Abstract
Endogenous agonists of transient receptor potential vanilloid-1 (TRPV1) (endovanilloids) are implicated as mediators of lung injury during inflammation. This study tested the hypothesis that endovanilloids produced following lipopolysaccharide (LPS) treatment activate TRPV1 and cause endoplasmic reticulum stress/GADD153 expression in lung cells, representing a mechanistic component of lung injury. The TRPV1 agonist nonivamide induced GADD153 expression and caused cytotoxicity in immortalized and primary human bronchial, bronchiolar/alveolar, and microvascular endothelial cells, proportional to TRPV1 mRNA expression. In CF-1 mice, Trpv1 mRNA was most abundant in the alveoli, and intratracheal nonivamide treatment promoted Gadd153 expression in the alveolar region. Treatment of CF-1 mice with LPS increased Gadd153 in the lung, lactate dehydrogenase (LDH) in bronchoalveolar lavage (BAL) fluid, and lung wet-to-dry weight ratio. Cotreating mice with LPS and the TRPV1 antagonist LJO-328 reduced Gadd153 induction and LDH in BAL but did not inhibit increases in lung wet-to-dry ratio. In Trpv1−/− mice treated with LPS, Gadd153 induction and LDH in BAL were reduced relative to wild-type mice, and the wet-to-dry weight ratios of lungs from both wild-type and Trpv1−/− mice decreased. Organic extracts of blood collected from LPS-treated mice were more cytotoxic to TRPV1-overexpressing cells compared with BEAS-2B cells and extracts from control mice, however, most pure endovanilloids did not produce cytotoxicity in a characteristic TRPV1-dependent manner. Collectively, these data indicate a role for TRPV1, and endogenous TRPV1 agonists, in ER stress and cytotoxicity in lung cells but demonstrate that ER stress and cytotoxicity are not essential for pulmonary edema.
Keywords: capsaicin, lipopolysaccharides, lung injury, endovanilloids, transient receptor potential vanilloid-1
lung injury can arise from inflammatory responses elicited by diverse stimuli, including chemical exposures, trauma, endotoxic and hemorrhagic shock, sepsis, and burn injury (24, 25, 44, 45). Limited advances in the treatment of lung injury and the more severe clinical manifestation, acute respiratory distress syndrome (ARDS), mandate a better understanding of the underlying biochemical processes that contribute to lung injury during systemic inflammation (24, 44, 45).
Transient receptor potential vanilloid-1 (TRPV1) is a calcium-selective ion channel that is activated by capsaicin and its synthetic analog nonivamide as well as temperatures >42°C and H+ (pH <6.2 at 37°C) (7). TRPV1 also is purportedly activated by various inflammatory mediators, including hydrogen sulfide (H2S) (1, 5, 41), anandamide (AEA), 2-arachidonoyl glycerol (2-AG), leukotriene B4 (LTB4) (19), 12(S)- and 15(S)-hydroperoxyeicosatetraenoic acids (HPETEs), 5(S)- and 15(S)-hydroxyeicosatetraenoic acids (HETEs) (11, 19, 30, 50), N-arachidonoyl amino acids such as N-arachidonoyl taurine (N-AT) (34), and N-arachidonoyl dopamine (NADA) (10, 11); these substances are often referred to as endovanilloids (42). TRPV1 is also potentiated by many endovanilloids and/or inflammatory mediators, meaning that low concentrations of these substances can facilitate channel opening by other chemical agonists or at physiological temperature or mildly acidic pH. A common characteristic of many endovanilloids is that they are produced during inflammation (3–6, 9, 14, 15, 20, 22, 23, 28, 29, 33, 46–49), leading to the hypothesis that certain endovanilloids may couple inflammation with lung damage via TRPV1-mediated processes.
Numerous studies implicate TRPV1 and endovanilloids, particularly H2S, as mediators of lung injury due to sepsis, burn injury, and lipopolysaccharide (LPS) exposure (1, 3–5, 9, 22, 27, 29, 35, 36, 41, 47–49). It has been shown that pretreatment of mice with capsaicin to obliterate Trpv1-expressing neurons, cotreatment with NK1 receptor antagonists that block the action of substance P and neurokinin A released from Trpv1-expressing sensory nerves, preprotachykinin A gene deletion, and cotreatment with the TRPV1 antagonist capsazepine attenuate lung injury by multiple inflammatory conditions.
A role for ER stress has also been suggested for LPS-induced lung injury. Endo et al. (13) demonstrated that lung injury following intraperitoneal LPS treatment coincided with the induction of proapoptotic Gadd153 expression, and Gadd153−/− mice were less susceptible to injury. Studies by our group have identified GADD153 as a mediator of lung cell death in vitro following treatment with TRPV1 agonists, including the endovanilloid AEA (39, 40), suggesting that the results presented by Endo et al. (13) may have involved Trpv1 activation and endoplasmic reticulum (ER) stress elicited by endogenous TRPV1 agonists produced in response to the LPS treatment. Thus, the hypothesis of this study is that endogenous TRPV1 agonists produced during inflammation activate TRPV1 in lung cells, cause ER stress and GADD153 expression, and promote lung injury.
MATERIALS AND METHODS
Chemicals.
Nonivamide (N-[3-methoxy-4-hydroxybenzyl]nonanamide), a capsaicin analog, LPS (Escherichia coli O111:B4), NaHS, and AEA were purchased from Sigma-Aldrich (St. Louis, MO). 2-AG, LTB4, 12(S)HpETE, 15(S)HpETE, 5(S)HETE, NADA, and N-AT were purchased from Cayman Chemicals (Ann Arbor, MI), and LJO-328 (31) was synthesized and supplied by Lee. PCR primers were synthesized by the University of Utah DNA Core Facility.
Cell culture.
Immortalized human bronchial epithelial cells (BEAS-2B) were purchased from American Type Culture Collection (Manassas, VA), and TRPV1-overexpressing BEAS-2B (TRPV1-OE) cells were generated as described previously (32). BEAS-2B and TRPV1-OE cells were cultured in LHC-9 media (Invitrogen, Carlsbad, CA). Primary human lung microvascular endothelial (HMVEC-L), small airway epithelial cells (SAEC), and normal human bronchial epithelial (NHBE) cells were purchased from Lonza (Walkersville, MD). HMVEC-L cells were cultured in supplemented EGM-MV media, SAEC cells in SAGM media, and NHBE cells in BEGM media (Lonza). Culture flasks for SAEC, NHBE, BEAS-2B, and TRPV1-OE cells were coated with LHC basal media fortified to 30 μg/ml collagen, 10 μg/ml fibronectin, and 10 μg/ml BSA. Cells were maintained between 30 and 90% maximum density and were subcultured using trypsin.
Cytotoxicity/dose-response assays.
Cells were subcultured into 96-well plates and grown to ∼90% confluence. Cells were treated for up to 24 h at 37°C with increasing concentrations of test agent or blood extract. Treatment solutions were prepared in LHC-9 media for BEAS-2B and TRPV1-OE cells and in OptiMEM I (Invitrogen) for NHBE, HMVEC-L, and SAEC cells. Cell viability was quantified using the Dojindo Cell Counting kit-8 (Dojindo Laboratories, Gaithersburg, MD).
Real-time quantitative PCR analysis of TRPV1 and GADD153 expression in human cells.
Cells were subcultured into six-well cell culture plates, grown to ∼90% density, and treated. Total RNA was extracted from cells using the Invitrogen PureLink Micro-to-Midi Total RNA Purification kit (Invitrogen). Total RNA (2.5 μg) was reverse transcribed into cDNA using Superscript III (Invitrogen). Real-time quantitative PCR (RT-qPCR) was performed using 1 μl cDNA (diluted 1:100) and RT2 SYBR Green qPCR Master Mix (SA Biosciences, Frederick, MD) on a Chromo 4 Real Time Detection System (Bio-Rad, Hercules, CA) using MJ Opticon Monitor 3 software. The PCR program used consisted of a 10-min incubation at 95°C, followed by 40 cycles of 95°C for 15 s, 55°C for 30 s, and then 72°C for 30 s. Experiments were performed in triplicate with a copy number standard curve for both the reference gene [β2-macroglobulin (β2M)] and the genes of interest (TRPV1 and GADD153). Primer sequences were (5′→3′): human (h) β2M sense GATGAGTATGCCTGCCGTGTG and antisense CAATCCAAATGCGGCATCT; hTRPV1 sense CTGCGGACCCACTCCAAAA and antisense CCTCGTGAGGGCAATCCAC; and hGADD153 sense AGAACCAGGAAACGGAAACAGA and antisense TCTCCTTCATGCGCTGCTTT.
Multiplex RT-PCR analysis of GADD153 expression in human cells.
Quantification of GADD153 and β-actin was performed as previously described (40). The PCR primers were (5′→3′): hGADD153 sense GACCTGCAAGAGGTCCTGTC and antisense TCGCCTCTACTTCCCTGGTC (395 nt) and hβ-actin sense GACAACGGCTCCGGCATGTGGCA and antisense TGAGGATGCCTCTCTTGCTCTG (183 nt).
Animals.
Experimental protocols were approved by the University of Utah Institutional Animal Care and Use Committee. Experiments were performed on 20- to 25-g male CF-1, C57BL/6, and C57BL/6 Trpv1−/− mice. CF-1 mice were purchased from Charles River Laboratories (Wilmington, MA). Trpv1−/− mice were purchased from Jackson Laboratories (Bar Harbor, MA), and breeding colonies for the C57BL/6 and Trpv1−/− mice were maintained at the University of Utah vivarium.
Airway dissection.
CF-1 mice were terminally anesthetized by intraperitoneal injection of pentobarbital (100 mg/kg). The lungs were exposed, the trachea was cannulated, and the lungs were inflated with ∼400 μl RNALater solution (Ambion, Austin, TX). The trachea was tied closed with surgical silk, and, after 5 min, the entire respiratory tract (trachea to lungs) was removed and stored in RNALater at 4°C until dissection <48 h after removal. The samples were trimmed to remove the heart and other tissues and dissected in a bath of RNALater. The trachea, main bronchi, bronchioles from the main bronchus into the parenchyma, and alveolar regions adjacent to the bronchioles were collected from the left lobe. Whole lung samples were from the right cranial and middle lobes. Tissues were stored at −80°C until analysis.
RT-qPCR analysis of Trpv1, Gadd153, SP-A, and Ccsp expression in mouse lung.
Total RNA was isolated using the Trizol Plus Total RNA isolation kit (Invitrogen). mRNA (1 μg) from each sample was amplified using the Message Amp II RNA amplification kit (Ambion). The aRNA (1 μg) was converted to cDNA using the Superscript III cDNA synthesis kit (Invitrogen). qPCR was performed using 1 μl cDNA (undiluted for TRPV1, diluted 1:50 for all others), RT2 SYBR Green qPCR Master Mix (SA Biosciences), and a Roche Lightcycler 480 instrument. The PCR program used consisted of a 10-min incubation at 95°C, followed by 40 cycles of 95°C for 15 s and 63°C for 60 s. Copy number standards were used for all genes. Clara cell specific protein (Ccsp) and surfactant protein-A (SP-A) transcript levels were used to confirm enrichment of bronchioles and alveolar tissue, and glyceraldehyde phosphate dehydrogenase (GAPDH) mRNA was used to normalize expression data. Primers were (5′→3′): mouse (m) Ccsp sense ATGAAGATCGCCATCACAATCAC and antisense GGATGCCACATAACCAGACTCT; mSP-A sense GAGGAGCTTCAGACTGCACTC and antisense AGACTTTATCCCCCACTGACAG; mTrpv1 sense CATCTTCACCACGGCTGCTTAC and antisense CAGACAGGATCTCTCCAGTGAC; mGadd153 sense GAACGAGCGGAAAGTGGCA and antisense CATGCGGTCGATCAGAGCC; and mGapdh sense AGGTCGGTGTGAACGGATTTG and antisense TGTAGACCATGTAGTTGAGGTCA.
LPS-induced inflammation.
Intraperitoneal injection of LPS was used as the proinflammatory stimulus. LPS was prepared in 0.9% saline or in 98.4% saline, 1% ethanol, 0.5% Tween 20, and 0.1% DMSO for experiments where LJO-328 cotreatment was used. Mice were injected intraperitoneally with vehicle, 10 mg/kg (CF-1 mice) or 15 mg/kg (C57BL/6 and TRPV1−/− mice), LPS with or without LJO-328 (5 mg/kg), and 5 mg/kg LJO-328 alone. Animals were terminally anesthetized by intraperitoneal injection of pentobarbital (100 mg/kg) and exsanguinated via cardiac puncture 12 and 18 h posttreatment.
Quantification of extravascular lung water.
Mice were killed, and the left lobe from each animal was weighed and dehydrated for 72 h at 55°C. The lungs were weighed again, and the wet-to-dry ratios were calculated.
Quantification of lactate dehydrogenase and protein in bronchoalveolar lavage fluid.
The trachea was cannulated with a blunt-ended 22-gauge needle attached to a 1-ml syringe, lungs were infused gently with ∼0.4 ml of sterile saline, and the fluid was repeated two times. The fluid was clarified by centrifugation at 1,000 g for 2 min at 4°C, and the supernatant was assayed for lactate dehydrogenase (LDH) activity using the TOX-7 Assay Kit (Sigma-Aldrich, St. Louis, MO). Bronchoalveolar lavage (BAL) protein was determined using the bicinchoninic acid assay (Pierce, Rockford, IL).
Quantification of Gadd153 expression in mouse lungs using multiplex PCR.
Lungs were removed and trimmed to remove the heart, trachea, bronchi, and connective tissues. Lungs were minced in ice-cold RNALater (Qiagen, Valencia, CA), incubated at 4°C for 24 h, and stored at −80°C until analysis. Tissue was homogenized, and total RNA was isolated using the Invitrogen PureLink Micro-to-Midi Total RNA Purification System. Total RNA (2.5 μg) was reverse transcribed into cDNA using Superscript III (Invitrogen). cDNA corresponding to Gadd153 and β-actin was amplified by PCR using 1 μl of the cDNA synthesis reaction and GoTaq Green PCR Master Mix using the following primers (5′→3′): mGadd153 sense GCAGCCATGGCAGCTGAGTCCCTGCCTTCC and antisense CAGACTCGAGGTGATGCCCACTGTTCATGC; and mβ-actin sense GTGACGAGGCCCAGAGCAAGAG and antisense AGGGGCCGGACTCATCGTACTC. The PCR program and product analysis procedure was the same as previously described for human GADD153 (40).
Blood/endovanilloid extracts.
Blood collected from three CF-1 mice was pooled, and 1 ml was diluted two times with PBS and extracted with 4 ml n-butyl chloride. The organic layer was clarified by centrifugation and collected. This procedure was repeated using hexane, and the combined organic extracts were dried under nitrogen gas. The residue was reconstituted with 60 μl DMSO and diluted to 3 ml with LHC-9 media. Cells were treated with LHC-9 media with increasing proportions of extract for 24 h at 37°C to determine cytotoxicity. LJO-328 was included at a concentration of 20 μM when used.
Statistical analysis.
Results are represented as means ± SE or SD (as noted in the legends for Figs. 1–6), and data were analyzed for statistical differences using ANOVA (α = 0.05) with post hoc testing or one-tailed unpaired t-tests (P ≤ 0.05), as indicated in the legends for Figs. 1–6.
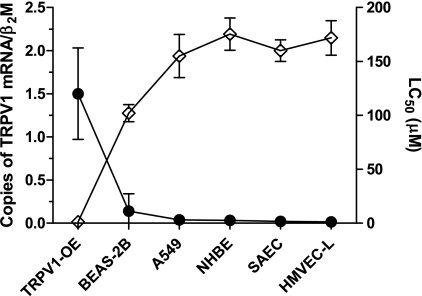
Fig. 1.
Quantification of transient receptor potential vanilloid-1 (TRPV1) mRNA values in human lung cells and corresponding concentration at which 50% loss in viability (lethality) was observed (LC50) for nonivamide. Copies of TRPV1 are represented relative to the gene β2-macroglobulin (β2M, ●; y-axis on left). LC50 values (◊; y-axis on right) were determined from dose-response curves from 24 h treatment of cells with nonivamide. BEAS-2B, human bronchial epithelial cells; HMVEC-L, human lung microvascular endothelial cells; NHBE, normal human bronchial epithelial cells; SAEC, small airway epithelial cells; TRPV1-OE, TRPV1-overexpressing BEAS-2B cells; A Pearson R value of −0.94 (P = 0.005) was obtained from correlation analysis showing a significant relationship between TRPV1 mRNA abundance and the LC50 values. Data represent means and SD of a minimum of 3 [quantitative PCR (qPCR)] or 9 (cytotoxicity) replicates.
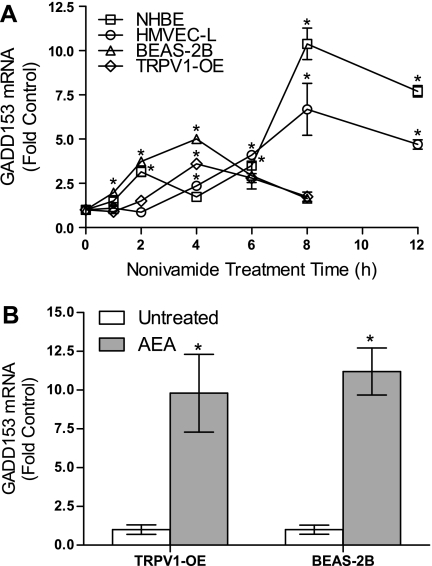
Fig. 2.
A: temporal changes in GADD153 mRNA expression in NHBE, HMVEC-L, BEAS-2B, and TRPV1-OE cells treated with LC50 concentrations of nonivamide. Data are means and SD for changes in GADD153 PCR product band density relative to β-actin, normalized to the 0-h, untreated control (n = 3). B: changes in GADD153 mRNA expression in TRPV1-OE and BEAS-2B cells treated with 25 μM anandamide (AEA) for 4 h. Data are normalized to the untreated sample. *Significant increases in expression using 1-way ANOVA (α = 0.05) with Dunnett's posttest (P < 0.05).
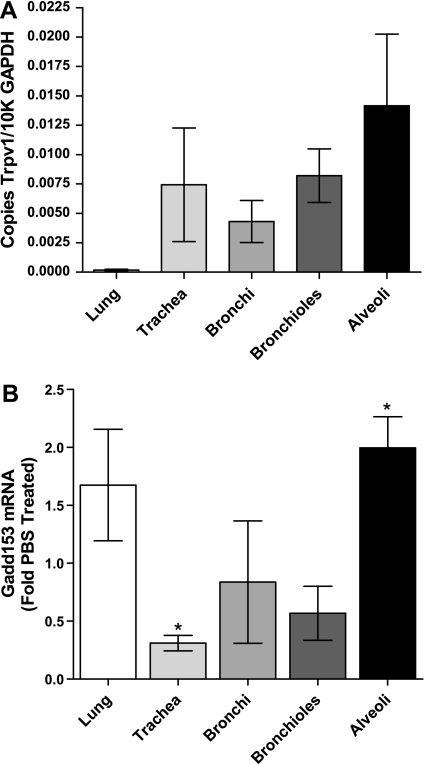
Fig. 3.
Analysis of Trpv1 and Gadd153 expression in mouse lungs using RT-qPCR. A: expression of Trpv1 in different anatomical regions of the mouse respiratory tract (n = 9). B: expression of Gadd153 4 h post-it instillation of sterile PBS (white bars) or 0.5 mg/kg nonivamide in PBS containing 4% vol/vol ethanol (gray-scaled bars) (n = 3). *Statistical difference (P < 0.05) relative to the corresponding PBS control value for the respective region using Student's t-test (n = 3).
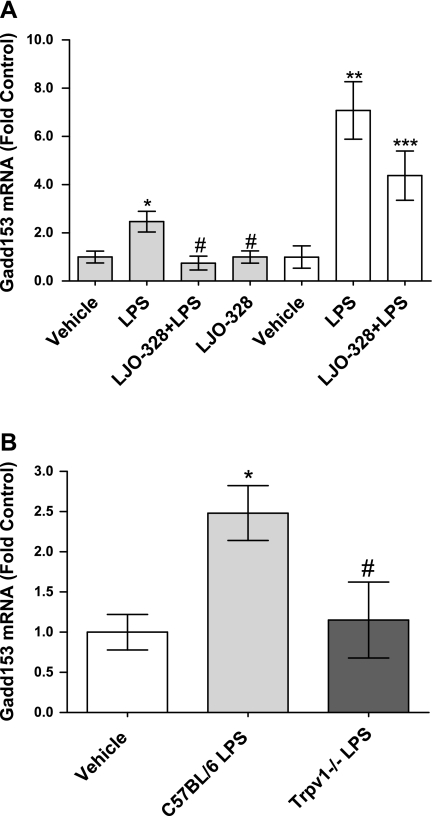
Fig. 4.
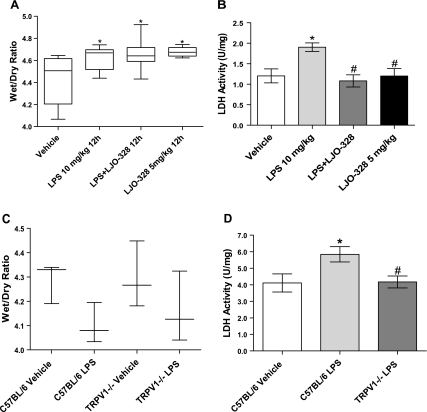
A: changes in Gadd153 mRNA abundance in lipopolysaccharide (LPS)-treated CF-1 mouse lungs 12 h (gray bars) and 18 h (white bars) after LPS treatment. Doses were vehicle, LPS (10 mg/kg ip), LPS (10 mg/kg ip) + LJO-328 (5 mg/kg ip), and LJO-328 (5 mg/kg ip) alone. *Statistical increases relative to the vehicle control (n = 15). #Decrease from LPS treatment (n = 15) in LPS + LJO-328-treated (n = 10) and LJO-328 only (n = 10) groups. ** and ***Statistical differences between the 18-h control (n = 5) compared with LPS treatment (n = 6) and LJO-328 + LPS (n = 6). B: changes in Gadd153 mRNA abundance in LPS-treated C57BL/6 and Trpv1−/− mouse lungs 12 h after LPS treatment. Vehicle injection (white bar, n = 5), LPS (15 mg/kg ip) (light gray bar, n = 5), and LPS (15 mg/kg ip) in Trpv1−/− mice (dark gray bar, n = 3). All data are means and SE, and statistical analysis was performed using 1-way ANOVA (α = 0.05) with Dunnett's posttest (P < 0.05). *Increase relative to the vehicle control group. #Decrease relative to the LPS-treated group.
Fig. 5.
A: quantification of CF-1 mouse lung wet-to-dry weight 12 h following vehicle injection (n = 6), LPS (10 mg/kg ip) (n = 6), LPS (10 mg/kg ip) + LJO-328 (5 mg/kg ip) (n = 6), and LJO-328 (5 mg/kg ip) only (n = 6). B: changes in lactate dehydrogenase (LDH) activity in bronchoalveolar lavage (BAL) of CF-1 mouse lungs 12 h following vehicle injection (white bars, n = 5), LPS (10 mg/kg ip) (light gray bars, n = 5), LPS (10 mg/kg ip) + LJO-328 (5 mg/kg ip) (dark gray bars, n = 5), and LJO-328 (5 mg/kg ip) only (black bars, n = 5). C: changes in lung wet-to-dry weight in C57BL/6 and Trpv1−/− mouse lungs 12 h after saline injection of C57BL/6 mice (n = 3), LPS (15 mg/kg ip) in C57BL/6 mice (n = 5), saline injection of Trpv1−/− mice (n = 3), and LPS (15 mg/kg ip) in Trpv1−/− mice (n = 3). D: changes in LDH activity in BAL of C57BL/6 and Trpv1−/− mice 12 h after saline injection of C57BL/6 mice (white bars, n = 5), LPS (15 mg/kg ip) in C57BL/6 mice (light gray bars, n = 5), and LPS (15 mg/kg) in Trpv1−/− mice (black bars, n = 3). Data are means and SE. *Significant increase relative to the vehicle control group. #Decrease relative to the LPS-treated group using 1-way ANOVA (α = 0.05) with Dunnett's posttest (P < 0.05).
Fig. 6.
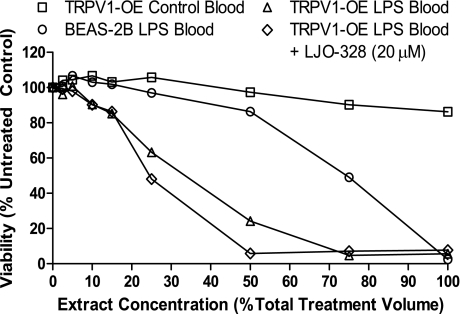
Cytotoxicity of n-butyl chloride + hexane extracts from control and LPS-treated CF-1 mouse blood in TRPV1-OE and BEAS-2B cells, with and without LJO-328 cotreatment (20 μM). Each treatment represents a single treatment well, using extracts prepared from blood pooled from three mice using a 24-h treatment time. Data are from a single experiment, but this trend was replicated on multiple occasions using extract preparations from single and multiple mice from different experiments.
RESULTS
TRPV1 expression and lung cell sensitivity to TRPV1 agonists.
TRPV1 mRNA was quantified in TRPV1-OE, BEAS-2B, A549, NHBE, SAEC, and HMVEC-L human lung cells using RT-qPCR. NHBE, SAEC, and HMVEC-L primary cells were selected because they are from regions of the lung that exhibit differential damage in various models of lung injury and in ARDS patients, with HMVEC-L, SAEC, and A549 cells representing cells from the most susceptible region, the alveoli. TRPV1 mRNA expression was highest in TRPV1-OE cells followed, in rank order, by BEAS-2B, A549, NHBE, SAEC, and HMVEC-L cells; TRPV1 mRNA levels highly correlated with the concentration at which 50% loss in viability (lethality) was observed (LC50) for nonivamide (Pearson R value = −0.94, P = 0.005; Fig. 1). The LC50 values for NHBE, HMVEC-L, and SAEC cells were ∼160–175 μM compared with ∼135, 100, and 1 μM for A549, BEAS-2B, and TRPV1-OE cells, respectively.
Induction of GADD153 in cultured human lung cells.
GADD153 mRNA was induced significantly in NHBE, HMVEC-L, BEAS-2B, and TRPV1-OE cells in a time-dependent manner following treatment with nonivamide, with the kinetics of GADD153 induction reflecting the potency of nonivamide as a toxin (Fig. 2A). Similarly, the endovanilloid AEA caused a 10- to 12-fold increase in GADD153 in TRPV1-OE and BEAS-2B cells at 8 h after treatment (Fig. 2B).
Trpv1 expression and Gadd153 induction by nonivamide treatment in mouse respiratory tissue.
Quantification of Trpv1 mRNA in the respiratory tract of CF-1 mice revealed expression throughout the respiratory tract (Fig. 3A). The highest expression was observed in alveolar tissue where SP-A mRNA was enriched ∼4- to 17-fold relative to the bronchiolar, bronchial, and tracheal samples, and Ccsp was ∼1/7th that of the bronchioles. Intratracheal administration of nonivamide (0.5 mg/kg) increased Gadd153 expression approximately twofold in the lung and, specifically, in the alveolar region (Fig. 3B). Gadd153 mRNA was reduced in the trachea, presumably because of locally higher doses of nonivamide and epithelial cell loss, as documented in rats exposed to capsaicin aerosols at similar doses (32).
Induction of Gadd1533 in mouse lungs following LPS treatment.
Gadd153 mRNA increased ∼2.5-fold at 12 h after LPS treatment of CF-1 mice and increased ∼7-fold at 18 h (Fig. 4A). Cotreatment of CF-1 mice with LPS and LJO-328 prevented Gadd153 induction at 12 h, with a sustained but lower effect at 18 h, suggesting clearance of LJO-328 and persistent inflammation in LPS-treated mice. Treatment with LJO-328 alone had no effect on Gadd153 expression (Fig. 4A). Gadd153 induction (∼2.5-fold) was also observed in C57BL/6 lungs following LPS treatment, which was attenuated in Trpv1−/− mouse lungs (Fig. 4B).
Quantification of LPS-induced lung injury via Trpv1 activation and ER stress.
In CF-1 mice, LPS treatment produced mild lung injury indicated by an increase in lung wet-to-dry weight ratio (Fig. 5A) and increased LDH in BAL fluid (Fig. 5B). LJO-328 cotreatment reduced the LPS-induced increase in LDH in BAL (Fig. 5A), but changes in the lung wet-to-dry weight ratio were equal for the LPS and LPS + LJO-328 treatment groups, both of which were above control levels (Fig. 5B). In C57BL/6 mice, LPS treatment also increased LDH in BAL fluid, but not in Trpv1−/− mice (Fig. 5D), and comparable decreases in lung wet-to-dry weight ratios were observed between the C57BL/6 and Trpv1−/− mice (Fig. 5D).
Analysis of endovanilloids in LPS-treated mouse blood.
Increased H2S in BAL was not observed, as predicted (22). However, organic extracts of blood from LPS-treated mice exhibited approximately twofold greater toxicity to TRPV1-OE cells compared with BEAS-2B cells, and these extracts were substantially more toxic than equivalent extracts of blood from control mice (Fig. 6). However, cytotoxicity was not attenuated by LJO-328 cotreatment.
Role of TRPV1 in the cytotoxicity of pure endovanilloids.
AEA was slightly more toxic to TRPV1-OE cells than BEAS-2B cells at 5 and 25 μM, and AEA toxicity was attenuated by LJO-328 cotreatment at the 5 μM AEA level (Table 1). However, dose-response analysis of AEA toxicity in multiple lung cells failed to show significant differences between BEAS-2B, TRPV1-OE, and other lung cells. NADA was more toxic to TRPV1-OE cells at 5 and 25 μM, and its cytotoxicity was partially inhibited by LJO-328 at these concentrations, similar to nonivamide (Table 1). 5(S)HETE was also more toxic to TRPV1-OE than BEAS-2B cells at 50 μM, but cytotoxicity was not prevented by LJO-328; rather, it was exacerbated. Finally, 2-AG exhibited slightly higher toxicity to TRPV1-OE cells at 5 μM, with a trend toward inhibition of cell death by LJO-328. Dose-response analysis confirmed that TRPV1-OE cells were more sensitive to 2-AG at concentrations >50 μM, but, like 5(S)HETE, toxicity was unexpectedly increased by LJO-328 cotreatment.
Table 1.
Screening for cytotoxicity of multiple purported endovanilloids in BEAS-2B, TRPV1-OE, and TRPV1-OE cells cotreated with the TRPV1 antagonist LJO-328 (20 μM)
| Viability, %untreated control* |
|||||||
|---|---|---|---|---|---|---|---|
| 5 μM |
25 μM |
50 μM |
|||||
| TRPV1 Agonist | Cell Type | − | + | − | + | − | + |
| Nonivamide# | BEAS-2B# | 108 ± 3^‡ | ND | ND | ND | ND | ND |
| TRPV1-OE# | 8 ± 2‡ | 81 ± 11†‡ | ND | ND | ND | ND | |
| AEA | BEAS-2B | 95 ± 4‡ | ND | 25 ± 2†‡ | ND | 0 ± 0 | ND |
| TRPV1-OE | 90 ± 3‡ | 102 ± 2†‡ | 18 ± 1‡ | 20 ± 2 | 0 ± 0 | 1 ± 0 | |
| 2-AG | BEAS-2B | 103 ± 6‡ | ND | 94 ± 4‡ | ND | 89 ± 5 | ND |
| TRPV1-OE | 93 ± 5‡ | 102 ± 13‡ | 98 ± 3‡ | 115 ± 1‡ | 86 ± 4 | 45 ± 9† | |
| LTB4 | BEAS-2B | 93 ± 2 | ND | 113 ± 5 | ND | 99 ± 1 | ND |
| TRPV1-OE | 103 ± 4 | 95 ± 2 | 103 ± 1 | 94 ± 3 | 111 ± 9 | 92 ± 3† | |
| 5(S)HETE | BEAS-2B | 101 ± 5 | ND | 68 ± 6 | ND | 38 ± 6^‡ | ND |
| TRPV1-OE | 95 ± 1 | 76 ± 4† | 61 ± 3 | 49 ± 2† | 10 ± 3‡ | 0 ± 0† | |
| 12(S)HpETE | BEAS-2B | 102 ± 1 | ND | 74 ± 2^ | ND | 15 ± 1 | ND |
| TRPV1-OE | 95 ± 1 | 73 ± 5† | 86 ± 1 | 49 ± 9† | 11 ± 2 | 3 ± 1 | |
| 15(S)HpETE | BEAS-2B | 103 ± 2 | ND | 96 ± 1^ | ND | 65 ± 1 | ND |
| TRPV1-OE | 99 ± 1 | 77 ± 2† | 104 ± 3 | 67 ± 2† | 61 ± 1 | 45 ± 2† | |
| NADA | BEAS-2B | 63 ± 2^‡ | ND | 8 ± 1^‡ | ND | 0 ± 0 | ND |
| TRPV1-OE | 23 ± 2‡ | 76 ± 2†‡ | 0 ± 0‡ | 1 ± 1 | 0 ± 0 | 0 ± 0 | |
| N-AT | BEAS-2B | 89 ± 1 | ND | 37 ± 15 | ND | 0 ± 0 | ND |
| TRPV1-OE | 90 ± 8 | 88 ± 2 | 48 ± 5 | 60 ± 14 | 0 ± 0 | 0 ± 0 | |
| H2S (as NaHS) | BEAS-2B | 105 ± 5 | ND | 108 ± 4 | ND | 105 ± 1 | ND |
| TRPV1-OE | 96 ± 5 | 100 ± 8 | 96 ± 5 | 98 ± 10 | 95 ± 1 | 99 ± 2 | |
TRPV1, transient receptor potential vanilloid-1; BEAS-2B, bronchial epithelial cells; TRPV1-OE, TRPV1-overexpressing BEAS-2B cells; AEA, anandamide; 2-AG, 2-arachidonoyl glycerol; LTB4, leukotriene B4; 5(S)HETE, 5(S)-hydroxyeicosatetraenoic acid; (S)HpETE, (S)-hydroperoxyeicosatetraenoic acid; NADA, N-arachidonoyl dopamine; N-AT, N-arachidonoyl taurine; H2S, hydrogen sulfide.
Viability was assessed following 24 h treatment (n = 3 experiments). inus and +, Absence or presence of LJO-328 at 20 μM in treatment media.
The concentration of nonivamide was 1.5 μM and is included as an example for the pattern that an agonist should resemble if cytotoxicity occurs in a TRPV1-dependent manner. ND, not determined.
Statistical difference between BEAS-2B vs. TRPV1-OE.
Statistical difference between TRPV1-OE in the absence vs. presence of LJO-328 using 2-way ANOVA with a Bonferroni posttest (α = 0.05).
Entries in bold text are consistent with a role for TRPV1 in mediating the cytotoxicity of these agents.
DISCUSSION
The major findings of this study are: 1) lung cells from the conducting airways, alveoli, and pulmonary capillaries of humans and mice express TRPV1 mRNA and exhibit ER stress when exposed to the TRPV1 agonists nonivamide and AEA, as well as other agonists as previously reported (40); 2) systemic inflammation elicited by LPS treatment of mice promotes ER stress/Gadd153 expression in the lung via an apparent Trpv1-dependent mechanism; this effect correlates with increases in LDH in BAL fluid; 3) pulmonary edema elicited by LPS, measured by the wet-to-dry weight ratio, appears to be independent of ER stress/Gadd153 induction under the experimental conditions used, and pulmonary edema may be exacerbated by inhibition or genetic deletion of TRPV1; and 4) multiple endovanilloids produced during inflammation cause cytotoxicity in vitro, but not in a traditional TRPV1-dependent manner.
A goal of this study was to characterize the functional consequences of TRPV1 activation by endogenous agonists in lung cell types known to be injured during systemic inflammation. TRPV1 expression and responses to the prototypical TRPV1 agonist nonivamide were evaluated. SAEC, HMVEC-L, and NHBE primary lung cells were equally sensitive to nonivamide (LC50 ∼160–175 μM), consistent with the low level of TRPV1 expression (Fig. 1), and all lung cell types responded similarly to nonivamide by increasing the expression of GADD153 mRNA (Fig. 2A). Additionally, the endovanilloid AEA induced GADD153 in BEAS-2B and TRPV1-OE cells (Fig. 2B), indicating that ER stress/GADD153 induction is a conserved response to TRPV1 agonists and that Gadd153 could be used as a marker for Trpv1 activation and ER stress in mouse lungs during inflammation.
Trpv1 expression and changes in Gadd153 mRNA expression caused by nonivamide treatment were quantified in the mouse respiratory tract. TRPV1 mRNA was expressed in greatest abundance in alveolar tissue (Fig. 3A), and, following nonivamide treatment, Gadd153 was induced in alveolar tissue (Fig. 3B). These data confirmed Gadd153 as a marker for Trpv1 activation in the region of the lung that is primarily damaged during inflammation.
It was hypothesized that endogenous TRPV1 agonists could couple systemic inflammation with lung injury via activation of TRPV1 in lung cells. As shown in Figs. 4 and 5, LPS-induced ER stress/Gadd153 induction was attenuated significantly by pharmacological inhibition of Trpv1 using LJO-328 and in Trpv1−/− mice; these findings are highly correlated with a reduction of LDH in BAL fluid (Fig. 5, B and D) and demonstrate a role for Trpv1 in lung injury.
LPS also increased the lung tissue wet-to-dry weight ratio relative to controls, indicating pulmonary edema. However, inhibition of Trpv1 using LJO-328 cotreatment had no effect on the wet-to-dry weight ratio (Fig. 5, A and C), and LJO-328 treatment alone increased the wet-to-dry weight ratio comparable to LPS alone (Fig. 5A), suggesting a deleterious effect of Trpv1 inhibition on the regulation of pulmonary vascular homeostasis. There are two possible explanations for the contradictory results for ER stress/Gadd153 induction and BAL LDH vs. changes in tissue wet-to-dry weight. First, LPS treatment stimulates systemic vascular leakage, resulting in a lower circulating blood volume and a higher plasma oncotic pressure that could have reduced the magnitude of change in the wet-to-dry weight ratio in the absence of volume resuscitation. Accordingly, any protective effect from Trpv1 inhibition could have been masked. In support of this concept, we have consistently observed lower wet-to-dry weight ratios in a mouse model of third-degree burn injury when animals are not volume resuscitated (Dull, unpublished observations). Alternatively, inhibition of TRPV1, while protective against ER stress and cytotoxicity, may be detrimental with respect to maintaining vascular function throughout the body. Specifically, it has been shown that rats treated with LPS (10 mg/kg iv) and cotreated with the TRPV1 antagonist capsazepine (43), and Trpv1−/− mice with peritoneal sepsis (8), exhibited exacerbated LPS-induced hypotension, peritoneal edema, and greater mortality. Similarly, inhibition of somatostatin release from Trpv1-expressing neurons in the airways of LJO-328-treated and Trpv1−/− mice could also exacerbate systemic vascular water loss, based on studies showing that the release of this neuropeptide from intranasal LPS (and presumably endovanilloid)-stimulated airway sensory neurons is anti-inflammatory and protective against pulmonary and systemic injury (12, 17, 18). As such, a reduction in edema with TRPV1 inhibition would not be expected, rather it would be exacerbated, as implied by Fig. 5. Regardless, the collective results in Figs. 4 and 5 indicate that Trpv1 is activated in lung cells during inflammation, leading to ER stress and lung cell cytotoxicity, as indicated by Gadd153 induction and LDH in BAL fluid, respectively, but that these processes are not critical determinants of pulmonary edema.
Finally, the identities of specific endovanilloids responsible for ER stress/Gadd153 induction in mouse lungs were pursued. The presence of elevated concentrations of cytotoxic endovanilloids in blood of LPS-treated mice was implied by results presented in Fig. 6 where blood extracts from LPS-treated mice exhibited substantially greater toxicity in TRPV1-OE cells compared with BEAS-2B cells, and to comparable extracts from control mice. However, the cytotoxicity of the extract was not attenuated by LJO-328 cotreatment, due potentially to the extracts being chemically complex, containing multiple toxicants that caused cell death via activation of TRPV1 at binding sites that were not affected by LJO-328, via TRPV1-independent mechanisms, or a combination of both.
Subsequently, specific chemicals identified in the literature as endovanilloids were tested for TRPV1-dependent toxicity in lung cells (Table 1). 2-AG, NADA, and 5(S)HETE exhibited slightly greater toxicity in TRPV1-OE cells, consistent with a role for TRPV1, but only NADA cytotoxicity was attenuated by LJO-328, and NADA may or may not be relevant with respect to the effects of systemic inflammation on lung cells. None of the other purported endovanilloids tested in Table 1 exhibited properties consistent with TRPV1-dependent cell death, which was particularly surprising for H2S, based on reports that it is a mediator of lung injury via activation of TRPV1-expressing sensory nerves (1, 3–5, 9, 22, 27, 29, 35, 36, 41, 47–49). These data raise questions as to whether H2S truly acts through TRPV1 to promote lung injury or if it acts through TRPA1 (26, 37), which is coexpressed by the TRPV1-expressing C-fiber neurons that regulate neurogenic inflammation and that would be eliminated by capsaicin pretreatment/C-fiber depletion protocols previously used to implicate Trpv1 as a mediator of lung injury by H2S. TRPA1 is also activated by oxidants, electrophilic lipids, and many other inflammatory mediators (2), making it a likely alternative candidate or parallel mediator of lung injury where substance P-expressing airway C-fibers are involved. Collectively, from these studies, it is concluded that unique TRPV1 agonists couple inflammation with ER stress and cytotoxicity but that neither the major circulating endovanilloids presumed to be present in the blood extracts used in Fig. 6 nor the pure endovanilloids studied in Table 1 are the agonists principally responsible for the observations in lung tissue, despite possibilities that the lack of inhibition of cytotoxicity by these test substances can be rationalized by an inability of LJO-328 to effectively antagonize their activity in vitro where mixed modes of TRPV1 activation may occur, as shown for several antagonists using capsaicin, heat, protons (16, 21), and AEA (personal communication with Lee). Thus, there remains a need to identify and characterize additional endogenous agonists that couple TRPV1 activation in lung cells with inflammation and to characterize other potential parallel pathways leading to lung injury during inflammation.
In summary, the collective results support the hypothesis that TRPV1 participates in the pathogenesis of LPS/inflammation-induced lung/lung cell damage, but the identity(ies) of the putative TRPV1 agonist(s) that elicit TRPV1-dependent responses in lung cells remain unknown. It is proposed that selective inhibition of TRPV1 and/or specific agonist synthesis pathways may represent a feasible approach to prevent select components of lung injury, such as cytotoxicity via the ER stress response pathway, but that such therapies will require site-specific delivery, based on results that systemic Trpv1 inhibition is associated with adverse consequences, including increased lung (and other tissue) injury, morbidity, and mortality (8, 12, 17, 18, 38, 43).
GRANTS
This work was supported by National Institutes of Health Grants HL-069813 and ES-017431 and by a seed grant from the Department of Anesthesiology, University of Utah School of Medicine. Development and synthesis of LJO-328 was supported by Grant R11–2007-107–02001-0 from the National Research Foundation of Korea (J. Lee).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Alan R. Light and Ron Hughen for maintaining C57BL/6 and Trpv1−/− mouse colonies, Dr. Robert Paine III (Chief, Pulmonary Critical Care Medicine, University of Utah) for technical review of this manuscript, and Dr. Laura Van Winkle (University of California-Davis) for technical advice on the airway dissection and mRNA analysis procedures.
REFERENCES
- 1. Ang SF, Moochhala SM, Bhatia M. Hydrogen sulfide promotes transient receptor potential vanilloid 1-mediated neurogenic inflammation in polymicrobial sepsis. Crit Care Med 38: 619–628 [DOI] [PubMed] [Google Scholar]
- 2. Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23: 360–370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatia M, Slavin J, Cao Y, Basbaum AI, Neoptolemos JP. Preprotachykinin-A gene deletion protects mice against acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 284: G830–G836, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Bhatia M, Wong FL, Fu D, Lau HY, Moochhala SM, Moore PK. Role of hydrogen sulfide in acute pancreatitis and associated lung injury. FASEB J 19: 623–625, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Bhatia M, Zhi L, Zhang H, Ng SW, Moore PK. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am J Physiol Lung Cell Mol Physiol 291: L896–L904, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Bottoms GD, Adams HR. Involvement of prostaglandins and leukotrienes in the pathogenesis of endotoxemia and sepsis. J Am Vet Med Assoc 200: 1842–1848, 1992 [PubMed] [Google Scholar]
- 7. Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24: 487–517, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 21: 3747–3755, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol 146: 498–505, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol 143: 251–256, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Petrocellis La, DM V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci 77: 1651–1666, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Elekes K, Helyes Z, Nemeth J, Sandor K, Pozsgai G, Kereskai L, Borzsei R, Pinter E, Szabo A, Szolcsanyi J. Role of capsaicin-sensitive afferents and sensory neuropeptides in endotoxin-induced airway inflammation and consequent bronchial hyperreactivity in the mouse. Regul Pept 141: 44–54, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem (Tokyo) 138: 501–507, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Fink MP. Prostaglandins and sepsis: still a fascinating topic despite almost 40 years of research. Am J Physiol Lung Cell Mol Physiol 281: L534–L536, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fink MP, O'Sullivan BP, Menconi MJ, Wollert PS, Wang H, Youssef ME, Fleisch JH. A novel leukotriene B4-receptor antagonist in endotoxin shock: a prospective, controlled trial in a porcine model. Crit Care Med 21: 1825–1837, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist-induced hyperthermia. J Neurosci 30: 1435–1440, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Helyes Z, Elekes K, Nemeth J, Pozsgai G, Sandor K, Kereskai L, Borzsei R, Pinter E, Szabo A, Szolcsanyi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol 292: L1173–L1181, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Helyes Z, Pinter E, Sandor K, Elekes K, Banvolgyi A, Keszthelyi D, Szoke E, Toth DM, Sandor Z, Kereskai L, Pozsgai G, Allen JP, Emson PC, Markovics A, Szolcsanyi J. Impaired defense mechanism against inflammation, hyperalgesia, and airway hyperreactivity in somatostatin 4 receptor gene-deleted mice. Proc Natl Acad Sci USA 106: 13088–13093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97: 6155–6160, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kadoi Y, Hinohara H, Kunimoto F, Saito S, Goto F. Cannabinoid antagonist AM 281 reduces mortality rate and neurologic dysfunction after cecal ligation and puncture in rats. Crit Care Med 33: 2629–2636, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lázár JG, Laxmikant, Khairathkar-Joshi Neelima, Blumberg Peter M, Szallasi Arpad Screening TRPV1 antagonists for the treatment of pain: lessons learned over a decade. Exp Opin Drug Disc 4: 159–180, 2009 [DOI] [PubMed] [Google Scholar]
- 22. Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Maccarrone M, De Petrocellis L, Bari M, Fezza F, Salvati S, Di Marzo V, Finazzi-Agro A. Lipopolysaccharide downregulates fatty acid amide hydrolase expression and increases anandamide levels in human peripheral lymphocytes. Arch Biochem Biophys 393: 321–328, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Matute-Bello M, Ga MA. Animal Models of Acute Lung Injury. ATS Website http://www.thoracic.org/sections/clinical-information/critical-care/critical-care-research/animal-models-of-acute-lung-injury.html, 2007 [DOI] [PMC free article] [PubMed]
- 26. Miyamoto R, Otsuguro KI, Ito S. Time- and concentration-dependent activation of TRPA1 by hydrogen sulfide in rat DRG neurons. Neurosci Lett 499: 137–142, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Ng SW, Zhang H, Hegde A, Bhatia M. Role of preprotachykinin-A gene products on multiple organ injury in LPS-induced endotoxemia. J Leukoc Biol 83: 288–295, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Palace GP, Horgan MJ, Malik AB. Generation of 5-lipoxygenase metabolites following pulmonary reperfusion in isolated rabbit lungs. Prostaglandins 43: 339–349, 1992 [DOI] [PubMed] [Google Scholar]
- 29. Puneet P, Hegde A, Ng SW, Lau HY, Lu J, Moochhala SM, Bhatia M. Preprotachykinin-A gene products are key mediators of lung injury in polymicrobial sepsis. J Immunol 176: 3813–3820, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Reilly CA. Neurogenic inflammation: the role of TRP channels in the lung. In: Comprehensive Toxicology, edited by Yost GS. New York, NY: Elsevier, 2010, p. 129–150 [Google Scholar]
- 31. Reilly CA, Johansen ME, Lanza DL, Lee J, Lim JO, Yost GS. Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J Biochem Mol Toxicol 19: 266–275, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci 73: 170–181, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roche M, Kelly JP, O'Driscoll M, Finn DP. Augmentation of endogenous cannabinoid tone modulates lipopolysaccharide-induced alterations in circulating cytokine levels in rats. Immunology 125: 267–271, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry 45: 9007–9015, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Sio SW, Moochhala S, Lu J, Bhatia M. Early protection from burn-induced acute lung injury by deletion of preprotachykinin-A gene. Am J Respir Crit Care Med 181: 36–46, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Sio SW, Puthia MK, Lu J, Moochhala S, Bhatia M. The neuropeptide substance P is a critical mediator of burn-induced acute lung injury. J Immunol 180: 8333–8341, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–399, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Than M, Nemeth J, Szilvassy Z, Pinter E, Helyes Z, Szolcsanyi J. Systemic anti-inflammatory effect of somatostatin released from capsaicin-sensitive vagal and sciatic sensory fibres of the rat and guinea-pig. Eur J Pharmacol 399: 251–258, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Thomas KC, Ethirajan M, Shahrokh K, Sun H, Lee J, Cheatham TE, 3rd, Yost GS, Reilly CA. Structure-activity relationship of capsaicin analogs and transient receptor potential vanilloid 1-mediated human lung epithelial cell toxicity. J Pharmacol Exp Ther 337: 400–410, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thomas KC, Sabnis AS, Johansen ME, Lanza DL, Moos PJ, Yost GS, Reilly CA. Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J Pharmacol Exp Ther 321: 830–838, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123–1131, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Van Der Stelt M, Di Marzo V. Endovanilloids putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem 271: 1827–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Wang Y, Novotny M, Quaiserova-Mocko V, Swain GM, Wang DH. TRPV1-mediated protection against endotoxin-induced hypotension and mortality in rats. Am J Physiol Regul Integr Comp Physiol 294: R1517–R1523, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369: 1553–1564, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Yamaji K, Sarker KP, Kawahara K, Iino S, Yamakuchi M, Abeyama K, Hashiguchi T, Maruyama I. Anandamide induces apoptosis in human endothelial cells: its regulation system and clinical implications. Thromb Haemost 89: 875–884, 2003 [PubMed] [Google Scholar]
- 47. Zhang H, Hegde A, Ng SW, Adhikari S, Moochhala SM, Bhatia M. Hydrogen sulfide up-regulates substance p in polymicrobial sepsis-associated lung injury. J Immunol 179: 4153–4160, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Zhang H, Zhi L, Moore PK, Bhatia M. Role of hydrogen sulfide in cecal ligation and puncture-induced sepsis in the mouse. Am J Physiol Lung Cell Mol Physiol 290: L1193–L1201, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Zhang J, Sio SW, Moochhala S, Bhatia M. Role of hydrogen sulfide in severe burn injury-induced inflammation in mice. Mol Med 16: 417–424, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999 [DOI] [PubMed] [Google Scholar]