Abstract
Acute lung injury is a principal cause of morbidity and mortality in response to mustard gas (SM) inhalation. Obstructive, fibrin-containing airway casts have recently been reported in a rat inhalation model employing the SM analog 2-chloroethyl ethyl sulfide (CEES). The present study was designed to identify the mechanism(s) causing activation of the coagulation cascade after CEES-induced airway injury. Here we report that CEES inhalation elevates tissue factor (TF) activity and numbers of detached epithelial cells present in lavage fluid (BALF) from rats after exposure (18 h). In vitro studies using 16HBE cells, or with rat BALF, indicated that detached epithelial cells could convert factor X (FX) to the active form FXa when incubated with factor VII and could elicit rapid clotting of plasma. In addition, immunocytochemical analysis demonstrated elevated cell surface (TF) expression on CEES-exposed 16HBE cells as a function of time. However, total cell TF expression did not increase. Since membrane surfaces bearing TF are important determinants of clot initiation, anticoagulants directed against these entities were tested for ability to limit plasma clotting or FX activation capacity of BALF or culture media. Addition of tifacogin, a TF pathway inhibitor, effectively blocked either activity, demonstrating that the procoagulant actions of CEES were TF pathway dependent. Lactadherin, a protein capable of competing with clotting factors for phospholipid-binding sites, was partially effective in limiting these procoagulant actions. These findings indicate that TF pathway inhibition could be an effective strategy to prevent airway obstruction after SM or CEES inhalation.
Keywords: tissue factor pathway inhibitor, rat, lactadherin
sulfur mustard [bis(2-chloroethyl)sulfide; also known as SM] is a vesicant and chemical weapon that was used in several conflicts during the 20th century (37). It remains a threat because of the large stockpiles present in many countries and relative ease of manufacture. Exposure to SM vapor or droplets can affect the eyes, skin, and lung. The laryngeal and tracheobronchial airway epithelium are especially susceptible to injury. With high-level exposures, SM can injure more distal regions of the lung. Following inhalation, there is a latent period of 4–12 h before symptoms begin. Pain and cough with progressive rhinosinusitis and tracheobronchitis can be followed by hemorrhagic pulmonary edema and respiratory failure within 20–48 h, depending on the dose inhaled (30, 32, 34). Pathological features in these individuals and in mustard-exposed animals include mucosal hyperemia, airway epithelial sloughing, and edema, each of which can contribute to acute airway obstruction (18, 40, 41). Despite over 90 years of study, emergency protocols for acute SM exposure consist only of supportive treatment, with no effective antidote.
The mechanism behind the vesicant properties of SM involves an intermolecular cyclization step whereby a chlorine group is liberated from the molecule, resulting in the formation of a sulfonium derivative (42). In this state, SM becomes a strong electrophile, enabling reactions with numerous nucleophilic sites on and within cells, including membranes (43), DNA (9, 24)- and thiol-containing peptides and proteins (17). A similar conversion step also occurs at the second terminal chlorine of SM, enabling the formation of persistent lesions and cross-links. Chloroethyl ethyl sulfide (CEES) is a mustard analog differing from SM by the absence of the second chlorine at the terminal four-carbon position. Thus CEES causes only monofunctional alkylating reactions, whereas SM is bifunctional. Nonetheless, CEES is capable of DNA and protein alkylation (27) and retains some of the vesicant and inflammatory properties of SM (13). Lower toxicity along with commercial availability allow for CEES to be used as a model for SM in most laboratory settings.
Extravascular fibrin deposition can be a prominent feature of many forms of acute lung injury (22). Similarly, in a recently developed rat model of CEES inhalation, we found that occlusion of bronchial airways develops within <24 h after exposure (39). These obstructive lesions contain prominent fibrin, the level of which indicates that coagulation substrates must be exiting the vasculature and entering the airways to form the casts. Dye injection and microdissection studies have indicated that coagulation substrates enter the airways by leakage from the bronchial circulation.
The coagulation process serves to preserve circulating blood elements by minimizing extravasation upon injury. This is achieved by clot formation, a dynamic process regulated by pathways that initiate, amplify, and then modulate thrombus formation. Initiation of coagulation is primarily driven by tissue factor (TF). TF binds and activates factor VII (FVII) to factor VIIa, which then induces proteolytic activation of factor X (FX) to FXa. Assembly of FXa with cofactors on negatively charged phospholipids from cell membranes results in rapid conversion of prothrombin to thrombin, causing fibrin formation. In lung, nonvascular expression of TF leads to localized zones of fibrin deposition at sites of injury or infection (20, 21). In addition, cell surface expression of TF is constitutive in basal epithelial cells of tracheobronchial airways (15). This could help limit extravasation into airways when lung injury also encompasses blood vessels in the underlying submucosa. However, displacement or destruction of TF-bearing cells during injury also could extend these zones into the airway lumen.
The purpose of the present study was to define the mechanism causing activation of the coagulation cascade after CEES-induced airway epithelial injury. Working with either rat bronchoalveolar lavage (BAL) fluid (BALF) or human bronchial epithelial (16HBE) cell cultures, we demonstrated that CEES exposure causes epithelial cell apoptosis and detachment. In these studies we found that both cell culture media and BALF obtained after CEES exposure demonstrated elevated TF activity and enhanced capability to convert FX to FXa and could elicit rapid clotting of plasma. Addition of tifacogin (23), a TF pathway inhibitor, effectively blocked either activity and demonstrated that these procoagulant actions were TF dependent. Such findings indicated that TF pathway inhibition could be an effective strategy to prevent airway obstruction after SM inhalation.
MATERIALS AND METHODS
Cell culture and reagents.
16HBE cells were obtained from the ATCC (Manassas, VA) and grown on collagen-coated 100-mm dishes using Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA). Exposure of cultured cells to CEES (TCI America, Portland, OR) was performed by making initial dilutions in DMSO, followed by adding 10 μl of the CEES-DMSO solution into 10 ml of DMEM in a 50 ml conical tube. This media/CEES solution was then sonicated for 3 s and the proper volume immediately added to the respective culture. The concentration of DMSO was 0.1% in all experiments. Tifacogin, provided by Novartis (Basel, Switzerland), was produced in Escherichia coli and is a recombinant form of the endogenous TF pathway inhibitor (TFPI) glycoprotein. It is distinguished from endogenous TFPI by an alanine at the NH2-terminus and lack of glycosylation.
Animals and CEES exposure protocol.
Male Sprague-Dawley rats (250–300 g) from Harlan (Indianapolis, IN) were acclimated to Denver altitude for at least 1 wk with free access to food and water. Temperature was maintained between 20 and 24°C and lights were controlled on a 12-h on-off cycle. The National Jewish Institutional Animal Care Committee (IACUC) approved all experimental protocols. Just prior to CEES exposure, rats received a single intraperitoneal injection of ketamine (50 mg/kg), xylazine (5 mg/kg), and acepromazine (1 mg/kg) for anesthesia and sedation. After induction of anesthesia, rats were loaded into polycarbonate tubes and placed in a Jaeger nose-only inhalation system (CH Technologies, Westwood, NJ). Dry compressed air 6 l/min was mixed with 6 l/min additional compressed air and ethanolic CEES (92.5% ethanol-7.5% CEES; vol/vol) and delivered to a bioaerosol nebulizing generator (BANG) by means of a syringe pump (Razel Scientific, St. Albans, VT). Animals were exposed for 15 min, after which the BANG flow rate was decreased to zero, and diluting air increased to 12 l/min. Rats were then sequentially removed from the tube restraints and returned to their cages. Inhaled dose was estimated by connection of a liquid trap containing 500 μM glutathione (GSH) to an exposure port on the inhalation system and collection of the CEES aerosol as it was drawn through an inlet tube at 1 l/min. Declines in reduced GSH within the trap, as a function of CEES deposition, were determined by a nonenzymatic assay using Ellman's reagent. CEES deposition was ∼14 mg and was calculated by comparison of trap sample absorbance to standard curve values obtained by incubating GSH (500 μM) with known concentrations of CEES. By use of Guyton's formula to estimate respiratory minute volume (rat weight 300 g) the estimated inhaled dose of CEES was ∼1.94 mg over the 15-min exposure.
Immunocytochemistry for cytokeratin, E-cadherin, acetylated tubulin, CD68, and cleaved caspase 3-positive cells in BAL.
Rats were exposed for 15 min to ethanol alone or 7.5% CEES in ethanol (vol/vol). After recovery (18 h), rats were euthanized (pentobarbital, 130 mg/kg ip; Sleepaway, Fort Dodge Animal Health, Fort Dodge, IA), and lungs were immediately lavaged with two 5-ml aliquots of cold phosphate-buffered saline (PBS). Cells were pelleted by gentle centrifugation (800 g) and resuspended in 2 ml of PBS. Then 200-μl aliquots from each sample were deposited onto microscope slides by use of a Cytospin 2 (Shandon Scientific, Cheshire, UK), submerged in 4% paraformaldehyde for 10 min, and rinsed with PBS. Slides were permeabilized in 0.4% Triton X-100 solution for 20 min, followed by addition of blocking buffer containing 0.4% Triton X-100 and 10% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS for 60 min. After block, slides were incubated (2 h) with a monoclonal anti-cytokeratin, pan-fluorescein isothiocyanate (FITC) antibody (Sigma Aldrich no. F3418) at 1:100 (14 μg/ml) dilution. As a control, additional slides were treated with a 1:400 (14 μg/ml) solution of mouse IgG primary antibody (Jackson ImmunoResearch Laboratories), followed by a donkey anti-mouse FITC (Jackson Laboratories). Slides were processed similarly for acetylated tubulin (AT), CD68, TF, E-cadherin, and cleaved caspase 3 (CC3) staining, by using the following reagents: rabbit polyclonal CC3 (Abcam; Cambridge, MA) at 1:300 dilution (0.7 μg/ml); rabbit IgG control (0.7 μg/ml; DAKO; Carpinteria, CA); donkey anti-rabbit Alexa Fluor 549 (Invitrogen; Carlsbad, CA); mouse monoclonal acetylated α-tubulin at 1:500 (5 μg/ml; Abcam); mouse IgG (5 μg/ml; Jackson); donkey anti-mouse FITC-conjugated (Jackson); mouse anti-rat CD68 (Millipore Billerica, MA) at 1:300 dilution; rabbit anti-human TF at 1:300 (no. 4502; American Diagnostica); and mouse anti-human E-cadherin (BD Biosciences; no. 610182). Images were captured via a Leica fluorescence microscope with a ×10 objective. A total of five fields were captured for each sample. The percent of total cells staining positively for cytokeratin, AT, and CC3 was determined by calculating the total number of fluorescent-positive cells divided by the total number of 4,6-diamidino-2-phenylindole (DAPI)-stained cells in each of the five fields.
TF activity assay.
TF activity was assayed as FVII-dependent activation of FX. Five microliters of BALF or culture media were incubated in a 96-well plate for 45 min at 37°C with 45 μl of HBSA (137 mM NaCl, 5.38 mM KCl, 5.55 mM glucose, 10 mM HEPES, 0.1% BSA) and 50 μl of HBSA containing 20 nM FVII, 300 nM FX, and 12 mM CaCl2. Then 25 μl of 25 mM EDTA were added to cease FXa generation, and 25 μl of S-2765 FXa substrate (6 mM; Chromogenix; Bedford, MA) were added to each well and kinetic readings of absorbance were recorded every 20 s. The rate of absorbance increase between 60 and 120 s was determined and TF was expressed relative to the activity of Innovin recombinant human thromboplastin reagent (Dade, Miami, FL), by use of a user-defined equation: y = (Vmax × X)/(Km + X) in the nonlinear curve-fitting setting of Prism graphing software (GraphPad Software). A standard curve of serial dilutions of the Innovin was assayed during each experiment. The activity of 1× (manufacturer's specifications) was designated as 1,000 units of relative TF activity. For microparticle determinations, nonfrozen media or BALF (100 μl) were assayed by each of the following procedures: 1) noncentrifuged; 2) centrifuged at 3,700 g for 10 min and pellet resuspended in 100 μl of HBS; 3) centrifuged at 3,700 for 10 min, supernatant removed and further centrifuged at 100,000 g for 30 min, and pellet resuspended in 100 μl of HBS. For comparison of adherent cell and media fraction TF activity, 5 × 105 16HBE cells in 100-mm dishes were exposed to DMSO alone or CEES. After 18 h, medium was collected, of which 100 μl was processed according to either step 2 or step 3 above. Then 5-μl sample aliquots were tested in the activity assay. For adherent cell determination, remaining cells were gently rinsed and scraped into 1 ml of HBSA. After sonication for 10 s, this fraction was further diluted 1:10 in HBSA and 5 μl of this volume were simultaneously analyzed alongside the processed media samples.
MTT cytotoxicity assay.
16HBE cells were seeded into 96-well fibronectin-coated plates at a density of 16,000 cells/well and allowed to adhere overnight. Cells were treated with CEES (0–1,000 μM) for 18 h, after which media was replaced with serum and phenol red-free DMEM. MTS (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide; Sigma Aldrich, St. Louis, MO) a tetrazolium reagent, was added directly to each well and incubated for 2 h at 37°C. The MTS tetrazolium compound is converted to formazan by actively respiring cells. Absorbance was measured at 570 nm with a reference at 650 nm in a Spectra Max 340 plate reader (Molecular Devices, Sunnydale, CA). Mean absorbance was quantified from six independent wells and expressed as a percentage of the value for DMSO-treated controls.
Measurement of caspase activity.
16HBE cells were seeded at a density of 3 × 105 in fibronectin-coated six-well plates and grown overnight. Cultures were exposed to DMSO or 750 μM CEES in 3 ml of DMEM for 6, 12, or 18 h, after which media were collected and stored on ice. Remaining adherent cells were then rinsed once with PBS and harvested by scraping into a final volume of 3 ml of PBS, and 100-μl aliquots of either media or adherent cells were transferred to a 96-well plate and incubated with 100 μl of Caspase Glo 3/7 reagent (Promega; Madison, WI) buffer at 37°C. After 1 h, luminescence readings were taken using a Synergy2 plate reader (Biotek, Winooski, VT).
Clotting time measurements.
Samples consisting of 8 μl of either undiluted cell media or lavage diluted 1:1 (vol/vol) in saline were tested for their ability to accelerate clotting of recalcified plasma by addition into 96-well plates containing the following: 50 μl of platelet-poor plasma diluted 1:1 in saline; 92 μl of saline. Clotting reactions were then initiated by addition of 50 μl of 30 mM CaCl2. Absorbance values at 405 nm were measured at 20-s intervals for 12 min in a SpectraMax 340 plate reader set at 37°C. A well was considered clotted at the first time point when the absorbance reached within 0.003 absorbance units of the maximal value during the 12-min run. Platelet-poor plasma was obtained by direct cardiac puncture of anesthetized rats. Blood from five rats was collected into separate syringes containing 3.2% sodium citrate (volume citrate solution: blood = 1:9) and centrifuged at 2,000 g for 15 min, and the upper ¾ of the plasma was then pooled, aliquoted, and frozen at −80°C.
Western blot analysis for TF or FX.
16HBE cultures were exposed to DMSO alone or DMSO containing CEES for 4, 8 or 24 h. Cells were harvested by scraping at 4°C in 50 mM Tris (pH 8.0), 120 mM NaCl, and 0.5% Nonidet P-40 supplemented with 2 μg/ml of aprotinin and 100 μg/ml of phenylmethylsulfonyl fluoride. Cell lysate was cleared by centrifugation, and protein concentration was determined by Bradford assay. The lysates were boiled in 3× Laemmli buffer [1× = 62.5 mM Tris (pH 6.8), 2% SDS, 10% glycerol, and 0.5% bromphenol blue]. Proteins were separated by size on 4–15% gradient SDS-polyacrylamide gels and transferred to nitrocellulose. Membranes were blocked for 1 h in PBS containing 5% nonfat dry milk followed by overnight incubation with the primary antibody, rabbit anti-human TF (1:1,000; American Diagnostica, Stamford, CT), or rabbit anti-human β-actin (1:3,000; Sigma, St. Louis, MO). Nonspecific interactions were removed by a wash in Tris-buffered saline with 0.5% Tween 20 (TBS-T) for 1 h followed by incubation with goat anti-rabbit secondary antibody (1:5,000; Southern Biotechnology, Birmingham, AL). For FX immunoblotting, BALF was centrifuged (800 g), and a 30-μl aliquot was added to 6 μl of 5× Laemmli buffer, briefly centrifuged, and gel loaded. To solubilize protein from cast, 30 mg was dissolved in 4 M guanidine HCl, protein precipitated by use of trichloroacetic acid, and dissolved in 250 mM Tris (pH 8.0). FX antibodies used are identical to those used in FX cleavage experiments. All blots were washed again in TBS-T for 1 h, and the conjugates were visualized by chemiluminescence (Amersham, Arlington Heights, IL). Band pixel intensity on blot images was measured using ImageJ software (NIH). After background correction values were normalized to β-actin as a control for loading.
FX cleavage assay.
Three microliters of noncentrifuged BALF or media obtained after CEES or vehicle exposure were incubated with FX (32 ng) and FVII (300 ng) (Enzyme Research Laboratories, South Bend, IN) in 0.015 M Tris·HCl, 0.15 M NaCl, 0.005 M CaCl2, pH 7.4, at 37°C in a final volume of 20 μl. After 45 min, 5 μl of nonreducing Laemmli buffer was added to each reaction, and the entire volume of each sample was immediately loaded onto a 4–15% gradient acrylamide gel. SDS-page gel electrophoresis and Western blot analysis were performed as described above, except for use of nonreducing electrophoretic conditions and use of rabbit anti-human FX primary (Haematologic Technologies, Essex Junction, VT) and goat anti-rabbit secondary (Southern Biotech, Birmingham, AL). For anticoagulant experiments, BSA, tifacogin, or lactadherin (0.2–20 μg/ml) were incubated with BAL or media fluid samples for 15 min, after which 3 μl was removed and tested in the FX cleavage assay.
Lactadherin purification.
A lactadherin expression plasmid (plasmid 15,003) was obtained from Addgene (Cambridge, MA). The plasmid was transformed into competent DH5α E. coli by heat shock, and bacteria expanded in ampicillin-containing Luria-Bertani medium. Plasmid DNA was recovered by using a Qiagen EndoFree plasmid purification kit (Valencia, CA), and transfected into HK293 epithelial cells by use of calcium phosphate. After 36 h of growth, cells were lysed and lactadherin purified by use of Talon His-tag purification resin (Clontech, Montainview, CA) in a gravity flow column. Western blotting and Ponceau staining verified successful isolation.
Immunocytochemistry for TF.
16-HBE cells were plated on Labtek chamber slides at a density of 20,000 cells per chamber. After 24 h, cells were exposed to 750 μM CEES for various times, rinsed, and blocked with PBS containing 1% FBS for 20 min. Slides were incubated with a FITC-conjugated mouse anti-TF human antibody (Acris, Herford, Germany) for 30 min (1:500), rinsed with PBS, and fixed in 4% paraformaldehyde. After a final rinse, slides were mounted and images were captured using a Leica fluorescence microscope with a ×40 objective. Fluorescence quantification of TF was performed by ImageJ software using images displaying only green channel intensity. The background fluorescent intensity of each image was subtracted from the mean pixel intensity of each cell region to obtain final values. Fifty cells were analyzed for each condition.
Statistics.
Statistical analyses were performed with Prism 4 software. Means were compared by one-way analysis of variance followed by Tukey's test for multiple comparisons, and P values less than 0.05 were considered significant.
RESULTS
Airway obstruction and epithelial cell injury after CEES inhalation.
CEES elicits acute lung injury, with symptoms developing within a few hours of inhalation. In studies performed in rats, we have previously observed that CEES inhalation leads to the development of a procoagulant syndrome in the conducting airways, which can lead to their obstruction by casts containing prominent fibrin (38). A representative lesion revealed during airway microdissection 18 h after 7.5% CEES inhalation is pictured in Fig. 1A. At this CEES concentration, rat airways are usually less than 100% occluded, and BAL procedures can be performed with only modest restriction toward fluid introduction and retrieval. Cast formation was associated with the appearance of increased levels of protein in BALF, and, specifically, of proteins such as IgM and fibrinogen/fibrin that normally are present only in the circulating blood. Advancement of the frequency and magnitude of these lesions can cause both impaired gas exchange and acute mortality.
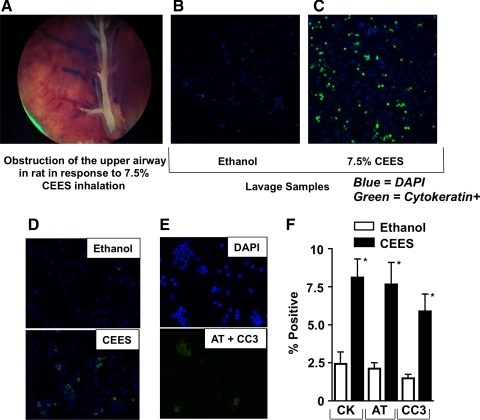
Fig. 1.
A: bright-field image (×10 magnification) of a large occlusive cast present in a central bronchus of a rat after 2-chloroethyl ethyl sulfide (CEES; 7.5%) exposure (18 h). B and C: immunocytochemical detection of cytokeratin-positive epithelial cells (green) in bronchoalveolar lavage fluid (BALF) samples from rats 18 h after inhalation of diluent only (ethanol) or diluent containing 7.5% CEES (vol/vol). BALF samples also were stained with 4,6-diamidino-2-phenylindole (DAPI) to demonstrate nuclei (blue). D: immunocytochemical detection of acetylated tubulin (AT)-positive cells (green) in BALF obtained 18 h after ethanol exposure (top) or 7.5% CEES (bottom). E: representative image of immunofluorescence labeled CEES BALF stained with DAPI (blue), AT (green), or cleaved caspase 3 (CC3; red). DAPI staining of this region is shown independently (top) to demonstrate that positive staining for CC3 (bottom) is a consistent feature of AT-positive cells. F: quantification of cytokeratin (CK), AT-, or CC3-expressing cell populations in BALF. Columns show the average percentage of positive staining cells from cytospins. N = 6 rats per group. *P < 0.05 vs. ethanol control.
Cytokeratins are a family of keratin-containing intermediate filament proteins found exclusively in the cytoplasmic cytoskeleton of epithelial tissue. Immunocytochemical evaluation of BALF for presence of cytokeratin-positive cells indicate that CEES-exposed rats have increased levels of airway epithelial cell detachment, compared with animals inhaling only ethanol vehicle (Fig. 1, B and C). Quantification of immunostaining (Fig. 1F) indicated that 2.44 ± 0.7% of all DAPI-positive cells were also cytokeratin positive in control groups, whereas 8.1 ± 3.2% were positive in the CEES-exposed groups. A similar distribution of AT staining was also evident in BALF from ethanol control (2.1 ± 0.9%) and CEES-exposed (7.7 ± 3.8%) populations (Fig. 1, D and F), As shown in Fig. 1D, the subcellular location of AT staining was limited to one hemisphere, presumably along the terminal plate of basal bodies, the site at which cilia are anchored. These data suggest that ciliated respiratory epithelial cells are among the cell population that detaches from the airway epithelial lining in response to CEES. Increased levels of CC3-positive cells in CEES BALF (Fig. 1, E and F) indicate that apoptotic injury is associated with this cell detachment.
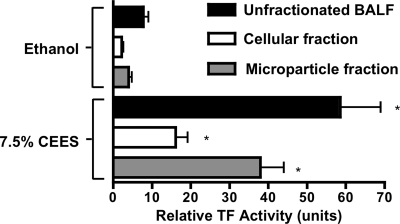
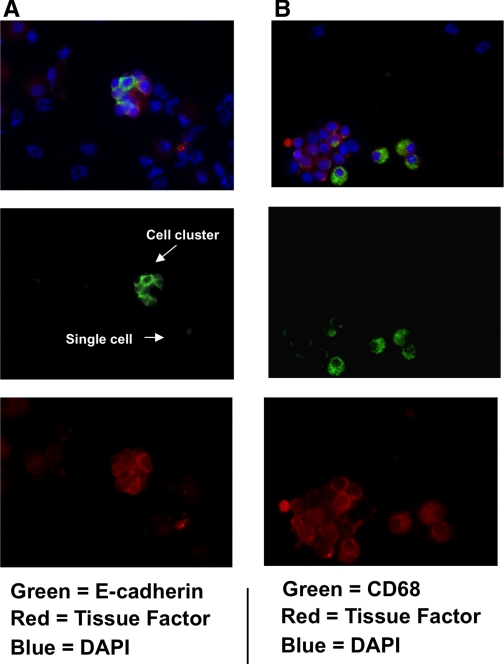
TF activity in BALF was measured to determine its association with airway clotting in response to CEES. These experiments were performed by incubating ethanol (control) or 7.5% CEES BALF for 18 h with FX and FVII and then measuring the amidolysis of a FXa substrate to estimate the amount of FXa generation. As shown in Fig. 2, ethanol BALF had a relative TF activity of 7.79 ± 2.60 units. In response to CEES exposure, this activity increased significantly to 58.53 ± 20.96 units. BALF samples were also fractionated by centrifugation into cellular and microparticle (MP) fractions. The former contain cells and other matter that pellets at 3,700 g, the latter contained residual particles that pellet at 100,000 g, after the initial removal of the cell fraction. Consistent with the elevations observed in whole BALF, TF activity also increased in both the cellular fractions (ethanol: 2.15 ± 0.9; CEES: 16.07 ± 6.3 units) and the MP fractions (ethanol: 3.97 ± 1.7; CEES: 37.95 ± 12.09 units) derived from BALF after CEES inhalation. Immunofluorescent staining of cytospins was also performed to determine what cell types in BALF express TF. Colocalization of TF to either CD68 or E-cadherin (Fig. 3) confirmed that macrophages and epithelial cells in BALF are both TF positive.
Fig. 2.
Effect of CEES inhalation on tissue factor (TF) activity in rat airways. BALF was recovered from rats 18 h after exposure to vehicle (ethanol) or 7.5% CEES and fractionated by centrifugation. The “cellular” fraction (3,700 g pellet) contains cell populations present in BALF, whereas the “microparticle” fraction contains small particles pelleted by ultracentrifugation (100,000 g) after first removing the cellular fraction. Each fraction was assayed for TF activity as described in methods. Data represent means ± SE of 3 determinations for each individual BALF sample (n = 4 rats per ethanol or CEES group). *P < 0.05 compared with corresponding ethanol-exposed control fraction. TF activity is expressed relative to the activity of recombinant human TF (Innovin; 1× strength designated as 1,000 units arbitrary).
Fig. 3.
Determination of the phenotype of TF-positive cells in BALF after CEES exposure. A: immunofluorescent imaging of TF expression (red) on epithelial cells was assessed by colocalization with E-cadherin (green). Images of numerous E-cadherin+ bodies indicate that epithelial cells are present in clusters and as individual cells. In B, TF expression (red) on macrophages was assessed by its colocalization with CD68 (green). In both A and B, the cytospin samples were also stained with DAPI (blue) to demonstrate all nucleated cell populations. Green and red channels were split into separate channels (middle and bottom rows) to assist in visualizing colocalization of TF to each respective cell type. Magnification ×40.
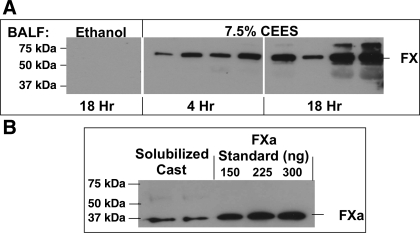
Coagulation FX is the substrate for proteolytic activity on cell surfaces. Its conversion to FXa is responsible for initiating coagulation. Western blotting was performed to determine the presence of FX in BALF. As shown in Fig. 4A, FX content in BALF from ethanol control rats is low. In contrast, BALF obtained 4 or 18 h after 7.5% CEES inhalation showed a time-dependent increase in protein levels. FXa, however, was not detected in any BALF samples. Additional Western blot analysis of proteins obtained after solubilization of solid cast material from CEES-exposed rats did demonstrate the presence of FXa. Since proteolytic activity present on cell surfaces is necessary to invoke the FX-dependent initiation phase of coagulation, our findings of increased epithelial detachment and elevated FX in BAL lead us to suspect a potential role for injured epithelial cells in the pathogenesis of mustard-induced airway coagulopathy.
Fig. 4.
Effect of CEES inhalation on factor X (FX) expression in rat airways. A: Western blot analysis of BALF obtained from rats at 4 or 18 h after exposure to vehicle (ethanol; 18 h) or 7.5% CEES, demonstrated increased FX expression after CEES exposure. The active form (FXa), however, was undetectable in BALF. BALF samples from ethanol-exposed (18 h) and CEES-exposed (18 h) rat lungs were loaded onto 1 gel, whereas those from CEES-exposed (4 h) rats were loaded onto a second gel. The 2 gels were electrophoresed together in the same device and identically transferred. After gel transfer, each membrane was then placed onto a single X-omat screen and the image was captured on a single piece of film. For each exposure condition, findings representative of the range of FX detected are shown. B: FXa was detected in airway casts from CEES-exposed lungs. Rat FXa migrates at 37 kDa and was confirmed by comparison to purified preparations that are shown in the three lanes at far right.
Effect of CEES on airway epithelial cell viability.
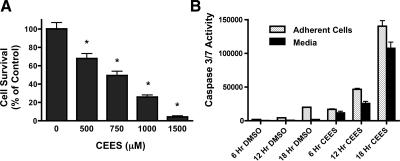
16HBE cells were plated at a density of 1 × 106 cells per 100-mm dish and exposed to varying CEES concentrations. After 24 h, MTT assays were performed to determine the viability of each culture (Fig. 5A). Compared with DMSO (diluent for CEES; 100% survival) alone, exposure to CEES significantly decreased viability at all concentrations tested. Exposure to 500 μM CEES caused moderate declines in cell viability (68.5 ± 18.46% percentage of control), whereas more severe declines were evident with dose escalation [(750 μM: 49.38 ± 18.13%) (1 mM: 25.88 ± 8.98%) (1.5 mM: 4.24 ± 4.44%) percentage of control, respectively]. An intermediate concentration of CEES (750 μM) was selected for further study because it exerted a LD50 effect in 16HBE cultures, despite the short half-life of CEES in aqueous medium. Caspase activation in response to CEES also was investigated. CEES exposure at this concentration caused a time-dependent increase in caspase 3 and 7 activity, which was detectable in both adherent cell fractions and in culture media (Fig. 5B). At 6 h after exposure, CEES elicited a sixfold increase in caspase 3 and 7 relative to DMSO exposure, the majority of which was retained in the adherent fraction. At 12 h, further increases in caspase activity were evident in both the adherent cell and media fractions relative to 6 h. By 18 h, caspase 3/7 activity in media was maximal, consistent with the extensive cell detachment occurring in these cultures.
Fig. 5.
A: cytotoxicity of in vitro exposure to CEES. 16HBE cultures were exposed to diluent (DMSO alone) or diluent containing increasing concentrations of CEES (500, 750, 1,000, or 1,500 μM). After 24 h, cell viability was determined by MTT assay. Values represent means ± SD of 3 independent experiments. *Statistical difference from diluent exposure (0 μM CEES). B: effect of CEES exposure on caspase 3/7 activation. 16HBE cells were exposed to either diluent (DMSO alone) or diluent containing CEES (750 μM) for 6, 12, or 18 h. At each time point, cultures were separated into samples consisting of the adherent cell fraction (stippled bar) or the media fraction (solid bars) and tested for caspase 3/7 activity. Each bar represents average values from 9 replicate cultures exposed to CEES in 3 independent experiments. *P < 0.05 compared with diluent (DMSO) control.
Medium from CEES-exposed epithelial cells decreases plasma clotting time.
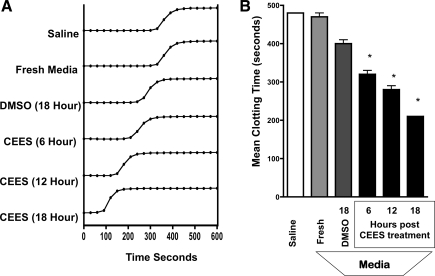
The procoagulant activity of medium recovered from DMSO or CEES-exposed epithelial cell cultures was determined by its addition to naive rat plasma in the presence of CaCl2. Clotting times of these samples were determined by monitoring the sigmoidal increase in absorbance at 405 nm, which is proportional to sample viscosity and translucence. Absorbance values eventually reach a plateau phase, at which time the sample has attained a semisolid clotted state that was visible to the naked eye. Shown in Fig. 6A are representative clotting curves obtained after the addition of saline, fresh medium, or medium obtained from 16HBE cell cultures at varying time periods after DMSO or CEES exposure. A consistent observation was the progressive shortening in the time required to reach the plateau phase of clotting as a function of CEES exposure duration. The mean time to clot for each treatment condition was determined by obtaining samples from three replicate cultures and is illustrated in Fig. 6B. Plasma samples treated with saline only exhibited a clotting time of 487 ± 15.0 s after saline addition. Clotting times did not significantly change in samples reacted with identical volumes of fresh media (470 ± 17.8 s). In contrast, clotting times were significantly shortened by media additions obtained at 6 h (320 ± 17.3 s), 12 h (280 ± 17.3 s), or 18 h (210 ± 0 s) after CEES exposure, compared with 18 h DMSO-exposed samples (400 ± 15.3 s).
Fig. 6.
Effect of CEES exposure on clotting time of plasma induced by culture media obtained from 16HBE cells. Cells were exposed to diluent (DMSO alone) or diluent containing CEES (750 μM) for 6, 12, or 18 h. Small volumes of media were then used in a “time to clot” assay by addition to naive plasma in the presence of CaCl2. A: histograms demonstrating the time-dependent increase in absorbance at 405 nm, which occurs as a function of fibrin clot formation. B: bars represent the mean clotting time in seconds ± SD of plasma treated with saline, fresh media, or media obtained 6, 12, or 18 h after diluent or CEES exposure. Time to clot was quantified by determining the time at which absorbance values plateaued. *P < 0.05 and denotes significant difference from DMSO-exposed control samples.
Medium from epithelial cells exposed to CEES causes cleavage of FX.
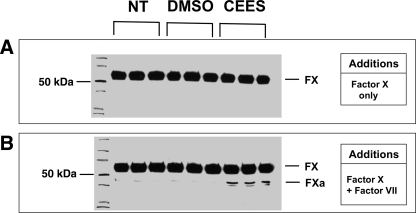
Membranes bearing lipidated TF provide the key cellular surface for triggering the initiation phase of coagulation. To determine whether media from CEES-exposed cultures exhibited TF-dependent procoagulant activity, we performed an assay to measure the conversion of FX to FXa. In these studies airway epithelial cell cultures were exposed to DMSO alone, or DMSO containing 750 μM CEES. After 6 h, media obtained from these cultures was then added to reactions containing only FX (Fig. 7A). As shown by Western blotting, these samples did not result in any FXa formation when incubated in the absence of FVII. In contrast, identical samples to which FVII had been added (Fig. 7B) did result in FX to FXa conversion. Media obtained from DMSO-exposed cultures generated only very little FXa, whereas much higher levels of FXa were generated in response to media added from CEES-exposed cultures.
Fig. 7.
Effect of culture media from CEES-exposed 16HBE cells on FX cleavage. 16HBE cultures were exposed either to no treatment (NT), diluent (DMSO) alone, or diluent containing CEES (750 μM). After 6 h, small volumes of media were obtained from these cultures and further incubated in buffer containing FX only (A) or buffer containing FX and factor VII (FVII) (B). After incubation (45 min), the reaction was stopped by adding sample loading buffer, and FX-to-FXa conversion was determined by Western blot. Blots shown were representative of 4 independent experiments.
Increased epithelial cell surface TF expression due to CEES exposure.
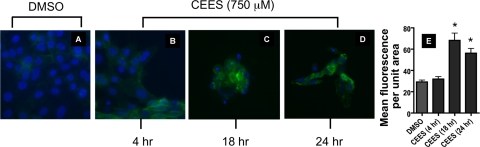
Evaluation of epithelial cell surface TF expression by immunocytochemistry was performed. Exposure to CEES (750 μM) resulted in strong staining at 18 and 24 h after exposure (Fig. 8, C and D) compared with DMSO control. Increased TF staining was particularly evident in those cells exhibiting less dense nuclear material as evidenced by a decreased intensity of DAPI counterstaining.
Fig. 8.
Effect of CEES exposure on cell surface expression of TF detected by immunocytochemistry. 16HBE cells were exposed to diluent (DMSO) or CEES (750 μM). Cells were fixed after 4, 18, or 24 h of exposure (A–D) and stained with a FITC-conjugated antibody against TF (green staining). Nuclei were detected by DAPI staining (blue). Fluorescence quantification of TF is plotted for DMSO control and CEES samples. Columns represent mean fluorescence values collected from a minimum of 50 cellular regions using images displaying only green channel intensity. *Statistically significant change compared with DMSO (P < 0.05).
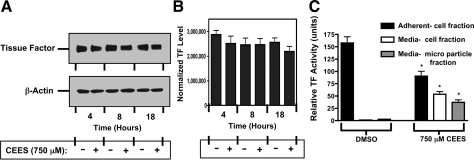
CEES exposure does not alter total cellular TF expression.
Western blotting for TF was done to determine whether changes in overall cell protein expression occurred in response to CEES. 16HBE cultures were exposed to DMSO or 750 μM CEES in DMSO for 4, 8, or 18 h, followed by cell lysis. Quantitation of Western blots obtained from three independent experiments and normalized against β-actin expression (Fig. 9, A and B, and data not shown), revealed that TF protein levels did not change significantly in response to CEES exposure. Levels of TF activity in media and in adherent cell fractions retained on the culture plate were also determined after 18 h exposure to DMSO or 750 μM CEES (Fig. 9C). In DMSO-treated cultures, the vast majority of TF activity was present in the adherent cells on the plate (157.6 ± 21.7 units of activity), with the media cell fraction (1.38 ± 0.26 units) and MP fraction (2.19 ± 0.13) possessing lower activity. In response to CEES exposure, retained adherent cells had less TF activity (90.4 ± 16.8 units) than that observed in control. Levels of TF activity in the media increased, however, in both the cell fraction (53.5 ± 7.77 units) and MP fraction (37.6 ± 10.1 units). The sum of TF activity in the three compartments, and subsequent statistical comparison, demonstrated that CEES exposure did not cause an overall increase in total activity relative to control.
Fig. 9.
Total cellular expression of TF following CEES exposure. A: untreated or CEES-exposed (750 μM) 16HBE cells were harvested after 4, 8, or 18 h of exposure and analyzed for TF abundance by Western blot. Expression of β-actin was measured to confirm that comparable levels of protein were loaded from all samples. The blot is from a representative experiment (n = 3 repetitions). B: bars represent mean density ± SD (arbitrary units) of 3 separate blots after quantification by imaging densitometry and normalization to β-actin. No statistical difference was observed between groups as assessed by ANOVA. C: effect of CEES exposure on the release of TF. 16HBE cell cultures were exposed to DMSO or 750 μM CEES for 18 h and separated into an adherent cell (solid bar) fraction, a fraction of pelleted floating cells from media (open bar), or microparticles (shaded bar). Material in each fraction was washed and resuspended at equal volume and assayed for TF as described in methods. Data are the means ± SD for 4 determinations. * P < 0.05 compared with corresponding DMSO control.
Effect of lactadherin and tifacogin on the clotting activity of culture media.
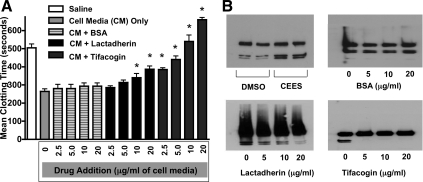
Experiments using either lactadherin or tifacogin were performed to determine their relative potential to inhibit the procoagulant activity of cell culture media obtained after CEES exposure. Lactadherin is a naturally occurring glycoprotein that can interfere with the function of clotting factors at cell membrane surfaces by binding to phospholipids, whereas tifacogin is a recombinant form of TF pathway inhibitor. In clotting time experiments (Fig. 10A), plasma treated with saline only demonstrated a mean clot time of 505 ± 41.2 s. In contrast, addition of culture media from three replicate CEES-exposed 16HBE cultures reduced mean plasma clotting time to 264 ± 32.8 s. Incubation of the media from CEES-exposed cells with varying amounts of BSA for 15 min prior to its addition to plasma did not significantly change its procoagulant activity [BSA (2.5 μg/ml: 280 ± 40 s); (5 μg/ml: 273 ± 41.6 s); (10 μg/ml: 293.3 ± 30.5 s); (20 μg/ml: 293.3 ± 30.5 s)]. Incubation of media with lactadherin demonstrated a modest normalization (e.g., slowing) in clotting time [lactadherin (2.5 μg/ml: 285 ± 19.1 s); (5 μg/ml: 313 ± 23.1 s) (10 μg/ml: 340 ± 40.0 s) (20 μg/ml: 386.7 ± 30.5 s)]. Incubation with tifacogin was more effective than exposure to either BSA or lactadherin, significantly increasing clotting time at all concentrations tested [tifacogin (2.5 μg/ml: 385 ± 19.1 s); (5 μg/ml: 440 ± 34.6 s) (10 μg/ml: 540 ± 60.0 s) (20 μg/ml: 660 ± 20.0 s)]. Each of these interventions was also tested for its potential to inhibit FX cleavage. As shown in Fig. 10B (top left), culture media obtained 18 h after CEES exposure was robust in its capacity to cleave FX compared with media obtained after DMSO exposure only. Pretreatment of the CEES-exposed media with BSA (top right) did not alter its ability to cleave FX cleavage. By contrast, pretreatment with lactadherin (bottom left) decreased the amount of FX generated by this medium in a concentration-dependent fashion. Pretreatment with tifacogin (bottom right) completely abolished FXa generation when FX was incubated with media obtained from CEES-exposed epithelial cell cultures.
Fig. 10.
Impact of lactadherin and recombinant TF pathway inhibitor (rTFPI; tifacogin) on the procoagulant activity of 16HBE cell media following CEES exposure. A: cell media (CM) obtained 18 h after CEES exposure was incubated with varying concentrations of bovine serum albumin (BSA), lactadherin or tifacogin and tested in a time to clot assay by its addition to naive plasma. Each bar represents the mean clotting time in seconds ± SD for each treatment, as well as plasma treated with saline only or culture media (18 h post-CEES exposure) not incubated with anticoagulants. Time to clot was quantified by determining the time at which absorbance values plateaued. *P < 0.05, significant difference from the CEES-exposed cell media (shaded bar; control for this experiment). B: Western blot analysis for FX cleavage to FXa product. Incubation of factors X and VII with media obtained after CEES exposure (18 h) generated greater FX-to-FXa conversion compared with DMSO-exposed (18 h) cultures (top left). Incubation of CEES-exposed cell media with BSA (top right), lactadherin (bottom left), or tifacogin (bottom right) prior to its addition to FX and FVII demonstrated variable capacity to inhibit FX-to-FXa conversion.
Effect of tifacogin on procoagulant activity of BAL.
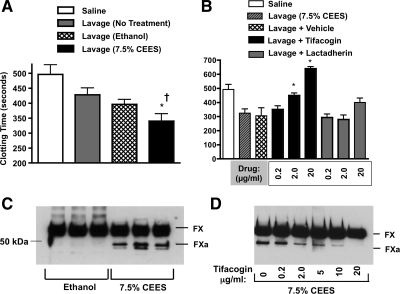
Lavage samples obtained from rats exposed to 7.5% CEES had significantly greater procoagulant activity compared with BAL from naive or ethanol-exposed rats (Fig. 9A). Similar to results obtained from cell media, incubation (15 min) of BALF from CEES-exposed rats with tifacogin (0.2 to 20 μg/ml) significantly diminished its procoagulant activity in the clotting assay (Fig. 11B). At the highest tifacogin concentration used (20 μg/ml), the clotting times of plasma exposed to BAL from CEES-exposed rats increased above the levels observed in nontreated control plasma, an indication that all procoagulant activity had been neutralized. The anticoagulant activity of lactadherin was also tested. At 0.2 or 2.0 μg/ml concentrations, it was incapable of reversing rapid clotting caused by BAL. At high concentration (20 μg/ml), lactadherin showed a trend toward increasing clot time, but these changes were nonsignificant compared with lavage (7.5% CEES) only samples. The impact of BALF on FX cleavage was also evaluated. As shown in Fig. 11C, BALF obtained after CEES inhalation demonstrated greater FXa generation than ethanol BALF samples. Tifacogin was also tested for ability to block FX cleavage induced by CEES BALF (Fig. 11D). Pretreatment of BAL with concentrations of tifacogin ranging from 0.2 to 20 μg/ml demonstrated dose-dependent ability to limit FXa generation. At the high concentration (20 μg/ml), tifacogin abolished BALF-induced FX cleavage, similar to our findings in cell culture medium.
Fig. 11.
CEES BALF has elevated procoagulant activity, which can be attenuated by tifacogin. A: BALF was obtained from rats 18 h after no treatment, inhalation of diluent alone (ethanol), or inhalation of diluent containing CEES (7.5%, vol/vol). Small volumes of BALF were included in a time to clot assay by adding them to naive plasma in the presence of CaCl2. Bars represent the mean clotting time ± SD in seconds. *P < 0.05 compared with BALF from untreated animals. †P < 0.05 compared with BALF from rats exposed to diluent (ethanol). B: effect of tifacogin or lactadherin on clotting time effect of rat BALF obtained after 7.5% CEES exposure. *P < 0.05 compared with BALF treated with vehicle only (control, “Lavage + Vehicle”). C: Western blot analysis demonstrating that BALF from CEES-exposed rats generates greater FX-to-FXa conversion compared with BALF obtained after ethanol exposure. D: incubation of 7.5% CEES BALF with tifacogin prior to its addition to FX and FVII showed a dose-dependent capacity to inhibit FX cleavage.
DISCUSSION
The present studies demonstrate that exposure of 16HBE cells to CEES gives rise to a procoagulant medium environment fully capable of both FX activation and acceleration of blood coagulation. These studies in cell culture complement our rat model of CEES inhalation by indicating that epithelial injury observed in response to CEES may be a direct contributor to airway coagulopathy. The use of tifacogin, and to a lesser extent lactadherin, as anticoagulants demonstrated efficacy in inhibition of CEES-induced procoagulant activity in culture media and lavage and provides a rationale for interventions directed against TF-driven blood coagulation in response to mustard inhalation.
The early onset of hemorrhage and edema seen in animal models of SM and CEES implies a severe disruption of the vascular and airway barriers (3, 26). More recently it has been recognized that occlusive airway casts comprised primarily of fibrin develop in the airways as a consequence of CEES inhalation in rats. Excepting these reports, the link between airway coagulopathy and mustard-induced respiratory failure is not well established. Other animal models exhibiting pathophysiological similarities to CEES inhalation include those involving airway burns and smoke inhalation (38, 28). Studies utilizing these latter models have found that extensive leak occurs from the bronchial circulation into the airways, which can lead to life-threatening obstruction. Additionally, these studies have shown that use of aerosolized anticoagulants, including activated protein C, or combined heparin and recombinant antithrombin, are effective in improving pulmonary function after injury (12, 28).
Despite extensive studies of SM/CEES exposure over more than a century, the mechanisms underlying airway injury have not been fully elucidated. This is likely due in part to the potent alkylating properties of SM vesicants, which permit chemical interaction with many cellular biomolecules. Recent work with SM indicates that numerous apoptotic markers are prominent during cell death after exposure (11). Studies with CEES also report similar apoptotic changes (16). Our findings in 16HBE cell cultures indicate that the effector caspases 3 and 7 are activated in a time-dependent fashion in response to CEES. As cells detach and die, a sharp increase in caspase activity can be detected in the media. Coinciding with this caspase activation, the culture media also becomes increasingly procoagulant. Release of injured epithelial cells also occurs in the airways in response to CEES, as evidenced by our findings of increased cytokeratin-positive bodies present in BAL. Immunostaining for acetylated tubulin indicates that ciliated epithelia represent a significant fraction of the detached cells. It appears possible, if not likely, that the same cells may have expressed both antigens, since the percentage of both were similar in BALF after CEES inhalation. Although not specifically tested for, cells originating from distal airway zones could also be represented by BALF sampling. As free-floating cell types are likely to become trapped in cast material, our counting of cytokeratin-positive cells in BALF from CEES-exposed rats is likely to be an underestimation. Additional studies on BALF demonstrating the colocalization of E-cadherin and TF suggest that dislocation of TF-bearing cellular material from airway epithelium into the lumen may help initiate coagulation at locations beyond the initial sites of injury. The destruction of these epithelial cells, or macrophages, may then give rise to the increased TF activity that is observed in cellular and MP fractions after CEES exposure.
FX is a vitamin K-dependent plasma glycoprotein that plays a pivotal role in hemostasis. In its zymogen form it can be activated by either the TF-FVIIa complex or the factor IXa-VIIIa pairing, resulting in the proteolytic cleavage of FX on its heavy chain. Such cleavage splits off the carboxy terminal end, releasing the activation peptide (FXa), which can subsequently convert prothrombin to thrombin in the presence of phospholipids. In the present study, FX was found to be elevated in BALF in response to CEES exposure. Detection of FXa in solubilized casts obtained after exposure further implicates its central role in airway clot initiation. In media obtained from CEES-exposed cultures, the addition of FX and FVII resulted in cleavage of FX into FXa. If FVII was omitted from this reaction, there was no FX cleavage, supporting the role for TF. Incubation of procoagulant media with tifacogin, an inhibitor of TF pathway-dependent coagulation, also abolished any subsequent FXa generation in this assay. This finding more firmly supports a role for TF in mustard-induced airway coagulation.
In accord with these results from the FXa generation assay, cell culture media or BAL obtained after CEES exposure each exhibited markedly enhanced blood clotting capabilities. The clotting assay employed in these studies can be viewed as an extension of the FXa generation assay, in that it encompasses all initiation and propagation steps in the clotting process leading up to fibrin formation. Incubation of BAL or culture media with tifacogin markedly blocked their ability to accelerate clotting. Interestingly, tifacogin used at high concentration prolonged clotting times for durations longer than those observed for plasma not incubated with any cell culture media. It has previously been reported that naive plasma contains low levels of TF (19). Thus any endogenous TF present in the plasma employed in our assay was likely being inhibited by tifacogin, in addition to any TF introduced by culture media and BAL samples.
TFPI is a serine protease inhibitor capable of binding active sites on both TF/FVIIa complex and FXa. By virtue of these interactions it is believed to be the only endogenous regulator of the TF-dependent pathway of coagulation. Its presence can be expected to be low in airways during acute lung injury (7). Tifacogin is a recombinant form of TFPI that has demonstrated a strong positive outcome on mortality in a baboon sepsis model (10). In a clinical trial of patients with severe sepsis, however, tifacogin did not confer protection and was associated with an increased risk of bleeding (1, 23). Whether these different outcomes stem from an inability to control for all heterogeneous elements that comprise septic syndromes in human patients remains uncertain. For example, it is unclear whether intervention was able to begin sufficiently early, before established multiple organ failure, in the human trial.
Our findings that TF drives coagulation in CEES-exposed epithelial cultures draw a different conclusion from studies performed in endothelial or monocytic cells (25, 35). These latter studies suggest that procoagulant activity derived from these apoptotic cell types is dictated by the phospholipid content of their membrane environment, rather than TF expression. It should be noted, however, that these cell types express comparatively low levels of TF relative to lung epithelial cells. Our findings, however, are in agreement with recent cell-based models of coagulation, which suggest that the extrinsic and intrinsic pathways of coagulation are codependent, with the TF/FVII complex serving as the critical initiator of coagulation in vivo (8, 29). In macrophage populations in lung, TF expression is believed to be dependent on their degree of maturation (31). Cells of advanced maturity display highest activity, whereas less mature macrophages exhibit less. Our studies demonstrating colocalization of CD68 and TF indicate that macrophage populations in BALF are also a contributing source of TF, in addition to epithelium.
Lactadherin (MFG-E8) is a glycoprotein originally isolated from milk that is secreted by mammary epithelium, dendritic cells, and macrophages. Its expression in mammalian cells produces two glycosylation variants of 52 and 47 kDa (PAS-6 and PAS-7, respectively) (4). The anticoagulant properties of lactadherin stem from its ability to bind phosphatidylserine, -inositol, and -glycerol on cell membranes (33). Such binding has been shown to interfere with the activity of FVIIIa-FIXa and FVIIa-TF complexes, by hindering the positioning of substrates at cell membrane surfaces. In the present cell media studies, lactadherin was moderately effective in blocking the conversion of FX to FXa and rapid plasma clotting caused by CEES-exposed media. The molarity of lactadherin used in these studies equaled 111 (5 μg/ml), 222 (10 μg/ml), or 444 (20 μg ml) nM, respectively. As an anticoagulant it was not as effective as tifacogin, which at its lowest concentration 131.5 nM (5 μg/ml) was more effective than all concentrations of lactadherin tested. A possible explanation for these differences is the inability of lactadherin to suppress all phospholipid-binding sites in circumstances of extensive cell death. Similarly, lactadherin also exhibits a greater affinity for relatively intact membrane surfaces, as opposed to smaller portions of membrane. Its limited effectiveness in the present study could be the result of the elevated MP levels observed in culture and BALF in response to CEES. Lastly, lactadherin, unlike tifacogin, is neither a direct nor irreversible inhibitor and therefore may be incapable of completely abolishing FVII-TF complex or FXa activity.
The availability of TF at the cell surface is thought to be posttranslationally regulated by mechanisms of encryption and deencryption. In encrypted form, TF is believed to be unavailable for interaction with FVII. Purported mechanisms of encryption include TF receptor dimerization, internalization into the membrane, or caveoli (5, 6). Conversely, TF deencryption has been shown to involve PS exposure to the outer cell membrane, and would seem to be the most logical explanation in situations involving apoptotic injury (14). Whether some or all of these processes regulate TF availability on epithelial cells is uncertain. Our present studies indicate that 16HBE cells have increased surface TF expression in response to CEES exposure. Whether this increased surface presentation is mediated by a deencryption process, or simply reflects enhanced exposure of the TF molecule due to structural membrane injury or apoptosis, is unknown. What is evident, however, is that apoptosis and cell destruction in response to CEES exposure give rise to 16HBE cell detachment and MP formation in our culture model. A similar injury process may occur in the lung in response to CEES inhalation, as evidenced by the large increase in TF activity detected in the MP component of BALF. The cellular origin of these BALF MPs is not fully defined, but our present studies colocalizing TF with CD68 and E-cadherin suggest that macrophages and epithelial cells are two likely candidates.
In conclusion, our in vitro and in vivo experiments demonstrate that cell culture media and airway surface liquid become procoagulant as a function of exposure to the SM analog CEES. Either fluid type was found to be capable of FXa generation in the presence of FVII, and both could elicit rapid clotting of plasma. Addition of tifacogin, a TFPI, effectively blocked these procoagulant activities. Dissemination of procoagulant matter derived from apoptotic TF-expressing cells after mustard inhalation may promote airway obstruction by extending the zones of FXa generation and thrombin activation from local sites of injury further into the airway lumen. Therapeutic interventions directed against cell surface coagulation initiation appear to be capable of limiting this response, making them attractive as possible rescue agents for inhalation injury caused by SM exposure.
GRANTS
This research is supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Environmental Health Sciences, Grant Number U54 ES015678.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We recognize Raymond Birge for submission of the lactadherin expression construct into the Addgene repository. Susan Reynolds and Jorge DiPaola are acknowledged for helpful discussions.
REFERENCES
- 1. Abraham E, Reinhart K, Opal S, Demeyer I, Doig C, Rodriguez AL, Beale R, Svoboda P, Laterre PF, Simon S, Light B, Spapen H, Stone J, Seibert A, Peckelsen C, De Deyne C, Postier R, Pettilä V, Artigas A, Percell SR, Shu V, Zwingelstein C, Tobias J, Poole L, Stolzenbach JC, Creasey AA; OPTIMIST Trial Study Group Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: a randomized controlled trial. JAMA 290: 238–247, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Ait-Oufella H, Kinugawa K, Zoll J, Simon T, Boddaert J, Heeneman S, Blanc-Brude O, Barateau V, Potteaux S, Merval R, Esposito B, Teissier E, Daemen MJ, Lesèche G, Boulanger C, Tedgui A, Mallat Z. Lactadherin deficiency leads to apoptotic cell accumulation and accelerated atherosclerosis in mice. Circulation 115: 2168–2177, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Allon N, Amir A, Manisterski E, Rabinovitz I, Dachir S, Kadar T. Inhalation exposure to sulfur mustard in the guinea pig model: Clinical, biochemical and histopathological characterization of respiratory injuries. Toxicol Appl Pharmacol 241: 154–162, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Bovine PAS-6/7 binds alpha v beta 5 integrins and anionic phospholipids through two domains. Biochemistry 36: 5441–5446, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Bach RR. Mechanism of tissue factor activation on cells. Blood Coagul Fibrinolysis 9: S37–S43, 1998 [PubMed] [Google Scholar]
- 6. Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol 26: 456–461, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Bastarache JA, Wang L, Wang Z, Albertine KH, Matthay MA, Ware LB. Intra-alveolar tissue factor pathway inhibitor is not sufficient to block tissue factor procoagulant activity. Am J Physiol Lung Cell Mol Physiol 294: L874–L881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carraway MS, Welty-Wolf KE, Miller DL, Ortel TL, Idell S, Ghio AJ, Petersen LC, Piantadosi CA. Blockade of tissue factor: treatment for organ injury in established sepsis. Am J Respir Crit Care Med 167: 1200–1209, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Crathorn AR, Roberts JJ. Mechanism of the cytotoxic action of alkylating agents in mammalian cells and evidence for the removal of alkylated groups from deoxyribonucleic acid. Nature 211: 150–153, 1966 [DOI] [PubMed] [Google Scholar]
- 10. Creasey AA, Chang AC, Feigen L, Wun TC, Taylor FB, Hinshaw LB. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest 91: 2850–2860, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dillman JF, Phillips CS, Dorsch LM, Croxton MD, Hege AI, Sylvester AJ, Moran TS, Sciuto AM. Genomic analysis of rodent pulmonary tissue following bis-(2-chloroethyl) sulfide exposure. Chem Res Toxicol 18: 28–34, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Enkhbaatar P, Cox RA, Traber LD, Westphal M, Aimalohi E, Morita N, Prough DS, Herndon DN, Traber DL. Aerosolized anticoagulants ameliorate acute lung injury in sheep after exposure to burn and smoke inhalation. Crit Care Med 35: 2805–2810, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Gautum A, Vijayaraghavan R, Sharma M, Ganesan K. Comparative toxicity studies of sulfur mustard (2,2′-dichloro diethyl sulfide) and monofunctional sulfur mustard (2-chloroethyl ethyl sulfide), administered through various routes in mice. J Med CBR Def 4: 1–21, 2006 [Google Scholar]
- 14. Greeno EW, Bach RR, Moldow CF. Apoptosis is associated with increased cell surface tissue factor procoagulant activity. Lab Invest 75: 281–289, 1996 [PubMed] [Google Scholar]
- 15. Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 25: 139–48, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Han S, Espinoza LA, Liao H, Boulares AH, Smulson ME. Protection by antioxidants against toxicity and apoptosis induced by the sulphur mustard analog 2-chloroethylethyl sulphide (CEES) in Jurkat T cells and normal human lymphocytes. Br J Pharmacol 141: 795–802, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herriot RM, Anson ML, Northrop J. Reaction of enzymes and proteins with mustard gas (bis(beta-chloroethyl)sulfide). J Gen Physiol 30: 185–210, 1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosseini K, Moradi A, Mansouri A, Vessal K. Pulmonary manifestations of mustard gas injury: a review of 61 cases. Ir J Med Sci 14: 20–26, 1989 [Google Scholar]
- 19. Hugel B, Socié G, Vu T, Toti F, Gluckman E, Freyssinet JM, Scrobohaci ML. Elevated levels of circulating procoagulant microparticles in patients with paroxysmal nocturnal hemoglobinuria and aplastic anemia. Blood 93: 3451–3456, 1999 [PubMed] [Google Scholar]
- 20. Idell S, Peters J, James KK, Fair DS, Coalson JJ. Local abnormalities of coagulation and fibrinolytic pathways that promote alveolar fibrin deposition in the lungs of baboons with diffuse alveolar damage. J Clin Invest 84: 181–193, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idell S, Peterson BT, Gonzalez KK, Gray LD, Bach R, McLarty J, Fair DS. Local abnormalities of coagulation and fibrinolysis and alveolar fibrin deposition in sheep with oleic acid-induced lung injury. Am Rev Respir Dis 138: 1282–1294, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med 31: S213–S220, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Laterre PF, Opal SM, Abraham E, LaRosa SP, Creasey AA, Xie F, Poole L, Wunderink RG. A clinical evaluation committee assessment of recombinant human tissue factor pathway inhibitor (tifacogin) in patients with severe community-acquired pneumonia. Crit Care 13: R36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lawley PD, Brookes P. Molecular mechanism of the cytotoxic action of difunctional alkylating agents and of resistance to this action. Nature 206: 480–483, 1965 [DOI] [PubMed] [Google Scholar]
- 25. Lechner D, Kollars M, Gleiss A, Kyrle PA, Weltermann A. Chemotherapy induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J Thromb Haemost 5: 520–527, 2007 [DOI] [PubMed] [Google Scholar]
- 26. McClintock SD, Hoesel LM, Das SK, Till GO, Neff T, Kunkel RG, Smith MG, Ward PA. Attenuation of half sulfur mustard gas-induced acute lung injury in rats. J Appl Toxicol 26: 126–131, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Mozier NM, Hoffman JL. Biosynthesis and urinary excretion of methyl sulfonium derivatives of the sulfur mustard analog, 2 chloroethyl sulfide, and other thioethers. FASEB J 4: 3329–3333, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Murakami K, McGuire R, Cox RA, Jodoin JM, Bjertnaes LJ, Katahira J, Traber LD, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock 18: 236–241, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Nemerson Y. The tissue factor of blood coagulation. Semin Hematol 29: 170–176, 1992 [PubMed] [Google Scholar]
- 30. Papirmeister B, Feister AJ, Robinson SI, Ford RD. Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Boca Raton, FL: CRC, 1991, p. 106 [Google Scholar]
- 31. Rothberger H, McGee MP, Lee TK. Tissue factor activity. A marker of alveolar macrophage maturation in rabbits. Effects of granulomatous pneumonitis. J Clin Invest 73: 1524–1531, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rusch GM, Garrett R, Tobin P, Falke E, Lu PY. The development of acute exposure guideline levels for hazardous substances. Drug Chem Toxicol 25: 339–348, 2002 [DOI] [PubMed] [Google Scholar]
- 33. Shi J, Gilbert GE. Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid binding sites. Blood 101: 2628–2636, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Somani SM, Babu SR. Toxicodynamics of sulfur mustard. Int J Clin Pharmacol Ther Toxicol 27: 419–435, 1989 [PubMed] [Google Scholar]
- 35. Stampfuss JJ, Censarek P, Bein D, Schrör K, Grandoch M, Naber C, Weber AA. Membrane environment rather than tissue factor expression determines thrombin formation triggered by monocytic cells undergoing apoptosis. J Leukoc Biol 83: 1379–1381, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 104: 4–10, 1980 [DOI] [PubMed] [Google Scholar]
- 37. Szinicz L. History of chemical and biological warfare agents. Toxicology 214: 167–181, 2005 [DOI] [PubMed] [Google Scholar]
- 38. Traber DL, Hawkins HK. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol 29: 295–302, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Veress LA, O'Neill HC, Hendry-Hofer TB, Loader JE, Rancourt RC, White CW. Airway obstruction due to bronchial vascular injury after sulfur mustard analog inhalation. Am J Respir Crit Care Med 182: 1352–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warthin AS, Weller CV. The lesions of the respiratory and gastrointestinal tract produced by mustard gas (dichlorethyl sulphide). J Lab Clin Med 4: 229–264, 1919 [Google Scholar]
- 41. Winternitz MC. Anatomical changes in the respiratory tract initiated by irritating gases. Military Surgeon 44: 476–493, 1919 [Google Scholar]
- 42. Yang YC, Szafraniec LL, Beaudry WT, Ward JR. Kinetics and mechanism of the hydrolysis of 2-chloroethyl sulfides. J Org Chem 14: 3293–3297, 1988 [Google Scholar]
- 43. Zhang Z, Peters BP, Monteiro-Riviere NA. Assessment of sulfur mustard interaction with basement membrane components. Cell Biol Toxicol 11: 89–101, 1995 [DOI] [PubMed] [Google Scholar]