Abstract
Expression and function of Kv7 (KCNQ) voltage-activated potassium channels in guinea pig and human airway smooth muscle cells (ASMCs) were investigated by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), patch-clamp electrophysiology, and precision-cut lung slices. qRT-PCR revealed expression of multiple KCNQ genes in both guinea pig and human ASMCs. Currents with electrophysiological and pharmacological characteristics of Kv7 currents were measured in freshly isolated guinea pig and human ASMCs. In guinea pig ASMCs, Kv7 currents were significantly suppressed by application of the bronchoconstrictor agonists methacholine (100 nM) or histamine (30 μM), but current amplitudes were restored by addition of a Kv7 channel activator, flupirtine (10 μM). Kv7 currents in guinea pig ASMCs were also significantly enhanced by another Kv7.2–7.5 channel activator, retigabine, and by celecoxib and 2,5-dimethyl celecoxib. In precision-cut human lung slices, constriction of airways by histamine was significantly reduced in the presence of flupirtine. Kv7 currents in both guinea pig and human ASMCs were inhibited by the Kv7 channel blocker XE991. In human lung slices, XE991 induced robust airway constriction, which was completely reversed by addition of the calcium channel blocker verapamil. These findings suggest that Kv7 channels in ASMCs play an essential role in the regulation of airway diameter and may be targeted pharmacologically to relieve airway hyperconstriction induced by elevated concentrations of bronchoconstrictor agonists.
Keywords: KCNQ voltage-activated potassium channel, precision-cut lung slice, bronchoconstrictor signal transduction, asthma therapy
in asthma and other lung diseases, airways can become hyperconstricted because of increases in local bronchoconstrictor concentrations [e.g., histamine released from mast cells (13, 42) or acetylcholine released as a neurotransmitter (2)]. These substances activate Gq/11-coupled receptors on airway smooth muscle cells (ASMCs) (37) in the walls of the bronchioles, resulting in contraction of the ASMCs and hence constriction of the airways. Activation of Gq/11-coupled receptors on ASMCs leads to the release of intracellular Ca2+ stores and/or an increase in Ca2+ influx across the plasma membrane (14, 21, 38, 39). The resulting elevation of cytosolic calcium concentration ([Ca2+]cyt) stimulates contraction of ASMCs. Ca2+ influx via voltage-sensitive Ca2+ channels (VSCCs) contributes to bronchoconstrictor signal transduction (21, 39), particularly at low agonist concentrations (1), although the physiological and clinical relevance of this contribution remains controversial (15).
A well-established mechanism for stimulation of Ca2+ influx is by inhibiting potassium (K+) channels, which can depolarize the membrane to the voltage range required for activation of VSCCs. We recently implicated such a mechanism in the stimulation of vascular smooth muscle contraction (6, 28). The activity of vascular Kv7.5 channels was found to be suppressed by the pituitary hormone arginine vasopressin, and this effect was proposed to mediate its physiological vasoconstrictor actions (6, 28). Pharmacological activators of Kv7 channels, including retigabine (ethyl N-[2-amino-4-[(4-fluorophenyl)methylamino]phenyl]carbamate), flupirtine (ethyl-N-[2-amino-6-(4-fluorophenylmethylamino)pyridin-3-yl]carbamate), and S-1 ((S)-N-[1-(3-morpholin-4-yl-phenyl)-ethyl]-3-phenyl-acrylamide), were found to relax vascular smooth muscle and dilate arteries (20, 28, 31, 45, 46). We also recently discovered that the cyclooxygenase-2 (COX-2) inhibitor celecoxib (Celebrex) and its COX-2-independent analog 2,5-dimethylcelecoxib (DMC) are potent activators of vascular smooth muscle Kv7 channels and effective dilators of rat mesenteric and basilar arteries (4, 31). However, there are no reports to date that Kv7 channels are expressed or functional in ASMCs.
In the present study we explored the possibility that Kv7 channels are expressed and functional in ASMCs and that their activity can be inhibited by bronchoconstrictor agonists. We also examined the efficacy of clinically used Kv7 channel activators in opposing the effects of bronchoconstrictors at the cellular and tissue level. Our findings support an important role of Kv7 channels in ASMCs as mediators of bronchoconstrictor signal transduction and as potential therapeutic targets to relieve ASMC hypercontraction.
MATERIALS AND METHODS
All animal studies were approved by the Loyola University Chicago Institutional Animal Care and Use Committee and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (1996, National Academy of Sciences, Washington, DC). The uses of human tissues were reviewed and approved by the Loyola University Chicago Institutional Review Board for the Protection of Human Subjects.
Isolation of Airway Myocytes
Adult male guinea pigs (300–400 g) were euthanized with pentobarbital sodium (150 mg/kg ip). Lungs together with heart and trachea were then immediately excised and placed in ice-cold dissecting solution containing (in mM) 145 NaCl, 4.7 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 2 CaCl2 × 2H2O, 2 pyruvic acid, 0.02 EDTA dihydrate, 3 MOPS, 5 d-glucose, and 1% fatty acid-free BSA; pH adjusted to 7.4 with NaOH on ice. Methods for isolation of guinea pig ASMCs were essentially as described by Janssen and Sims (16). Briefly, connective tissue, vasculature, and innervation were removed from primary and secondary bronchi. Bronchial segments 3–5 mm in length were transferred to ice-cold physiological saline solution (PSS) containing (in mM) 140 NaCl, 5.36 KCl, 0.34 Na2HPO4, 0.44 KH2PO4, 1.2 MgCl2, 0.05 CaCl2, 10 HEPES, 10 d-glucose, pH adjusted to 7.2 with NaOH on ice, 298 mosmol/l. The segments were cut open and the epithelium was removed by use of a cotton-tipped applicator. Each bronchial segment was then cut into strips ∼1 mm wide. The bronchial strips were transferred into PSS (pH adjusted to 7.2 with NaOH at 37°C) supplemented with BSA (1 mg/ml; fraction V, Roche Diagnostics USA), collagenase Type VIII (400 units/ml; Sigma), papain (30 units/ml; Worthington), and dl-dithiothreitol (1 mM; Sigma). The strips were then incubated for 45–60 min at 37°C. After enzymatic digestion, the tissue was washed three to five times with ice-cold PSS and gently triturated with a fire-polished Pasteur pipette to release individual myocytes.
Human tracheal tissue was obtained from discarded lung transplant donor tissue, and trachealis muscles were dissected from the trachea in ice-cold dissecting solution and cut into strips ∼2 mm in diameter. Strips of muscle were then transferred to ice-cold PSS and then into PSS at 37°C (pH adjusted to 7.2 with NaOH at 37°C) supplemented with BSA (1 mg/ml; fraction V, Roche Diagnostics USA), collagenase Type VIII (950 units/ml; Sigma), papain (38 units/ml; Worthington), and dl-dithiothreitol (1 mM; Sigma). The strips were then incubated for 45 min at 37°C. After enzymatic digestion, the tissue was washed three to five times with ice-cold PSS and gently triturated to release individual myocytes.
Freshly isolated ASMCs were kept on ice until use. The cells were then dispensed onto a glass coverslip base of the recording chamber and allowed to adhere for at least 15 min at room temperature.
Precision-Cut Lung Slices
Human donor lungs that were not suitable for transplantation were obtained from the Regional Organ Bank of Illinois within 24 h after removal from the donor. The right middle lobe was inflated with low-temperature-melting Type IX-A agarose [1.5% in Hank's balanced salt solution (HBSS) supplemented with 20 mM HEPES] at 37°C, then cooled to 4°C to solidify the agarose. Precision-cut lung slices (250 μm thick) containing cross sections of 0.05–0.5 mm airways were made by use of a Leica VT 1200S vibratome while maintaining the lung at 4°C. Human lung tissue removed during lobectomy or transplant procedures (from chronic obstructive pulmonary disease patients) was also used in some experiments; in these cases, parenchyma was infused by multiple injections with low melting point agarose (2% in HBSS/HEPES) at 37°C, then cooled to 4°C for 30 min to solidify the agarose. Lung slices (1 mm thick) containing cross sections of 0.1–0.5 mm airways were made by using a rat brain slicer (Zivic Instruments) while maintaining the lung tissue at 4°C.
Lung slices were incubated for at least 12 h in serum-free F-12/DMEM tissue culture medium supplemented with ITS (insulin-transferrin-selenium, Mediatech, Manassas, VA) and antibiotics at 37°C under 5% CO2. Lung slices generally remained viable for at least 4 days. Slices containing airways were used only if 1) the airway was approximately circular, 2) beating cilia were observed (indicating an intact epithelium), and 3) the airway wall and all parenchymal attachments were intact.
To measure airway constriction/dilation, a lung slice was mounted in a perfusion chamber on the stage of an inverted microscope (Olympus IX-71) and visualized via a ×10 objective. A cross section of a small bronchiole (0.05–1.5 mm in diameter) was positioned in the center of the microscopic field and the lung slice was then equilibrated in control medium (in mM: 140 NaCl, 5.36 KCl, 1.2 MgCl2, 2 CaCl2, 10 HEPES, 10 d-glucose, pH 7.3, 298 mosmol/l) at room temperature for at least 30 min. Control medium plus or minus drugs was continuously superfused over the lung slice via a gravity-fed perfusion system at a rate of ∼5 ml/min, controlled by a four-way valve. Experiments were conducted at room temperature. Images were captured with a 12-bit digital camera (Hamamatsu Orca) at 5-s intervals. Luminal area was measured by image analysis using Simple-PCI software (Hamamatsu, Sewickley, PA). Summarized measurements of luminal area of the airway in each experiment represent the average for the last 1–5 min of drug application or washout (total 12–60 measurements in each case) and for 5 min of control recording.
Patch-Clamp
The whole cell perforated patch configuration was used to measure membrane currents under voltage-clamp conditions. All experiments were performed at room temperature with continuous perfusion of bath solution as described previously (4, 6, 28). The standard bath solution for ASMCs contained (in mM) 140 NaCl, 5.36 KCl, 1.2 MgCl2, 2 CaCl2, 10 HEPES, 10 d-glucose, pH 7.3, 298 mosmol/l. Standard internal (pipette) solution for ASMCs contained (in mM) 135 KCl, 5 NaCl, 10 HEPES, 0.05 K2EGTA, 1 MgCl2, 20 d-glucose, pH 7.2, 298 mosmol/l.
Voltage-clamp command voltages were generated by using an EPC10 amplifier under control of PATCHMASTER software (HEKA, Pfalz, Germany). Amphotericin B (120 μg/ml) was added to the internal solution for membrane patch perforation. For recordings of total voltage-activated K+ currents (Kvtotal), series resistance after amphotericin perforation was below 30 MΩ and was compensated by 60%. Whole cell currents were digitized at 5 kHz and filtered at 2.9 kHz. Kvtotal in ASMCs was recorded by application of 5-s voltage steps from −64 mV holding voltage to test voltages ranging from −84 to +50 mV. Peak (maximum) current amplitude was measured within the first 500 ms of the voltage steps using the “Online Analysis” tool provided by PatchMaster software to determine the current-voltage relationship for the transient component of Kvtotal. To obtain end-pulse sustained K+ current, the last 6,000 data points recorded during each voltage step (corresponding to 1,000-ms recording time) were averaged and normalized by cell capacitance. Stable currents were recorded for at least 15 min prior to drug application.
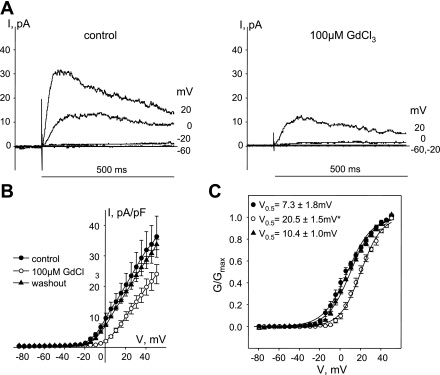
To isolate Kv7 currents, 100 μM GdCl3 was added to external solutions. Application of 100 μM GdCl3 reversibly shifted activation of the transient component of Kvtotal from 7.3 ± 1.8 to 20.5 ± 1.5 mV (Fig. 1, n = 3, P < 0.001, one-way ANOVA) similar to the shift observed in 4-aminopyridine-sensitive Kv currents in rat mesenteric and basilar artery myocytes (28, 31). Isolated Kv7 currents were recorded by application of 5-s voltage steps from a −4 mV or −64 mV holding voltage to test voltages ranging from −84 to +16 mV, with 10-s intervals between steps. Series resistance compensation was not required for recording Kv7 currents in isolation because of their small current amplitudes. Time courses of the effects of drugs or physiological agonists were recorded while measuring Kv7 currents at −20 mV holding voltage. A selective irreversible inhibitor of Kv7 currents (10 μM of linopirdine or 10 μM of XE991) was added at the end of each experiment.
Fig. 1.
Shift of voltage-dependent activation of transient delayed-rectifier K+ currents in guinea pig airway smooth muscle cells (ASMCs) by Gd3+. A: representative current (I) traces recorded during the first 500 ms of 5-s voltage steps at −60, −20, 0, and +20 mV as indicated in control (left) and in the presence of 100 μM GdCl3 (right). B: current-voltage (V; I-V) relationships of peak K+ currents recorded in ASMCs before (control, ●, n = 3), after 10-min treatment with 100 μM GdCl3 (○, n = 3), and after washout of GdCl3 for 10 min (▴, n = 3). C: averaged fractional conductance plots calculated from peak K+ currents measured in ASMCs in control (●) and in the presence of 100 μM GdCl3 (○) and after washout of 100 μM GdCl3 (n = 3) fitted to a Boltzmann distribution [voltage of half-maximal activation (V0.5) = 7.3 ± 1.8 mV in control, V0.5 = 20.5 ± 1.5 mV in the presence of 100 μM GdCl3, V0.5 = 10.4 ± 1.0 mV after washout of 100 μM GdCl3, n = 3]. G/Gmax, the fraction of maximal conductance. *Significant difference from control, P < 0.001, 1-way ANOVA.
To analyze the voltage dependence of channel activation, the conductance was calculated as described by Wickenden et al. (44) from the peak current for Kvtotal or from end-pulse sustained currents and normalized to maximum conductance for each experiment [according to the equation G = I/(V − EK), where I is the current, V is the step voltage, and EK is the reversal potential for potassium (−86 mV)]. Normalized conductances were fitted by a Boltzmann distribution: G/Gmax = 1/{1+exp[(V0.5 − V)/s]}, where G/Gmax is the fraction of maximal conductance, V0.5 is the voltage of half-maximal activation, and s is the slope factor.
qRT-PCR
Quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) was used to determine the expression of KCNQ1–5 in guinea pig ASMCs and human trachealis muscle.
Guinea pig ASMCs.
ASMCs were isolated as described above from smooth muscle dissected free from the entire trachea. After trituration of the tissue, remaining undigested pieces were removed and ASMCs were pelleted by centrifugation (5 min at 2,000 g). Total RNA was extracted from ASMCs by use of RNeasy Plus Mini Kit (Qiagen, Valencia, CA). RNA was quantified with use of a NanoDrop spectrophotometer, and the mRNA was reverse transcribed with a Bio-Rad iScript cDNA synthesis kit. The cDNA thus obtained was amplified in a qRT-PCR reaction by using primers specific for guinea pig KCNQ1–3,5 (34) and KCNQ4 (26) (Table 1). Quantitative expression was performed with Applied Biosystems 7300 Gene Expression System in 25-μl reactions consisting of 12.5 μl Maxima SYBR Green/ROX qPCR Master Mix (Fermentas, Burlington, Ontario, Canada), cDNA derived from 25–250 ng mRNA, and 5 pmol each primer. Cycle parameters were typically 95°C for 15 s, followed by 60°C for 30 s (40 cycles), followed by a dissociation step to confirm a single PCR product. PCR products were also verified by DNA sequencing. Standard curves were plotted by 10-fold serial dilution of known amounts of cDNA for each target. Primer efficiencies were determined from the slope of the standard curve dilution series for each of the KCNQ targets, by using the formula Efficiency = −1 + 10(−1/slope).
Table 1.
Guinea pig primers
| Gene | Primer Sequence | Product Size, bp |
|---|---|---|
| KCNQ 1 | F:5′-ATTGTCCTGGTGGTGTTCTTTG-3′ | 206 |
| R:5′-CCCCTGATGGCTGATGTGG-3′ | ||
| KCNQ 2 | F:5′-TCTACGCCACCAACCTGTC-3′ | 79 |
| R:5′-TACATGGGCACCGTGACC-3′ | ||
| KCNQ 3 | F:5′-CTTGAAAACCGTCATCTGC-3′ | 124 |
| R:5′-CAAGTTCACAGGGTCGTG-3′ | ||
| KCNQ 4 | F:5′-GGGCCTCTCTAAGACTCAAG-3′ | 235 |
| R:5′-AGGTGTCCTGCTGAATACTG-3′ | ||
| KCNQ 5 | F:5′-CGTCCGCACTCAGAAGTC-3′ | 137 |
| R:5′-TCCAATGTACCAGGCTGTG-3′ |
The correlation coefficients for all standard curves were >0.99, and slope values gave efficiencies ≥90% in all cases. The target copy number in a sample was estimated by extrapolating the PCR threshold cycle to known target values on the standard curve. The mRNA levels of KCNQ1–5 were averaged from four male guinea pigs (250–350 g) with each reaction performed in duplicate.
RNA extracted from one guinea pig ASMC sample was reverse transcribed as described above for end point PCR to confirm PCR product sizes for each primer pair. cDNA was amplified by using Platinum Taq PCR Mix (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol and conventional PCR cycle parameters of 95°C for 1 min, 50°C for 1 min (30 cycles), and final extension at 72°C for 5 min. The PCR products were separated by agarose gel electrophoresis and the gel was visualized and imaged by use of a BioImaging System (UVP, Upland, CA).
Human trachealis.
RNA was extracted from human donor lung trachealis muscle (cut into 2 mm × 5 mm pieces; 4–5 pieces per human sample) by the acid-guanidinium-phenol extraction method (7). Reverse transcription was carried out as above, and qRT-PCR was performed with primer pairs specific for human KCNQ genes (35) (Table 2). Each reaction was performed in triplicate for at least four individuals, and KCNQ1–5 transcript copy numbers were estimated as described above for guinea pig, by extrapolating to standard curves constructed for each of the human KCNQ gene targets.
Table 2.
Human Primers
| Gene | Primer Sequence | Product Size, bp |
|---|---|---|
| KCNQ 1 | F:5′-AACCTCATGGTGCGCATCA-3′ | 100 |
| R:5′-CCGCGATCCTTGCTCTTTT-3′ | ||
| KCNQ 2 | F:5′-GGAAACCGTTCTGTGTGATTGAC-3′ | 128 |
| R:5′-TCCGCAGAATCTGCAGGAA-3′ | ||
| KCNQ 3 | F:5′-CCACGCCAAAACACAAGAAGT-3′ | 100 |
| R:5′-TGATGTGGATGGTCTGGCTACA-3′ | ||
| KCNQ 4 | F:5′-GCGACCGTACGACGTGAAG-3′ | 100 |
| R:5′-CAATTTGGTCCACCCGAGTT-3′ | ||
| KCNQ 5 | F:5′-TCCCTGAGCACACAAAATTGG-3′ | 102 |
| R:5′-CCCGCAGACCAGATTCGA-3′ |
F = Forward; R = Reverse
Immunostaining
Freshly dispersed ASMCs were plated on 12-mm diameter coverslips and allowed to adhere for 1 h at room temperature. Cells were fixed for 15 min with 2% paraformaldehyde in phosphate-buffered saline (PBS) and permeabilized with 0.5% Triton X-100 in PBS for 20 min at room temperature. After blocking with Image-iT signal enhancer (Invitrogen) for 30 min at room temperature, coverslips were incubated with rabbit anti-KCNQ1 polyclonal antibody (ab65092, Abcam, 1:1,000 dilution), rabbit anti-KCNQ2 polyclonal antibody (AB5577, Millipore, 1:1,000 dilution), rabbit anti-KCNQ3 polyclonal antibody (AB5483, Chemicon, 1:100 dilution), rabbit anti-KCNQ4 polyclonal antibody (H-130, Santa Cruz, 1:50 dilution), or rabbit anti-KCNQ5 polyclonal antibody (AB5599, Chemicon, 1:500 dilution) in blocking buffer (0.25% Triton X-100 plus 3% goat serum and 1% bovine serum albumin in PBS) at 4°C with slow agitation over night. After three 15-min washes with blocking buffer, coverslips were incubated with secondary antibody (goat anti-rabbit Alexa Fluor 488 diluted 1:400) in blocking buffer for 2 h. Cell images were acquired with a laser scanning confocal microscope (Radiance 2000 MP; Bio-Rad, Hercules, CA) and a ×40 oil-immersion objective (N.A. ¼ 1.3). Images were acquired in line-scan mode using Laser Sharp 2000 software. Coverslips incubated with secondary antibody (Alexa Fluor 488) but without primary antibody had no detectable fluorescence at the gain and laser intensity used. Anti-KCNQ2 and anti-KCNQ3 antibodies preincubated for 1 h with commercially available blocking peptides (at 1:1 ratio) were used as negative controls for antibody specificity.
Statistics.
SigmaStat (Systat Software, Point Richmond, CA) was used for all statistical analyses. Paired Student's t-test was used for comparisons of parameters measured before and after treatments. Comparisons among multiple treatment groups were evaluated by ANOVA followed by a Holm-Sidak post hoc test. Cumulative concentration-response data were analyzed by repeated-measures ANOVA and post hoc Holm-Sidak test. Differences associated with P values ≤0.05 were considered statistically significant.
Materials
Flupirtine, linopirdine, collagenase, histamine, acetyl-β-methylcholine chloride (methacholine), and verapamil were from Sigma-Aldrich (St. Louis, MO). JNJ303 and LR-3 (L-364,373) were from Tocris (Ellisville, MO). Celecoxib was from LKT Laboratories (Saint Paul, MN). Retigabine dihydrochloride was from LGM Pharma (Boca Raton, FL). XE991 dihydrochloride was from Ascent Scientific (Princeton, NJ). Amphotericin B was from Calbiochem (San Diego, CA). Papain was from Worthington Biochemical (Lakewood, NJ). Low-melting-point agarose was from GIBCO (Invitrogen). 2,5-Dimethyl-celecoxib was generously provided by Dr. Axel Schönthal (University of Southern California).
RESULTS
Expression of Multiple Kv7 Subtypes in ASMCs from Guinea Pig and Human Lungs
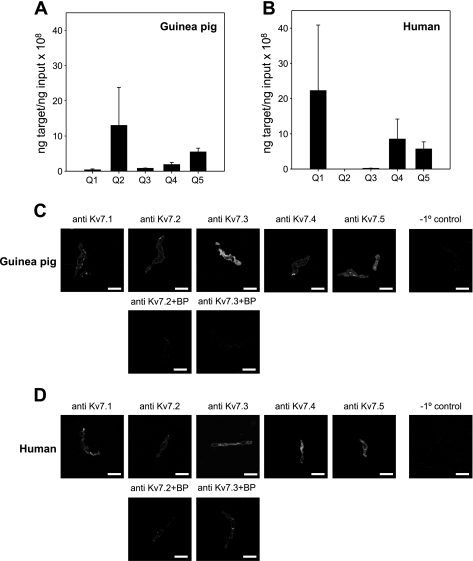
Real-time RT-PCR from guinea pig ASMCs isolated by enzymatic digestion revealed KCNQ2 > KCNQ5 > KCNQ4 > KCNQ3 ≫ KCNQ1 expression (Fig. 2A). The pattern was more variable in human trachealis muscle, which showed abundant expression of KCNQ1 relative to guinea pig, no detectable expression of KCNQ2 or KCNQ3, and modest expression of KCNQ4 and KCNQ5 mRNAs (Fig. 2B). Immunohistochemistry in ASMCs isolated from guinea pig or human trachealis muscle support the expression of multiple Kv7 subtypes in ASMCs from human and guinea pig (Fig. 2, C and D).
Fig. 2.
Multiple KCNQ subtypes are expressed in guinea pig and human ASMCs. Expression levels of mRNAs for KCNQ1–5 were estimated by quantitative real-time RT-PCR in guinea pig airway myocytes (A) and human trachealis muscle (B). Average target mRNA (ng) ± SE from n = 4 guinea pigs and from n = 4 human trachealis muscle samples. C and D, top rows: immunostaining of guinea pig trachealis myocytes (C) and human trachealis myocytes (D) with anti-Kv7.1–7.5 antibodies. C and D, bottom rows: images of the guinea pig (C) and human (D) ASMCs stained with Kv7.2 and Kv7.3 antibodies preincubated with the corresponding blocking peptide (anti-Kv7.2 + BP; anti-Kv7.3 + BP). Cells processed for imaging without primary antibody (−1° control) show no detectible fluorescence in ASMCs at the same gain and intensity settings (right).
Kv7 Currents in Freshly Dissociated Guinea Pig and Human ASMCs
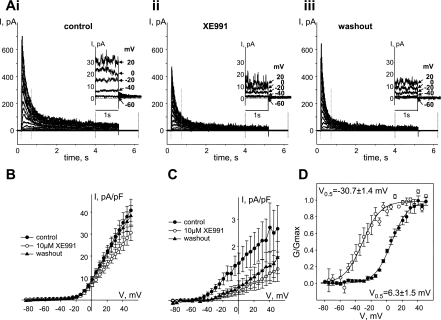
To detect Kv7 currents and estimate their contribution to the Kvtotal in ASMCs, we employed methods recently developed to measure these currents in vascular smooth muscle cells (6, 28, 31). This involves perforated-patch voltage-clamp techniques (to avoid rundown of Kv7 currents) and application of long (5 s) voltage steps from a −64 mV holding potential to record the slowly activating, noninactivating component of voltage-activated potassium currents. As shown in Fig. 3, this protocol elicited outward currents with a transient component that peaked within 500 ms and a sustained component that persisted to the end of the 5-s voltage steps. Application of XE991, a relatively selective inhibitor of Kv7 channels, had a modest effect on the transient component, reversibly decreasing peak currents by ∼27% only at voltages positive to −4 mV (P < 0.05, paired Student's t-test, Fig. 3, A and B). In contrast, the sustained component of the current (measured during the last 1 s of the 5-s voltage steps) was irreversibly inhibited by XE991 (by 71% in the voltage range from −30 to +30 mV; P < 0.05, paired Student's t-test, Figs. 1C and 3A, insets). In the physiological voltage range from −60 to −30 mV, the sustained component of current was completely abolished in the presence of XE991.
Fig. 3.
XE991 distinguishes between transient Kv currents and noninactivating Kv7 currents in guinea pig ASMCs. A: representative traces of total voltage-activated K+ currents (Kvtotal) recorded before (control) (i), during treatment with 10 μM XE991 (ii), and after washout of XE991 (iii). Currents were evoked with a 5-s voltage-step protocol from −64 mV holding voltage. Insets: last 2.2 s of the selected traces recorded at −60, −40, −20, 0, and +20 mV (as indicated by arrows) on an expanded scale for clarity. B: I-V relationships of peak K+ currents (from first 500 ms of the traces, indicated by vertical dotted gray lines in A) recorded in ASMCs before (control, ●, n = 4), after 10-min treatment with 10 μM XE991 (○, n = 4), and after 10-min washout of XE991 (▲, n = 4). C: I-V relationships of sustained K+ currents (averaged from last 1 s of the traces) recorded in ASMCs before (control, ●, n = 4), after 10-min treatment with 10 μM XE991 (○, n = 4), and after 10-min washout of XE991 (▲, n = 4). D: averaged fractional conductance plots fitted to a Boltzmann distribution calculated from the transient component (peak K+ currents reversibly blocked by 10 μM XE991; ●, n = 4, V0.5 = 6.3 ± 1.5 mV) and sustained component of K+ currents irreversibly blocked by 10 μM XE991 (○, n = 4, V0.5 = −30.7 ± 1.4 mV).
To compare the voltage dependence of the reversibly blocked XE991-sensitive transient component and the irreversibly blocked XE991-sensitive sustained component, activation curves were generated for XE991-sensitive peak currents (control current minus current recorded in the presence of XE991 as shown on Fig. 3B) and XE991-sensitive sustained current (control current minus current recorded after washout of XE991 as shown on Fig. 3C). Boltzmann fits of the voltage dependences of activation of both components revealed that V0.5 of the transient current component that was reversibly blocked by XE991 (6.3 ± 1.5 mV, n = 4, Fig. 3D) was not significantly different from the V0.5 of the transient component of Kvtotal (7.3 ± 1.8 mV, n = 3, Fig. 1). These values are similar to those previously reported for 4-aminopyridine-sensitive delayed-rectifier potassium currents measured in rat mesenteric artery smooth muscle cells [5.1 ± 2.1 mV (28)]. On the other hand, the V0.5 of the XE991-sensitive sustained current component (−30.7 ± 1.4 mV, n = 4, Fig. 3D) is in the good agreement with the values reported for Kv7 currents in smooth muscle cells from mesenteric and basilar arteries [around −34 mV (28, 31)].
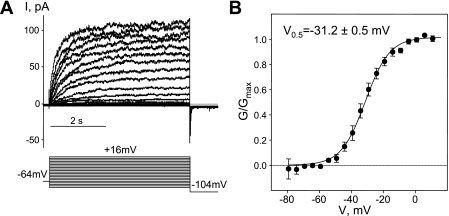
The long (5-s) voltage-step protocol is intended to minimize the transient current through Kv1 and Kv2 family potassium channels and enable recording of Kv7 currents in relative isolation. Nevertheless, at voltages positive to −30 mV an XE991-insensitive window current contributes ∼30% to the total currents measured (Fig. 3C). This window current significantly contaminated our measurements of Kv7 currents, narrowing the voltage range at which the Kv7 currents could be recorded in isolation. To increase the voltage range at which reasonably pure Kv7 currents could be recorded, we included 100 μM GdCl3 in the external solution. We had previously found that GdCl3 at this concentration was able to block nonselective cationic conductance and voltage-dependent Ca2+ conductances in A7r5 smooth muscle cells without effecting Kv7.5 currents (6). In the present study, application of 100 μM GdCl3 reversibly shifted the activation of the transient component of Kvtotal from 7.3 ± 1.8 to 20.5 ± 1.5 mV (Fig. 1) resetting the threshold for activation for these currents to around −10 mV (Fig. 1). Moreover, a subset of ASMCs (∼50%) exhibited only Kv7-like currents in the presence of 100 μM of GdCl3 at voltages up to +16 mV [based on the slow kinetics of activation with no apparent inactivation during 5-s voltage steps (Fig. 4A) and voltage-dependent activation with a very negative threshold (around −60 mV) fitted by a single Boltzmann function, with V0.5 around −31 mV (Fig. 4B; Fig. 5, C and E)]. The V0.5 value was similar to that measured for the sustained component of Kvtotal that was irreversibly blocked by XE991 (−30.7 ± 1.4 mV, n = 4, Fig. 3D).
Fig. 4.
Guinea pig ASMCs express functional KCNQ channels. A: representative traces of the current recorded in the presence of 100 μM GdCl3 using a 5-s voltage-step protocol depicted below [from −64 mV holding voltage, cell capacitance (C) = 15 pF]. B: averaged fractional conductance plots calculated from steady-state Kv7 currents fitted to a Boltzmann distribution (V0.5 = −31.0 ± 1.5 mV, n = 13).
Fig. 5.
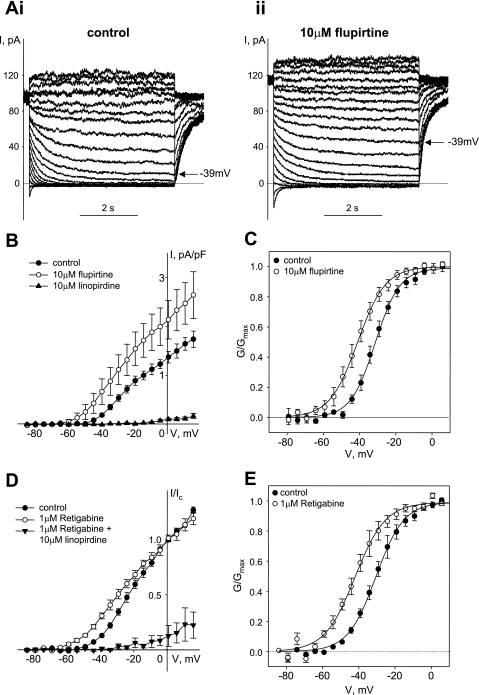
Pharmacology of Kv7 currents in guinea pig ASMCs. A: representative traces of Kv7 currents recorded in control (i) and in the presence of 10 μM flupirtine (ii) with a voltage-step protocol from −4 mV holding voltage. B: I-V relationships of Kv7 currents recorded in ASMCs before (control, ●, n = 5), after 5-min treatment with 10 μM flupirtine (○, n = 5), and after 5-min treatment with linopirdine (10 μM, ▲, n = 5). C: averaged fractional conductance plots calculated from steady-state Kv7 currents measured in ASMCs in control (●) and in the presence of 10 μM flupirtine (○) (n = 10) fitted to a Boltzmann distribution (V0.5 = −30.9 ± 1.4 mV in control, V0.5 = −41.0 ± 2.3 mV in the presence of 10 μM flupirtine; n = 10, P < 0.001, paired Student's t-test). D: I-V relationships of Kv7 currents normalized to control currents measured at +1 mV before (control, ●, n = 4), after 5-min treatment with 1 μM retigabine (○, n = 4), and after 5-min treatment with 10 μM linopirdine in the presence of 1 μM retigabine (▲, n = 5). E: averaged fractional conductance plots calculated from steady-state Kv7 currents measured in ASMCs in control (●) and in the presence of 1 μM retigabine (○) fitted to a Boltzmann distribution (V0.5 = −30.2 ± 1.6 mV in control, V0.5 = −41.5 ± 2.4 mV in the presence of 1 μM retigabine; n = 3, P < 0.05, paired Student's t-test).
To ensure a minimal contamination by transiently activating delayed-rectifier potassium currents, we combined the use of GdCl3 with a voltage-step protocol similar to what is commonly used to record Kv7 currents in neurons [M-currents (17)]. Long voltage steps (5 s) were applied from a −4 mV holding voltage because transiently activating delayed-rectifier potassium currents tends to inactivate at this more depolarized holding potential, reducing their contribution to total currents. Kv7 currents recorded by use of this voltage-step protocol were reversibly enhanced by the selective activators of Kv7.2–7.5 channels: flupirtine and retigabine (Fig. 5). The increase in current amplitude was accompanied by a shift of the activation curve to more negative voltages (Fig. 5, C and E), which was reversed on washout of either flupirtine or retigabine (not shown). The Kv7 currents were also completely and irreversibly suppressed by the Kv7 channel blockers linopirdine (Fig. 5, B and D) and XE991 (Figs. 6B and 8, B, D, and E).
Fig. 6.
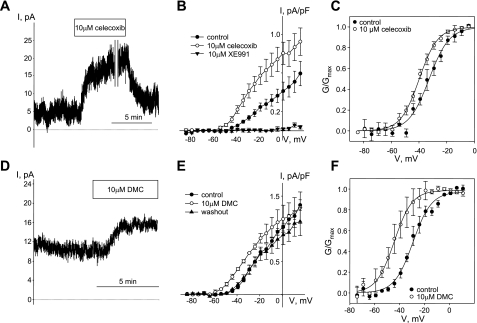
Celecoxib and 2,5-dimethyl-celecoxib (DMC) enhanced Kv7 currents in guinea pig ASMCs. A: time course of celecoxib (10 μM)-induced enhancement of endogenous Kv7 currents recorded in ASMCs at −20 mV holding voltage (C = 23 pF, representative of 5 similar experiments). A break in the recording (10 min) is indicated by 2 vertical gray lines. B: I-V relationships of Kv7 currents recorded from −4 mV holding voltage in ASMCs before (control, ●), during treatment with 10 μM celecoxib (○), and after 5-min treatment with the Kv7 channel blocker XE991 (10 μM, ▲, n = 4). C: averaged fractional conductance plots calculated from steady-state Kv7 currents measured in ASMCs in control (●) and in the presence of 10 μM celecoxib (○) fitted to a Boltzmann distribution (V0.5 = −32.4 ± 2.2 mV in control, V0.5 = −39.8 ± 1.5 mV in the presence of 10 μM celecoxib; n = 5, P < 0.05, paired Student's t-test). D: time course of Kv7 current enhancement by DMC (10 μM) recorded at −20 mV holding voltage (C = 13 pF, representative of 3 similar experiments). E: I-V relationships of Kv7 currents recorded in ASMCs before (control, ●), during treatment with 10 μM DMC (○), and after 10 min of washout (▲, n = 3). F: averaged fractional conductance plots calculated from steady-state Kv7 currents measured in ASMCs in control (●) and in the presence of 10 μM DMC (○) fitted to a Boltzmann distribution (V0.5 = −28.7 ± 2.2 mV in control, V0.5 = −43.1 ± 4.6 mV in the presence of 10 μM DMC; n = 3, P < 0.05, paired Student's t-test).
Fig. 8.
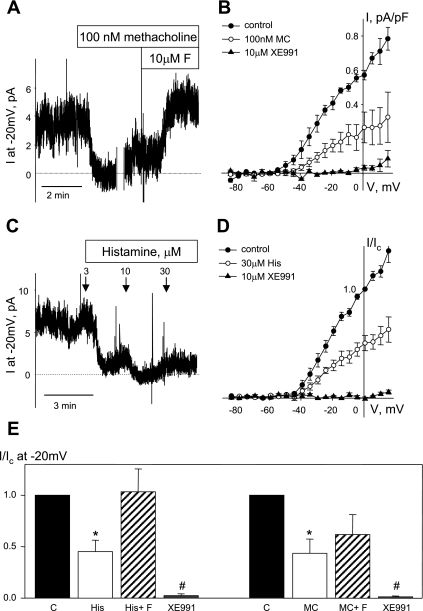
Suppression of Kv7 currents by bronchoconstrictor agonists in guinea pig ASMCs and their restoration by the Kv7 channel activator flupirtine. A: the time course of Kv7 current inhibition was recorded in ASMCs, at −20 mV holding voltage in control (2 min) following by methacholine application (100 nM for 3 min). Time course was disrupted for 10 min for recording of steady-state I-V curves (time break indicated by vertical gray lines). After an additional 1-min recording, flupirtine (10 μM) was applied in the presence of methacholine (C = 21 pF, representative of 4 similar experiments). B: average I-V relationships of Kv7 currents recorded from −4 mV holding voltage in ASMCs before (control, ●, n = 4), during treatment with 100 nM methacholine (○, n = 4), and in the presence of XE991 (10 μM, ▲, n = 3) applied in the end of each experiment to confirm that recorded current are indeed Kv7 currents. C: dose-dependent suppression of Kv7 currents recorded at −20 mV holding voltage was observed upon application of histamine (3–30 μM, indicated by arrows). D: average I-V relationships of Kv7 currents recorded from −4 mV holding voltage and normalized to control currents recorded at +1 mV in control (●, n = 4), in the presence of 30 μM histamine (○, n = 4) and in the presence of XE991 (10 μM, ▲, n = 4). E: normalized currents recorded at −20 mV in control (solid bars), in the presence of agonists (open bars: His, 30 μM histamine, n = 4; MC, 100 nM methacholine, n = 4); in the presence of agonists plus 10 μM flupirtine (hatched bars: His+F, 10 μM flupirtine in the presence of 30 μM histamine, n = 4; MC+F, 10 μM flupirtine in the presence of 100 nM methacholine, n = 4) and in the presence of 10 μM XE991 (n = 4). Ic, control current measured before treatment. *Significant difference from control (P < 0.05, 1-way ANOVA). #Significant difference from all other treatment (P < 0.05, 1-way ANOVA).
Recently we discovered that celecoxib enhances Kv7 currents in A7r5 rat aortic smooth muscle cells and rat mesenteric artery myocytes independently of its inhibitory actions on COX-2 (4). Here we observed a similar robust enhancement of Kv7 currents in guinea pig airway myocytes during treatment with celecoxib (10 μM) (Fig. 6, A and B). Current amplitude was partially restored upon washout of celecoxib (Fig. 6A), and currents were blocked completely upon application of 10 μM XE991, a selective Kv7 channel blocker (Fig. 6B). Application of celecoxib (10 μM) induced a negative shift of the activation curve (7.3 ± 1.2 mV, P < 0.05, paired Student's t-test, n = 5) (Fig. 6C). A structural analog of celecoxib, DMC, lacking COX-2 inhibitory activity (41), also reversibly enhanced Kv7 currents in guinea pig ASMCs (Fig. 6, D and E). The DMC-induced increase in current amplitude was accompanied by a 14.5 ± 2.5 mV negative shift of the activation curve (P < 0.05, paired Student's t-test, n = 3) (Fig. 6F).
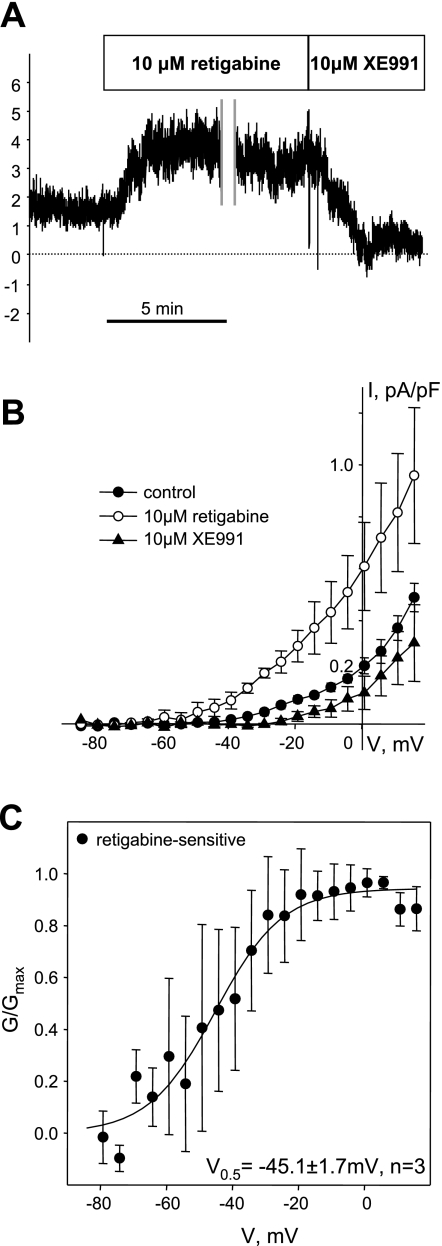
Kv7 currents were also successfully recorded from a small number of isolated human ASMCs by using the 5-s voltage-step protocol applied from a −4 mV holding voltage in the presence of 100 μM GdCl3 (total n = 6, Fig. 7). Current densities (0.10 ± 0.01 pA/pF at −20 mV, n = 6) were relatively small compared with Kv7 currents in guinea pig ASMCs (0.6 ± 0.1 pA/pF at −20 mV, n = 24). Nevertheless, currents in human ASMCs were significantly enhanced by 10 μM retigabine (∼3-fold increase over control at −20 mV) and significantly inhibited by 10 μM XE991 (∼70% inhibition from control at −20 mV) (Fig. 7, A and B). Because of the small current amplitudes and small numbers of successful recordings, evaluation of the activation curve was limited to the retigabine-sensitive component of the current (retigabine-sensitive component of the current was derived for each experiment by subtracting control currents from the currents recorded in the presence of 10 μM of retigabine, n = 3). The conductance (calculated as described in materials and methods), was plotted against voltage and fitted with the Boltzmann equation, revealing a V0.5 = −45.1 ± 1.7 mV (Fig. 7C).
Fig. 7.
Kv7 currents in human ASMCs. A: time course of endogenous Kv7 current recorded in a human ASMC at −20 mV holding voltage before and during treatment with retigabine (10 μM) followed by XE991 (10 μM) (C = 16 pF, representative of 3 similar experiments). Vertical gray lines indicate a 10-min break to record I-V relationships from −4 mV holding voltage in the presence of retigabine. B: average I-V relationships of Kv7 currents measured in control (●, n = 5) in the presence of 10 μM retigabine (○, n = 3) and in the presence of XE991 (10 μM, ▲, n = 3). C: averaged fractional conductance plot calculated from retigabine sensitive portion of sustained Kv7 currents (currents measured in the presence of retigabine minus control currents) fitted to a Boltzmann distribution (V0.5 = −45.1 ± 1.7 mV, n = 3). Currents measured before retigabine treatment were subtracted from currents measured in the presence of retigabine at the same voltages.
Suppression of Kv7 Currents by Bronchoconstrictor Agonists Is Reversed by Kv7 Channel Activators
Neuronal and vascular Kv7 currents have been found to be inhibited upon activation of Gq/11-coupled receptors (10, 25, 29). We tested the ability of Gq/11-coupled bronchoconstrictor agonists methacholine and histamine to suppress Kv7 currents in guinea pig ASMCs. Application of 100 nM methacholine induced rapid and near complete inhibition of Kv7 currents, although the currents were partially restored within 1–2 min to about 40% of the control levels while methacholine was still present (Fig. 8A). The sustained effect of methacholine (100 nM) was an ∼60% reduction of Kv7 currents at all voltages positive to −24 mV (Fig. 8, B and E; P < 0.05, paired Student's t-test). The Kv7 channel activator flupirtine (10 μM) was able to restore the amplitude of Kv7 currents remaining after suppression by methacholine to 64% of control (Fig. 8, B and E). Histamine, another bronchoconstrictor agonist, also induced suppression of Kv7 currents in airway myocytes in a dose-dependent manner (Fig. 8C). Histamine-induced Kv7 current inhibition was transient at agonist concentrations from 1–10 μM, but application of 30 μM histamine induced sustained current inhibition at all voltages positive to −29 mV (Fig. 6, C–E). Subsequent addition of flupirtine (10 μM) in the continued presence of 30 μM histamine restored remaining Kv7 current amplitude to the values measured prior to histamine treatment (Fig. 8E).
Functional Kv7 Channels Are Required for Maintenance of Resting Diameter of Small Airways in Human Lung Slices
To investigate the physiological relevance of functional Kv7 channels in human airways we used precision cut lung slices. Small round airways lined with intact epithelia identified by beating cilia constricted reproducibly and dose dependently in response to application of bronchoconstrictor agonists histamine (Fig. 9) and methacholine (not shown). Application of 50 nM histamine induced a 41 ± 10% constriction of small airways (as estimated by reduction of luminal area). The histamine-induced constriction was slightly but significantly attenuated (29 ± 10%, P = 0.014, paired Student's t-test) in the presence of 100 μM flupirtine, an activator of Kv7.2–7.5 channels (Fig. 9C).
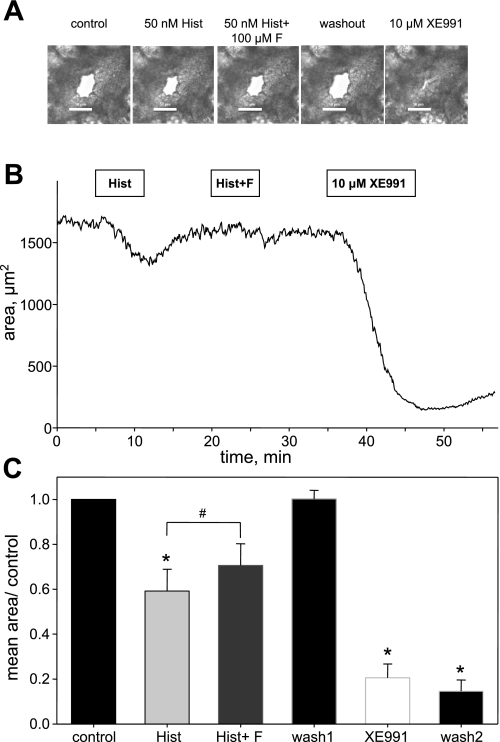
Fig. 9.
Functional Kv7 channels are required for maintaining resting diameter of small airways in human lung slices. A: representative images of a small airway before treatment (control), in the presence of 50 nM histamine applied for 5 min (50 nM Hist), in the presence of 50 nM histamine applied with 100 μM flupirtine for 5 min (50 nM Hist + 100 μM F), following a 10-min washout of histamine and flupirtine (washout), and in the presence of 10 μM XE991 applied for 10 min. B: corresponding representative time course of changes in luminal area of the same small airway. Both 50 nM histamine (Hist) and 50 nM histamine with 100 μM flupirtine (Hist + F) were applied for 5 min followed by a 10-min washout in each case; 10 μM XE991 was applied for 10 min. C: summarized bar graph of average airway luminal area measured in multiple lung slices from 2 human subjects. Luminal area measured during the last min of treatment with 50 nM histamine (Hist), 50 nM histamine with 100 μM flupirtine (Hist + F), or 10 μM XE991 was normalized to control area measured before treatments; *significant difference from control (1-way ANOVA, P < 0.001, n = 6–8); #significant difference between histamine alone and histamine plus flupirtine treatment groups (paired Student's t-test, P < 0.05, n = 6).
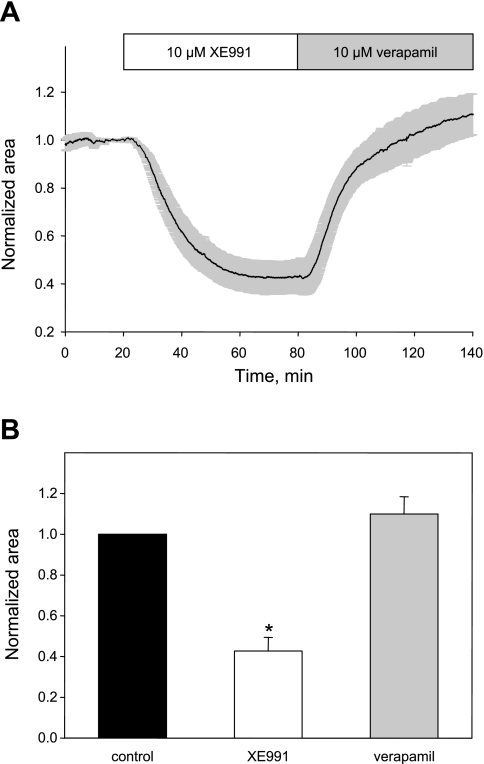
Application of 10 μM of XE991, a potent and selective inhibitor of Kv7 channels, consistently induced near total constriction of small airways (46–89 μm in diameter) in human lung slices (79 ± 6% constriction, n = 6; Fig. 9). In larger airways (121–242 μm in diameter), the effect of XE991 was qualitatively similar, although the airway only partially occluded (57 ± 7% constriction, n = 4; Fig. 10A). The bronchoconstrictor effect of XE991 was not reversed by washing out XE991 for up to 45 min (Fig. 9) but was fully reversed by treatment with a calcium channel blocker, verapamil (10 μM), with a time constant of 15.5 ± 0.1 min (estimated from a single exponential fit of the relaxation time course; Fig. 10B), implicating L-type VSCCs as downstream effectors in the actions of XE991.
Fig. 10.
Constriction of human airways by Kv7 channel blocker XE991 is reversed by the calcium channel blocker verapamil. A: average time course (mean ± SE) of constriction of human airway by XE991 (10 μM, open bar) and its subsequent reversal by addition of the calcium channel blocker verapamil (10 μM, shaded bar) normalized to average control area measured during 20 min of control recording for each experiment. B: summary of effects of XE991 and verapamil on human airway luminal area (n = 4 slices containing airways of diameters ranging from 122 to 242 μm in diameter). *Significant difference from control and verapamil (P < 0.001, 1-way ANOVA).
DISCUSSION
Our results reveal for the first time that KCNQ (Kv7) voltage-activated potassium channels are expressed in guinea pig and human ASMCs. We confirmed their function by measuring potassium currents with the expected electrophysiological and pharmacological characteristics of Kv7 currents. Kv7 currents were suppressed by Gq/11-coupled bronchoconstrictor agonists, but the currents could be restored by drugs that are selective Kv7 channel activators. In precision-cut human lung slices, a Kv7 channel blocker induced profound airway constriction, which was reversed by addition of a calcium channel blocker. Drugs that enhanced Kv7 currents attenuated bronchoconstrictor-induced airway constriction, revealing an important contribution of these channels to maintenance of airway diameter and providing evidence to support a novel pharmacological strategy targeting these channels for the relief of airway hyperconstriction.
Kv7 Channels in ASMCs
The Kv7 family of voltage-activated K+ channels is encoded by five genes (KCNQ1–5). Unlike other classes of voltage-activated K+ channels, Kv7 channels are known to activate at very negative voltages (around −60 mV) and function to stabilize resting membrane voltages in many types of excitable cells (29). They were first identified in the brain, where they modulate neuronal excitation (17). More recently, Kv7 channels have been identified in vascular smooth muscle cells (VSMCs) (6, 19, 20, 28, 31, 35, 45, 46) as well as visceral and myometrial smooth muscle (18, 32, 36). Our findings here indicate that Kv7 channels are also expressed and functional in ASMCs and that their activity can be modulated in opposite directions by bronchoconstrictor agonists and clinically used Kv7 channel activators.
The expression pattern in human ASMCs was similar to that reported in vascular smooth muscle in mouse and rat arteries (expression of KCNQ1, KCNQ4, and KCNQ5, with little or no detectable KCNQ2 or KCNQ3) (5, 20, 45). In human arteries, only KCNQ2 was undetectable (35). The expression pattern for KCNQ1 and KCNQ2 mRNAs differed between guinea pig and human ASMCs. KCNQ2 was the most abundant KCNQ transcript in guinea pig ASMCs, but was undetectable in human ASMCs, whereas KCNQ1 was most abundant in human and barely detectable in guinea pig. It remains to be determined how these differences come about and whether the differential expression of KCNQ genes between guinea pig and human ASMCs has a functional impact on regulation of airway diameter. Immunofluorescence detection of Kv7 channel proteins also indicated the presence of multiple Kv7 subtypes in both human and guinea pig ASMCs, with similar expression patterns between these species, although these methods are not quantitative and the findings are highly dependent on the quality of available antibodies and should therefore be interpreted with caution.
Any of the expressed Kv7 channel protein subtypes could potentially contribute to the K+ currents measured in guinea pig or human ASMCs or to the bronchiolar constriction in human lung slices. Considering that 1) robust currents were detected in guinea pig myocytes where KCNQ1 expression was virtually undetectable; 2) selective activators of Kv7.2–7.5 subtypes, flupirtine and retigabine, robustly enhanced the currents in both guinea pig and human ASMCs; and 3) flupirtine relaxed the airways in human lung slices, it is likely that some combination of Kv7.2–5 subtypes forms functional channels that contribute to the regulation of airway diameter.
Our laboratory previously found that the activity of vascular Kv7.5 channels is suppressed by the pituitary hormone arginine vasopressin to mediate its physiological vasoconstrictor actions (6, 28). The suppression of ASMC Kv7 currents by both methacholine and histamine suggests that these channels may also be common signal transduction intermediates in the actions of Gq/11-coupled bronchoconstrictor agonists. The signaling pathway involved in regulation of ASMC Kv7 channels remains to be determined. There is abundant evidence demonstrating that hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) results in suppression of neuronal Kv7 currents, whereas protein kinase C (PKC) activation has been implicated in the suppression of VSMC Kv7 currents (6, 28). PIP2 hydrolysis leading to diacylglycerol production and PKC activation are likely downstream events following binding of either methacholine to M3 muscarinic acetylcholine receptors or histamine to H1 receptors on ASMCs (38). Studies of human bronchial smooth muscle contractility indicated that activation of PKC induces sustained contraction of human ASMCs and that this effect is dependent on activation of L-type VSCCs (40). Similarly, both carbachol (a muscarinic receptor agonist) and a membrane-permeant diacylglycerol analog were found to induce sustained elevation of [Ca2+]cyt in bovine airway myocytes, and these effects were abolished in the presence of an L-type VSCC blocker (22). PKC-dependent suppression of Kv7 currents may therefore be a plausible mechanism to induce membrane depolarization for activation of L-type VSCCs, Ca2+ influx, and ASMC contraction.
Flupirtine restored Kv7 current amplitudes to near control values following suppression of these currents by histamine or methacholine. This may represent an opposing effect of flupirtine on channels that were inhibited by the bronchoconstrictor agonists or an enhancement of the remaining currents conducted by the fraction of channels that were not inhibited. In either case, it suggests that Kv7 channel activators may have therapeutic utility in relieving the actions of bronchoconstrictor agonists.
Airway Hypersensitivity in Asthma
A diagnosis of asthma is generally made when bronchoconstrictor agonists that act directly on ASMCs induce constriction of airways at lower than normal concentrations, or when airways constrict with a lower threshold of indirect bronchial provocation (e.g., exercise or inhalation of cold air) (3, 8). It remains to be determined why asthma sufferers have increased sensitivity to multiple direct and indirect stimuli. A reduction of K+ channel activity and the associated increase in electrical resistance in the plasma membranes of ASMCs could be a mechanism for sensitization of ASMCs to multiple bronchoconstrictor stimuli. According to Ohm's law, voltage is proportional to the product of current and resistance. Therefore, an increase in resistance would enable a small current to produce a larger change in voltage. In other words, a reduction in outward K+ currents would increase membrane resistance and thereby serve to sensitize ASMCs to depolarizing stimuli (depolarizing stimuli include small increases in inward current, e.g., due to activation of Cl− or cation channels in response to bronchial provocation). Thus bronchial provocation would tend to induce greater membrane depolarization and activation of VSCCs when K+ channel activity is reduced. Such a mechanism is consistent with the observation that the sensitivity of porcine trachealis muscle to histamine-induced contraction was enhanced in the presence of a nonspecific K+ channel blocker (tetraethylammonium, 10 mM) and that this effect was abolished by the calcium channel blocker verapamil (33). If ASMC Kv7 channel activity were reduced in asthma, bronchial provocation by pharmacological or mechanical stimulation would more effectively depolarize the cells to activate L-type voltage-sensitive Ca2+ channels and enhance airway constriction. The converse would also hold: increasing Kv7 channel activity would reduce ASMC electrical excitability and decrease the sensitivity to bronchial provocation.
Pharmacological Targeting of Kv7 Channels in ASMCs
The COX-2 inhibitor celecoxib (Celebrex) and its COX-2-independent analog DMC were recently found to enhance the activity of Kv7 channels in VSMCs (4). Both of these agents also significantly enhanced Kv7 currents in guinea pig ASMCs (Fig. 6). Celecoxib was previously shown to inhibit other Kv channel subtypes [Kv2.1, Kv1.5, and Kv4.3 channels (12, 27)] and to distinguish among Kv7 family members, enhancing Kv7.5 currents (4) and inhibiting Kv7.1 currents (27). In the present study, both celecoxib and DMC induced a significant negative shift of the activation curve that was not observed previously for endogenous Kv7.5 or overexpressed Kv7.5 currents in VSMCs (4). This finding may indicate that functional channels in ASMCs differ from homomeric Kv7.5, perhaps involving Kv7.2, Kv7.3, or Kv7.4 subtypes or heteromeric channels composed of two or more Kv7 subtypes. Celecoxib actions on these other types of Kv7 channels as well as the mechanisms of its action have yet to be determined.
Both celecoxib and DMC are also robust inhibitors of VSCC (4). Their double action as enhancers of Kv7.2–7.5 channels and blockers of L-type Ca2+ channels may be beneficial for reducing airway hypercontraction in asthma. However, the clinical utility of celecoxib for treatment of asthma patients is still controversial. Retrospective analyses of clinical trials revealed celecoxib to be safe even in asthma patients with aspirin hypersensitivity (24, 43). However, Daham et al. (9) recently found that celecoxib had an asymmetric impact on prostanoid formation, decreasing the formation of bronchodilator prostaglandins while maintaining increased levels of bronchoconstrictor prostaglandins in asthma patients. DMC would presumably avoid the loss of bronchodilatory prostaglandins since it does not inhibit COX-2 enzymes (23); it might therefore be a more effective bronchodilator than celecoxib, retaining its Kv7 channel-activating and calcium channel-blocking actions, but without affecting prostanoid production.
During the past 30 years, a number of clinical trials were conducted to evaluate the efficacy of L-type Ca2+ channel blockers (CCBs) in the treatment of asthma. The results were inconsistent, although almost all the clinical trials revealed at least a subset of patients who benefited from treatment with either verapamil or nifedipine (11). Taken as a whole, the clinical trials revealed that asthma patients benefit from treatment with CCBs, but the efficacy of these agents is limited because of adverse side effects associated with systemic administration and limitations of formulation that prevent delivery of effective doses of verapamil or nifedipine by inhalation (11).
Some of the limitations associated with CCB therapy may be overcome by using K+ channel activators, which are expected to have the same ultimate effect of reducing Ca2+ influx in ASMCs. Flupirtine and celecoxib have well-established clinical safety profiles when administered systemically, and it may also be possible to develop inhalational formulations of these drugs, which would reduce off-target effects. Furthermore, if the Kv7 channel subunit stoichiometry in ASMCs differs from that of channels found in other tissues, it may be possible to develop drugs that have selectivity for ASMC Kv7 channels to provide improved therapeutic indices. A recent review by Malerba et al. (30) highlights the potential for use of activators of KATP channels or BKCa channels to treat asthma but notes that attempts to develop suitable drugs targeting those channels in the airways has so far not met with success. Flupirtine significantly attenuated histamine-induced constriction of human airways and several Kv7.2–7.5 channel activators (retigabine, flupirtine, celecoxib, and DMC) were effective in enhancing Kv7 currents in guinea pig ASMCs. Kv7 channel activators have yet to be tested as asthma therapies, but our findings here raise hope that these drugs may be effective bronchodilators.
Summary
In summary, our results reveal a fundamental mechanism for regulating airway diameter: the activity of Kv7 potassium channels in ASMCs under resting conditions is essential to maintain the relaxed state of the airways. These channels are inhibited by the Gq/11-coupled bronchoconstrictor agonists methacholine and histamine; suppression of Kv7 channel activity is sufficient to induce airway constriction. Our results also provide evidence that drugs that enhance Kv7 channel activity are effective bronchodilators that may be useful for the relief of airway hyperconstriction in asthma or other airway diseases.
GRANTS
This work was supported by R01 HL089564 (to K. L. Byron) and by intramural funds from Loyola University Chicago.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Barnes PJ. Clinical studies with calcium antagonists in asthma. Br J Clin Pharmacol 20: 289S–298S, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Belmonte KE. Cholinergic pathways in the lungs and anticholinergic therapy for chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 297–304, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Brannan JD. Bronchial hyperresponsiveness in the assessment of asthma control. Chest 138: 11S–17S, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Brueggemann LI, Mackie AR, Mani BK, Cribbs LL, Byron KL. Differential effects of selective cyclooxygenase-2 inhibitors on vascular smooth muscle ion channels may account for differences in cardiovascular risk profiles. Mol Pharmacol 76: 1053–1061, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brueggemann LI, Mackie AR, Martin JL, Cribbs LL, Byron KL. Diclofenac distinguishes among homomeric and heteromeric potassium channels composed of KCNQ4 and KCNQ5 subunits. Mol Pharmacol 79: 10–23, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brueggemann LI, Moran CJ, Barakat JA, Yeh JZ, Cribbs LL, Byron KL. Vasopressin stimulates action potential firing by protein kinase C-dependent inhibition of KCNQ5 in A7r5 rat aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 292: H1352–H1363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987 [DOI] [PubMed] [Google Scholar]
- 8. Cockcroft DW. Direct challenge tests. Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138: 18S–24S, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Daham K, Song WL, Lawson JA, Kupczyk M, Gülich A, Dahlén SE, FitzGerald GA, Dahlén B. Effects of celecoxib on major prostaglandins in asthma. Clin Exp Allergy 41: 36–45 [DOI] [PubMed] [Google Scholar]
- 10. Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6: 850–862, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Fish JE. Calcium channel antagonists in the treatment of asthma. J Asthma 21: 407–418, 1984 [DOI] [PubMed] [Google Scholar]
- 12. Frolov RV, Berim IG, Singh S. inhibition of delayed rectifier potassium channels and induction of arrhythmia: a novel effect of celecoxib and the mechanism underlying it. J Biol Chem 283: 1518–1524, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol 71: 489–507, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J 30: 114–133, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Janssen LJ. Ionic mechanisms and Ca2+ regulation in airway smooth muscle contraction: do the data contradict dogma? Am J Physiol Lung Cell Mol Physiol 282: L1161–L1178, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Janssen LJ, Sims SM. Acetylcholine activates non-selective cation and chloride conductances in canine and guinea-pig tracheal myocytes. J Physiol 453: 197–218, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci 1: 21–30, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Jepps TA, Greenwood IA, Moffatt JD, Sanders KM, Ohya S. Molecular and functional characterization of Kv7 K+ channel in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol 297: G107–G115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi S, Balan P, Gurney AM. Pulmonary vasoconstrictor action of KCNQ potassium channel blockers. Respir Res 7: 31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Joshi S, Sedivy V, Hodyc D, Herget J, Gurney AM. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 329: 368–376, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jude JA, Wylam ME, Walseth TF, Kannan MS. Calcium signaling in airway smooth muscle. Proc Am Thorac Soc 5: 15–22, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kajita J, Yamaguchi H. Calcium mobilization by muscarinic cholinergic stimulation in bovine single airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 264: L496–L503, 1993 [DOI] [PubMed] [Google Scholar]
- 23. Kardosh A, Wang W, Uddin J, Petasis NA, Hofman FM, Chen TC, Schonthal AH. Dimethyl-celecoxib (DMC), a derivative of celecoxib that lacks cyclooxygenase-2-inhibitory function, potently mimics the anti-tumor effects of celecoxib on Burkitt's lymphoma in vitro and in vivo. Cancer Biol Ther 4: 571–582, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kowalski ML, Makowska J. Use of nonsteroidal anti-inflammatory drugs in patients with aspirin hypersensitivity: safety of cyclo-oxygenase-2 inhibitors. Treat Respir Med 5: 399–406, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci 9: 305–310, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Liang GH, Jin Z, Ulfendahl M, Jarlebark L. Molecular analyses of KCNQ1–5 potassium channel mRNAs in rat and guinea pig inner ears: expression, cloning, and alternative splicing. Acta Otolaryngol (Stockh) 126: 346–352, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Macías Á, Moreno C, Moral-Sanz J, Cogolludo Á, David M, Alemanni M, Pérez-Vizcaíno F, Zaza A, Valenzuela C, González T. Celecoxib blocks cardiac Kv1.5, Kv43 and Kv71 (KCNQ1) channels: effects on cardiac action potentials. J Mol Cell Cardiol 49: 984–992, 2010 [DOI] [PubMed] [Google Scholar]
- 28. Mackie AR, Brueggemann LI, Henderson KK, Shiels AJ, Cribbs LL, Scrogin KE, Byron KL. Vascular KCNQ potassium channels as novel targets for the control of mesenteric artery constriction by vasopressin, based on studies in single cells, pressurized arteries, and in vivo measurements of mesenteric vascular resistance. J Pharmacol Exp Ther 325: 475–483, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mackie AR, Byron KL. Cardiovascular KCNQ (Kv7) potassium channels: physiological regulators and new targets for therapeutic intervention. Mol Pharmacology 74: 1171–1179, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Malerba M, Radaeli A, Mancuso S, Polosa R. The potential therapeutic role of potassium channel modulators in asthma and chronic obstructive pulmonary disease. J Biol Reg Homeost Agents 24: 123–130, 2010 [PubMed] [Google Scholar]
- 31. Mani BK, Brueggemann LI, Cribbs LL, Byron KL. Activation of vascular KCNQ (Kv7) potassium channels reverses spasmogen-induced constrictor responses in rat basilar artery. Br J Pharmacol 164: 237–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McCallum LA, Pierce SL, England SK, Greenwood IA, Tribe RM. The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med 15: 577–586, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell HW. Electromechanical effects of tetraethylammonium and K+ on histamine-induced contraction in pig isolated tracheal smooth muscle. Lung 165: 129–142, 1987 [DOI] [PubMed] [Google Scholar]
- 34. Navarro-Lopez J, Jimenez-Diaz L, Geranton SM, Ashmore JF. Electrophysiological and molecular analysis of Kv7/KCNQ potassium channels in the inferior colliculus of adult guinea pig. J Mol Neurosci 37: 263–268, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA. Expression and function of the K+ channel KCNQ genes in human arteries. Br J Pharmacol 162: 42–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol 282: G277–G287, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol 378: 149–169, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Penn RB, Benovic JL. Regulation of heterotrimeric G protein signaling in airway smooth muscle. Proc Am Thorac Soc 5: 47–57, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perez-Zoghbi JF, Karner C, Ito S, Shepherd M, Alrashdan Y, Sanderson MJ. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol Ther 22: 388–397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rossetti M, Savineau JP, Crevel H, Marthan R. Role of protein kinase C in nonsensitized and passively sensitized human isolated bronchial smooth muscle. Am J Physiol Lung Cell Mol Physiol 268: L966–L971, 1995 [DOI] [PubMed] [Google Scholar]
- 41. Schönthal AH, Chen TC, Hofman FM, Louie SG, Petasis NA. Celecoxib analogs that lack COX-2 inhibitory function: preclinical development of novel anticancer drugs. Expert Opin Investig Drugs 17: 197–208, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Smith N, Johnson FJ. Early- and late-phase bronchoconstriction, airway hyper-reactivity and cell influx into the lungs, after 5'-adenosine monophosphate inhalation: comparison with ovalbumin. Clin Exp Allergy 35: 522–530, 2005 [DOI] [PubMed] [Google Scholar]
- 43. West PM, Fernandez C. Safety of COX-2 inhibitors in asthma patients with aspirin hypersensitivity. Ann Pharmacother 37: 1497–1501, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Wickenden AD, Zou A, Wagoner PK, Jegla T. Characterization of KCNQ5/Q3 potassium channels expressed in mammalian cells. Br J Pharmacol 132: 381–384, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yeung SY, Pucovsky V, Moffatt JD, Saldanha L, Schwake M, Ohya S, Greenwood IA. Molecular expression and pharmacological identification of a role for K(v)7 channels in murine vascular reactivity. Br J Pharmacol 151: 758–770, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol 588: 3277–3293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]