Hagimoto N, Miyazaki H, Kaneko Y, Kawasaki M, Maeyama T, Hara N. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunol 117: 202–208, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci USA 98: 2604–2609, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 399: 597–601, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godzich M, Hodnett M, Frank JA, Su G, Pespeni M, Angel A, Howard MB, Matthay MA, Pittet JF. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia-reperfusion injury in rats. FASEB J 20: 1519–1521, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Gropper MA, Wiener-Kronish J. The epithelium in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care 14: 11–15, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Guerrero C, Lecuona E, Pesce L, Ridge KM, Sznajder JI. Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol 281: L79–L85, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gurkan OU, O'Donnell C, Brower R, Ruckdeschel E, Becker PM. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am J Physiol Lung Cell Mol Physiol 285: L710–L718, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Hatakeyama T, Pappas PJ, Hobson RW, 2nd, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol 574: 275–281, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess W, Bruckner JB, von Faber du Faur J, Schmidt D, Tarnow J. [The haemodynamic effects of dobutamine and dopamine in patients with coronary artery disease A study performed under general anaesthesia (author's transl)]. Anaesthesist 28: 316–321, 1979. [PubMed] [Google Scholar]
- 21.Hollenberg SM, Guglielmi M, Parrillo JE. Discordance between microvascular permeability and leukocyte dynamics in septic inducible nitric oxide synthase deficient mice. Crit Care 11: R125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotchkiss CE, Hall PD, Cline JM, Willingham MC, Kreitman RJ, Gardin J, Latimer A, Ramage J, Feely T, DeLatte S, Tagge EP, Frankel AE. Toxicology and pharmacokinetics of DTGM, a fusion toxin consisting of a truncated diphtheria toxin (DT388) linked to human granulocyte-macrophage colony-stimulating factor, in cynomolgus monkeys. Toxicol Appl Pharmacol 158: 152–160, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Curr Drug Targets 8: 509–514, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Jonkam CC, Bansal K, Traber DL, Hamahata A, Maybauer MO, Maybauer DM, Cox RA, Lange M, Connelly RL, Traber LD, Djukom CD, Salsbury JR, Herndon DN, Enkhbaatar P. Pulmonary vascular permeability changes in an ovine model of methicillin-resistant Staphylococcus aureus sepsis. Crit Care 13: R19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaner RJ, Crystal RG. Pathogenesis of high altitude pulmonary edema: does alveolar epithelial lining fluid vascular endothelial growth factor exacerbate capillary leak? High Alt Med Biol 5: 399–409, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol 22: 657–664, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Kosmidou I, Karmpaliotis D, Kirtane AJ, Barron HV, Gibson CM. Vascular endothelial growth factors in pulmonary edema: an update. J Thromb Thrombolysis 25: 259–264, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Kroll J, Waltenberger J. VEGF-A induces expression of eNOS and iNOS in endothelial cells via VEGF receptor-2 (KDR). Biochem Biophys Res Commun 252: 743–746, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Leaver SK, Evans TW. Acute respiratory distress syndrome. BMJ 335: 389–394, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marwick JA, Edirisinghe I, Arunachalam G, Stevenson CS, Macnee W, Kirkham PA, Rahman I. Cigarette smoke regulates VEGFR2-mediated survival signaling in rat lungs. J Inflamm 7: 11, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay MA. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest 122: 340S–343S, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, Macintyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta-2 agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184: 561–568, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthay MA, Calfee CS. Therapeutic value of a lung protective ventilation strategy in acute lung injury. Chest 128: 3089–3091, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Matthay MA, Clerici C, Saumon G. Invited review: Active fluid clearance from the distal air spaces of the lung. J Appl Physiol 93: 1533–1541, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Matthay MA, Robriquet L, Fang X. Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2: 206–213, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Matthay MA, Uchida T, Fang X. Clinical acute lung injury and acute respiratory distress syndrome. Curr Treat Options Cardiovasc Med 4: 139–149, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319–327, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medford AR, Douglas SK, Godinho SI, Uppington KM, Armstrong L, Gillespie KM, van Zyl B, Tetley TD, Ibrahim NB, Millar AB. Vascular endothelial growth factor (VEGF) isoform expression and activity in human and murine lung injury. Respir Res 10: 27, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods 14: 157–167, 1985. [DOI] [PubMed] [Google Scholar]
- 40.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest 101: 2567–2578, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel GP, Grahe JS, Sperry M, Singla S, Elpern E, Lateef O, Balk RA. Efficacy and safety of dopamine versus norepinephrine in the management of septic shock. Shock 33: 375–380, 2010. [DOI] [PubMed] [Google Scholar]
- 42.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med 173: 281–287, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Pham I, Uchida T, Planes C, Ware LB, Kaner R, Matthay MA, Clerici C. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 283: L1133–L1142, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Pyne-Geithman GJ, Caudell DN, Cooper M, Clark JF, Shutter LA. Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro. Neurocrit Care 10: 225–231, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qvist J, Brynjolf I, Munck O. The effects of dopamine and noradrenaline on cardiovascular function in patients with acute respiratory failure. Eur J Anaesthesiol 11: 107–110, 1994. [PubMed] [Google Scholar]
- 46.Sarkar C, Chakroborty D, Mitra RB, Banerjee S, Dasgupta PS, Basu S. Dopamine in vivo inhibits VEGF-induced phosphorylation of VEGFR-2, MAPK, and focal adhesion kinase in endothelial cells. Am J Physiol Heart Circ Physiol 287: H1554–H1560, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Sinha S, Vohra PK, Bhattacharya R, Dutta S, Mukhopadhyay D. Dopamine regulates phosphorylation of VEGF receptor 2 by engaging Src-homology-2-domain-containing protein tyrosine phosphatase 2. J Cell Sci 122: 3385–3392, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tassiopoulos AK, Carlin RE, Gao Y, Pedoto A, Finck CM, Landas SK, Tice DG, Marx W, Hakim TS, McGraw DJ. Role of nitric oxide and tumor necrosis factor on lung injury caused by ischemia/reperfusion of the lower extremities. J Vasc Surg 26: 647–656, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 290: L209–L221, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Ware LB, Kaner RJ, Crystal RG, Schane R, Trivedi NN, McAuley D, Matthay MA. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. Eur Respir J 26: 101–105, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1376–1383, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369: 1553–1564, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, Simms BT, Mizgerd JP, Carmeliet P, Karumanchi SA, Aird WC. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J Exp Med 203: 1447–1458, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S, Rahman M, Qi Z, Herwald H, Thorlacius H. Simvastatin regulates CXC chemokine formation in streptococcal M1 protein-induced neutrophil infiltration in the lung. Am J Physiol Lung Cell Mol Physiol 300: L930–L939, 2011. [DOI] [PubMed] [Google Scholar]
- 56.Zheng H, Chen XL, Han ZX, Zhang Z, Wang SY, Xu QL. Ligustrazine attenuates acute lung injury after burn trauma. Burns 31: 453–458, 2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.