Abstract
Phosphodiesterase (PDE) 4 inhibitors are potent anti-inflammatory drugs with antihypertensive properties, and their therapeutic role in bronchopulmonary dysplasia (BPD) is still controversial. We studied the role of PDE4 inhibition with piclamilast on normal lung development and its therapeutic value on pulmonary hypertension (PH) and right ventricular hypertrophy (RVH) in neonatal rats with hyperoxia-induced lung injury, a valuable model for premature infants with severe BPD. The cardiopulmonary effects of piclamilast treatment (5 mg·kg−1·day−1) were investigated in two models of experimental BPD: 1) daily treatment during continuous exposure to hyperoxia for 10 days; and 2) late treatment and injury-recovery in which pups were exposed to hyperoxia or room air for 9 days, followed by 9 or 42 days of recovery in room air combined with treatment started on day 6 of oxygen exposure until day 18. Prophylactic piclamilast treatment reduced pulmonary fibrin deposition, septum thickness, arteriolar wall thickness, arteriolar vascular smooth muscle cell proliferation and RVH, and prolonged survival. In the late treatment and injury-recovery model, hyperoxia caused persistent aberrant alveolar and vascular development, PH, and RVH. Treatment with piclamilast in both models reduced arteriolar wall thickness, attenuated RVH, and improved right ventricular function in the injury recovery model, but did not restore alveolarization or angiogenesis. Treatment with piclamilast did not show adverse cardiopulmonary effects in room air controls in both models. In conclusion, PDE4 inhibition attenuated and partially reversed PH and RVH, but did not advance alveolar development in neonatal rats with hyperoxic lung injury or affect normal lung and heart development.
Keywords: right ventricular hypertrophy, pulmonary hypertension, bronchopulmonary dysplasia, oxidative stress, piclamilast
the preterm lung is highly susceptible to injury during resuscitation, mechanical ventilation, and proinflammatory mediators that may interfere with signaling pathways required for normal lung development, and this may progress toward bronchopulmonary dysplasia (BPD), a chronic lung disease (4). The hallmark in BPD is alveolar enlargement caused by an arrest in alveolar and vascular development. Serious complicating factors in the perinatal period are inflammation and oxidative stress and, at later stages, pulmonary hypertension (PH), due to elevated pulmonary artery pressure and pulmonary vascular resistance that increase afterload of the right ventricle (RV) and ultimately lead to RV hypertrophy (RVH) and associated cardiac disease (4, 21, 22). PH is characterized by persistent vasoconstriction and structural remodeling of the pulmonary blood vessels, including increased proliferation of vascular smooth muscle cells, which ultimately lead to high mortality in the absence of appropriate treatment due to right heart failure in children and adults (1, 3, 19, 33, 36).
Agents that elevate intracellular cGMP or cAMP levels exert therapeutic effects in experimental models of PH (10, 11, 20, 31). Phosphodiesterases (PDEs) inactivate the second messengers of important pulmonary vasodilator agents, including prostacyclin and nitric oxide, by hydrolysis. cAMP and its downstream target protein kinase A inhibit the extracellular signal-regulated kinase activation and suppress the proliferation of pulmonary fibroblasts, vascular smooth muscle cells, airway epithelial cells, and inflammatory cells (35). Among the 11 families of PDEs, the major cAMP-metabolizing enzymes are attributed to the PDE4 family (7, 14, 15, 35), which consists of four genes (A–D) that are expressed in all immunocompetent cells, pulmonary artery smooth muscle cells (27), fibroblasts, and endothelial and epithelial cells (35). Our laboratory recently demonstrated, in a hyperoxia-induced neonatal lung injury rat model, that PDE4 inhibition improved survival and reduced lung injury by attenuating pulmonary inflammation (12).
The effect of PDE4 inhibition on cardiac disease in experimental BPD has to be elucidated, whereas the role of PDE4 inhibition on alveolarization in neonatal hyperoxic lung injury and in normal lung development in rodents is controversial (12, 26, 39). Therefore, we studied the cardiopulmonary effects of the second generation PDE4 inhibitor piclamilast in neonatal rats with severe hyperoxia-induced BPD, which is complicated by PH and RVH, using two different treatment strategies: 1) a prophylactic treatment strategy (early concurrent treatment); and 2) a more clinically relevant strategy, in which treatment was started after injury was induced (late treatment and injury-recovery). We demonstrated that prophylactic PDE4 inhibition with piclamilast in hyperoxia-induced neonatal lung injury improved angiogenesis and attenuated PH and RVH, but did not induce septation in the enlarged alveoli. In the injury-recovery model, neonatal exposure to hyperoxia for 9 days induced persistent alveolar simplification, PH, and RVH in young adult rats. Late treatment with piclamilast reversed established PH and RVH, but did not advance alveolar and pulmonary vascular development. Piclamilast treatment did not exert adverse effects on normal lung and heart development in both models, despite postnatal growth retardation.
MATERIALS AND METHODS
Full methodological details are available in the online supplement. (The online version of this article contains the supplemental data.)
Animals
The research protocol was approved by the Institutional Animal Care and Use Committee of the Leiden University Medical Center. Neonatal rat pups were pooled and distributed over two experimental groups (N = 12): an oxygen and oxygen-piclamilast group, and two room air-exposed control groups injected either with saline or piclamilast. The oxygen concentration, body weight, evidence of disease, and mortality were monitored daily.
Early concurrent treatment.
Pups were continuously exposed to 100% oxygen for 10 days (Fig. 1A). From day 2, pups received either 100 μl piclamilast (5.0 mg·kg−1·day−1; a gift from Nycomed, Konstanz, Germany) in 0.9% saline (containing 0.05–0.1% DMSO), or daily 0.9% saline (containing 0.05–0.1% DMSO; 100 μl), subcutaneously. Except for the survival experiments, lung and heart tissue were collected on days 1, 3, 6, and 10. Separate experiments were performed for collection of lung and heart tissue for pulmonary fibrin deposition and RT-PCR (N = 12) and histology (N = 12).
Fig. 1.
A: in the early concurrent treatment model for experimental bronchopulmonary dysplasia (BPD), neonatal rat pups were exposed to 100% oxygen (O2; solid bars) or room air (RA) directly after birth (day 1) until day 10. Treatment of RA and O2 pups with piclamilast (pic; 5 mg·kg−1·day−1; shaded bars; RA-pic and O2-pic, respectively) or 0.01% DMSO in 0.9% NaCl (open bars) was started on day 2 until day 10. †Lung and heart tissues were harvested on days 1, 3, 6, and 10. B: in the late treatment and recovery model for experimental BPD, neonatal rat pups were exposed to O2 (solid bars) or RA directly after birth (day 1) until day 9. Hereafter, pups were allowed to recover in RA up to day 51. Treatment with pic (shaded bars) or DMSO in 0.9% NaCl (open bars) was started on day 6 until day 18. †Lung and heart tissues were harvested on days 9 (end-hyperoxic period), 18 (end-treatment period), and 51. Right ventricular (RV) function was determined on day 18.
Late treatment and recovery.
Lung injury and recovery were investigated by exposing pups to hyperoxia for 9 days, followed by recovery in room air for 9 or 42 days (Fig. 1B). After 6 days of hyperoxia, daily injections with 100 μl piclamilast (5.0 mg·kg−1·day−1) in 0.9% saline (containing 0.05–0.1% DMSO) or 100 μl 0.9% saline (containing 0.05–0.1% DMSO) were started and continued throughout a 9-day recovery period in room air. Lung and heart tissues were collected for histology at the end of the 9-day hyperoxia period (N = 8), after a 9-day recovery period in room air (N = 8), and after 6-wk of recovery in room air (N = 8).
Tissue Preparation
Lungs and heart were snap-frozen in liquid nitrogen for real-time RT-PCR or fibrin deposition assay and fixed in formalin for histology studies, as previously described (10, 11).
Histology
Formalin-fixed, paraffin-embedded, 4-μm-thick heart and lung sections were stained with hematoxylin and eosin. Lungs were immunostained additionally with anti-α-smooth muscle actin (ASMA; 1:10,000), anti-von Willebrand factor (vWF; 1:4,000), anti-tenascin-C (TN-C; 1:500), or anti-Ki67 (1:125) using standard methods (10, 11). Quantitative morphometry was performed by two independent researchers blinded to the treatment strategy, as previously described (10, 41).
Fibrin Detection Assay
Quantitative fibrin deposition in lung tissue homogenates was determined by Western blotting, as described previously (11, 38).
Cyclic AMP Assay
The cyclic AMP concentration was determined in lung tissue homogenates using a cyclic AMP EIA kit (581001.1, Cayman Chemical, Ann Arbor, MI), according to the manufacturer's instructions.
Real-time RT-PCR
Total RNA isolation from lung and heart tissue homogenates, first-strand cDNA synthesis, and real-time quantitative PCR were performed as described previously (10, 38). Primers are listed in Table 1.
Table 1.
Sequences of oligonucleotides used as forward and reverse primers for real-time RT-PCR
| Gene Product | Forward Primer | Reverse Primer |
|---|---|---|
| ANP | 5′-CCAGGCCATATTGGAGCAAA-3′ | 5′-AGGTTCTTGAAATCCATCAGATCTG-3′ |
| BNP | 5′-GAAGCTGCTGGAGCTGATAAGAG-3′ | 5′-TGTAGGGCCTTGGTCCTTTG-3′ |
| ECE-1 | 5′-GCCCACCCTGGGTCTCA-3′ | 5′-AGCACCAGACCTGTGCGAAT-3′ |
| ET-1 | 5′-TGTGCTCACCAAAAAGACAAGAA-3′ | 5′-GGTACTTTGGGCTCGGAGTTC-3′ |
| ETA | 5′-CACGACCAAGTTCATGGAGTTTT-3′ | 5′-AGGGCATGCAGAAGTAGAATCC-3′ |
| ETB | 5′-CAGGATTCTGAAGCTCACCCTTT-3′ | 5′-TCCAAAACCAGCAAAAAACTCA-3′ |
| IL-6 | 5′-ATATGTTCTCAGGGAGATCTTGGAA-3′ | 5′-TGCATCATCGCTGTTCATACAA-3′ |
| TF | 5′-CCCAGAAAGCATCACCAAGTG-3′ | 5′-TGCTCCACAATGATGAGTGTT-3′ |
| VEGFA | 5′-GCGGATCAAACCTCACCAAA-3′ | 5′-TTGGTCTGCATTCACATCTGCTA-3′ |
| VEGFR2 | 5′-CCACCCCAGAAATGTACCAAAC-3′ | 5′-AAAACGCGGGTCTCTGGTT-3′ |
| β-Actin | 5′-TTCAACACCCCAGCCATGT-3′ | 5′-AGTGGTACGACCAGAGGCATACA-3′ |
ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; ECE-1, endothelin converting enzyme-1; ET, endothelin; IL-6, interleukin-6; TF, tissue factor; VEGF, vascular endothelial growth factor.
Hemodynamic Measurements
On day 18, RV pressure-volume loops were determined as previously described (18). After anesthetized rats were mechanically ventilated, a combined pressure-conductance catheter (model FT212, SciSense, London, Ontario, Canada) was introduced via the apex into the RV and positioned toward the pulmonary valve. The catheter was connected to a signal processor (FV898 Control Box, SciSense), and RV pressures and volumes were recorded digitally and analyzed. After hemodynamic measurements, the heart was removed, fixed in buffered formaldehyde, and processed for histology.
Statistical Analysis
Values are expressed as means ± SE. Differences between groups (≥3) were analyzed with ANOVA, followed by Tukey's multiple-comparison test. For comparison of survival curves, Kaplan-Meier analysis, followed by a log rank test, was performed. Differences in the number of RVs positive for TN-C were analyzed with contingency table analysis, followed by Fisher's exact test. GraphPad Prism 5 (GraphPad Software, La Jolla, CA) was used for statistical analysis. Differences at P values < 0.05 were considered statistically significant.
RESULTS
Effects of Piclamilast on Growth and Survival
Prophylactic treatment model.
At birth, on postnatal day 1, mean body weight of the preterm rat pups was 5.2 g (Fig. 2A). In room air-exposed control pups treated with 5.0 mg·kg−1·day−1 of piclamilast, growth was significantly retarded from day 5 onward compared with room air- and oxygen-exposed controls (P < 0.05; Fig. 2A). Mean body weight of room air-exposed controls was 21.2 g, and that of oxygen-exposed pups was 15.2 g on day 10. Piclamilast treatment significantly reduced body weight in room air- and oxygen-exposed pups to 13.2 and 10.6 g, respectively (Fig. 2B). After 10 days of oxygen exposure, 77% of the oxygen-exposed control pups survived vs. 100% of the pups of the other experimental groups (P < 0.001; Fig. 2C).
Fig. 2.
Growth (A), body weight (B and D), and survival (C and E) at day 10 after early concurrent treatment (N = 12; A–C) and after late treatment and recovery (N = 8; D and E) on days 9, 18, and 51 in RA controls (open bars, ◊), RA pups treated with 5.0 mg·kg−1·day−1 pic (RA-pic; hatched bars, ♦), age-matched O2-exposed controls (solid bars, ▵), and O2 pups treated with 5.0 mg·kg−1·day−1 pic (O2-pic; shaded bars, ▴). Growth and body weight are expressed as means ± SE. C: Kaplan-Meier survival curve of O2-pic rat pups (▵), age-matched, O2-exposed controls (▵), RA-exposed controls (◊), and RA-pic pups (♦) during the first 10 days after birth (N = 12). Survival data are expressed as percentage ± SE of pups surviving at the observed time point. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. age-matched O2-exposed controls. Δ P < 0.05, ΔΔ P < 0.01, and ΔΔΔ P < 0.001 vs. RA-pic pups. φ P < 0.001 vs. recovery on postnatal day (pd) 18.
Late treatment and injury-recovery model.
On day 9, mean body weight of room air pups was 17.8 ± 0.4 g (Fig. 2D). On day 18, mean body weight was 32 ± 0.8 g and increased to 190 ± 7 g into adulthood on day 51. Mean body weight after 9 days of hyperoxia exposure was 13.3 ± 0.5 g. A recovery period of 42 days (day 51) in room air resulted in a significant difference between room air-exposed and oxygen-exposed controls (190 ± 7 vs. 165 ± 8 g, P < 0.01). Treatment of room air controls and oxygen-exposed pups with piclamilast did not have a significant effect on mean body weight.
On days 9, 18, and 51, all room air-exposed pups survived (Fig. 2E). Exposure to hyperoxia for 9 days resulted in a 73% survival, which increased to 90% after treatment with piclamilast during the last 3 days of hyperoxia (P < 0.001). Eighty percent of the pups that recovered in room air after hyperoxic lung injury survived until day 18, and >95% of the 18-day survivors were still alive on day 51. Survival on days 18 and 51 was not affected by piclamilast treatment.
Effects of Piclamilast on Lung Airway Development
Prophylactic treatment model.
Lung development proceeds from the saccular stage at birth toward the alveolar stage on day 10 (Fig. 3A). Treatment with piclamilast for 10 days during normal neonatal development did not result in differences in alveolar septum thickness, pulmonary vessel density, alveolar crest (Fig. 3, B and E–G), and arteriolar medial wall thickness (Fig. 4, B and I) compared with room air-exposed controls. Oxygen exposure for 10 days resulted in lung edema, a heterogeneous distribution of enlarged air spaces, which were surrounded by septa with increased thickness (1.8-fold, P < 0.001; Fig. 3, C and F), a marked reduction in pulmonary vessel density (1.5-fold, P < 0.001 on day 6 and 3.8-fold, P < 0.001 on day 10; Fig. 3, C and E) and number of alveolar crests (3.7-fold, P < 0.001; Fig. 3G), and an increase in arteriolar medial wall thickness (2.8-fold, P < 0.001 on day 10; Fig. 4, C and I). Piclamilast treatment partially improved alveolar development during hyperoxia by thinning of alveolar septa (31%, P < 0.01; Fig. 3, D and F), increasing pulmonary vessel density (58.1%, P < 0.05 on day 10; Fig. 3, D and E), and reducing arteriolar medial wall thickness (48.8%, P < 0.001 on day 10; Fig. 4, D and I), but did not improve alveolar enlargement, determined by the number of alveolar crests per tissue ratio (Fig. 3G) compared with oxygen exposure for 10 days. To investigate the role of vascular smooth muscle cell proliferation in the hyperoxia-induced increase in medial wall thickness, we quantified the number of cells positive for the proliferation marker Ki67 (Fig. 4, E–H) in the ASMA-positive layer of small arterioles (Fig. 4, A–D). Treatment with piclamilast for 10 days during normal neonatal development did not result in differences in the number of Ki67-positive cells relative to arteriolar diameter (Fig. 4J) or medial wall thickness (Fig. 4K) compared with room air-exposed controls. Oxygen exposure for 10 days resulted in a persistent increase in the number of Ki67-positive cells from day 3 onward (1.6-fold, P < 0.01 on day 3; 1.8-fold, P < 0.05 on day 6 and 2.5-fold, P < 0.01 on day 10; Fig. 4, G and J) compared with room air-exposed controls, which could be prevented by piclamilast treatment (Fig. 4, H and J). The hyperoxia-induced increase in Ki67-positive cells in the ASMA-positive layer of small arterioles on days 6 and 10, but not on day 3, could be explained by an increase in medial wall thickness (Fig. 4K).
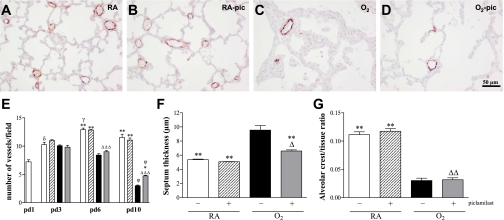
Fig. 3.
Lung sections stained for von Willebrand factor (A–D) and lung morphometry (E–G) of RA controls (A and open bars), RA-pic pups treated with 5.0 mg·kg−1·day−1 pic (B and hatched bars), age-matched O2-exposed controls (C and solid bars), and O2-pic pups treated with 5.0 mg·kg−1·day−1 pic (D and shaded bars) on day 10 (A–D, F, and G) or on days 1, 3, 6, and 10 (E) after early concurrent treatment. Pictures were taken at a ×200 magnification. Lung morphometry, including the quantifications of number of pulmonary vessels (E), septum thickness (F), and alveolar crest per tissue ratio (G), was determined on paraffin sections in RA and O2 pups daily injected either with saline or pic. Values are means ± SE (N = 12). *P < 0.05 and **P < 0.001 vs. age-matched, O2-exposed controls. Δ P < 0.05, ΔΔ P < 0.01, and ΔΔΔ P < 0.001 vs. RA-pic pups. φ P < 0.001 vs. pd6. γ P < 0.01 vs. pd3. δ P < 0.001 vs. pd1.
Fig. 4.
Serial lung sections stained for α-smooth muscle actin (ASMA; A–D) and for the proliferation marker Ki67 (E–H), and lung morphometry (I–K) of RA controls (A and E, open bars), RA-pic (B and F, hatched bars), age-matched, O2-exposed controls (C and G, solid bars), and O2-pic pups (D and H, shaded bars) on day 10 (A–H) or on days 1, 3, 6, and 10 (I–K) after early concurrent treatment. Pictures were taken at a ×1,000 magnification. Lung morphometry, including the quantifications of medial wall thickness (I) and number of Ki67-positive cells in the ASMA-positive layer of small arterioles relative to vessel diameter (J) or medial wall thickness (K), was determined on paraffin sections in RA and O2 pups daily injected either with saline or pic. Values are means ± SE (N = 12, I; and N = 6, J and K). Arrows in G indicate Ki67-positive cells in the ASMA-positive layer of the arteriole. *P < 0.05, **P < 0.01 and ***P < 0.001 vs. age-matched, O2-exposed controls. φ P < 0.001 vs. pd6.
Late treatment and injury-recovery model.
Treatment of room air-exposed pups with piclamilast had no adverse effects on vascular (Fig. 5A) and alveolar development (Fig. 5B) and medial wall thickness (Fig. 5C) on days 9, 18, and 51. Continuous neonatal exposure to hyperoxia for 9 days resulted in enlarged alveoli, demonstrated by a 2.7-fold decrease in the number of alveolar crests (P < 0.001; Fig. 5B), and disturbed vascular development, demonstrated by a 2.5-fold reduction in blood vessel density (P < 0.001; Fig. 5A) and a 2.4-fold increase in medial wall thickness (P < 0.001; Fig. 5C) compared with room air controls. Piclamilast treatment during the last 3 days of the injurious hyperoxic period from day 6 to day 9 did not improve alveolarization, vascular development, and medial wall thickness. A recovery period of 9 days in room air after hyperoxia-induced lung injury had a minor beneficial effect on the number of alveolar crests (P < 0.001; Fig. 5B) and blood vessel density (P < 0.001; Fig. 5A), but no effect on medial wall thickness (Fig. 5C). Treatment with piclamilast did not improve alveolarization and vascular development, but reduced significantly medial wall thickness by 42.7% (P < 0.001; Fig. 5C) compared with nontreated hyperoxia-exposed pups at the end of the recovery period on day 18. A recovery period of 42 days in room air after hyperoxia-induced lung injury had only a minor beneficial effect on the number of blood vessels on day 51 (P < 0.01; Fig. 5A), but no improvement on alveolarization or medial wall thickness. Treatment with piclamilast did not improve alveolarization and vascular development, but reduced medial wall thickness by 45.9% (P < 0.001; Fig. 5C) compared with nontreated hyperoxia-exposed pups at the end of the recovery period on day 51.
Fig. 5.
Quantification of number of pulmonary vessels (A), alveolar crest per tissue ratio (B), and medial wall thickness (C) determined on paraffin sections after late treatment and recovery on days 9, 18, and 51 in RA controls (open bars), RA-pic pups treated with 5.0 mg·kg−1·day−1 pic (hatched bars), age-matched, O2-exposed controls (solid bars), and O2-pic pups treated with 5.0 mg·kg−1·day−1 pic (shaded bars). Values are means ± SE (N = 8). *P < 0.001 vs. age-matched, O2-exposed controls. Δ P < 0.01 and ΔΔ P < 0.001 vs. RA-pic pups. δ P < 0.05, δδ P < 0.01, and δδδ P < 0.001 vs. recovery on pd9. φ P < 0.05, φφ P < 0.01, and φφφ P < 0.001 vs. recovery on pd18.
Effects of Piclamilast on Pulmonary Fibrin Deposition and cAMP Levels
Prophylactic treatment model.
Pulmonary fibrin deposition, a sensitive marker for tissue damage in hyperoxia-induced lung injury, was studied in lung tissue homogenates (Fig. 6A). Fibrin deposition was at reference levels during normal neonatal pulmonary development on day 10 in the absence or presence of piclamilast treatment (<25 ng fibrin/mg tissue) and increased ninefold in lungs of pups exposed to 100% oxygen for 10 days (P < 0.01). Piclamilast therapy attenuated hyperoxia-induced fibrin deposition by 80% (P < 0.01), as demonstrated previously (12).
Fig. 6.
Western blot analysis of fibrin deposition (A) and quantification of cyclic AMP (cAMP; B) in lung homogenates of RA controls (open bars), RA-pic pups treated with 5.0 mg·kg−1·day−1 pic (hatched bars), age-matched, O2-exposed controls (solid bars), and O2-pic pups treated with 5.0 mg·kg−1·day−1 pic (shaded bars) on day 10 after early concurrent treatment. Values are means ± SE (N = 12). **P < 0.01 and ***P < 0.001 vs. age-matched, O2-exposed controls. ΔΔΔ P < 0.001 vs. RA-exposed controls.
Exposure to hyperoxia for 10 days decreased the cyclic AMP levels in lung homogenates 1.5-fold (P < 0.01), compared with room air controls (Fig. 6B). Treatment with piclamilast did not restore the hyperoxia-induced decrease in cAMP levels and had no effect on cAMP levels during normal development in room air controls.
mRNA Expression in Lung Tissue
Prophylactic treatment model.
Treatment with piclamilast for 10 days during normal neonatal development resulted in a decrease in mRNA expression (Fig. 7) of endothelin converting enzyme-1 (ECE-1) by 1.3-fold (F), endothelin (ET) receptor A (ETA) by 1.4-fold (G) and ETB by 1.4-fold (H), but did not result in significant differences in mRNA expression of the inflammatory protein interleukin-6 (IL-6; A), the procoagulant protein tissue factor (TF; B), the growth factor vascular endothelial growth factor A (VEGFA; C), and its receptor vascular endothelial growth factor receptor-2 (VEGFR2; D). Ten days of oxygen exposure resulted in an increase in mRNA expression of IL-6 by 128-fold (P < 0.001), TF by 4.6-fold (P < 0.001), and endothelin-1 (ET-1) by 7.2-fold (P < 0.001), and a decrease in mRNA expression of VEGFA by 2.2-fold (P < 0.001), VEGFR2 by 2.7-fold (P < 0.001), ECE-1 by 1.3-fold (P < 0.05), ETA by 2.2-fold (P < 0.001), and ETB by 2.0-fold (P < 0.001). Treatment with piclamilast of oxygen-exposed pups for 10 days resulted in a reduction of the mRNA expression of IL-6 by 70% (P < 0.001) and TF by 54% (P < 0.001) and an increase in mRNA expression of VEGFA by 63% (P < 0.001), VEGFR2 by 102% (P < 0.05), and ETA by 56% (P < 0.001) compared with oxygen-exposed pups.
Fig. 7.
Relative mRNA expression in lungs, determined with RT-PCR, of genes related to inflammation [interleukin-6 (IL-6); A], coagulation [tissue factor (TF); B], alveolar growth [vascular endothelial growth factor A (VEGFA), C; and VEGF receptor 2 (VEGFR2); D], and the endothelin (ET) signaling pathway [ET-1, E; endothelin converting enzyme-1 (ECE-1), F; ETA, G; and ETB, H] on day 10 after early concurrent treatment. Experimental groups include RA controls (open bar), RA-pic rat pups treated with 5.0 mg·kg−1·day−1 pic (hatched bar), age-matched O2-exposed controls (solid bar), and O2-pic rat pups treated with 5.0 mg·kg−1·day−1 pic (shaded bar). Values are means ± SE (N = 12). *P < 0.05 and ***P < 0.001 vs. age-matched, O2-exposed controls. Δ P < 0.05, ΔΔ P < 0.01, and ΔΔΔ P < 0.001 vs. RA-exposed controls.
Heart Development and RVH
Prophylactic treatment model.
At birth the ratio between RV and left ventricular (LV) free wall thickness was 0.44, decreased to 0.23 on day 3, and did not change hereafter until day 10 (Fig. 8A). Treatment of control pups with piclamilast did not result in any differences in cardiac characteristics (Fig. 8, A–C, and Table 2). Exposure to hyperoxia resulted in a gradual development of RVH, as demonstrated by a 1.5-fold (P < 0.05) and a 2.1-fold (P < 0.001) increase in the ratio between RV and LV free wall thickness and a 1.6-fold (P < 0.05) and a 2.2-fold (P < 0.001) increase in RV free wall thickness on days 6 and 10, respectively, which was completely prevented by treatment with piclamilast (Fig. 8, A and B, and Table 2). Exposure to hyperoxia showed a tendency toward an increase in interventricular septum (IVS) thickness, but did not have a significant effect on LV free wall and IVS thickness (Fig. 8B and Table 2). Because the hyperoxia-induced increase in RV free wall thickness was highest on day 10, we also studied the weight ratio RV/(LV + IVS) as an additional marker of RVH on day 10 (Fig. 8C). Exposure to hyperoxia for 10 days resulted in a 1.5-fold increase in the RV/(LV + IVS) weight ratio (P < 0.001; Fig. 8C), which was prevented by treatment with piclamilast. Extracellular expression of TN-C, a marker of myocardial overload, was visible in the RV only after exposure to hyperoxia (Supplemental Fig. S1C, Table 3). Piclamilast treatment decreased the number of RVs positive for TN-C by 90% (P < 0.05, Supplemental Fig. S1D, Table 3). Extravascular TN-C expression was absent in room air-exposed controls injected with either saline or 5.0 mg·kg−1·day−1 of piclamilast (Supplemental Fig. S1, A and B, Table 3).
Fig. 8.
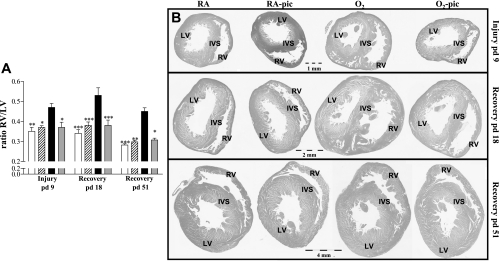
Ventricular free wall thickness, indicated as the RV-to-left ventricle (LV) ratio (A) and RV hypertrophy depicted as the ratio RV/[LV + interventricular septum (IVS)] (C) in RA-exposed controls (open bars), RA-pic pups treated with pic (5.0 mg·kg−1·day−1; hatched bar), age-matched, O2-exposed controls (solid bar), and O2-pic pups treated with pic (5.0 mg·kg−1·day−1; shaded bar) on day 10 (C) or on days 1, 3, 6, and 10 (A and B) after early concurrent treatment. Values are means ± SE (N = 12). B: paraffin heart sections stained with hematoxylin and eosin at a × 40 magnification in RA controls, RA-pic, O2 controls, and O2-pic on days 1, 3, 6, and 10. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. age-matched, O2-exposed controls. δ P < 0.01 vs. pd3. φ P < 0.01 vs. pd6.
Table 2.
Cardiac characteristics in early concurrent treatment
| RA |
O2 |
||||
|---|---|---|---|---|---|
| Day | Saline | Piclamilast | Saline | Piclamilast | |
| RV free wall thickness | 1 | 85 ± 8 | NA | NA | NA |
| 3 | 59 ± 3 | 54 ± 4 | 58 ± 3 | 47 ± 3 | |
| 6 | 71 ± 4* | 65 ± 7** | 112 ± 5δ | 63 ± 4** | |
| 10 | 85 ± 7*** | 85 ± 4*** | 184 ± 13φ | 111 ± 4***,φφ | |
| IVS thickness | 1 | 169 ± 10 | NA | NA | NA |
| 3 | 216 ± 7 | 212 ± 12 | 191 ± 7 | 186 ± 7 | |
| 6 | 267 ± 11 | 213 ± 10** | 309 ± 14δ | 250 ± 8 | |
| 10 | 289 ± 26 | 302 ± 10φ | 324 ± 13 | 264 ± 6* | |
| LV free wall thickness | 1 | 200 ± 11 | NA | NA | NA |
| 3 | 257 ± 12 | 256 ± 13 | 252 ± 5 | 231 ± 8 | |
| 6 | 289 ± 12 | 253 ± 9 | 303 ± 11 | 270 ± 14 | |
| 10 | 329 ± 26 | 336 ± 5φ | 343 ± 9 | 367 ± 7φφ | |
Values are means ± SE in μm/. RA, room air; RV, right ventricle; LV, left ventricle; IVS, interventricular septum; NA, not applicable.
P < 0.05,
P < 0.01, and
P < 0.001 vs. age-matched O2-exposed controls.
P < 0.001 vs. postnatal day 3.
P < 0.01 and
P < 0.001 vs. postnatal day 6.
Table 3.
Protein expression of TNC in the RV free wall
| Treatment | Saline-positive TNC | n | Piclamilast-positive TNC | n |
|---|---|---|---|---|
| RA | 0 | 12 | 0 | 10 |
| O2 | 11 | 12 | 1* | 12 |
n, No. of animals. TNC, tenascin-C.
P < 0.05 vs. age-matched O2-exposed controls.
Late treatment and injury-recovery model.
Treatment of room air-exposed control pups with piclamilast had no effect on cardiac characteristics (Fig. 9 and Table 4). Nine days of hyperoxic lung injury resulted in a 1.3-fold increase in the ratio RV/LV wall thickness (P < 0.01; Fig. 9) and a 1.4-fold increase in RV free wall thickness compared with room air controls (P < 0.05; Table 4). A recovery period of 9 (day 18) or 42 days (day 51) did not reduce hyperoxia-induced RVH in the nontreated pups, but treatment with piclamilast attenuated hyperoxia-induced RVH on days 9, 18, and 51 (Fig. 9 and Table 4).
Fig. 9.
A: RV hypertrophy depicted as the RV/LV ratio, after late treatment and recovery on days 9, 18, and 51 in RA controls (open bars), RA-pic pups treated with 5.0 mg·kg−1·day−1 pic (hatched bars), age-matched, O2-exposed controls (solid bars), and O2-pic pups treated with 5.0 mg·kg−1·day−1 pic (shaded bars). B: paraffin heart sections stained with hematoxylin and eosin at × 40 magnification in RA controls, RA-pic, O2 controls, and O2-pic on days 9, 18, and 51. Values are means ± SE (N = 8). *P < 0.05, **P < 0.01, and ***P < 0.001 vs. age-matched, O2-exposed controls.
Table 4.
Cardiac characteristics in late treatment and recovery
| RA |
O2 |
||||
|---|---|---|---|---|---|
| Day | Saline | Piclamilast | Saline | Piclamilast | |
| RV free wall thickness | 9 | 100 ± 7* | 107 ± 4 | 142 ± 10 | 118 ± 9 |
| 18 | 96 ± 9*** | 110 ± 5*** | 179 ± 9 | 140 ± 10* | |
| 51 | 96 ± 2*** | 98 ± 2*** | 160 ± 7 | 113 ± 4** | |
| IVS thickness | 9 | 284 ± 7 | 288 ± 11 | 299 ± 15 | 288 ± 13 |
| 18 | 265 ± 16 | 278 ± 8 | 283 ± 21 | 319 ± 16 | |
| 51 | 293 ± 6 | 292 ± 8 | 299 ± 8 | 286 ± 13 | |
| LV free wall thickness | 9 | 281 ± 7 | 289 ± 11 | 306 ± 11 | 334 ± 9 |
| 18 | 277 ± 14* | 292 ± 5 | 331 ± 19 | 366 ± 14Δ | |
| 51 | 342 ± 5δ | 329 ± 6 | 358 ± 12 | 368 ± 7 | |
Values are means ± SE in μm/.
P < 0.05,
P < 0.01, and
P < 0.001 vs. age-matched O2-exposed controls.
P < 0.001 vs. RA-exposed controls.
P < 0.001 vs. postnatal day 18.
Cardiac mRNA Expression
Prophylactic treatment model.
Exposure to hyperoxia for 10 days increased RV mRNA expression for the natriuretic peptides, atrial natriuretic peptide (ANP; 13-fold; P < 0.001, Fig. 10A) and brain natriuretic peptide (BNP; 17-fold; P < 0.001, Fig. 10B), compared with room air controls. Treatment with piclamilast during hyperoxia decreased the expression of ANP (by 54%; P < 0.05) and BNP (by 64%; P < 0.001) compared with that in oxygen-exposed controls.
Fig. 10.
Relative mRNA expression in the RV free wall and LV, including the IVS (LV + IVS), determined with RT-PCR, of atrial natriuretic peptide (ANP; A) and brain natriuretic peptide (BNP; B) in RA-exposed controls (open bars), RA-pic pups treated with pic (5.0 mg·kg−1·day−1; hatched bar), age-matched, O2-exposed controls (solid bar), and O2-pic pups treated with pic (5.0 mg·kg−1·day−1; shaded bar) on day 10 after early concurrent treatment. Values are means ± SE (N = 12). *P < 0.05 and **P < 0.001 vs. age-matched, O2-exposed controls. ΔP < 0.001 vs. RA-exposed controls.
Exposure to hyperoxia for 10 days increased mRNA expression in the LV + IVS for the natriuretic peptides, ANP (3.7-fold; P < 0.001, Fig. 10A) and BNP (3.1-fold; P < 0.001, Fig. 10B), compared with room air controls. In LV + IVS, treatment with piclamilast decreased the expression of BNP (by 30%; P < 0.05) compared with oxygen-exposed controls.
RV Function
Late treatment and injury-recovery model.
Treatment of room air-exposed control pups with piclamilast slowed down relaxation (P < 0.05) on day 18 (Table 5). After a hyperoxic period of 9 days and 9 days of recovery, peak RV pressure and end-systolic RV pressure were elevated (P < 0.001), compared with room air controls, demonstrating hyperoxia-induced PH. Volumetric indexes and cardiac output were maintained, despite the increased afterload. Systolic function reflected by ejection fraction tended to be improved. Treatment with piclamilast reduced hyperoxia-induced peak RV pressure and end-systolic pressure by 20% (P < 0.05) compared with oxygen-exposed controls, demonstrating that piclamilast attenuated hyperoxia-induced PH on day 18.
Table 5.
RV function in late treatment and recovery at postnatal day 18
| RA |
O2 |
|||
|---|---|---|---|---|
| RV | Saline | Piclamilast | Saline | Piclamilast |
| General hemodynamics | ||||
| Stroke volume, μl/g | 1.12 ± 0.10 | 1.18 ± 0.14 | 1.27 ± 0.13 | 1.07 ± 0.12 |
| Cardiac index, ml·g−1·min−1 | 0.34 ± 0.03 | 0.28 ± 0.03 | 0.41 ± 0.05 | 0.26 ± 0.03* |
| Stroke work, mmHg·μl−1·g−1 | 15.5 ± 1.5*** | 19.2 ± 2.1** | 42.4 ± 6.5 | 27.7 ± 3.4 |
| Systolic function | ||||
| Peak RV pressure, mmHg | 17.4 ± 0.8*** | 22.0 ± 1.5*** | 43.5 ± 3.7 | 34.9 ± 1.9*,ΔΔ |
| End-systolic pressure, mmHg | 15.3 ± 0.9*** | 20.2 ± 1.4*** | 39.1 ± 3.3 | 32.1 ± 1.9*,ΔΔ |
| End-systolic volume, μl/g | 1.18 ± 0.15 | 0.88 ± 0.13 | 1.06 ± 0.13 | 0.83 ± 0.27 |
| Ejection fraction, % | 49.2 ± 3.7 | 57.5 ± 4.6 | 54.3 ± 3.6 | 63.6 ± 7.0 |
| Diastolic function | ||||
| End-diastolic pressure, mmHg | 2.8 ± 0.5 | 3.3 ± 0.5 | 4.9 ± 0.4 | 4.9 ± 0.7Δ |
| End-diastolic volume, μl/g | 2.30 ± 0.18 | 2.06 ± 0.18 | 2.33 ± 0.22 | 1.90 ± 0.27 |
| Relaxation time constant, ms | 17.7 ± 1.5 | 23.3 ± 1.1***,Δ | 15.6 ± 1.0 | 24.8 ± 1.4*** |
Note: All volumetric indexes were indexed for body weight.
P < 0.05,
P < 0.01, and
P < 0.001 vs. age-matched O2-exposed controls.
P < 0.05 and
P < 0.01 vs. RA-exposed controls.
DISCUSSION
Our data demonstrate that prophylactic treatment with piclamilast, a specific second generation PDE4 inhibitor, prolongs survival and prevents cardiopulmonary disease in neonatal rat pups with hyperoxia-induced chronic lung disease, a valuable model for severe BPD in preterm infants (38). Piclamilast treatment of experimental BPD prevented the development of PH and RVH and attenuated lung inflammation, alveolar septum thickness, impaired angiogenesis, and vascular arteriolar remodeling. Early exposure to hyperoxia for 9 days in the neonatal period causes persistent alveolar simplification, PH, and RVH in neonatal and adult rats. Piclamilast treatment reverses established PH and RVH in hyperoxia-exposed rat pups and in adult survivors of hyperoxic lung injury in an injury-recovery model, but does not reverse hyperoxia-induced alveolar enlargement in neonatal and adult rats.
Neonatal lung injury by exposure to 100% oxygen is a valuable rat model for severe BPD in preterm infants (38), suffering from an overwhelming inflammatory response accompanied by complications such as PH and RVH, that are characteristic for premature infants with “old” BPD. Exposure to lower oxygen concentration results in a milder injurious response in rodents with fewer pulmonary vascular and cardiac complications that reflects “new” BPD in premature infants, nowadays seen in neonatal intensive care units (41).
Prophylactic piclamilast treatment improved hyperoxia-induced RVH, as shown by reduced thickness and weight of the RV, and reduced ANP, BNP, and extracellular TN-C expression in the RV, markers that are upregulated under myocardial stress conditions (5, 40). Although PDE4 is expressed in the mammalian heart, the therapeutic effects of PDE4 inhibitors in cardiac disease are still unclear (29). The beneficial effect of piclamilast on the heart can be explained indirectly by a decrease in pulmonary arteriolar wall thickness, resulting in less vasoconstriction and PH and, as a result, reduced RVH. Vasoconstriction and remodeling of pulmonary blood vessels with proliferation of smooth muscle cells and fibroblasts in pulmonary vessels are important contributors to PH (2, 19). In this study, we demonstrate that the hyperoxia-induced increase in medial wall thickness of pulmonary arterioles is preceded by an increase in vascular smooth muscle cell proliferation that can be blocked by PDE4 inhibition with piclamilast, suggesting an important role of vascular smooth muscle cell proliferation in the development of PH. The importance of smooth muscle cells in the therapeutic effect of PDE3 and PDE4 inhibitors in PH is supported by 1) a reduced proliferation of pulmonary artery smooth muscle cells by cAMP (30); 2) a high activity of PDE3 and PDE4 in these cells (28); and 3) the beneficial effects of PDE3 and/or PDE4 inhibitors on vascular remodeling, vasoconstriction, and RVH in experimental models in vivo, including hyperoxia-induced neonatal lung injury (this study), monocrotaline- or hypoxia-induced PH (20, 28), bleomycin-induced pulmonary fibrosis (8), and in vitro studies with human pulmonary artery smooth muscle cells (16). Studies with prostacyclin analogs, which induce relaxation of vascular smooth muscle by stimulating the production of cAMP and, subsequently, inhibiting the proliferation of smooth muscle cells, have shown a decrease in pulmonary arterial hypertension in vitro and in vivo (6, 32). Because PDE4 inhibition reduces the expression of ET-1, a potent vasoconstrictor and stimulator of the proliferation of vascular smooth muscle cells in adult lung injury animal models of (8, 9), we studied mRNA expression of ET-1, its converting enzyme ECE-1, and both receptors ETA and ETB in experimental BPD. Piclamilast treatment does not affect the hyperoxia-induced increase in ET-1 and decrease in ECE-1 and ETB expression, and even partially restores ETA expression, which is associated with smooth muscle cell proliferation and contraction (19). These data suggest that signaling of ET-1 via ETA and ETB does not play a significant role in the beneficial effects of piclamilast on PH and lung damage in hyperoxia-induced lung injury. Since proinflammatory cytokines and chemokines can stimulate pulmonary artery smooth muscle cell proliferation (17), the therapeutic effect of PDE4 inhibition on PH in experimental BPD may, at least partially, take place via inhibition of the inflammatory response, an important contributor to experimental BPD in this and previous studies on neonatal hyperoxia-induced lung disease, which is significantly inhibited in the prophylactic experimental BPD model by the PDE4 inhibitors piclamilast and rolipram (12, 26).
A small beneficial effect of piclamilast treatment on vascularization, associated with an increased expression of the angiogenic factor VEGFA and its receptor VEGFR2, was observed on day 10 in the prophylactic model, but not at any other time point, and not in the injury recovery model. However, a similar effect on alveolarization was absent, which was unexpected, because both processes are linked to each other. After 10 days of exposure to hyperoxia, the lung, including the vascular endothelium, is seriously damaged, resulting in a decrease in expression of vWF. Piclamilast treatment protects the lung and its vascular bed from damage during the hyperoxic insult by attenuating the inflammatory process and vascular remodeling, thereby attenuating the reduction in vWF expression.
Hyperoxia-induced RVH, which can be detected from neonatal day 6 onward, precedes the detection of PH, determined by arteriolar wall thickness, which can be detected from neonatal day 9 onward. Since RVH is a direct consequence of PH, these unexpected results can be explained by increased vasoconstriction rather than proliferation of vascular smooth muscle cells in small pulmonary arterioles and/or a reduction of the pulmonary vascular bed, as demonstrated by a decrease in pulmonary vascular density from day 6 onward.
Recent data on PDE4 inhibition in hyperoxia-induced neonatal lung injury show conflicting effects on alveolarization in rodents (25). In neonatal rats with an ongoing lung injury due to oxidative stress and inflammation, piclamilast or rolipram did not protect against impaired alveolarization (this study and Refs. 12, 26), but in a less aggressive model of hyperoxic lung injury in neonatal mice, PDE4 inhibition with cilomilast enhanced lung alveolarization (39). These contradictory findings may be explained by differences in oxidative stress (100 vs. 85% oxygen), duration of hyperoxia (10 vs. 28 days), start of treatment (starting on day 1 vs. 14), the PDE4 inhibitor used (rolipram, piclamilast and cilomilast), and species (rats vs. mice). In contrast with cilomilast treatment, rolipram and piclamilast treatment reduces body weight gain, which will decrease lung volumes and absolute alveolar surface area (24). The observed growth retardation in neonatal rats after piclamilast administration can be explained, at least in part, by a decrease in food intake, which is possibly due to reduced passage, although we did not systemically evaluate this. However, we did not find any effect of PDE4 inhibition on normal, lung development, i.e., alveolarization and angiogenesis were normal, despite a reduction in body weight gain compared with untreated normal rats (this study and Refs. 12, 26). PDE4 inhibitors have adverse effects in adult patients that are associated with the central nervous system, including emesis, nausea, and vomiting (23). Although piclamilast in particular, and nonspecific PDE inhibitors in general, such as theophylline, are not known to have adverse effects on neurodevelopment (37), it will be important to evaluate this potential before piclamilast could be considered in clinical trials in neonates (13, 34).
Piclamilast treatment does not have a significant effect on total cAMP levels in lung tissue under normoxia and hyperoxia in rat pups. Because local differences in cAMP levels are not revealed by whole lung tissue measurements, the expected PDE4 inhibitor-induced increase in cAMP levels in vascular smooth muscle cells are probably compensated by a decrease in other cell types by the complex interaction of different stimuli and enzymes that lead to the generation and inactivation of cAMP and/or cGMP by numerous PDEs (14, 15).
Treatment of oxygen-exposed rat pups with piclamilast resulted in a decrease in end-systolic and peak systolic RV pressure. However, the effect of piclamilast on heart pressure was less than expected from our morphometric data on lung arteriolar wall thickness and RVH (RV free wall thickness, relative to the LV). This discrepancy may be explained by 1) a reduction of the pulmonary vascular bed, as demonstrated by a persistent decrease in hyperoxia-induced vascular density that cannot be restored after piclamilast treatment; and/or 2) a direct effect of piclamilast on the myocardium, resulting in enhanced contractility of the RV. We hypothesize that this direct effect on the heart of piclamilast, being a PDE4 inhibitor, may be explained by an increase in intracellular cAMP levels in cardiomyocytes. Elevated cAMP levels after β-adrenergic-dependent signaling are associated with a positive inotropic effect, resulting in protein kinase A-dependent phosphorylation of multiple proteins involved in the regulation of the cytosolic Ca2+ concentration (29). Piclamilast-induced enhanced contractility of the RV can explain, at least in part, the limited increase in RV free wall thickness in piclamilast-treated oxygen-exposed rats, despite an elevated RV peak pressure.
Perspectives
Although in neonatal rats with hyperoxia-induced lung injury piclamilast has been shown to reduce PH and RVH, both major contributors to mortality or severe morbidity in patients with BPD, the mechanism by which piclamilast affects the developing organism needs to be better defined before clinical application should be considered.
GRANTS
This study was supported by National Institutes of Health Grants 1R01 HL092158 and 1R01 ES015330 (F. J. Walther).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.P.d.V., F.J.W., P.S., A.v.d.L., and G.T.W. conception and design of research; Y.P.d.V., E.H.L., P.S., M.M., and G.T.W. performed experiments; Y.P.d.V., E.H.L., P.S., M.M., and G.T.W. analyzed data; Y.P.d.V., E.H.L., P.S., A.v.d.L., and G.T.W. interpreted results of experiments; Y.P.d.V., E.H.L., M.M., and G.T.W. prepared figures; Y.P.d.V. and G.T.W. drafted manuscript; Y.P.d.V., F.J.W., E.H.L., P.S., A.v.d.L., and G.T.W. approved final version of manuscript; F.J.W., P.S., A.v.d.L., and G.T.W. edited and revised manuscript.
Supplementary Material
REFERENCES
- 1. Abman SH. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 91: 283–290, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol 661: 323–335, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Abman SH. Pulmonary hypertension in children: a historical overview. Pediatr Crit Care Med 11, Suppl 2: S4–S9, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946–1955, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Boerma M, van der Wees CG, Vrieling H, Svensson JP, Wondergem J, van der LA, Mullenders LH, van Zeeland AA. Microarray analysis of gene expression profiles of cardiac myocytes and fibroblasts after mechanical stress, ionising or ultraviolet radiation. BMC Genomics 6: 6, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Clapp LH, Finney P, Turcato S, Tran S, Rubin LJ, Tinker A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol 26: 194–201, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem 278: 5493–5496, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Cortijo J, Iranzo A, Milara X, Mata M, Cerda-Nicolas M, Ruiz-Sauri A, Tenor H, Hatzelmann A, Morcillo EJ. Roflumilast, a phosphodiesterase 4 inhibitor, alleviates bleomycin-induced lung injury. Br J Pharmacol 156: 534–544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Franceschi L, Platt OS, Malpeli G, Janin A, Scarpa A, Leboeuf C, Beuzard Y, Payen E, Brugnara C. Protective effects of phosphodiesterase-4 (PDE-4) inhibition in the early phase of pulmonary arterial hypertension in transgenic sickle cell mice. FASEB J 22: 1849–1860, 2008 [DOI] [PubMed] [Google Scholar]
- 10. de Visser YP, Walther FJ, Laghmani EH, Boersma H, van der Laarse A, Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Visser YP, Walther FJ, Laghmani EH, van der Laarse A, Wagenaar GT. Apelin attenuates hyperoxic lung and heart injury in neonatal rats. Am J Respir Crit Care Med 182: 1239–1250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Visser YP, Walther FJ, Laghmani EH, van Wijngaarden S, Nieuwland K, Wagenaar GT. Phosphodiesterase-4 inhibition attenuates pulmonary inflammation in neonatal lung injury. Eur Respir J 31: 633–644, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Donohue PK, Gilmore MM, Cristofalo E, Wilson RF, Weiner JZ, Lau BD, Robinson KA, Allen MC. Inhaled nitric oxide in preterm infants: a systematic review. Pediatrics 127: e414–e422, 2011 [DOI] [PubMed] [Google Scholar]
- 14. Essayan DM. Cyclic nucleotide phosphodiesterases. J Allergy Clin Immunol 108: 671–680, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fan CK. Phosphodiesterase inhibitors in airways disease. Eur J Pharmacol 533: 110–117, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Growcott EJ, Spink KG, Ren X, Afzal S, Banner KH, Wharton J. Phosphodiesterase type 4 expression and anti-proliferative effects in human pulmonary artery smooth muscle cells. Respir Res 7: 9, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guignabert C, Raffestin B, Benferhat R, Raoul W, Zadigue P, Rideau D, Hamon M, Adnot S, Eddahibi S. Serotonin transporter inhibition prevents and reverses monocrotaline-induced pulmonary hypertension in rats. Circulation 111: 2812–2819, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol 291: H2424–H2430, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 351: 1425–1436, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Izikki M, Raffestin B, Klar J, Hatzelmann A, Marx D, Tenor H, Zadigue P, Adnot S, Eddahibi S. Effects of roflumilast, a phosphodiesterase-4 inhibitor, on hypoxia- and monocrotaline-induced pulmonary hypertension in rats. J Pharmacol Exp Ther 330: 54–62, 2009 [DOI] [PubMed] [Google Scholar]
- 21. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet 367: 1421–1431, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 365: 167–175, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Massaro D, Teich N, Maxwell S, Massaro GD, Whitney P. Postnatal development of alveoli. Regulation and evidence for a critical period in rats. J Clin Invest 76: 1297–1305, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehats C, Bourbon J, Jarreau PH. Does PDE4 inhibition improve alveolarisation in hyperoxia-exposed immature rodents? Eur Respir J 33: 1236, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Mehats C, Franco-Montoya ML, Boucherat O, Lopez E, Schmitz T, Zana E, Evain-Brion D, Bourbon J, Delacourt C, Jarreau PH. Effects of phosphodiesterase 4 inhibition on alveolarization and hyperoxia toxicity in newborn rats. PLos One 3: e3445, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Millen J, MacLean MR, Houslay MD. Hypoxia-induced remodelling of PDE4 isoform expression and cAMP handling in human pulmonary artery smooth muscle cells. Eur J Cell Biol 85: 679–691, 2006 [DOI] [PubMed] [Google Scholar]
- 28. Phillips PG, Long L, Wilkins MR, Morrell NW. cAMP phosphodiesterase inhibitors potentiate effects of prostacyclin analogs in hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol 288: L103–L115, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Rao YJ, Xi L. Pivotal effects of phosphodiesterase inhibitors on myocyte contractility and viability in normal and ischemic hearts. Acta Pharmacol Sin 30: 1–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rybalkin SD, Bornfeldt KE. Cyclic nucleotide phosphodiesterases and human arterial smooth muscle cell proliferation. Thromb Haemost 82: 424–434, 1999 [PubMed] [Google Scholar]
- 31. Schermuly RT, Kreisselmeier KP, Ghofrani HA, Yilmaz H, Butrous G, Ermert L, Ermert M, Weissmann N, Rose F, Guenther A, Walmrath D, Seeger W, Grimminger F. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am J Respir Crit Care Med 169: 39–45, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Schermuly RT, Yilmaz H, Ghofrani HA, Woyda K, Pullamsetti S, Schulz A, Gessler T, Dumitrascu R, Weissmann N, Grimminger F, Seeger W. Inhaled iloprost reverses vascular remodeling in chronic experimental pulmonary hypertension. Am J Respir Crit Care Med 172: 358–363, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med 11, Suppl 2: S79–S84, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tin W, Wiswell TE. Drug therapies in bronchopulmonary dysplasia: debunking the myths. Semin Fetal Neonatal Med 14: 383–390, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 157: 351–370, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, Haworth SG. Development and pathology of pulmonary hypertension. J Am Coll Cardiol 54: S3–S9, 2009 [DOI] [PubMed] [Google Scholar]
- 37. von Poblotzki M, Rieger-Fackeldey E, Schulze A. Effects of theophylline on the pattern of spontaneous breathing in preterm infants less than 1000 g of birth weight. Early Hum Dev 72: 47–55, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ. Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 36: 782–801, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Woyda K, Koebrich S, Reiss I, Rudloff S, Pullamsetti SS, Ruhlmann A, Weissmann N, Ghofrani HA, Gunther A, Seeger W, Grimminger F, Morty RE, Schermuly RT. Inhibition of phosphodiesterase 4 enhances lung alveolarisation in neonatal mice exposed to hyperoxia. Eur Respir J 33: 861–870, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto K, Dang QN, Kennedy SP, Osathanondh R, Kelly RA, Lee RT. Induction of tenascin-C in cardiac myocytes by mechanical deformation. Role of reactive oxygen species. J Biol Chem 274: 21840–21846, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, Tanswell AK. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med 170: 1188–1196, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.