Abstract
Epidemiological studies have shown that maternal preeclampsia (PE) increases the risk of bronchopulmonary dysplasia (BPD), but the underlying mechanism is unknown. Soluble vascular endothelial growth factor receptor-1 (soluble VEGFR1, known as soluble fms-like tyrosine kinase 1, or sFlt-1), an endogenous antagonist of vascular endothelial growth factor (VEGF), is markedly elevated in amniotic fluid and maternal blood in PE. Therefore, we hypothesized that antenatal exposure to excess sFlt-1 disrupts lung development through impaired VEGF signaling in utero, providing a mechanistic link between PE and BPD. To determine whether increased sFlt-1 in amniotic fluid is sufficient to cause sustained abnormalities of lung structure during infancy, sFlt-1 or saline was injected into amniotic sacs of pregnant Sprague-Dawley rats at 20 days of gestation (term, 22 days). After birth, pups were observed through 14 days of age for study. We found that intra-amniotic sFlt-1 treatment decreased alveolar number, reduced pulmonary vessel density, and caused right and left ventricular hypertrophy in 14-day-old rats. In addition, intra-amniotic sFlt-1 treatment suppressed activation of lung VEGF receptor-2 and increased apoptosis in endothelial and mesenchymal cells in the newborn lung. We conclude that exposure to excess sFlt-1 in amniotic fluid during late gestation causes sustained reductions in alveolarization and pulmonary vascular growth during infancy, accompanied by biventricular hypertrophy suggesting pulmonary and systemic hypertension. We speculate that impaired VEGF signaling in utero due to exposure of high amniotic fluid levels of sFlt-1 in PE disrupts lung growth and contributes to the increased risk of BPD in infants born to mothers with PE.
Keywords: bronchopulmonary dysplasia, preeclampsia, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1, pulmonary hypertension
bronchopulmonary dysplasia (BPD) is the chronic lung disease of infancy that follows premature birth and is characterized by impaired lung growth due to early lung injury (53). BPD is a common and severe complication of preterm birth (19, 59, 66). Infants with BPD have prolonged respiratory insufficiency and are at high risk for pulmonary hypertension (39, 65), lung infections (51, 78), recurrent hospitalizations (63), exercise intolerance (30, 74, 75), and adverse neurodevelopmental outcome (3). Past studies have identified significant contributions to the pathogenesis of BPD from prematurity, side effects of respiratory support (9, 18, 52), and complications of neonatal illness (5, 27, 44). Although the use of antenatal steroids, surfactant, and improvements in ventilator and oxygen therapies have markedly altered the clinical course of preterm infants, the incidence of BPD has not decreased in the very premature infants during the past decade (59, 66). Late respiratory complications of premature birth and BPD generally reflect the effects of persistent abnormalities in distal lung structure, which include decreased alveolarization and a dysmorphic pulmonary vascular bed (14).
Recently, the impact of an adverse intrauterine environment on impairing neonatal lung growth and worsening respiratory outcomes of preterm infants has been recognized by both clinical (8, 10, 28, 31, 41, 54, 55) and experimental studies (29, 47, 69, 71). Among diverse antenatal factors, preeclampsia (PE) is shown to be independently associated with a high risk for BPD, but the underlying mechanism is unknown (28, 31, 40, 79).
PE is the most common complication of pregnancy and is due to maternal endothelial dysfunction that leads to hypertension, proteinuria, and other complications (61, 62). Moreover, maternal PE is a frequent cause of preterm birth (7), accounting for nearly 20% of delivery before 28 wk gestational age (49), and significantly increases perinatal and neonatal morbidities (61, 62). Placental overproduction of soluble vascular endothelial growth factor (VEGF) receptor-1 (soluble fms-like tyrosine kinase-1, also known as sFlt-1), which inhibits VEGF signaling mainly through trapping free VEGF (38, 60), causes maternal endothelial dysfunction and plays a central role in the pathogenesis of PE (37, 43, 46, 48). The magnitude of excess sFlt-1 in maternal serum not only correlates with the severity of PE (48, 73), but serum levels of sFlt-1 in the mother are also inversely related to gestational age and birth weight of the newborn (73). In addition to high maternal serum levels, sFlt-1 is markedly increased in amniotic fluid during the second and third trimesters of pregnancies complicated by PE (64, 76, 77). Whether exposure of the fetal lung to excess intra-amniotic sFlt-1 can impair lung VEGF signaling and contribute to the pathogenesis of BPD is unknown. VEGF, a key regulator of angiogenesis (12, 56), plays a critical role in directing vascular and alveolar growth in the developing lung. Previous studies have shown that VEGF inhibition during the early neonatal period results in persistent abnormalities of alveolar and pulmonary vascular structures into and beyond infancy, which are characteristic of pathological changes in human BPD (32, 42, 67). However, whether transient impairment of VEGF signaling in the fetus is sufficient to cause sustained disruption in postnatal lung growth has not been studied.
To determine mechanisms that may link PE with a high risk for BPD, we hypothesized that antenatal exposure to excess sFlt-1 disrupts normal lung development through impaired VEGF signaling in utero. We further hypothesized that fetal exposure to elevated levels of intra-amniotic sFlt-1 is sufficient to cause sustained abnormalities of lung structure into infancy. We report that intra-amniotic sFlt-1 treatment during late gestation impaired lung VEGF signaling and increased apoptosis of lung cells in newborn rats, which were followed by reductions in alveolarization and lung vascular growth along with biventricular hypertrophy during infancy. These findings demonstrate biological plausibility underlying epidemiological evidence that links PE with BPD.
MATERIALS AND METHODS
Animals
All procedures and protocols were approved by the Animal Care and Use Committee at the University of Colorado Health Sciences Center. Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and maintained in room air at Denver's altitude (1,600 meters; barometric pressure, 630 mmHg; inspired oxygen tension, 122 mmHg) for at least 1 wk before giving birth. Animals were fed ad libitum and exposed to day-night cycles alternatively every 12 h. Rats were killed with an intraperitoneal injection of pentobarbital sodium (0.3 mg/g body wt; Fort Dodge Animal Health, Fort Dodge, IA).
Study Design
Intra-amniotic sFlt-1administration.
At 20 days gestation (term: 22 days), pregnant rats were prepared for receiving intra-amniotic injections. The timing of injection during the late canalicular stage of lung development in the rat was selected to parallel a similar stage of human lung development in 24- to 26-wk premature newborns who are at the highest risk for BPD. After premedication with buprenorphine (0.01–0.05 mg/kg, intramuscular injection), laparotomy was preformed on pregnant rats under general anesthesia with 1–2% isoflurane inhalation via a face mask (Anesthesia machine: Matrx by Midmark, model VIP3000). During anesthesia and laparotomy, pregnant rats were kept on a heating pad for preventing hypothermia. Pregnant rats were randomly assigned to saline control or sFlt-1 group; the saline group received 50 μl of normal saline per amniotic sac, and the sFlt-1 group received 1 μg of recombinant human sFlt-1-Fc (R&D Systems, Minneapolis, Minnesota) diluted to 50 μl with normal saline per sac. We established the dose of intra-amniotic sFlt-1 from preliminary studies in which sFlt-1 at lower doses (0.125–0.25 μg/sac) had a small but significant decrease in radial alveolar counts (RAC, 23% decrease; P < 0.05) in infant rats. However, right ventricle-to-body weight ratios are not increased at a dose <0.5 μg/sac (data not shown). As a result, the dose of intra-amniotic sFlt-1 at 1 μg/sac was chosen for this study. Under sterile preparation, a midline abdominal incision of 3–4 cm in length was made to expose the amniotic sacs for intra-amniotic injections. The amniotic sac closest to the right ovary was first identified and injected, and then in a counterclockwise sequence each sac was identified and injected with a maximum of 10 sacs injected per dam. Limiting sFlt-1 injections to 10 sacs per pregnant rat was to achieve a consistent total dose of sFlt-1 on the individual mother rats, given intra-amniotic sFlt-1 is absorbed into the maternal circulation through an intramembranous pathway, which is characterized by a microscopic network of fetal vasculature on the fetal surface of the placenta to mediate the transfer of intra-amniotic substances into fetal and maternal circulations (23–26). Similarly, considering the communicaiton between the amniotic cavity and maternal and fetal circualtions through the intramembranous pathway, intra-amniotic saline was given in separate litters to serve as controls. The total number of amniotic sacs in each mother rat was examined and recorded during laparotomy. The abdominal incision was closed with nylon sutures. Bupivacaine (1–2 mg/kg, subcutaneous injection) was applied over the incision wound for postoperative pain control. Pregnant rats were monitored closely to ensure arousal within 10 min after surgery, and rats were placed back to the cages and were monitored for activity, ability to drink and eat, and for signs of bleeding or infection.
Cesarean section.
Two days after intra-amniotic injections, cesarean section was performed on pregnant rats under general anesthesia with isoflurane inhalation, as described above. The fetus in the amniotic sac closest to the right ovary was first delivered, which was followed by delivery of the rest of the fetuses in a counterclockwise sequence, to identify fetuses exposed to intra-amniotic injections. The total number of amniotic sacs in each mother rat was further verified at the time of delivery. The main reason for performing cesarean section instead of allowing vaginal delivery is to identify the fetuses exposed to intra-amniotic injections, based on the order of the amniotic sacs and their anatomic locations related to the ovaries. All of the rat pups in the injected amniotic sacs were delivered within 5 min after the onset of anesthesia. Maternal rats were then killed with pentobarbital sodium. Newborn rats were immediately placed on a heating pad to avoid hypothermia and were dried manually with gauze sponges. Pups received no supplemental oxygen or artificial ventilation at birth. The survival rate at birth was recorded. Within 30 min after birth, the pups were weighed and placed with foster mother rats in regular cages. For the first 24 h of life, the newborn pups were monitored closely for mortality or signs of respiratory distress.
Rat lungs were harvested at birth for Western blot analysis and at birth and 14 days of age for histological assessment. Hearts were dissected and weighed at birth and 7 and 14 days of age. Three to nine rats were studied in each group for each measurement at each time point. Survival of the infant rats was monitored and recorded daily from birth throughout the study period. Survival rate was calculated as the number of survived pups divided by the number of sacs that received intra-amniotic injection in each given litter. Body weight was measured at birth and at the time of being killed for study measurements.
Study Measurements
Tissue for histological analysis.
Animals were killed with intraperitoneal pentobarbital sodium. A catheter was placed in the trachea, and the lungs were inflated with 4% paraformaldehyde and maintained at 20 cmH2O pressure for 60 min. A ligature was tightened around the trachea to maintain pressure, and then the tracheal cannula was removed. Lungs were then immersed in 4% paraformaldehyde at room temperature for 24 h for fixation. A 2-mm-thick transverse section was taken from the midplane of the right lower lobe and left lobe of the fixed lungs per animal, respectively, to process and embed in paraffin wax.
Immunohistochemistry.
Slides with 5-μm paraffin sections were stained with hematoxylin and eosin for assessing alveolar structures and with von Willebrand Factor (vWF), an endothelial cell-specific marker, for quantifying vessel density.
Morphometric analysis.
RAC, mean linear intercept (MLI), and other indexes of alveolar structure and pulmonary vessel density were determined by standard morphometric techniques, as outlined below. In each animal, at least 5 measurements were obtained for RAC, at least 10 images were processed for computer-assisted image analysis of alveolar structure, and at least 10 high-power fields were examined for measuring pulmonary vessel density.
RADIAL ALVEOLAR COUNTS.
Alveolarization was assessed by the RAC method of Emery and Mithal as described (16, 17). Respiratory bronchioles were identified as bronchioles lined by epithelium in one part of the wall. From the center of the respiratory bronchiole, a perpendicular line was dropped to the edge of the acinus connective tissues or septum or pleura, and the number of septae intersected by this line was counted.
MLI AND OTHER INDEXES OF ALVEOLAR STRUCTURE.
Measurements of MLI, nodal point density, and interstitial thickness were performed with a computer-assisted image analysis program. Briefly, the images were captured on a Zeiss Axioscope2 microscope, using the ×10 objective and captured as a high-resolution TIFF image by a MicroPublisher digital camera (2,560 × 1,940 pixel resolution; Qimaging, Burnaby, Canada). The fields with large airways or vessels were avoided. These images were processed as previously described (4).
PULMONARY VESSEL DENSITY.
Pulmonary vessel density was determined by counting vWF-stained vessels with an external diameter at 50 μm or less per high-power field. The fields containing large airways or vessels with external diameter >50 μm were avoided.
Indexes of Right Ventricular Hypertrophy and Left Ventricular Hypertrophy
The right ventricle (RV) and left ventricle plus septum (LV+S) were dissected and weighed. The ratios of RV to LV+S weights (RV/LV+S%) and RV/body weights (RV/BW%) were determined to evaluate right ventricular hypertrophy (RVH). The ratio of LV+S to body weight (LV+S/BW%) was determined to evaluate left ventricular hypertrophy (LVH).
Western blot analysis.
Frozen lung samples were homogenized in ice-cold radioimmunoprecipitation buffer (PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 10 mg/ml phenylmethylsulfonyl fluoride) with protease inhibitor (Pierce, Rockford, IL) and phosphatase inhibitor cocktail (Calbiochem, Gibbstown, NJ). Samples were centrifuged at 1,500 g for 20 min at 4°C to remove cellular debris. Protein content in the supernatant was determined by the Bradford method (11), using BSA as the standard. Next, 25 μg [for total VEGF, endothelial nitric oxide synthase (eNOS), and active caspase-3] or 50 μg [for total and phosphorylated vascular endothelial growth factor receptor 2 (VEGFR2)] of protein sample per lane was resolved by SDS-PAGE, and proteins from the gel were transferred to polyvinylidene difluoride membrane. Blots were blocked for 1 h or overnight in 5% nonfat dry milk in PBS with 0.1% Tween 20. For total VEGF and eNOS, the blots were incubated for 1 h at room temperature with rabbit anti-human polyclonal VEGF antibody (sc-507, 1:500; Santa Cruz Biotechnology) and mouse anti-human polyclonal eNOS (BD610297; BD Biosciences), respectively, in 5% nonfat dry milk in PBS with 0.1% Tween 20. For active caspase-3, the blots were incubated overnight at 4°C with rabbit cleaved caspae-3 antibody (Asp175; Cell Signaling). For VEGFR2, the blots were incubated overnight at 4°C with rabbit phosphorylated VEGFR2 antibody (Tyr1175; no. 2478; Cell Signaling) in 5% BSA; after finishing the detection of phosphorylated VEGFR2, the blots were incubated with stripping buffer (no. 46430; Thermo Scientific) and then incubated for 1 h at room temperature with rabbit VEGFR2 antibody (no. 2479; Cell Signaling) in 5% nonfat dry milk in PBS with 0.1% Tween 20. For secondary antibody, blots were incubated for 1 h at room temperature with a goat anti-rabbit IgG horseradish peroxidase (HRP) antibody (sc-2054; Santa Cruz Biotechnology) or goat anti-mouse HRP-conjugated antibody (no. 172–1011; Bio-Rad). After being washed, bands were visualized by enhanced chemiluminescence (ECL Advance kit; Amersham Pharmacia Biotech, Buckinghamshire, UK). For Western analysis of VEGF, recombinant mouse VEGF protein (Santa Cruz Biotechnology) was used as a positive control. On each blot, β-actin was detected as a housekeeping protein to compare expression between samples; mouse monoclonal β-actin antibody (Sigma A-5316; Sigma-Aldrich) was used for detecting β-actin. Densitometry was performed using Quantity One (Bio-Rad). Three to five animals were analyzed per study group.
Immunofluorescent colocalization.
To identify lung cells undergoing apoptosis, immunofluorescence staining was performed on 5-μm cryosections cut from the optimum cutting temperature-embedded lung blocks. The sections were blocked with BSA in PBS for 1 h and washed with PBS and then were incubated with anti-active caspase-3 antibody (1:50; AB3623; Chemicon International) along with either anti-vWF antibody (1:50; sc-8068; Santa Cruz Biotechnology), anti-vimentin antibody (1:50; sc-6260; Santa Cruz Biotechnology), antibody to α-smooth muscle actin (α-SMA) (1:50; ab18147; Abcam), or antibody to surfactant protein-C (SP-C) precursor (1:100; sc-7706; Santa Cruz Biotechnology) at 4°C overnight. Next, the sections were incubated with secondary antibodies at 1:500 (donkey anti-rabbit Alexa Fluor 594; donkey anti-goat or donkey anti-mouse 488; Molecular Probes, Eugene, OR) for 2 h at room temperature and washed. The sections were then mounted with 4′,6-diamidino-2-phenylindole (Vector Laboratories) and imaged with an Olympus IX71 fluorescence microscope (Olympus America, Center Valley, PA).
Statistical Analysis
Statistical analysis was performed with the InStat 3.0 software package (GraphPad Software, San Diego, CA). Statistical comparisons were made between groups using t-test or ANOVA with Newman-Keuls post hoc analysis for significance. P < 0.05 was considered significant.
RESULTS
Survival Rate and Body Weight
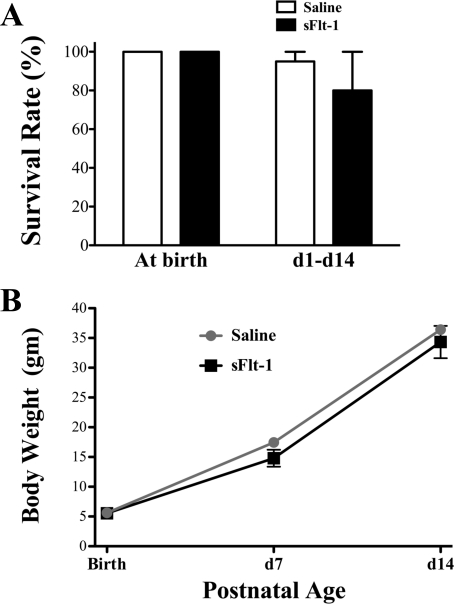
A total of three litters of fetal rats received intra-amniotic saline injections, and four litters received intra-amniotic sFlt-1 treatment. Both control and sFlt-1 groups had 100% survival at birth (Fig. 1A). In both groups, newborn rats appeared pink and vigorous without evidence of cyanosis or respiratory distress, as closely monitored for the first 24 h of life. In the two litters kept through day 14 in each group, the survival rate of sFlt-1 rats decreased to 80% at day 1, statistically not different from the 95% of survival rate in saline control. No mortality was found at and beyond day 2 in either group. There was no difference in body weights between sFlt-1 and saline rats at birth, and days 7 and 14 (Fig. 1B). The rate of body weight gain was similar between the two groups from birth through day 14.
Fig. 1.
Effects of intra-amniotic soluble fms-like tyrosine kinase 1 (sFlt-1) treatment on survival rate and body weight (BW) of infant rats, from birth through 14 days of age. A: both saline and sFlt-1 groups had 100% survival at birth; from day 1 to day 14, 80% of rats survived in the sFlt-1 group and 95% survived in the control (p > 0.05). B: there was no difference in body weight between sFlt-1 rats and saline controls at birth, and days 7 and 14.
Lung Histology and Morphometric Analyses
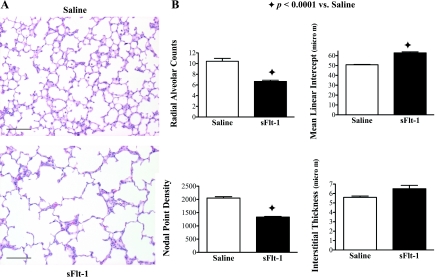
Figure 2 shows the effects of intra-amniotic sFlt-1 treatment on alveolarization in 14-day-old rats. The distal airspace appeared simplified with decreased septation and alveoli in sFlt-1 rats as compared with saline control (Fig. 2A). By morphometric analysis, sFlt-1 rats had decreased RAC (P < 0.0001), increased MLI (P < 0.0001), and decreased nodal point density (P < 0.0001) compared with saline control; interstitial thickness was not different between the two groups (Fig. 2B).
Fig. 2.
Effects of intra-amniotic sFlt-1 treatment on distal lung growth in 14-day-old rats; lung histology was stained with hematoxylin and eosin. Micrographs are representative and were obtained at the same magnification. Internal scale bar = 100 μm. sFlt-1 rats demonstrated simplified alveoli (A), decreased radial alveolar counts, increased mean linear intercept, and reduced nodal point density (B), compared with the control; n = 5–9 animals/group.
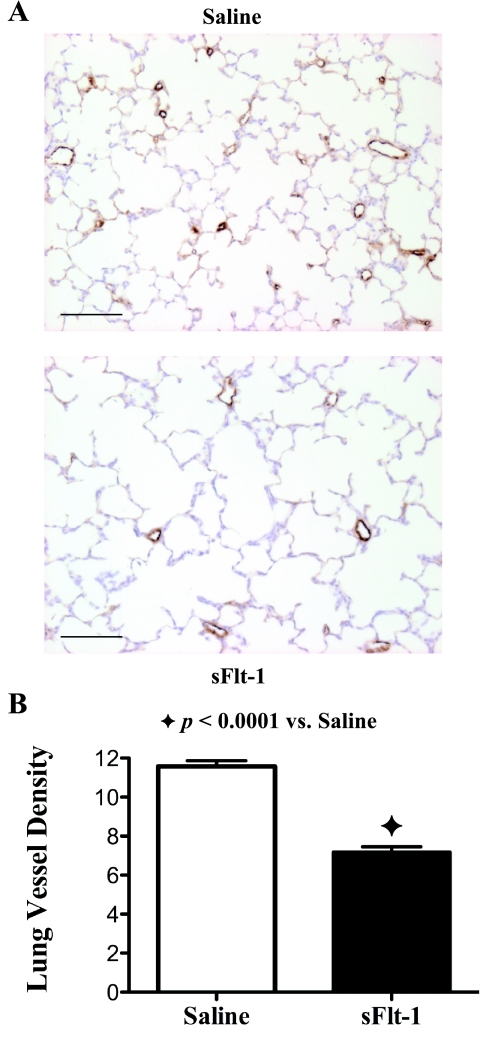
The effects of intra-amniotic sFlt-1 treatment on pulmonary vascular growth in 14-day-old rats are shown in Fig. 3. Pulmonary vessel density was decreased by 38% in sFlt-1 rats compared with saline control (P < 0.0001). There was no difference found between the male and female in the sFlt-1 group regarding lung histology and morphometric analyses.
Fig. 3.
Effects of intra-amniotic sFlt-1 treatment on pulmonary vessel density in lung histology stained with von Willebrand Factor (vWF) from 14-day-old rats. Micrographs are representative and were obtained at the same magnification. Internal scale bar = 100 μm. Pulmonary vessel density was reduced in sFlt-1 rats as shown by histology (A) and documented by morphometric analysis (P < 0.0001; B); n = 5–9 animals/group.
RVH and LVH Indexes
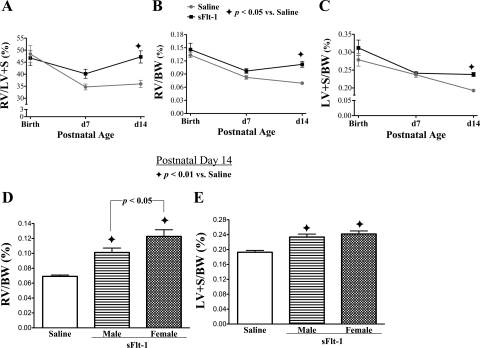
The effects of intra-amniotic sFlt-1 treatment on heart weights in infant rats are shown in Fig. 4. The RV/LV+S and RV/BW ratios (Fig. 4, A and B), both of which are proportional to the degree of RVH, were comparable between sFlt-1 rats and saline control at birth and at day 7, respectively. Both parameters in sFlt-1 rats increased above control values at day 14 (P < 0.05). Similarly, the LV+S/BW ratio (Fig. 4C), an index of LVH, was similar between sFlt-1 and saline groups at birth and at day 7, respectively, but then increased in sFlt-1 rats at day 14 to 19% above control values (p < 0.05). From birth through day 7, the RVH and LVH indexes decreased in both groups (Fig. 4, A–C). From day 7 to day 14, the RV/BW ratio tended to further decrease in the control, whereas it trended up in sFlt-1 rats (Fig. 4B). During this period, the LV+S/BW ratio nearly further decreased in the control, but it remained unchanged in sFlt-1 rats (Fig. 4C). The time course suggests neonatal onsets of progressive RVH and LVH in sFlt-1 rats. At day 14, the effect of intra-amniotic sFlt-1 treatment on increasing RV/BW% was more striking in female infant rats than male (Fig. 4D). There was no apparent gender-related difference on LV+S/BW% in sFlt-1 rats (Fig. 4E).
Fig. 4.
Effects of intra-amniotic sFlt-1 treatment on heart weights in infant rats. The ratios of the right ventricle (RV)/left ventricle + septum (LV + S) (A), RV/body weight (RV/BW) (B), and LV/BW (C) are shown at birth, and days 7 and 14. These indexes from the sFlt-1 group were at control values at birth and day 7 but then rose above control levels by day 14. RV/BW% in the sFlt-1 group was higher in female infant rats than males at day 14 (D), but there was no gender-related difference in LV+S/BW% at day 14 in sFlt-1 rats (E); n = 4–8 animals/group.
Effects of Intra-Amniotic sFlt-1 on VEGF Signaling in the Newborn Rat Lung
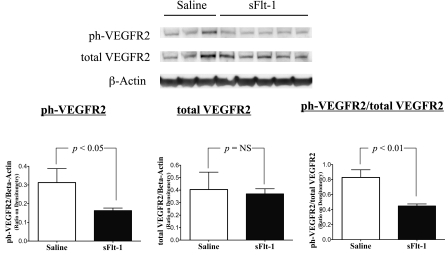
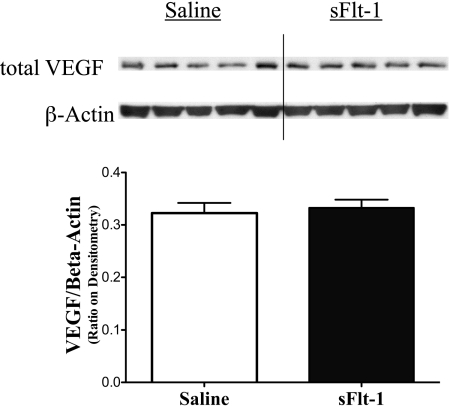
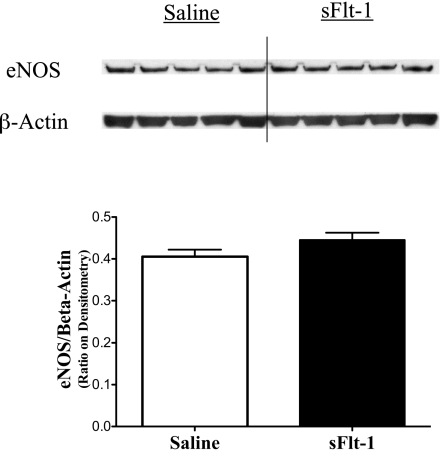
To assess the effects of intra-amniotic sFlt-1treatment on lung VEGF signaling at birth, phosphorylated VEGFR2, total VEGFR2, total VEGF, and eNOS proteins were measured by Western blot analyses on newborn rat lung homogenates. Lung phosphorylated VEGFR2 protein was decreased in sFlt-1 rats compared with saline control (P < 0.05; Fig. 5A), whereas lung total VEGFR2 protein was comparable between the two groups (Fig. 5B). The ratio of phosphorylated VEGFR2 to total VEGFR2 proteins in the sFlt-1 group was 46% below control values in the newborn rat lungs (P < 0.01; Fig. 5C). Total lung VEGF (Fig. 6) and eNOS protein contents (Fig. 7) did not differ between sFlt-1 and control groups at birth.
Fig. 5.
Effects of intra-amniotic sFlt-1 treatment on phosphorylated (ph) vascular endothelial growth factor receptor-2 (VEGFR2) and total VEGFR2 protein contents in the newborn rat lung. Compared with saline controls, sFlt-1 rats showed decreased ph-VEGFR2 (A), unchanged total VEGFR2 (B), and a decreased ratio of ph-VEGFR2 to total VEGFR2 proteins (C) in the lung at birth; n = 3–5 animals/group. NS, not significant.
Fig. 6.
Effects of intra-amniotic sFlt-1 treatment on protein contents of total VEGF in the newborn rat lung. Lung vascular endothelial growth factor (VEGF) protein contents were similar between sFlt-1 and control groups at birth; n = 5 animals/group.
Fig. 7.
Effects of intra-amniotic sFlt-1 treatment on protein contents of endothelial nitric oxide synthase (eNOS) in the newborn rat lung. Lung eNOS protein contents were similar between sFlt-1 and control groups at birth; n = 5 animals/group.
Effects of Intra-Amniotic sFlt-1 on Apoptosis in the Newborn Rat Lung
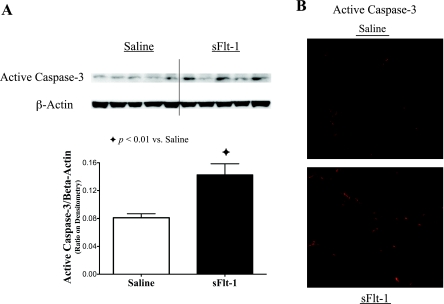
To evaluate to the effects of intra-amniotic sFlt-1 treatment on activity of apoptosis in newborn rat lungs, active caspase-3 protein was analyzed by Western blot on lung homogenates, and showed elevation by 76% in sFlt-1 rats (P < 0.01; Fig. 8A). Consistent with Western blot analysis, active caspase-3 signals remarkably increased in sFlt-1 rats as compared with saline control, in immunofluorescence staining on newborn rat lung sections (Fig. 8B).
Fig. 8.
Effects of intra-amniotic sFlt-1 treatment on active caspase-3 in the newborn rat lung. sFlt-1 rats showed increased protein contents of active caspase-3 in lung homogenates by Western blot analysis (P < 0.01; A) and increased active caspase-3 by immunofluorescence staining on lung sections in comparison with the controls (B); n = 5 animals/group in Western blot analysis. Micrographs of immunofluorescence staining are representative and were obtained at the same magnification, ×200.
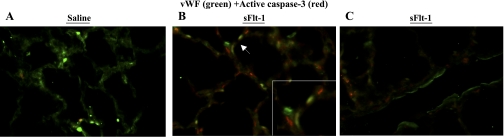
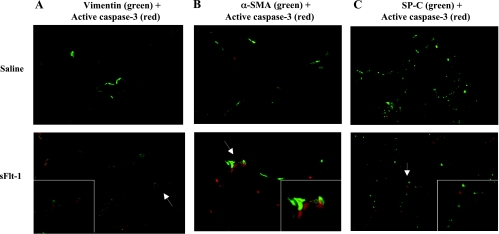
To identify which cells express active caspase-3 in the newborn lung, immunofluorescent staining was performed with active caspase-3 and one of the markers of variable lung cell types on newborn rat lung sections. Compared with scarce signals of active caspase-3 on vWF-positive cells in saline control (Fig. 9A), sFlt-1 rats demonstrated colocalization of vWF and active caspase-3 in microvessels (Fig. 9B) but not in larger pulmonary vessels (Fig. 9C). To further identify nonendothelial cells stained with active caspase-3 in the distal airspace of sFlt-1 rats (Fig. 9B), immunofluorescence staining was performed for active caspase-3 with vimentin, α-SMA, and SP-C precursor, respectively. Vimentin is expressed in mesenchymal and endothelial cells, α-SMA is a marker of myofibroblasts in distal airspace, and SP-C precursor is produced exclusively by type-2 alveolar epithelial cells. Many cells that were positive for active caspase-3 coexpressed vimentin in the interstitium of distal airspace (Fig. 10A), indicating the localization of active caspase-3 in mesenchymal cells in addition to endothelial cells. There was no colocalization of active caspase-3 and α-SMA (Fig. 10B), indicating that those active caspas-3 (+) mesenchymal cells are not myofibroblasts. Active caspase-3 staining was not found in lung cells positive for SP-C (Fig. 10C).
Fig. 9.
Immunofluorescence staining for vWF (green) and active caspase-3 (red) on lung sections from newborn rats. Micrographs are representative and were obtained at the same magnification, ×200. Compared with scarce signals of active caspase-3 (red) in the control (A), sFlt-1 rats demonstrated colocalization of vWF (green) and active caspase-3 (red) in microvessels (B, arrow; higher magnification in the inset) but not in larger pulmonary vessels (C). Some of those active casapse-3-postive cells were not stained with vWF (B).
Fig. 10.
Immunofluorescence staining on lung sections from newborn rats. Micrographs are representative and were obtained at the same magnification, ×200. In sFlt-1 rats, active caspase-3 (red) was localized in cells stained with vimentin (green; A) but not localized in cells stained with α-smooth muscle actin (α-SMA) (green; B) nor surfactant protein-C (SP-C) (green; C). The areas demonstrating positive (vimentin) and negative (α-SMA; SP-C) colocalization with active caspase-3 are indicated with arrows and shown in higher magnification in the insets. Control rats showed few cells positive for active caspase-3 in each set of immunofluorescence staining.
DISCUSSION
We found that excess sFlt-1 in amniotic fluid during the late canalicular and early saccular stage of lung development reduces VEGF signaling and increases apoptosis in the newborn rat lung, which is followed by sustained reductions in alveolarization and pulmonary vascular growth during infancy. In addition, infant rats that are exposed to excess intra-amniotic sFlt-1 develop RVH and LVH over time. These findings suggest that the increased risk for BPD and vascular dysfunction in infants with maternal PE may result from fetal exposure to elevated levels of intra-amniotic sFlt-1.
For more than a decade, epidemiological studies have shown an increased risk of BPD in preterm infants born to preeclamptic mothers (28, 31, 40, 79), but the underlying mechanism was unknown. The present study shows that excess intra-amniotic sFlt-1 is sufficient to cause sustained abnormalities of lung structure during infancy. This effect is likely mediated through impairing lung VEGF signaling in the fetus, leading to increased endothelial and mesenchymal apoptosis in the distal airspace of the newborn. These findings further demonstrate the critical role of VEGF signaling in directing vascular and alveolar growth in the developing lung. Moreover, the present study reveals that intact VEGF signaling is required to maintain both endothelial and mesenchymal cell survival during lung development. Our finding confirms and extends past studies that endothelial survival relies on VEGF signaling through phosphorylation of VEGFR2 (20) and that inhibition of VEGFR2 injures pulmonary endothelium in the fetus (80) and neonates (68). A recent study shows that VEGF mediates endothelial-mesenchymal cross-talk to drive morphogenesis of testis (15). Future work is needed to determine whether VEGF signaling directly regulates mesenchymal cells or through endothelial cells in the developing lung.
This is the first study demonstrating that primary inhibition of VEGF in the fetus results in sustained abnormalities of lung structure during infancy. Previous studies have shown that intra-amniotic endotoxin or antenatal dexamethasone treatment downregulates lung VEGF or VEGFR-2 expression at birth and impairs alveolarization through late infancy in rats (57, 69). The present study highlights that antenatal impairment of lung VEGF signaling may link the sustained disruption of infant lung growth with preceding intrauterine stimuli, such as chorioamnionitis, antenatal dexamethasone, and PE, in animal models. Past studies have shown that neonatal VEGF inhibition reduces angiogenesis as well as alveolar growth in the developing lung, but the mechanisms are poorly understood (22, 42, 50, 67, 81). With impaired VEGF signaling in utero, as shown in the current study, mesenchymal cell apoptosis could further compromise vascular growth in the developing lung, given mesenchymal cells are capable of differentiating into perivascular cells to stabilize newly forming endothelial channels (2, 45). Future study is needed to better characterize the α-SMA-negative mesenchymal lung cells that undergo apoptosis under suppression of VEGFR2 signaling, in order to delineate whether mesenchymal injury due to VEGF inhibition is directly involved in the impaired formation of secondary septae in the developing lung.
This rat model of PE demonstrates that excess sFlt-1 in amniotic fluid impairs lung VEGF signaling in the fetus. Following intra-amniotic sFlt-1 treatment, the reduction in phosphorylated VEGFR2 in the presence of intact total VEGFR2 indicates a shortage of lung VEGF to activate VEGFR2 in the newborn lung. As in clinical PE, excess intra-amniotic sFlt-1 may directly reach the fetal lung via fetal breathing, or through an intramembranous pathway and fetal swallowing to enter the fetal circulation and then arrive in the fetal lung. Bidirectional flow of amniotic fluid across human fetal trachea has been proven by Doppler velocimetry during normal fetal breathing movements (36). Moreover, fetal pulmonary aspiration of intra-amniotic sFlt-1 is supported by the finding that muscle paralysis inhibits lung deposition of intra-amniotic iron dextran in fetal rabbits, whereas iron accumulates in control fetal lungs (21). In addition, substances in the amniotic cavity can be transferred into the fetal circulation through an intramembranous pathway (23–26), which is characterized by a microscopic network of fetal vasculature on the fetal surface of the placenta (23, 24).
This study also reports the late development of RVH in infant rats treated with intra-amniotic sFlt-1, suggesting the progressive onset of pulmonary hypertension after exposure to the fetal environment of PE. Our findings are interesting not only because pulmonary hypertension can complicate BPD (39, 65) but also because offspring of mothers with PE are at risk for pulmonary vascular dysfunction later in life (33). Similarly, we found LVH in infant rats exposed to excess sFlt-1 in utero. Interestingly, a high incidence of systemic hypertension has been recognized in infants with BPD, but its etiology is unclear (1). Moreover, past studies have identified an increased risk of systemic vascular dysfunction in offspring of mothers with PE, but the mechanism remains unknown (33, 35, 58, 70, 72). As shown in a previous study, neonatal treatment with truncated sFlt-1 impairs the growth of renal glomerular capillaries in mice (22), suggesting that renal vascular injury may contribute to systemic hypertension or LVH following disruption of VEGF signaling during development. Future studies would determine whether systemic vascular abnormalities have existed early in those with maternal PE, contributing to hypertension in infants with BPD and predisposing to cardiovascular diseases later in life.
This study provides mechanistic insights underlying the risk of preterm infants for BPD in the setting of maternal PE. In one study on preterm infants with very low birth weight, maternal PE is associated with increased BPD at 36 wk corrected age although it did not increase oxygen requirement at 28 days postnatal age (40). These findings suggest that progressive worsening of lung disease can occur later in the neonatal course of preterm infants born to preeclamptic mothers, which is a pattern observed in clinical BPD (6, 13, 34). Moreover, the present study demonstrates that the fetal environment of PE is sufficient to cause sustained and late pulmonary structural abnormalities characteristic of human BPD, despite the absence of adverse postnatal stimuli, such as hyperoxia, mechanical ventilation, infection, or related problems. Collectively, these findings suggest the need for new strategies to prevent BPD, of which the incidence seems to have reached a plateau in the very premature infants (59, 66). Future research is required to investigate whether early intervention, before those high-risk infants become symptomatic for clinical deterioration, may rescue the preterm lung that has been injured before birth.
A potential limitation of this rat model is the lack of direct placental production of sFlt-1 to more comprehensively reproduce the fetal environment of PE. We speculate that it explains why the sFlt-1 rats did not have signs of intrauterine growth restriction (IUGR), which is a common complication of PE. On the other hand, the current study demonstrates impaired neonatal lung growth in the absence of reduced birth weight or decreased postnatal somatic growth, indicating that impaired lung growth following intra-amniotic sFlt-1 treatment is not due to IUGR or neonatal malnutrition in this model. Moreover, the present study highlights that excess intra-amniotic sFlt-1 per se is sufficient to impair lung VEGF signaling at birth. Considering the pharmacokinetics of sFlt-1 as administered via single intra-amniotic injections in this study, the concentrations of intra-amniotic sFlt-1 immediately after injections cannot be directly compared with the amniotic levels of sFlt-1 measured in patients diagnosed with PE. In brief, sFlt-1 injected into the amniotic cavity is absorbed through an intramembranous pathway into the fetal circulation and maternal circulation, whereby excess sFlt-1 is metabolized and excreted in the maternal compartment, gradually decreasing the concentrations of intra-amniotic sFlt-1 after single injections in this rat model. In patients diagnosed with PE, the levels of sFlt-1 in amniotic fluid are rather relatively steady, since sFlt-1 is constantly overproduced in the placenta.
We conclude that intra-amniotic sFlt-1 treatment during late gestation impairs VEGF signaling and increases apoptosis in the newborn rat lung, which is followed by persistent reductions in alveolarization and pulmonary vascular growth and the subsequent development of biventricular hypertrophy during infancy. We speculate that disruption of VEGF signaling in utero due to antenatal exposure of excess sFlt-1 contributes to an increased risk for BPD and the predisposition for pulmonary and systemic vascular dysfunction in infants with maternal PE.
GRANTS
This work was partly supported by grants from the National Heart, Lung, and Blood Institute, including T32 HL-07670 (for J. R. Tang) and RO1 HL-68702 (S. H. Abman).
DISCLOSURES
Dr. Karumanchi is a co-inventor on multiple patents held by the Beth Israel Deaconess Medical Center that are related to angiogenic factors for the use in diagnosis and therapy of preeclampsia. Dr. Karumanchi has financial interest in Aggamin LLC.
REFERENCES
- 1. Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed 87: F15–F18, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akeson AL, Wetzel B, Thompson FY, Brooks SK, Paradis H, Gendron RL, Greenberg JM. Embryonic vasculogenesis by endothelial precursor cells derived from lung mesenchyme. Dev Dyn 217: 11–23, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol 30: 227–232, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Bancalari E, Claure N, Gonzalez A. Patent ductus arteriosus and respiratory outcome in premature infants. Biol Neonate 88: 192–201, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bancalari E, Claure N, Sosenko IR. Bronchopulmonary dysplasia: changes in pathogenesis, epidemiology and definition. Semin Neonatol 8: 63–71, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Basso O, Rasmussen S, Weinberg CR, Wilcox AJ, Irgens LM, Skjaerven R. Trends in fetal and infant survival following preeclampsia. JAMA 296: 1357–1362, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Been JV, Rours IG, Kornelisse RF, Jonkers F, de Krijger RR, Zimmermann LJ. Chorioamnionitis alters the response to surfactant in preterm infants. J Pediatr 156: 10–15, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol 293: L1099–L1110, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Bose C, Van Marter LJ, Laughon M, O'Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics 124: e450–e458, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976 [DOI] [PubMed] [Google Scholar]
- 12. Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Charafeddine L, D'Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics 103: 759–765, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 8: 73–81, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proc Natl Acad Sci USA 108: 167–172, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1–postnatal lung growth. Thorax 37: 572–579, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2–intrauterine and early postnatal lung growth. Thorax 37: 580–583, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delemos RA, Coalson JJ, Gerstmann DR, Kuehl TJ, Null DM., Jr Oxygen toxicity in the premature baboon with hyaline membrane disease. Am Rev Respir Dis 136: 677–682, 1987 [DOI] [PubMed] [Google Scholar]
- 19. Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196: 147 e141–e148, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem 274: 16349–16354, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galan HL, Tennant LB, Marsh DR, Creasy RK. Paralysis of the preterm rabbit fetus inhibits the pulmonary uptake of intraamniotic iron dextran. Am J Obstet Gynecol 177: 42–49, 1997 [DOI] [PubMed] [Google Scholar]
- 22. Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 126: 1149–1159, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Gilbert WM, Brace RA. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 74: 748–754, 1989 [PubMed] [Google Scholar]
- 24. Gilbert WM, Eby-Wilkens E, Tarantal AF. The missing link in rhesus monkey amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 89: 462–465, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Gilbert WM, Newman PS, Brace RA. Potential route for fetal therapy: intramembranous absorption of intraamniotically injected furosemide. Am J Obstet Gynecol 172: 1471–1476, 1995 [DOI] [PubMed] [Google Scholar]
- 26. Gilbert WM, Newman PS, Eby-Wilkens E, Brace RA. Technetium Tc 99m rapidly crosses the ovine placenta and intramembranous pathway. Am J Obstet Gynecol 175: 1557–1562, 1996 [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez A, Sosenko IR, Chandar J, Hummler H, Claure N, Bancalari E. Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 128: 470–478, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Gortner L, Misselwitz B, Milligan D, Zeitlin J, Kollee L, Boerch K, Agostino R, Van Reempts P, Chabernaud JL, Breart G, Papiernik E, Jarreau PH, Carrapato M, Gadzinowski J, Draper E. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology 99: 112–117, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Gras-LeGuen C, Denis C, Franco-Montoya ML, Jarry A, Delacourt C, Potel G, Bourbon J, Roze JC, Jarreau PH. Antenatal infection in the rabbit impairs post-natal growth and lung alveolarisation. Eur Respir J 32: 1520–1528, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Halvorsen T, Skadberg BT, Eide GE, Roksund OD, Carlsen KH, Bakke P. Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study. Acta Paediatr 93: 1294–1300, 2004 [PubMed] [Google Scholar]
- 31. Hansen AR, Barnes CM, Folkman J, McElrath TF. Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr 156: 532–536, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Jakkula M, LeCras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607, 2000 [DOI] [PubMed] [Google Scholar]
- 33. Jayet PY, Rimoldi SF, Stuber T, Salmon CS, Hutter D, Rexhaj E, Thalmann S, Schwab M, Turini P, Sartori-Cucchia C, Nicod P, Villena M, Allemann Y, Scherrer U, Sartori C. Pulmonary and systemic vascular dysfunction in young offspring of mothers with preeclampsia. Circulation 122: 488–494, 2010 [DOI] [PubMed] [Google Scholar]
- 34. Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 46: 641–643, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke 40: 1176–1180, 2009 [DOI] [PubMed] [Google Scholar]
- 36. Kalache KD, Chaoui R, Bollmann R. Doppler assessment of tracheal and nasal fluid flow during fetal breathing movements: preliminary observations. Ultrasound Obstet Gynecol 9: 257–261, 1997 [DOI] [PubMed] [Google Scholar]
- 37. Karumanchi SA, Bdolah Y. Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 145: 4835–4837, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun 226: 324–328, 1996 [DOI] [PubMed] [Google Scholar]
- 39. Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, Mullen MP. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 120: 1260–1269, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Korhonen P, Tammela O, Koivisto AM, Laippala P, Ikonen S. Frequency and risk factors in bronchopulmonary dysplasia in a cohort of very low birth weight infants. Early Hum Dev 54: 245–258, 1999 [DOI] [PubMed] [Google Scholar]
- 41. Lal MK, Manktelow BN, Draper ES, Field DJ. Chronic lung disease of prematurity and intrauterine growth retardation: a population-based study. Pediatrics 111: 483–487, 2003 [DOI] [PubMed] [Google Scholar]
- 42. LeCras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 44. Liljedahl M, Bodin L, Schollin J. Coagulase-negative staphylococcal sepsis as a predictor of bronchopulmonary dysplasia. Acta Paediatr 93: 211–215, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277: 242–245, 1997 [DOI] [PubMed] [Google Scholar]
- 46. Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest 111: 600–602, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res 55: 287–295, 2004 [DOI] [PubMed] [Google Scholar]
- 48. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McElrath TF, Hecht JL, Dammann O, Boggess K, Onderdonk A, Markenson G, Harper M, Delpapa E, Allred EN, Leviton A. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 168: 980–989, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol 32: 420–427, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Meissner HC. Selected populations at increased risk from respiratory syncytial virus infection. Pediatr Infect Dis J 22: S40–S45, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol 298: L23–L35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease Bronchopulmonary dysplasia. N Engl J Med 276: 357–368, 1967 [DOI] [PubMed] [Google Scholar]
- 54. Payne MS, Goss KC, Connett GJ, Kollamparambil T, Legg JP, Thwaites R, Ashton M, Puddy V, Peacock JL, Bruce KD. Molecular microbiological characterization of preterm neonates at risk of bronchopulmonary dysplasia. Pediatr Res 67: 412–418, 2010 [DOI] [PubMed] [Google Scholar]
- 55. Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet 269: 40–44, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Risau W. Mechanisms of angiogenesis. Nature 386: 671–674, 1997 [DOI] [PubMed] [Google Scholar]
- 57. SanFeliciano L, Remesal A, Isidoro-Garcia M, Ludena D. Dexamethasone and betamethasone for prenatal lung maturation: differences in vascular endothelial growth factor expression and alveolarization in rats. Neonatology 100: 105–110, 2011 [DOI] [PubMed] [Google Scholar]
- 58. Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Pre-eclampsia and offspring's blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol 98: 1009–1014, 1991 [DOI] [PubMed] [Google Scholar]
- 59. Shah PS, Sankaran K, Aziz K, Allen AC, Seshia M, Ohlsson A, Lee SK, Lee SK, Shah PS, Andrews W, Barrington K, Yee W, Bullied B, Canning R, Cronin G, Dow K, Dunn M, Harrison A, James A, Kalapesi Z, Kovacs L, da Silva O, McMillan DD, Shah P, Ojah C, Peliowski A, Aziz K, Piedboeuf B, Riley P, Faucher D, Rouvinez-Bouali N, Sankaran K, Seshia M, Shivananda S, Cieslak Z, Synnes A, Walti H. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinato. doi: 10.1038/jp.2011.68. In press. [DOI] [PubMed] [Google Scholar]
- 60. Shibuya M. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct Funct 26: 25–35, 2001 [DOI] [PubMed] [Google Scholar]
- 61. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 365: 785–799, 2005 [DOI] [PubMed] [Google Scholar]
- 62. Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol 102: 181–192, 2003 [DOI] [PubMed] [Google Scholar]
- 63. Smith VC, Zupancic JA, McCormick MC, Croen LA, Greene J, Escobar GJ, Richardson DK. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr 144: 799–803, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol 122: 33–39, 2005 [DOI] [PubMed] [Google Scholar]
- 65. Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 67: 623–661, 2005 [DOI] [PubMed] [Google Scholar]
- 66. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O'Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID, 3rd, Watterberg KL, Saha S, Das A, Higgins RD. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L344–L351, 2004 [DOI] [PubMed] [Google Scholar]
- 68. Tang JR, Seedorf G, Balasubramaniam V, Maxey A, Markham N, Abman SH. Early inhaled nitric oxide treatment decreases apoptosis of endothelial cells in neonatal rat lungs after vascular endothelial growth factor inhibition. Am J Physiol Lung Cell Mol Physiol 293: L1271–L1280, 2007 [DOI] [PubMed] [Google Scholar]
- 69. Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, Balasubramaniam V, Abman SH. Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 299: L735–L748, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tenhola S, Rahiala E, Halonen P, Vanninen E, Voutilainen R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res 59: 320–324, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Ueda K, Cho K, Matsuda T, Okajima S, Uchida M, Kobayashi Y, Minakami H, Kobayashi K. A rat model for arrest of alveolarization induced by antenatal endotoxin administration. Pediatr Res 59: 396–400, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol 101: 529–533, 2003 [DOI] [PubMed] [Google Scholar]
- 73. Veas CJ, Aguilera VC, Munoz IJ, Gallardo VI, Miguel PL, Gonzalez MA, Lamperti LI, Escudero CA, Aguayo CR. Fetal endothelium dysfunction is associated with circulating maternal levels of sE-selectin, sVCAM1, and sFlt-1 during pre-eclampsia. J Matern Fetal Neonatal Med In press [DOI] [PubMed] [Google Scholar]
- 74. Vrijlandt EJ, Boezen HM, Gerritsen J, Stremmelaar EF, Duiverman EJ. Respiratory health in prematurely born preschool children with and without bronchopulmonary dysplasia. J Pediatr 150: 256–261, 2007 [DOI] [PubMed] [Google Scholar]
- 75. Vrijlandt EJ, Gerritsen J, Boezen HM, Duiverman EJ. Gender differences in respiratory symptoms in 19-year-old adults born preterm (Abstract). Respir Res 6: 117, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vuorela P, Helske S, Hornig C, Alitalo K, Weich H, Halmesmaki E. Amniotic fluid–soluble vascular endothelial growth factor receptor-1 in preeclampsia. Obstet Gynecol 95: 353–357, 2000 [DOI] [PubMed] [Google Scholar]
- 77. Wang CN, Chang SD, Peng HH, Lee YS, Chang YL, Cheng PJ, Chao AS, Wang TH, Wang HS. Change in amniotic fluid levels of multiple anti-angiogenic proteins before development of preeclampsia and intrauterine growth restriction. J Clin Endocrinol Metab 95: 1431–1441, 2010 [DOI] [PubMed] [Google Scholar]
- 78. Weisman LE. Populations at risk for developing respiratory syncytial virus and risk factors for respiratory syncytial virus severity: infants with predisposing conditions. Pediatr Infect Dis J 22: S33–S39, 2003 [DOI] [PubMed] [Google Scholar]
- 79. Withagen MI, Visser W, Wallenburg HC. Neonatal outcome of temporizing treatment in early-onset preeclampsia. Eur J Obstet Gynecol Reprod Biol 94: 211–215, 2001 [DOI] [PubMed] [Google Scholar]
- 80. Yamamoto Y, Shiraishi I, Dai P, Hamaoka K, Takamatsu T. Regulation of embryonic lung vascular development by vascular endothelial growth factor receptors, Flk-1 and Flt-1. Anat Rec (Hoboken) 290: 958–973, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Zhao L, Wang K, Ferrara N, Vu TH. Vascular endothelial growth factor co-ordinates proper development of lung epithelium and vasculature. Mech Dev 122: 877–886, 2005 [DOI] [PubMed] [Google Scholar]