Abstract
An early event in the pathogenesis of emphysema is the development of inflammation associated with accumulation of polymorphonuclear leukocytes (PMN) in small airways, and inflammatory cell recruitment from the circulation involves migration across endothelial and epithelial cell barriers. Platelet-activating factor (PAF) promotes transendothelial migration in several vascular beds, and we postulated that increased PAF production in the airways of smokers might enhance inflammatory cell recruitment and exacerbate inflammation. To examine this possibility, we incubated human lung microvascular endothelial cells (HMVEC-L) with cigarette smoke extract (CSE) and found that CSE inhibits PAF-acetylhydrolase (PAF-AH) activity. This enhances HMVEC-L PAF production and PMN adherence, and adherence is blocked by PAF receptor antagonists (CV3988 or ginkgolide B). CSE also inhibited PAF-AH activity of lung endothelial cells isolated from wild-type (WT) and iPLA2β knockout mice, and with WT cells, CSE enhanced PAF production and RAW 264.7 cell adherence. In contrast, CSE did not affect PAF production or RAW 264.7 cell adherence to iPLA2β-null cells, suggesting that iPLA2β plays an important role in PAF production by lung endothelial cells. These findings suggest that inhibition of PAF-AH by components of cigarette smoke may initiate or exacerbate inflammatory lung disease by enhancing PAF production and promoting accumulation of inflammatory cells in small airways. In addition, iPLA2β is identified as a potential target for therapeutic interventions to reduce airway inflammation and the progression of chronic lung disease.
Keywords: cigarette smoke extract, endothelium, phospholipase A2
smokers are at higher risk of heart attack, stroke, and chronic obstructive pulmonary disease (COPD). COPD is the fifth leading cause of death worldwide, and it is estimated that there are 15–17 million people with COPD in the U.S. alone (34). Human COPD includes emphysema and chronic bronchitis and is marked by several pathological features, such as small airway remodeling, vascular remodeling with pulmonary hypertension, and mucus overproduction (7, 15, 16). The progression of chronic lung diseases is marked by migration of circulating inflammatory cells into the airway, accumulation of neutrophils and macrophages, production of inflammatory mediators, and the release of proinflammatory cytokines and proteases (16). Activation of endothelial and epithelial cells in the airway and accumulation of leukocytes creates a milieu in which additional chemoattractant mediators are produced to perpetuate the influx of inflammatory cells that leads to tissue remodeling and destruction (16).
The molecular mechanisms by which inflammatory cells affect small airway remodeling are incompletely delineated at present (22, 41), but cigarette smoke promotes release of oxidants from neutrophils and macrophages, which may trigger or potentiate transforming growth factor-β release and collagen production (11, 32, 33).
Recruitment of circulating inflammatory cells into the airways involves migration across the endothelial and epithelial cell barriers, and such migration is promoted by mediators that include platelet-activating factor (PAF). PAF is a membrane phospholipid-derived mediator produced by endothelial cells that causes transient adherence of neutrophils (37). Net PAF production is governed by its relative rates of biosynthesis and degradation. The remodeling pathway for PAF synthesis is activated during inflammation and involves tightly coupled phospholipase A2 (PLA2)-catalyzed hydrolysis of membrane phospholipids to produce lyso-PAF (37, 44, 46) and acetylation of lyso-PAF at the sn-2 position catalyzed by acetyl-CoA:lyso-PAF acetyltransferase (21, 28, 47). The principal pathway for PAF degradation is its hydrolysis by the enzyme PAF-acetylhydrolase (PAF-AH). Components of cigarette smoke inhibit PAF-AH activity (9, 38), and this could result in PAF accumulation on the pulmonary endothelial cell surface in vivo and facilitate adherence and transmigration of circulating neutrophils.
We have previously reported that stimulation of human pulmonary microvascular endothelial cells (HMVEC-L) with protease-activated receptor agonists, e.g., tryptase, activates an intracellular group VI phospholipase A2 (iPLA2) and that this results in increased release of arachidonic acid, PAF production, and adherence of polymorphonuclear leukocytes (PMN) to HMVEC-L (42). We have also demonstrated that the iPLA2 isoform responsible for endothelial cell PAF production is the group VIA PLA2 (iPLA2β) and not the group VIB PLA2 (iPLA2γ) (44). In the studies reported in this article, we have examined the hypothesis that cigarette smoke may exacerbate inflammatory airway diseases by inhibiting PAF-AH activity, which results in increased endothelial cell PAF production and enhanced inflammatory cell recruitment to the lung.
MATERIALS AND METHODS
Materials.
HMVEC-L were obtained from Lonza (Walkersville, MD). Cigarette smoke extract (CSE) was obtained from Murty Pharmaceuticals (Lexington, KY). Methyl arachidonyl fluorophosphonate (MAFP) was obtained from Cayman Chemical (Ann Arbor, MI). [3H]acetic acid, sodium salt and hexadecyl-2-acetyl-sn-glyceryl-2-phosphorylcholine, 1-O-[acetyl-3H(N)] were obtained from PerkinElmer (Boston, MA). All other chemicals were obtained from Sigma Chemical (St. Louis, MO).
Endothelial cell culture.
HMVEC-L were grown to confluence in endothelial cell basal medium supplemented with growth factors (Lonza) and incubated at 37°C in an atmosphere of 95% O2-5% CO2. Cells from passages 3 and 4 were used for experiments.
The generation of mice deficient in iPLA2β has been described previously (4). Mice were housed in a pathogen-free facility, and studies were conducted under protocols approved by Saint Louis University Animal Care and Use Committee. Endothelial cells were isolated from mouse lung by collagenase digestion. The diced lung tissue was incubated in 1 mg/ml collagenase for 1 h at 37°C, and the digested tissue was passed through a cell strainer. Endothelial cells were isolated by incubation with anti-mouse platelet/endothelial cell adhesion molecule-1 coupled to magnetic beads. Cells were washed, resuspended in EGM-2MV cell culture medium (Lonza), and plated in 25-cm2 culture flasks. Nonadherent cells were removed the next day, and cells were grown to confluence and passaged at a 1:3 dilution. Cells from passages 3 and 4 were used for experiments. Isolation purity was verified by staining with anti-factor VIII antibody, and preparations with greater than 85% endothelial purity were used.
PLA2 activity.
The surrounding medium was removed from confluent endothelial cells and immediately replaced with ice-cold buffer containing 250 mM sucrose, 10 mM KCl, 10 mM imidazole, 5 mM EDTA, 2 mM DTT, and 10% glycerol (pH 7.8) (PLA2 assay buffer). Endothelial cells suspended in ice-cold PLA2 assay buffer were sonicated on ice six times for 10 s, and the sonicate was centrifuged at 14,000 g for 10 min. PLA2 activity in the supernatant was assessed by incubating the enzyme with a synthetic 100 μM (16:0, [3H]18:1) plasmenylcholine substrate (specific activity of 150 dpm/pmol, ∼68 μCi/μmol) in assay buffer containing 100 mM Tris, 4 mM EGTA, and 10% glycerol (pH 7.0) at 37°C for 5 min in a total volume of 200 μl. Reactions were initiated by adding the radiolabeled phospholipid substrate as a concentrated stock solution in ethanol. Reactions were terminated by the addition of butanol, and the released radiolabeled fatty acid was isolated by application of an aliquot of the butanol phase to channeled Silica Gel G plates, development in a petroleum ether-diethyl ether-acetic acid mixture (70:30:1, vol/vol/vol), and subsequent quantification by liquid scintillation spectrometry with appropriate quench correction.
Acetyl-CoA:lyso-PAF acetyltransferase activity.
HMVEC grown to confluence were removed from the tissue culture plate in ice-cold NaCl-PO4 buffer (139 mM NaCl, 5 mM Na2HPO4, 5 mM NaH2PO4·H2O, pH 7.4). Cellular protein (40 μg) was incubated with 40 μM 16:0 lyso-PAF, 200 μM [3H]acetyl-CoA (0.3 μCi/100 nmol) at 37°C for 15 min in buffer containing 4.2 mM HEPES (pH 7.4), 137 mM NaCl, 2.6 mM KCl, 1.3 mM CaCl2, 1 mM MgCl2, 1 mM DTT, and 0.25% (wt/vol) bovine serum albumin (BSA). Unreacted [3H]acetyl-CoA was removed using Dowex X1–8 resin columns, and [3H]acetyl-PAF was quantified by liquid scintillation spectrometry. Loss of [3H]acetyl-PAF was corrected by adding a known amount of [14C]palmitoyl-2-acetyl-sn-glycero-3-phosphocholine as an internal standard.
PAF-AH activity.
HMVEC-L or mouse lung endothelial cells grown to confluence in 35-mm dishes were removed from the tissue culture plate in 1.2 mM Ca2+-HEPES buffer and sonicated on ice. Cellular protein (25 μg) was incubated with 0.1 mM [3H]acetyl-PAF (10 mCi/mmol) for 30 min at 37°C. The reaction was stopped by adding 50 μl of 10 M acetic acid and 1.5 ml of 0.1 M sodium acetate. Released [3H]acetic acid was isolated by passing the reaction mixture through a C18 gel cartridge (Baker Chemical, Phillipsburg, NJ), and radioactivity was measured using a liquid scintillation counter.
PAF production.
HMVEC-L or mouse lung endothelial cells grown in 35-mm dishes were washed twice with Hanks' balanced salts solution (HBSS). Cells were incubated with 10 μCi [3H]acetic acid per well for 20 min. After experimental conditions, cell lipids were extracted using the method of Bligh and Dyer (10). The chloroform layer was concentrated under N2, resuspended in 9:1 CHCl3-MeOH, applied to a Silica Gel 60 TLC plate, and developed in chloroform-methanol-acetic acid-water (50:25:8:4 vol/vol/vol/vol). The region corresponding to [3H]PAF was scraped, and radioactivity was quantified using liquid scintillation spectrometry. Loss of PAF during extraction and chromatography was corrected by adding a known amount of [14C]PAF as an internal standard.
In selected experiments, human PAF was measured directly using an ELISA kit (Biotang, Waltham, MA). HMVEC-L monolayers were washed with ice-cold Dulbecco's phosphate-buffered saline (D-PBS) and frozen at −20°C. After two freeze-thaw cycles, aliquots of the suspension were added to microtiter plates with a biotin-conjugated polyclonal antibody specific for PAF. PAF content in samples was determined spectrophotometrically at 450 nm using a Synergy 2 microplate reader (Biotek, Winooski, VT).
Neutrophil adherence.
Blood (80 ml) was obtained from healthy adults and layered over an equal volume of Polymorphprep (Axis-Shield, Oslo, Norway) in 50-ml conical tubes. Tubes were spun at 500 g for 30 min at 20°C. The buffy coat at the sample-medium interface consisting of PMN was removed, washed, and resuspended in 5 ml of ice-cold HBSS, and cells were counted. HMVEC-L grown on a 12-mm plate were washed twice with HBSS. After appropriate pretreatment of PMN with CV3988 or ginkgolide B, 0.5 ml of PMN suspension (4 × 106 cells/ml) in HBSS was added to each of the wells and incubated for 10 min at room temperature. Media and unbound cells were removed and discarded. Plates were washed twice with prewarmed D-PBS. Adherent PMN and endothelial cells were lysed in 1 ml of 0.2% Triton X-100. For maximal binding, a 0.5-ml aliquot of PMN suspension plus 0.5 ml of 0.2% Triton X-100 was used. To measure myeloperoxidase activity, we added 400 μl of cell lysate to 1 ml of PBS, 1,200 μl of Hanks' buffer-BSA, and 200 μl of 3,3′-dimethoxybenzidine, and 200 μl of 0.05% H2O2 were added. After 15 min, 200 μl of 1% NaN3 were added to stop the reaction, and absorbance was measured using a 4050 UV/Visible spectrophotometer (Biochrom, Cambridge, UK) at 460 nm.
RAW 264.7 cell adherence.
RAW 264.7 cells were grown to confluence in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. Cell suspensions (10 × 106/ml) were labeled with 4 μg/ml calcein-AM for 45 min at 37°C. Cells were washed three times with HEPES buffer and resuspended at a concentration of 4 × 106/ml, and 0.5 ml was added to confluent mouse lung endothelial cell monolayers. At the end of the incubation time, nonadherent cells were removed and adherent cells and endothelial cells were lysed in 1 ml of 0.2% Triton X-100. Calcein fluorescence in each sample was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The percent RAW 264.7 cell adherence in each sample was calculated based on the fluorescence measured in 0.5 ml of RAW 264.7 cell suspension.
Cell surface expression of adhesion molecules.
HMVEC-L were grown to confluence in 16-mm culture dishes. Cells were incubated with CSE for selected time periods at 37°C in 95% O2-5% CO2. At the end of the incubation period, the medium was quickly removed and the cells were washed with PBS, immediately fixed in 1% paraformaldehyde, and incubated at 4°C overnight. Cells were washed three times with PBS and then blocked with Tris-buffered saline-Tween supplemented with 0.8% (wt/vol) BSA and 0.5% (wt/vol) fish gelatin for 1 h at room temperature. Cell cultures were incubated with appropriate primary antibodies (1:50; Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (1:5,000; Santa Cruz Biotechnology). Cultures were incubated in the dark for 30 min with 3,3′,5,5′-tetramethylbenzidine liquid substrate. Reactions were stopped by the addition of sulfuric acid, and color development was measured with a microtiter plate spectrophotometer at 450 nm.
Caspase activity.
Caspase activity was measured using a Caspase-Glo 3/7 assay kit (Promega, Madison, WI). After CSE treatment, endothelial cell monolayers were washed, trypsinized, and centrifuged. Cells (2.5 × 104) were resuspended in 75 μl of medium and aliquoted into 3 wells of a 96-well plate. An equal volume of Caspase-Glo was added to each well, and samples were incubated at room temperature for 30 min. Samples were analyzed for luminescence using a Victor 1420 multilabel counter (PerkinElmer, Waltham, MA). Medium-only samples were used as controls. Control luminescence was subtracted, and luminescence in each sample was calculated relative to that in untreated cell controls.
Cell viability assay.
Cell viability and CSE cytotoxicity were evaluated using the LIVE/DEAD viability/cytotoxicity kit (Molecular Probes, Eugene, OR). After CSE incubation, HMVEC-L were washed with D-PBS and incubated with 4 μM ethidium homodimer-1 and 2 μM calcein-AM for 45 min at 37°C. Fresh D-PBS was added to the cells, and fluorescence was examined using an Olympus IX81 motorized inverted microscope (Olympus, Center Valley, PA).
Statistical analysis.
All studies were repeated with at least four separate cell cultures. Data were analyzed using Student's t-test or one-way analysis of variance followed by post hoc analysis using Dunnett's test. Differences were regarded as significant at P < 0.05 and highly significant at P < 0.01. Data are means ± SE.
RESULTS
Endothelial cell PAF-AH activity is inhibited by CSE.
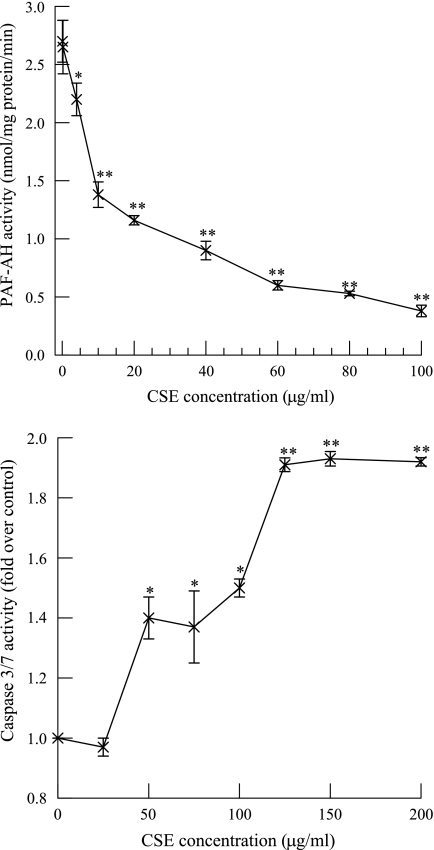
We incubated (8 h) HMVEC-L with increasing concentrations of CSE and measured PAF-AH activity. Significant inhibition of PAF-AH activity was observed with CSE concentrations >4 μg/ml (Fig. 1, top), and the IC50 was ∼11 μg/ml. Tithof et al. (54) have shown that polycyclic aromatic hydrocarbons found in cigarette smoke cause apoptosis in human coronary artery endothelial cells by a PLA2-dependent mechanism. We therefore incubated HMVEC-L with increasing concentrations of CSE for 8 h and measured caspase 3/7 activity (Fig. 1, bottom). No significant increase in caspase 3/7 activity at a CSE concentration of 25 μg/ml was observed, indicating that under those exposure conditions, apoptosis was not induced. We therefore used a CSE concentration of 20 μg/ml in all subsequent experiments to avoid inducing endothelial cell apoptosis.
Fig. 1.
Platelet-activating factor (PAF)-acetylhydrolase (PAF-AH) activity (top) and caspase 3/7 activity (bottom) measured in human lung microvascular endothelial cells (HMVEC-L) incubated with increasing concentrations of cigarette smoke extract (CSE) for 8 h. Data are means ± SE for 4 separate cell cultures. *P < 0.05; **P < 0.01 compared with control.
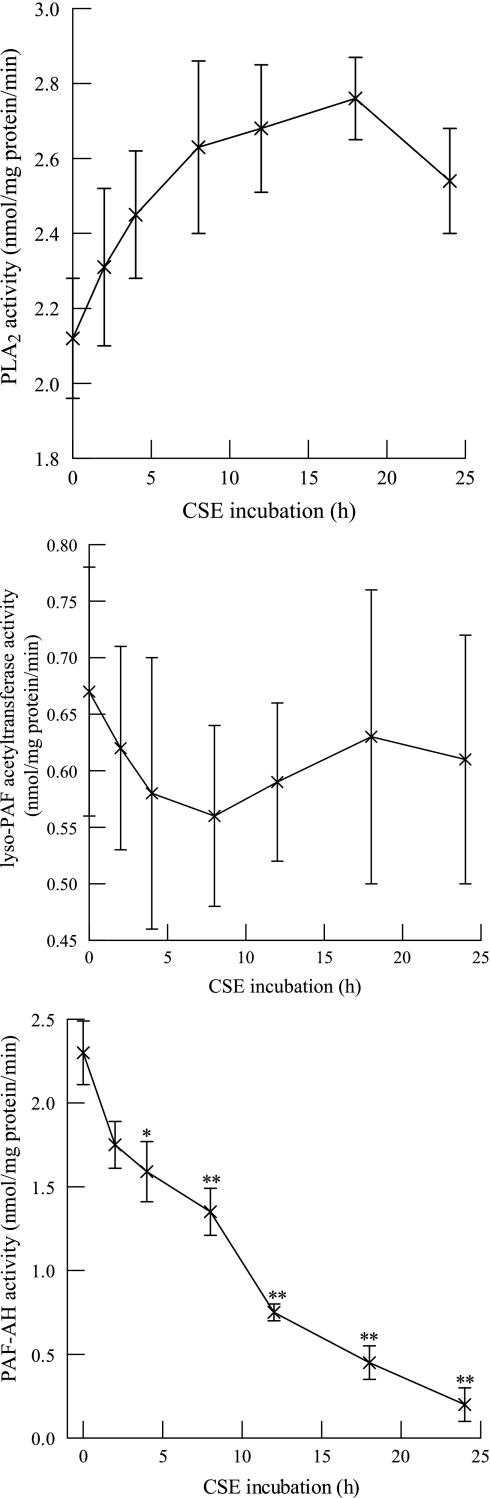
In subsequent experiments, we measured the activity of endothelial PAF synthesis (PLA2 and acetyl-CoA:lyso-PAF acetyltransferase) and degradation (PAF-AH) enzymes in response to CSE exposure. Incubation with CSE (20 μg/ml) had no significant effect on iPLA2 activity (Fig. 2, top) or acetyl-CoA:lyso-PAF acetyltransferase activity (Fig. 2, middle) but resulted in a time-dependent reduction in PAF-AH activity that was significant after 4 h and progressive over 24 h, when about 93% inhibition was achieved (Fig. 2, bottom). As a positive control, HMVEC-L were incubated with a known PAF-AH inhibitor (methyl arachidonyl fluorophosphonate, 5 μM, 10 min) (27), which achieved 98% inhibition of PAF-AH activity (0.05 ± 0.07 nmol·mg protein−1·min−1). Thus incubation with CSE results in a time- and concentration-dependent inhibition of endothelial cell PAF-AH activity but has little effect on PAF synthesis enzymes.
Fig. 2.
Phospholipase A2 (PLA2) activity (top), acetyl-CoA:lyso-PAF acetyltransferase activity (middle), and PAF-AH activity (bottom) measured in HMVEC-L incubated with CSE (20 μg/ml) for up to 24 h. Data are means ± SE for 6 separate cell cultures. *P < 0.05; **P < 0.01 compared with control.
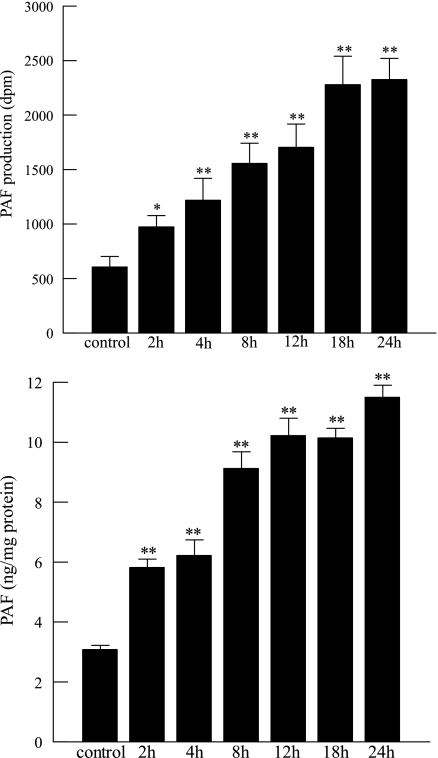
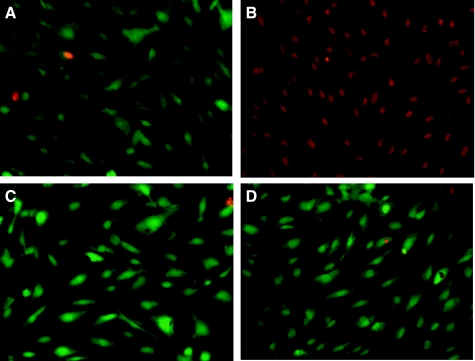
We have previously demonstrated that inhibition of PAF-AH with MAFP results in increased endothelial cell PAF production by preventing its degradation (27). In the present study, we measured PAF accumulation in HMVEC-L incubated with CSE (20 μg/ml) for increasing time intervals. A time-dependent increase in PAF measured by incorporated [3H]acetate or by ELISA was observed and found to be significant after 4 h and to have increased progressively over 24 h (Fig. 3). Inhibiting PAF-AH activity with CSE thus augments net PAF production by HMVEC-L. We examined HMVEC-L cell death over time and demonstrated that there was no significant cell death associated with enzyme inhibition and PAF accumulation at 20 μg/ml CSE over 24 h (Fig. 4).
Fig. 3.
PAF production measured as [3H]acetyl-PAF (top) or by ELISA assay (bottom) in HMVEC-L incubated with CSE (20 μg/ml) for up to 24 h. Data are means + SE for 4 separate cell cultures. *P < 0.05; **P < 0.01 compared with control.
Fig. 4.
HMVEC viability measured using the LIVE/DEAD viability/cytotoxicity assay kit (Invitrogen). Cells were left untreated (A) or incubated with CSE (20 μg/ml) for 4 (C) or 24 h (D). Cells were treated with methanol (5 min) as a positive control for cell death (B).
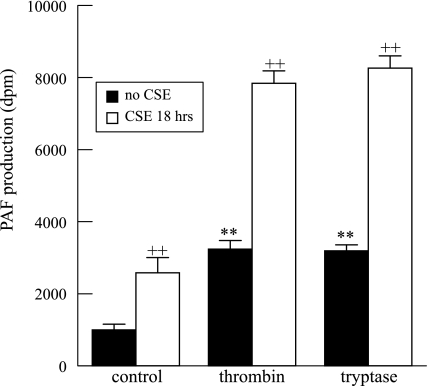
To determine whether inhibition of PAF-AH results in potentiation of agonist-stimulated increases in endothelial cell PAF production, we stimulated HMVEC-L that had been incubated with CSE (20 μg/ml, 18 h) with thrombin (1 IU/ml, 10 min) or tryptase (20 ng/ml, 10 min) and found that CSE increased PAF accumulation alone and potentiated the increases induced by thrombin or tryptase (Fig. 5).
Fig. 5.
PAF production in HMVEC-L in response to thrombin (1 IU/ml, 10 min) or tryptase (20 ng/ml, 10 min) was potentiated when HMVEC-L were exposed to CSE (20 μg/ml) for 18 h. Data are means + SE for 6 separate cell cultures. **P < 0.01 compared with unstimulated controls. ++P < 0.01, presence vs. absence of CSE.
CSE exposure results in increased PMN adherence to HMVEC-L.
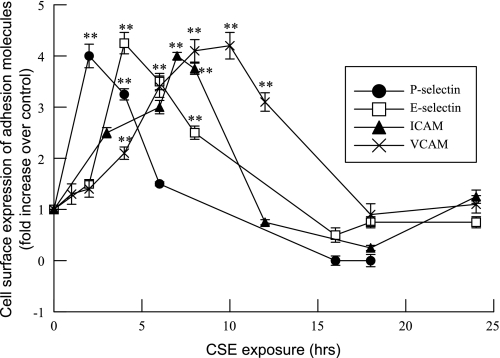
Increased endothelial cell PAF production is associated with increased PMN adherence to the endothelial cell wall (37, 44, 56). In addition to the PAF-PAF receptor interaction, adherence of PMN to activated endothelial cells and their transmigration across the endothelium also requires interactions between adhesion molecules on the endothelial cell surface and corresponding ligands on PMN (37). We examined the effects of CSE on HMVEC-L cell surface expression of adhesion molecules and observed sequential, transient expression of P-selectin, E-selectin, ICAM-1, and then VCAM-1, in that order, on the cell surface over time (Fig. 6).
Fig. 6.
Time course of changes in adhesion molecule expression in HMVEC-L incubated with CSE (20 μg/ml) for up to 24 h. Data are mean fold changes over control for cell surface expression of P-selectin, E-selectin, intercellular adhesion molecule (ICAM), and vascular cell adhesion molecule (VCAM). Data are means ± SE for 8 separate experiments. **P < 0.01 compared with control.
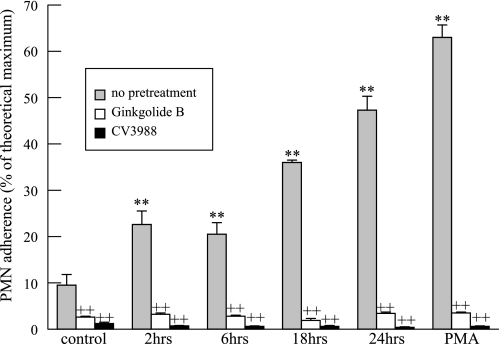
We isolated PMN from peripheral blood and incubated them with HMVEC-L that had been exposed to CSE. PMN adherence to HMVEC-L was found to increase progressively as a function of the duration of exposure to CSE (Fig. 7). As a positive control, HMVEC-L were incubated with PMA (100 nM, 20 min), which is known to stimulate HMVEC-L PAF production and PMN adherence. As expected, PMA did promote PMN adherence to HMVEC-L, and this was blocked by the PAF receptor antagonists ginkgolide B or CV3988 (10 μM, 10 min) (Fig. 7).
Fig. 7.
Polymorphonuclear leukocyte (PMN) adherence to HMVEC-L monolayers incubated with CSE (20 μg/ml) for up to 24 h. HMVEC-L were incubated with PMA (100 nM, 20 min) before the addition of PMN as a positive control. Where indicated, PMN were incubated with the PAF receptor antagonist CV3988 or ginkgolide B (10 μM, 10 min) before addition to HMVEC-L. PMN (2 × 106) were added to the wells for 10 min, and adherence was measured as myeloperoxidase activity and normalized to theoretical maximal binding. Data are means + SE for 4 separate cell culture experiments and PMN isolations. *P < 0.05; **P < 0.01 compared with control. ++P < 0.01, treated vs. nontreated PMN.
Together, these findings demonstrate that CSE increases cell surface expression of adhesion molecules in addition to promoting PAF production, and these effects cooperate to increase inflammatory cell adhesion to the endothelium. The fact that pretreating PMN with PAF receptor antagonists completely inhibited their adherence to the endothelium reflects the requirement that PAF on the endothelial cell surface interact with the PAF receptor on the PMN surface for adhesion to occur, even though other adhesion molecules are also expressed on the endothelial cell surface.
CSE does not increase PAF production in the absence of iPLA2β in mouse lung endothelial cells.
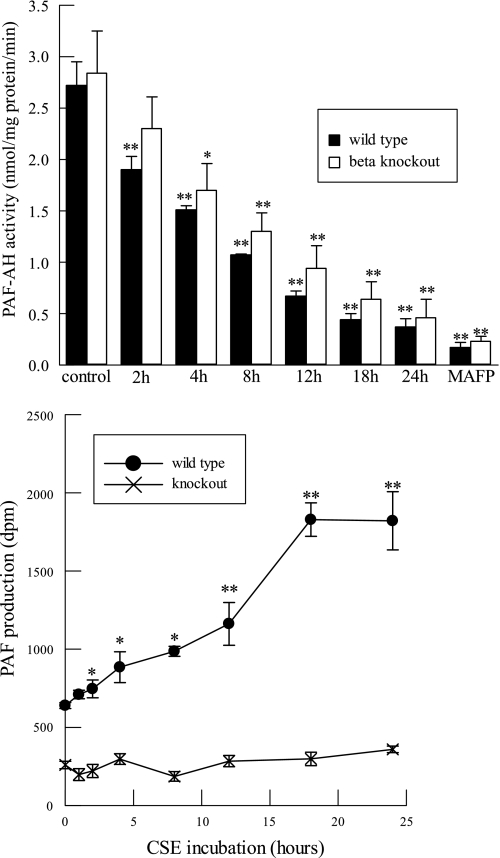
We have previously demonstrated that protease-activated receptor (PAR) agonists such as thrombin or tryptase stimulate human lung endothelial cell PAF production in a process that requires activation of group VIA PLA2 (iPLA2β) and that PAR agonists fail to stimulate PAF production by lung endothelial cells isolated from iPLA2β knockout (iPLA2β-KO) mice (46). Since the defect in iPLA2β-KO cells reflects a deficiency in PAF biosynthesis, it might be expected that inhibiting degradation with CSE would have little effect on net PAF production by iPLA2β-KO cells. To examine that possibility, we first incubated lung endothelial cells from wild-type (WT) and iPLA2β-KO mice with CSE and measured PAF-AH activity as a function of exposure time; we observed a progressive, time-dependent reduction in activity in cells of both genotypes with little difference between them (Fig. 8, top).
Fig. 8.
PAF-AH activity (top) and [3H]acetyl-PAF production (bottom) measured in lung endothelial cells isolated from wild-type or Ca2+-independent phospholipase A2β (iPLA2β)-knockout mice and incubated with CSE (20 μg/ml) for up to 24 h. Cells were incubated with methyl arachidonyl fluorophosphonate (MAFP; 5 μM, 10 min) as a positive control for inhibition of PAF-AH activity. Data are means ± SE for 6 separate cell cultures. *P < 0.05; **P < 0.01 compared with corresponding control.
We then measured PAF production under these conditions and found that incubation of WT lung endothelial cells with CSE resulted in progressive time-dependent accumulation of PAF, but with iPLA2β-KO cells no significant PAF accumulation occurred with increasing incubation intervals (Fig. 8, bottom). These data reflect the requirement for iPLA2β in lung endothelial PAF production and indicate that although iPLA2β-null cells express PAF-AH activity that is inhibited by CSE in the same manner as for WT cells, net PAF production by iPLA2β-null cells is not affected by CSE because they fail to synthesize PAF. Interference with PAF degradation thus fails to cause PAF accumulation with the iPLA2β-null cells. These results suggest that CSE might also fail to promote inflammatory cell adherence to iPLA2β-KO cells.
To test that possibility, we incubated RAW 264.7 cells, which represent a murine macrophage-like cultured cell line, with WT mouse lung endothelial cells that had been exposed to CSE or with control WT cells that had not been exposed. Exposure to CSE resulted in a significant increase in adherence of RAW 264.7 cells to WT lung endothelial cells over control levels (43.5 ± 2.6 vs. 11.7 ± 0.4%, n = 6, P < 0.01), and, as expected, adherence was suppressed to control levels by pretreatment with the PAF receptor antagonist ginkgolide B (13.4 ± 1.3%, n = 6), which reflects the requirement for PAF in the adherence process. In addition, pretreating the WT lung endothelial cells with the iPLA2β inhibitor (S)-bromoenol lactone [(S)-BEL, 5 μM, 10 min] also suppressed adherence of RAW 264.7 cells to control levels, which reflects the requirement for iPLA2β in PAF production. No increase in RAW 264.7 cell adherence was observed to lung endothelial cells isolated from iPLA2β-KO mice when incubated with CSE (5.7 ± 0.5 vs. 6.6 ± 1.1% for CSE-treated cells, n = 6).
DISCUSSION
Smoking is associated with increased morbidity and mortality and inflicts damage on multiple organ systems, including cardiovascular and lung tissue. In the U.S., the Centers for Disease Control has estimated annual smoking-attributable mortality, years of potential life lost (YPLL) for adults and infants, and productivity losses for adults. Between 1997 and 2001, cigarette smoking and passive exposure to tobacco smoke resulted in ∼438,000 premature deaths, 5.5 million YPLL, and $92 billion in productivity losses annually (1, 35). Cigarette smoke elicits the recruitment and adherence of circulating leukocytes to the vascular wall, which is an early event in inflammation and the pathogenesis of emphysema (16). Migration of circulating neutrophils to sites of tissue inflammation is a multistep process that involves the interaction of adhesion molecules and their receptors.
Neutrophil-endothelial cell adhesion requires the interaction of PAF on the surface of the endothelial cell with the PAF receptor on the surface of the neutrophils. Other events involved in adhesion include an initial tethering of PMN to selectin on the endothelial surface via PMN cell surface receptors (13, 25, 36). Firm adherence and transmigration are mediated by ICAM-1 and VCAM-1 expression on endothelial cells and their corresponding receptors on leukocytes (31, 39). Activation of leukocytes occurs via the interaction of endothelial cell PAF with the PAF receptor on the tethered cells, which then activates a program of adhesion and β-integrin-mediated leukocyte migration (14, 29, 43). PAF also primes leukocytes to interact with agonists they will encounter after migration from the vasculature (14, 29, 43). When endothelial cells fail to express PAF on their surface, PMN activation does not occur and they can be released to reenter the circulation.
PAF is implicated in the pathophysiology of a number of human diseases, including asthma, endotoxic shock, ischemic injury, diabetes, and hypertension (55). That PAF might be involved in cigarette smoke-induced tissue injury was first suggested in 1989 by a report that the plasma of smokers contained higher PAF concentrations than observed in nonsmokers (24). PAF is normally maintained at low concentrations by its rapid hydrolysis catalyzed by PAF-AH enzymes (26, 42, 50), which are calcium-independent phospholipases A2 (iPLA2) that preferentially hydrolyze phospholipids with short chain or oxidized fatty acids at the sn-2 position (26, 50), such as the sn-2 acetate residue of PAF. PAF-AH isoforms comprise PLA2 groups VII and VIII of the Dennis classification scheme (12, 45).
Group VIIA PAF-AH is a secreted enzyme found in the plasma. Groups VIIB, VIIIA, and VIIIB are intracellular PAF-AH enzymes (12, 45). Inhibition of endothelial cell PAF-AH activity results in increased endothelial cell surface expression of PAF and enhanced recruitment of circulating leukocytes and their adherence to the vascular wall (27). In the lung, these events are among the early inflammatory steps in the pathogenesis of emphysema (53). Previous studies have determined that cigarette smoke inhibits plasma PAF-AH activity in vitro (9, 38), although Lehr et al. (30) found no inhibition of circulating PAF-AH activity in hamsters exposed to cigarette smoke. In the studies described in this article, we have demonstrated that exposure of HMVEC-L to CSE extract results in a significant inhibition of PAF-AH activity, which indicates that intracellular PAF-AH isoforms are sensitive to inhibition by CSE.
In the present study, we used MAFP as a positive control for PAF-AH inhibition. Although MAFP was developed initially as a PLA2 inhibitor, we have demonstrated previously that it fails to inhibit thrombin-stimulated, membrane-associated endothelial cell iPLA2 activity, although MAFP does inhibit PAF-AH activity and thereby potentiates thrombin-stimulated PAF production (27). As we have previously reported, inhibition of PAF-AH alone by CSE is sufficient to result in a time-dependent increase in PAF production that correlates with PAF-AH inhibition in wild-type cells with intact PAF biosynthetic capabilities. To determine whether increased PAF production also reflected an additional effect of CSE to activate iPLA2 or acetyl-CoA: lyso-PAF acetyltransferase activity, we measured activity of these enzymes in HMVEC-L incubated with CSE over the course of 24 h and failed to detect changes in activity after any duration of CSE exposure examined. This corroborates our hypothesis that inhibition of PAF-AH by CSE alone is sufficient to increase HMVEC-L PAF production even without a concomitant activating stimulus for iPLA2, although activation of HMVEC-L iPLA2 with the PAR agonists thrombin or tryptase amplifies the increase in PAF production by CSE.
We have previously demonstrated that the majority of HMVEC-L PLA2 activity is membrane associated, Ca2+ independent, and preferentially hydrolyzes arachidonate-containing phospholipids (44). Stimulation of HMVEC-L iPLA2 activity with PAR agonists results in increased PAF production, and this is associated with enhanced PMN adherence and release of arachidonic acid and prostaglandin I2 (PGI2) (44). We also have demonstrated that pretreating HMVEC-L with the iPLA2β-selective inhibitor (S)-BEL completely inhibits thrombin- and tryptase-stimulated iPLA2 activity, PGI2 release, and PAF production (46). In addition, stimulation of lung endothelial cells from WT mice with the PAR agonists thrombin or tryptase results in increased PAF production, but lung endothelial cells from iPLA2β-KO mice fail to produce PAF under these conditions (46). These findings demonstrate that iPLA2β is required for endothelial cell PAF production in response to the PAR agonists thrombin or tryptase. In the present study, we have shown that lung endothelial cells isolated from WT or iPLA2β-KO mice exhibit similar levels of PAF-AH activity and that the activity is inhibited by CSE and MAFP in cells of both genotypes. Inhibiting PAF-AH activity in WT lung endothelial cells results in increased PAF production and adherence of mouse macrophages, but increased PAF production or macrophage adherence is not observed in iPLA2β-KO lung endothelial cells under these conditions. This indicates that inhibition of PAF-AH alone fails to increase PAF production when there is a deficiency in PAF biosynthesis, as in cells that lack iPLA2β. In future studies, we propose to determine whether the absence of iPLA2β decreases CSE-induced inflammatory cell recruitment to the lung in a mouse model of emphysema. These studies would indicate whether iPLA2β is a potential therapeutic target for smoking-induced inflammatory diseases.
Tobacco smoke is associated with many human diseases, including pulmonary and cardiovascular disease, and exerts a complex series of effects on release and inhibition of pro- and anti-inflammatory mediators. Cigarette smoke has been demonstrated to increase circulating levels of tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), IL-6, and IL-8 (8, 19) but is associated with decreased mononuclear cell production of IL-1β, IL-2, TNF-α, and interferon-γ (20). The net effect of cigarette smoke is to increase the PMN content of lung, and we propose that this results in part from increased endothelial cell PAF production. Smokers have higher levels of circulating PMN than nonsmokers, primarily as a result of increased catecholamine secretion that stimulate release of leukocytes and platelets into the circulation from the bone marrow (48). Smoking-induced impairment of immune function can lead to chronic pulmonary inflammation, with attendant remodeling and increased susceptibility to infection (3, 50). Innate cellular immune responses such as recruitment of neutrophils and macrophages are designed to promote clearance of injurious agents and to effect removal of particulates and resolution of inflammation. Neutrophils are recruited first and then assist in recruiting monocytes, which differentiate into tissue macrophages (6, 18). Although cigarette smoke enhances recruitment of these cells from the circulation, they are functionally impaired, and this can result in smoldering inflammation and increased susceptibility to chronic infection in smokers (49).
Endothelial cell dysfunction is a principal consequence of smoking and is linked to oxidant stress (5, 23), impaired nitric oxide production (51, 57), apoptosis (2, 17, 40), and inflammation. In the present study, we have demonstrated that inhibition of endothelial cell PAF-AH activity by CSE results in increased PAF production and then to inflammatory cell adhesion to the lung endothelium, and these effects occur at CSE concentrations below those required to trigger endothelial cell apoptosis. Inhibition of lung endothelial cell PAF-AH by cigarette smoke can result in increased pulmonary recruitment of inflammatory cells that contribute to chronic inflammation in the lungs of smokers. CSE-induced PAF production is blocked when endothelial cell iPLA2β is inhibited or absent, which reflects the requirement for iPLA2β as a potential target for therapeutic interventions to reduce airway inflammation and the progression of chronic lung disease.
GRANTS
The laboratory of J. Turk is supported by National Institutes of Health Grants R37 DK34388, P41 RR00954, P60 DK20579, and P30 DK56341.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.S., D.M.Y., P.R., and J.M. conception and design of research; J.S., D.M.Y., J.O.M., P.R., J.T., and J.M. performed experiments; J.S., D.M.Y., J.O.M., and J.M. analyzed data; J.S., D.M.Y., P.R., J.T., and J.M. interpreted results of experiments; J.S., D.M.Y., J.O.M., P.R., J.T., and J.M. approved final version of manuscript; P.R., J.T., and J.M. edited and revised manuscript; J.M. prepared figures; J.M. drafted manuscript.
REFERENCES
- 1. Adhikari B, Kahende J, Malarcher A, Pechacek T, Tong V. Smoking-attributable mortality, years of potential life lost, and productivity losses-United States, 2000–2004. MMWR Morb Mortal Wkly Rep 57: 1226–1228, 2008 [PubMed] [Google Scholar]
- 2. Aldonyte R, Brantly M, Block E, Patel J, Zhang J. Nuclear localization of active matrix metalloproteinase-2 in cigarette smoke-exposed apoptotic endothelial cells. Exp Lung Res 35: 59–75, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 34: J258–J265, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, Moley K, Turk J. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem 279: 38194–38200, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barbieri SS, Zacchi E, Amadio P, Gianellini S, Mussoni L, Weksler BB, Tremoli E. Cytokines present in smokers' serum interact with smoke components to enhance endothelial dysfunction. Cardiovasc Res 90: 475–483, 2011 [DOI] [PubMed] [Google Scholar]
- 6. Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 8: 183–192, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Berge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J 21: 46S–53S, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol 89: 1117–1119, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Bielicki JK, Knoff LJ, Tribble DL, Forte TM. Relative sensitivities of plasma lecithin:cholesterol acyltransferase, platelet-activating factor acetylhydrolase, and paraoxonase to in vitro gas-phase cigarette smoke exposure. Atherosclerosis 155: 71–78, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–7, 1959 [DOI] [PubMed] [Google Scholar]
- 11. Bridges RB, Fu MC, Rehm SR. Increased neutrophil myeloperoxidase activity associated with cigarette smoking. Eur J Respir Dis 67: 84–93, 1985 [PubMed] [Google Scholar]
- 12. Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50: S237–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67: 1033–1036, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Camussi G, Tetta C, Baglioni C. The role of platelet-activating factor in inflammation. Clin Immunol Immunopathol 57: 331–338, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J 29: 1224–1238, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol 294: L612–L631, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Hassoun PM. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol 44: 323–332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doherty DE, Downey GP, Worthen GS, Haslett C, Henson PM. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Invest 59: 200–213, 1988 [PubMed] [Google Scholar]
- 19. Glossop JR, Dawes PT, Mattey DL. Association between cigarette smoking and release of tumour necrosis factor-α and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology (Oxford) 45: 1223–1229, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hagiwara E, Takahashi KI, Okubo T, Ohno S, Ueda A, Aoki A, Odagiri S, Ishigatsubo Y. Cigarette smoking depletes cells spontaneously secreting Th(1) cytokines in the human airway. Cytokine 14: 121–126, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J Biol Chem 283: 11097–11106, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Hogg JC, Chu F, Utokaparch S, Woods R, Elliot WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, Pare PD. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Hsu CL, Wu YL, Tang GJ, Lee TS, Kou YR. Ginkgo biloba extract confers protection from cigarette smoke extract-induced apoptosis in human lung endothelial cells: role of heme oxygenase-1. Pulm Pharmacol Ther 22: 286–296, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Imaizumi T. Intravascular release of platelet-activating factor-like lipid (PAF-LL) induced by cigarette smoking. In: Proceedings of the Third International Conference on Platelet-Activating Factor and Structurally Related Alkyl Ether Lipids. Tokyo: 1989, p78 [Google Scholar]
- 25. Kansas GS. Selectins and their ligands: current concepts and controversies. Blood 88: 3259–3287, 1996 [PubMed] [Google Scholar]
- 26. Karasawa K. Clinical aspects of plasma platelet-activating factor-acetylhydrolase. Biochim Biophys Acta 1761: 1359–1372, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Kell PJ, Creer M, Crown K, Wirsig K, McHowat J. Inhibition of platelet-activating factor (PAF) acetylhydrolases by methyl arachidonyl fluorophosphonate potentiates PAF synthesis in thrombin-stimulated human coronary artery endothelial cells. J Pharmacol Exp Ther 307: 1163–1170, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Kihara Y, Yanagida K, Masago K, Kita Y, Hishikawa D, Shindou H, Ishii S, Shimizu T. Platelet-activating factor production in the spinal cord of experimental allergic encephalomyelitis mice via the group IVA cytosolic phospholipase A2-lyso-PAFAT axis. J Immunol 181: 5008–5014, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Kuijpers TW, Hakkert BC, Hart MHL, Roos D. Neutrophil migration across monolayers of cytokine-prestimulated endothelial cells: a role for platelet-activating factor and IL-8. J Cell Biol 117: 565–572, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest 99: 2358–2364, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689, 2007 [DOI] [PubMed] [Google Scholar]
- 32. MacNee W. Oxidants and COPD. Curr Drug Targets Inflamm Allergy 4: 627–641, 2005 [DOI] [PubMed] [Google Scholar]
- 33. MacNee W. Pulmonary and systemic oxidant/antioxidant imbalance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 50–60, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ 51: 1–16, 2002 [PubMed] [Google Scholar]
- 35. Mariolis P, Rock VJ, Asman K, Merritt R, Malarcher A, Husten C, Pechacek T. Tobacco use among adults—United States, 2005. MMWR Morb Mortal Wkly Rep 55: 1145–1148, 2006 [PubMed] [Google Scholar]
- 36. McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest 100, Suppl 11: S97–S103, 1997 [PubMed] [Google Scholar]
- 37. Meyer M, McHowat J. The role of platelet-activating factor in the adherence of circulating cells to the endothelium. In: Recent Research Developments in Physiology, edited by Pandalai SG. Tivandrum, India: Research Signpost, 2004, vol. 2, p. 129–147 [Google Scholar]
- 38. Miyaura S, Eguchi H, Johnston JM. Effect of a cigarette smoke extract on the metabolism of the proinflammatory autocoid, platelet-activating factor. Circ Res 70: 341–347, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol 6: 323–344, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nana-Sinkam SP, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, Choudhury Q, Cool C, Parr J, Moore MD, Bull TM, Voelkel NF, Geraci MW. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med 175: 676–685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pare PD, Wiggs BR, James A, Hogg JC, Bosken C. The comparative mechanics and morphology of airways in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 143: 1189–1193, 1991 [DOI] [PubMed] [Google Scholar]
- 42. Prescott SM, McIntyre TM, Zimmerman GA, Stafforini DM. Sol Sherry lecture in thrombosis: molecular events in acute inflammation. Arterioscler Thromb Vasc Biol 22: 727–733, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Prescott SM, Zimmerman GA, McIntyre TM. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci USA 81: 3534–3538, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rastogi P, McHowat J. Inhibition of calcium independent phospholipase A2 prevents inflammatory mediator production in pulmonary microvascular endothelium. Respir Physiol Neurobiol 165: 167–174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761: 1246–1259, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Sharma J, Turk J, McHowat J. Endothelial cell prostaglandin I2 and platelet-activating factor production are markedly attenuated in the calcium-independent phospholipase A2β knockout mouse. Biochemistry 49: 5473–5481, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:lyso-PAF acetyltransferase. J Biol Chem 282: 6532–65399, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Smith MR, Kinmonth AL, Luben RN, Bingham S, Day NE, Wareham NJ, Welch A, Khaw KT. Smoking status and differential white cell count in men and women in the EPIC-Norfolk population. Atherosclerosis 169: 331–337, 2003 [DOI] [PubMed] [Google Scholar]
- 49. Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2: 372–377, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2). Cardiovasc Drugs Ther 23: 73–83, 2009 [DOI] [PubMed] [Google Scholar]
- 51. Talukder MA, Johnson WM, Varadharaj S, Lian J, Kearns PN, El-Mahdy MA, Liu X, Zweier JL. Chronic cigarette smoking causes hypertension, increased oxidative stress, impaired NO bioavailability, endothelial dysfunction, and cardiac remodeling in mice. Am J Physiol Heart Circ Physiol 300: H388–H396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Taylor JD. COPD and the response of the lung to tobacco smoke exposure. Pulm Pharmacol Ther 23: 376–383, 2010 [DOI] [PubMed] [Google Scholar]
- 53. Tetley TD. Inflammatory cells and chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 4: 607–618, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Tithof PK, Elgayyar M, Cho Y, Guan W, Fisher AB, Peters-Golden M. Polycyclic aromatic hydrocarbons present in cigarette smoke cause endothelial cell apoptosis by a phospholipase A2-dependent mechanism. FASEB J 16: 1463–1464, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Tjoelker LW, Stafforini DM. Platelet-activating factor acetylhydrolases in health and disease. Biochim Biophys Acta 1488: 102–123, 2000 [DOI] [PubMed] [Google Scholar]
- 56. White MC, McHowat J. Protease activation of calcium-independent phospholipase A2 leads to neutrophil recruitment to coronary artery endothelial cells. Thromb Res 120: 597–605, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension 48: 278–285, 2006 [DOI] [PubMed] [Google Scholar]