Abstract
Mucociliary clearance is the primary innate physical defense mechanism against inhaled pathogens and toxins. Vectorial ion transport, primarily sodium absorption and anion secretion, by airway epithelial cells supports mucociliary clearance. This is evidenced by diseases of abnormal ion transport such as cystic fibrosis and pseudohypoaldosteronism that are characterized by changes in mucociliary clearance. Sodium absorption and chloride secretion in human bronchial epithelial cells depend on potassium channel activity, which creates a favorable electrochemical gradient for both by hyperpolarizing the apical plasma membrane. Although the role of basolateral membrane potassium channels is firmly established and extensively studied, a role for apical membrane potassium channels has also been described. Here, we demonstrate that bupivacaine and quinidine, blockers of four-transmembrane domain, two-pore potassium (K2P) channels, inhibit both amiloride-sensitive sodium absorption and forskolin-stimulated anion secretion in polarized, normal human bronchial epithelial cells at lower concentrations when applied to the mucosal surface than when applied to the serosal surface. Transcripts from four genes, KCNK1 (TWIK-1), KCNK2 (TREK-1), KCNK5 (TASK-2), and KCNK6 (TWIK-2), encoding K2P channels were identified by RT-PCR. Protein expression at the apical membrane was confirmed by immunofluorescence. Our data provide further evidence that potassium channels, in particular K2P channels, are expressed and functional in the apical membrane of airway epithelial cells where they may be targets for therapeutic manipulation.
Keywords: chloride secretion, cystic fibrosis, ion transport
the respiratory system is constantly challenged by inhaled pathogens, toxins, and particulate material. Turbulent airflow in the nasal passages and larger airways leads to deposition of most of these potentially harmful agents in the conducting airways, thereby protecting the alveolar epithelium. Removal of trapped debris and pathogens from conducting airways is accomplished primarily through mucociliary clearance (MCC). Normal MCC in the lungs comprises the organized propulsion of fluid and mucus on the airway surface from distal to proximal airways by ciliary beating. Pathogens and debris are eventually cleared from the respiratory tract by expectoration or swallowing.
Normal MCC is critically dependent on vectorial ion transport by airway epithelial cells, which modulates fluid balance at the airway surface. For example, patients with pseudohypoalderonism type I have loss of function mutations in the epithelial sodium (Na+) channel (ENaC) resulting in increased lung fluid and accelerated MCC (17). In contrast, patients with cystic fibrosis (CF) have severely impaired MCC due to loss of function mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), the predominant cAMP-dependent anion channel of airway epithelial cells (1, 5, 27). One hypothesis that is well supported both in vitro (23) and in vivo (22) regarding the underlying pathophysiology of CF lung disease is that absent CFTR function leads to hyperabsorption of Na+ via ENaC and concomitant paracellular passage of chloride (Cl−) leading to iso-osmotic volume loss of airway surface liquid (4). An alternative and not mutually exclusive hypothesis is that lack of CFTR leads to hyposecretion of fluid from either submucosal glands, surface epithelium, or both. Support for the latter hypothesis comes from ex vivo studies of submucosal glands (16) and from a novel, in vivo porcine model of CF (5).
In human airways epithelium, Na+ absorption, and Cl− secretion depend on favorable electrochemical driving forces. This is accomplished by the activity of the Na+-potassium (K+)-ATPase that uses energy released from ATP hydrolysis to move Na+ and K+ against their electrochemical gradients at the basolateral membrane and the presence of K+ channels that provide a conductive exit pathway out of the cell for K+, thus hyperpolarizing cell membrane potential (9). It is established that active ion transport by Cl− secreting epithelia is dependent on basolateral membrane K+ channels and electrical interdependence of the two membranes (31). However, data also support the presence and function of apical membrane K+ channels (3, 25). Many K+ channels from each of the three major topological classes (six-transmembrane, four-transmembrane, and two-transmembrane; Ref. 2) have been identified in airway epithelial cells and are thought to play a role in modulating ion transport as well as other vital epithelial functions (2).
Based on the observation that four transmembrane domain, two-pore K+ (K2P) channels are found at the apical plasma membrane of Calu-3 cells (8), which are derived from a lung adenocarcinoma (30), we hypothesized that these channels would also be found in the apical membrane of surface airway cells. Here, we demonstrate that Na+ absorption and Cl− secretion in normal human bronchial epithelial (HBE) cells are sensitive to inhibition by pharmacological agents that block K2P channels and that this sensitivity differs depending on the membrane to which the drugs are applied. Furthermore, we demonstrate by RT-PCR that mRNA transcripts from multiple KCNK genes that encode K2P channels can be identified in HBE cells, and we demonstrate by immunofluorescence that K2P channels are located in the apical domain of HBE cells. Taken together, these data support the hypothesis that K2P channels are expressed and function at the apical membrane of surface epithelial cells and, therefore, that inhaled K+ channel modulators might be useful as therapeutic agents for modulating MCC.
MATERIALS AND METHODS
Cell culture.
Normal HBE cells in frozen aliquots were purchased from Lonza (Walkersville, MD) and expanded in bronchial epithelial cell growth medium (BEGM) supplemented with Singlequots for BEGM (Lonza). When cells had reached ∼90% confluence, they were collected and frozen in liquid nitrogen at a density of 1 × 106 cells/ml. Frozen, passage 2 cells were then reexpanded in a 75-cm2 tissue culture flask in hormonally defined BEGM as before freezing. These cells were collected by trypsinization and seeded onto permeable supports (Transwell #3470; Costar) in media containing 50% DMEM in BEGM. The supplements were adjusted such that triiodothyronine was removed and the final retinoic acid concentration was set at 50 nM (all-trans retinoic acid; Sigma). Cells were seeded at a density of ∼8 × 104 per 0.33 cm2. HBEs were maintained submerged for the first 5–7 days in culture after which the apical medium was removed. The medium was refreshed twice weekly and the day before any experimentation. At all stages of culture, cells were maintained at 37°C in 5% CO2 in an air incubator. Under these conditions, HBEs formed a well-differentiated mucociliary phenotype with the classical ion transport phenotype associated with this tissue (12). A total of eight lots of HBE cells were used to complete these studies.
Solutions.
The normal bath solution for Ussing chamber experiments contained the following (in mM): 120 NaCl, 25 NaHCO3, 3.3 KH2PO4, 0.8 K2HPO4, 1.2 MgCl2, 1.2 CaCl2, and 10 glucose. All experiments in the Ussing chamber were performed in the presence of 95% O2-5% CO2 at 37°C to maintain physiological pH and temperature. For experiments in which a K+ gradient was generated, the normal bath solution served as the low-K+ solution and the high-K+ solution was created by equimolar replacement of 120 mM NaCl with 120 mM KCl. For experiments in which a Na+ gradient was generated, the normal bath solution served as the high Na+ solution and the low Na+ solution was created by equimolar replacement of 120 mM NaCl with 120 mM NMDG-gluconate. For experiments in which a Cl− gradient was generated, the normal bath solution served as the high Cl− solution and the low Cl− solution was created by equimolar replacement of 120 mM NaCl with 120 mM Na-gluconate.
Short circuit current measurements.
Short circuit current (Isc) measurements were performed as previously described (28). Briefly, Transwell inserts were mounted in a modified, vertical Ussing chamber (Physiologic Instruments, San Diego, CA), and the monolayers were voltage-clamped continuously to 0 mV after fluid resistance compensation using an automatic voltage clamp (VCC 600; Physiologic Instruments). Isc was digitized at 1 Hz, and data were stored on a computer hard drive using Acquire and Analyze software build 2.3.0 (Physiologic Instruments). Transepithelial resistance (Rt) was calculated automatically by the software using a 3-mV, 640-ms bipolar voltage pulse in between data record points.
Isc was allowed to stabilize at the beginning of each experiment and after each drug application. By convention, an upward deflection in the Isc tracing represents net serosal to mucosal anion movement (secretion) or mucosal to serosal cation movement (absorption). Where mean Isc data are presented, they are shown as change in Isc from the stable Isc recorded before and after each drug application.
Chemicals.
Bupivacaine (Sigma) and quinidine (Sigma) were dissolved in DMSO and applied at the indicated concentrations. Equivalent volumes of 100% DMSO were used as vehicle control. BaCl2 (Sigma) was dissolved in distilled, deionized water as a 1,000× stock. Forskolin (Calbiochem, EMD, San Diego, CA) was dissolved in DMSO as a 5,000× stock. Ouabain was dissolved in 100% EtOH as a 5,000× stock. Nystatin (Sigma), valinomycin (Calbiochem), and nigericin (Calbiochem) were dissolved in DMSO and used at the stated concentrations.
RT-PCR.
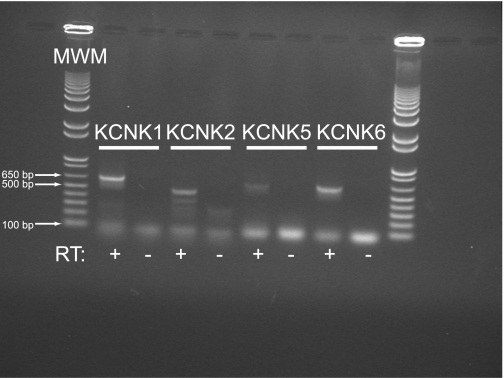
HBE cells were lysed in Trizol (Invitrogen), and total RNA was extracted per manufacturer's protocol. First-strand cDNA synthesis was performed using olig-dT primers and reverse transcription reagents from Applied Biosystems. Gene-specific PCR was carried out with the primer pairs detailed in Table 1, using Taq PCR Supermix (Invitrogen) and the following conditions: 94°C × 2 min (1 cycle), 94°C × 30 s, 58–60°C (optimized for each primer pair) × 30 s, 68°C × 1 min (40 cycles), and 68°C × 5 min (1 cycle). PCR products were separated on 1% agarose gels, and DNA was detected using ethidium bromide. Negative control PCR was performed using total RNA from reverse transcriptase negative first-strand synthesis reactions. Positive PCR samples from separate runs were consolidated on a single representative gel for presentation. A transcript was considered to be detectable if positive RT-PCR results were obtained from at least two separate donors.
Table 1.
Gene-specific primers used to identify KCNK transcripts by RT-PCR
| Gene | Forward Primer | Reverse Primer | Product Size |
|---|---|---|---|
| KCNK1 | 5′-CACCGTGCTCTCCACCACAGG-3′ | 5′-TTGCAGGGCCATCCACGCAG-3′ | 667 bp |
| KCNK2 | 5′-ATCGGAGCCACCGTGTTCAAAGC-3′ | 5′-CGCAGGCAGAGCCACAAAGAG-3′ | 503 bp |
| KCNK5 | 5′-GGTGGCCAGGGCGCTGCAAG-3′ | 5′-GGTGCAGGGCAGGAGGGCAG-3′ | 634 bp |
| KCNK6 | 5′-CTGGACGCCTTCGTGGAGCG-3′ | 5′-GTCGGACACGTGGCGGAAGG-3′ | 608 bp |
Immunohistochemistry.
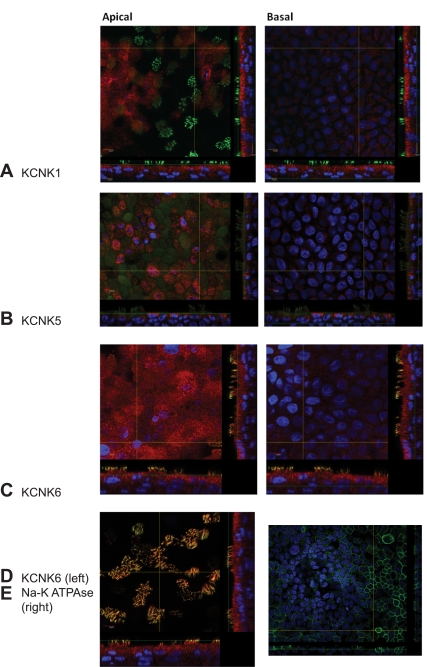
HBE cells were grown to maturity on Transwell permeable supports (usually achieved ∼4 wk after passage to permeable supports). Cells were washed three times with PBS followed by fixation in cold 4% paraformaldehyde for 20 min at room temperature. The permeable support was again washed three times with PBS before excision of the membrane containing the cells from its plastic supports. The membrane was then immersed in PBS with 0.3% Triton X-100, 5% normal goat serum, and 1% BSA for 60 min at room temperature to permeabilized the plasma membrane and block nonspecific binding sites. Following blocking, membranes were washed three times with PBS. Primary antibodies against KCNK1 (Sigma no. hpa016049; 1:200), KCNK5 (Novus Biologicals clone 2B4; 1:1,000), or KCNK6 (Abcam no. ab84208; 1:2,500) were applied overnight at 4°C in the presence of primary mouse antibody against type IV β-tubulin (KCNK1 and KCNK6) or primary rabbit antibody against all subtypes of β-tubulin (KCNK5). Following overnight incubation, membranes were washed with PBS and appropriate biotinylated goat IgG secondary antibody (1:750 in PBS) was applied for 60 min at room temperature. Unbound secondary antibody was removed by three PBS washes, and cy3-labeled avidin (streptavidin-Cy3; 1:750 in PBS; Sigma) plus a FITC-labeled fluorescent secondary antibody (1:100) against either mouse (KCNK1 and KCNK6) or rabbit (KCNK5) and Hoechst (1:10,000 in PBS) were applied for 60 min at room temperature. In this way, proteins of interest were detected by cy3 staining, whereas tubulin was counterstained with FITC and nuclei were labeled with Hoescht. Individual membranes were then mounted on glass slides, covered with a coverslip, and placed in the dark at 4°C until the mounting medium had cured (usually 10–14 days). Images were obtained on an Olympus IX81 confocal microscope using Fluoview software.
Data and statistical analysis.
Unless otherwise stated, values are means ± SE for all inserts tested. Fifty-percent inhibitory concentrations (IC50) were calculated using Prism 5 (GraphPad Software, San Diego, CA) by comparing log10[inhibitor] vs. normalized Isc. Statistical analysis was also performed with Prism 5. Comparisons between groups were made with either Student's t-test or with two-way ANOVA followed by Bonferroni posttest analysis for significance between groups as appropriate. Significance was defined as a P ≤ 0.05.
RESULTS
A total of 102 HBE inserts representing eight nondiseased, deceased donors were used for these studies. Each experiment was performed on cells from at least two donors using at least two biological replicates. At baseline, the mean Isc and Rt for all tested inserts were 16 ± 1 μA/cm2 and 728 ± 54.1 Ωcm2, respectively.
Concentration-response studies with bupivacaine and quinidine.
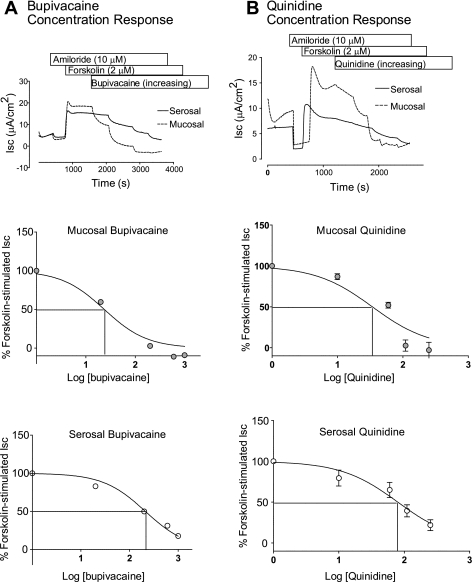
We first tested the hypothesis that pharmacological inhibitors of K2P channels could affect electrogenic, vectorial ion transport in HBE cells. Inserts were mounted in a modified, vertical Ussing chamber and bathed in symmetrical HCO3−-buffered solutions as described in materials and methods. Two order-of-addition protocols were performed to investigate the effects of the K2P channel blockers bupivacaine, a local anesthetic agent, and quinidine, a class IA antiarrhythmic agent (21).
First, amiloride and forskolin were sequentially added to promote CFTR-dependent Cl− secretion in the absence of ENaC-dependent Na+ absorption. Bupivacaine applied to either the mucosal or serosal bath inhibited forskolin-stimulated Isc, a measure of CFTR-dependent Cl− secretion in HBE cells (Fig. 1A). This inhibition was dose-dependent with IC50 values of 23.5 and 211 μM when bupivacaine was applied to the mucosal or serosal bath, respectively. Additionally, the maximum amount of forskolin-stimulated Isc inhibited by bupivacaine was dependent on the bath to which the bupivacaine was added with maximal inhibitions of 100% (mucosal addition) and 82% (serosal addition) of forskolin-stimulated Isc. Similar experiments were carried out with quinidine (Fig. 1B). Addition of quinidine to either the mucosal or serosal bath resulted in a dose-dependent inhibition of forskolin-stimulated Isc with IC50 values of 34 and 79 μM, respectively, and maximal inhibitions of 103 and 78% of forskolin-stimulate Isc, respectively.
Fig. 1.
Bupivacaine and quinidine inhibit forskolin-stimulated short circuit current (Isc). Well-differentiated human bronchial epithelial (HBE) cells were mounted in modified, vertical Ussing chambers. Amiloride and forskolin were sequentially followed by addition of either bupivacaine (A) or quinidine (B) in increasing concentrations (0, 20, 200, 600, and 1,000 μM). Top: representative Isc tracings demonstrating that both bupivacaine and quinidine inhibit forskolin-stimulated Isc and that these effects are dependent on whether the inhibitor is added to the mucosal or serosal bath. Middle: concentration-response curve comparing percentage of forskolin-stimulated Isc to concentration of inhibitor added to the mucosal bath. Bottom: concentration-response curve comparing percent of forskolin-stimulated Isc to concentration of inhibitor added to the serosal bath. Solid lines denote IC50.
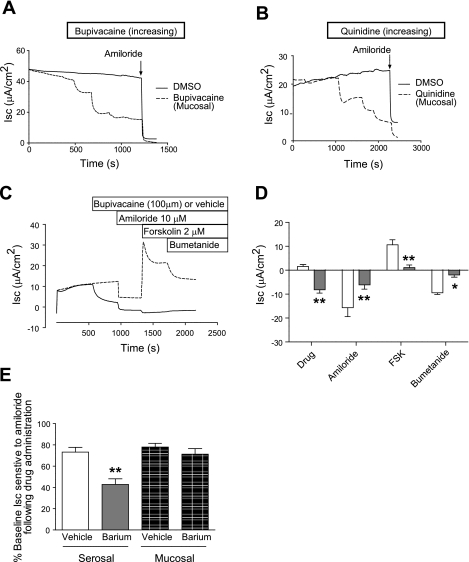
Next, either bupivacaine or quinidine was added to the mucosal bath of HBE cells before the addition of other pharmacological agents. Both bupivacaine (Fig. 2A) and quinidine (Fig. 2B) inhibited basal Isc in HBE cells with IC50 values of 70 and 47 μM, respectively, and maximal inhibition of 70% for each. Amiloride-sensitive Isc usually represents ∼70% of resting Isc in HBE cells. Therefore, we compared amiloride-sensitive Isc after a single addition of mucosal bupivacaine (100 μM) or DMSO. Mucosal addition of bupivacaine significantly inhibited amiloride-sensitive Isc (Fig. 2, C and D), and the continued presence of bupivacaine in the mucosal bath also prevented forskolin from stimulating Isc (Fig. 2C). We next performed a similar experiment using a single addition of BaCl2 (1 mM), an inhibitor of many K+ channels. BaCl2 applied to the serosal bath, but not the mucosal bath, significantly reduced amiloride-sensitive Isc (Fig. 2E).
Fig. 2.
Bupivacaine and quinidine inhibit amiloride-sensitive Isc. Well-differentiated HBE cells were mounted in modified, vertical Ussing chambers. Bupivacaine (A) or quinidine (B) was added to the mucosal bath in increasing concentrations (0, 20, 200, 600, and 1,000 μM) before the addition of amiloride. In a separate set of experiments (C, representative tracing), bupivacaine (100 μM) was added to the mucosal bath followed by the addition of amiloride, forskolin (FSK), and bumetanide. Note that bupivacaine significantly inhibited amiloride-sensitive Isc while almost completely eliminating forskolin-stimulated Isc (D, mean change in Isc with each condition; n = 5 vehicle and 6 bupivacaine-treated inserts representing 2 cell lots; **P < 0.01, *P < 0.05, by two-way ANOVA followed by Bonferroni posttests). E: barium (1 mM) added to the serosal bath, but not the mucosal bath, significantly inhibited amiloride-sensitive Isc (n = 4 inserts representing 2 cell lots for each condition; *P < 0.05, by one-way ANOVA followed by Tukey's multiple comparison test).
Permeabilization experiments.
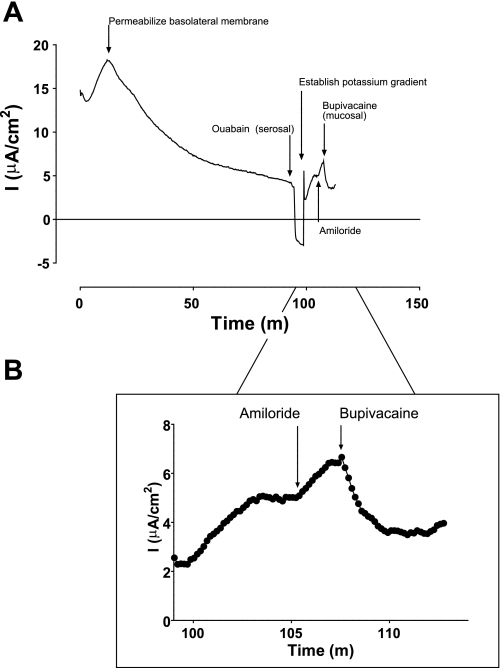
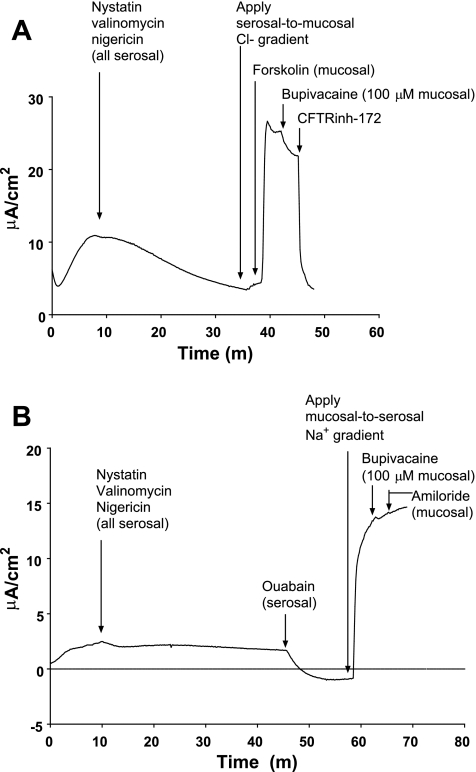
In response to our observation that pharmacological sensitivity of Isc to inhibitors differed depending on the bath to which each was applied, we performed permeabilization experiments to identify a K+ conductance at the apical membrane of HBE cells. To do so, HBE cells were mounted in modified, vertical Ussing chambers in symmetrical solutions, and nystatin (60 μM), nigericin (20 μM), and valinomycin (20 μM) were added to the serosal solution (26). Over 90 min following the addition of ionophores, Isc decreased towards, but did not reach, 0 μA/cm2. When Isc stabilized, ouabain was added to the serosal solution, causing Isc to reach or fall below 0 μA/cm2 (Fig. 3A). The mucosal solution was then exchanged with 12 vol (60 ml) of high-K+, low-Na+ solution, creating a serosal-to-mucosal Na+ gradient and a mucosal-to-serosal K+ gradient. Addition of amiloride to the mucosal solution caused an upward deflection of Isc, consistent with inhibition of Na+ diffusion down its serosal-to-mucosal concentration gradient, thus confirming permeabilization of the basolateral membrane (Fig. 3A). Finally, bupivacaine (100 μM) was added to the mucosal solution. Bupivacaine addition caused a downward deflection of Isc (−0.85 ± 0.15 μA; n = 6; P = 0.003, by paired t-test) consistent with inhibition of K+ diffusion down its concentration gradient through an apical membrane K+ conductance (Fig. 3, A and B, magnification). In separate experiments, we tested the hypotheses that bupivacaine would inhibit Cl− conductance through CFTR and Na+ conductance through ENaC in permeabilized HBE monolayers. These experiments demonstrated only minimal effects of bupivacaine on CFTR but were inconclusive with respect to the effects of bupivacaine on ENaC (Fig. 4).
Fig. 3.
A bupivacaine-sensitive K+ conductance at the apical membrane of HBE cells. Well-differentiated HBE cells were mounted in modified, vertical Ussing chambers followed by the addition of nystatin (60 μM), nigericin (20 μM), and valinomycin (20 μM). After ∼90 min, ouabain was added to silence the Na+-K+-ATPase. The mucosal solution was then exchanged for one containing low Na+ and high K+, creating opposing concentration gradients. Note that addition of amiloride to the mucosal bath caused an upward deflection in the current tracing, confirming that Na+ was moving from serosal to mucosal across the apical membrane. Subsequent addition of bupivacaine caused a downward deflection in the current tracing consistent with inhibition of an apical membrane K+ conductance. A: representative current tracing. B: detail of the current tracing following establishment of ionic gradients.
Fig. 4.
Effects of bupivacaine on isolated Cl− and Na+ gradients in permeabilized HBE cells. A: representative current tracing of experiment in which apical membrane Cl− conductance is isolated. Note that bupivacaine (100 mM) has only minimal effects on CFTR conductance compared with CFTRinh-172. At this dose in nonpermeabilized cells, bupivacaine inhibited virtually all forskolin-stimulated short-circuit current. B: representative current tracing in which apical membrane Na+ conductance is isolated. Note that neither bupivacaine nor amiloride has a substantial effect on diffusive Na+ current, suggesting that paracellular Na+ movement accounts for much of the observed current. Given this observation, we cannot firmly conclude that bupivacaine does not inhibit epithelial sodium (Na+) channel.
RT-PCR.
Our pharmacological data strongly suggested the presence of K2P channels in HBE cells. Therefore, we performed RT-PCR using oligo-dT primers for first-strand cDNA synthesis to identify mRNA from KCNK gene transcription. Using the gene-specific primers detailed in Table 1, we identified mRNA from KCNK1, KCNK2, KCNK5, and KCNK6 (Fig. 5). In each case, a band of the predicted size was detected in the reverse-transcriptase positive samples, but not in the reverse-transcriptase negative samples, confirming that amplification was not due to the presence of genomic DNA contamination in our samples. Multiple bands smaller than the predicted band were detected using the KCNK2 primers, possibly representing nonspecific priming of cDNA. Other KCNK transcripts were not reliably or reproducibly identified (data not shown). In addition, we identified KCNN4 and KCNQ1, both of which have previously been shown to regulate vectorial ion transport in airway epithelial cells (7, 24) (data not shown).
Fig. 5.
Multiple KCNK gene transcripts can be detected in HBE cells. Total RNA was isolated from mature, well-differentiated HBE cells. First-strand cDNA synthesis was accomplished using oligo-dT primers to preferentially target mRNA. PCR was performed using the gene-specific primers detailed in Table 1. Control reactions were performed with total RNA that was processed as for reverse transcription but in the absence of the reverse transcriptase enzyme (RT−). PCR products of the expected size were detected for KCNK1, KCNK2, KCNK5, and KCNK6. MWM, molecular weight marker.
Immunohistochemistry.
To confirm protein expression and evaluate cellular localization, we performed immunofluorescence studies. The protein encoded by each transcript that was identified by RT-PCR, except for KCNK2 for which only nonspecific signal was detected (data not shown), was also identified by immunofluorescence (Fig. 6). Moreover, immunofluorescence signal for each protein was found predominantly toward the apical membrane, though in slightly different patterns. For example KCNK1 and KCNK6 were found diffusely at the apical membrane, whereas KCNK5 staining was punctate and may have been subapical as previously described (8), although our immunofluorescence likely does not provide the resolution to detect whether a protein is in the apical membrane or resides in a subapical compartment. In addition to robust expression at the apical membrane of the cells, KCNK6 was uniquely found to coexpress with type IV tubulin (Fig. 5D), suggesting it is localized in cilia proper. For comparison and to highlight the apical staining of the KCNK channels, we also demonstrate immunofluorescence for the beta subunit of the Na+-K+-ATPase (Fig. 6E), which is expressed predominantly at the basolateral membrane.
Fig. 6.
Immunofluorescence confirms the presence of KCNK channels at the apical membrane. Using commercially available antibodies, we performed immunofluorescence studies on well-differentiated HBE cells. The channel of interest is represented in red, and beta-tubulin is counterstained in green. Nuclei are in blue. We detected each channel seen by RT-PCR except KCNK2. Each KCNK channel (A: KCNK1; B: KCNK5; and C: KCNK6) was found toward the apical aspect of the monolayer (left) more so than at the basal aspect of the monolayer (right). KCNK6 was uniquely identified in cilia where it colocalized with β-tubulin (D). Immunofluorescence for the β-subunit of the Na+-K+-ATPase (E) is demonstrated for comparison.
DISCUSSION
Our data demonstrate for the first time that K2P channels are expressed and functional in the apical membrane of HBE cells. This conclusion is supported by pharmacological studies that demonstrate a difference in sensitivity of Isc to bupivacaine at the apical and basolateral membranes; by permeabilization studies that demonstrate a consistent, bupivacaine-sensitive K+ conductance at the apical membrane; and by RT-PCR and immunofluorescence studies that respectively demonstrate mRNA expression and apically located protein expression of these channels. These data are in agreement with and extend the observations of Davis and Cowley (8) who found similar K2P expression patterns and pharmacological inhibition profiles in Calu-3 cells, a commonly studied airway epithelial cell line that bears resemblance to submucosal gland serous cells (30).
Membrane-dependent inhibition of forskolin-stimulated Isc by K2P channel inhibitors.
We demonstrate that the IC50 for bupivacaine and quinidine-mediated inhibition of forskolin-stimulated Isc were a full log and a half-log lower, respectively, at the apical vs. the basolateral membrane and that maximal inhibition of Isc was also greater when these drugs were applied to the apical membrane. Unlike the strong inhibition of Isc by bupivacaine and quinidine when applied to the apical membrane, we did not observe an effect of mucosal Ba2+ (Fig. 2E), consistent with the presence of K2P channels, which are relatively Ba2+ resistant (15), although it is possible that bupivacaine and quinidine pass into the cell and that Ba2+ cannot access this space.
These data are strongly suggestive of predominant channel expression at the apical membrane, although we cannot completely exclude the possibility that differences in membrane permeability or accessibility of the drug to the membrane account for some of the difference. For example, if the collagen matrix on which the cells are grown impeded drug access to the basolateral membrane, this might result in a higher IC50 for drug applied to the serosal bath, whether it was acting at the basolateral membrane or the apical membrane. In addition, our concentration-response curves are derived from experiments in Ussing chambers rather than on single channels in patch pipettes. Nonetheless, the IC50 values that we calculated are in general agreement with published values, particularly for those seen in TASK channels (21).
If channel number alone were different at the apical vs. basolateral membrane, one might expect to see differences in maximal inhibition but not necessarily a log-fold difference in IC50. Our immunofluorescence data suggest that K2P channels are distributed differently throughout the HBE monolayers both with respect to cell type and intracellular location. Therefore, different ratios of one channel to another at the apical vs. basolateral membranes may account for some of the observed differences in pharmacological inhibition. Because of these potential confounders, we proceeded to isolate apical membrane conductance by rendering the basolateral membrane permeable to cations and silencing the Na+-K+-ATPase (26). The results of these studies (Fig. 3) strongly support the conclusion that there is a bupivacaine-sensitive K+ conductance at the apical membrane.
The observation that application of sufficient bupivacaine or quinidine to the mucosal surface of amiloride-inhibited, forskolin-stimulated HBE cells causes Isc to go to zero, suggests that these channels play a role in maintaining hyperpolarization of apical membrane potential. In HBE cells grown under similar conditions, apical membrane potential when measured by microelectrode impalement in the presence of amiloride was approximately −60 mV (20). Such a negative apical membrane potential provides a large driving force for anion secretion because the apical membrane equilibrium potential for Cl− was measured to be approximately −25 mV in polarized human epithelial cells under similar ionic conditions, although it should be noted that these were nasal epithelial cells and not lower airway cells (33). CFTR activation by forskolin-mediated stimulation of cAMP/PKA signaling provides an apical membrane pathway for Cl− secretion. In response to the opening of this conductance and subsequent Cl− secretion, the apical membrane potential shifts toward the equilibrium potential for Cl−. However, the presence of K+ channels at the basolateral membrane, the apical membrane, or both maintains the apical membrane potential at a value more negative than the Cl− equilibrium potential thereby providing a driving force for sustained Cl− secretion. There is good evidence demonstrating the activity of basolateral membrane K+ channels that cause hyperpolarization of the apical membrane through interdependence of the apical and basolateral membranes (11, 13, 31). However, the presence and activity of basolateral membrane K+ channels are not mutually exclusive with the presence and activity of apical membrane channels. As pointed out by Davis and Cowley (8), mathematical modeling of Cl− secretion by Cook and Young (6) predicts that the presence of apical membrane K+ channels is optimal for anion secretion. Therefore, one possible interpretation of our data is that blocking apical membrane K+ channels in the presence of amiloride and forskolin shifts the apical membrane potential completely to the Cl− equilibrium potential resulting in virtual cessation of secretion seen as an Isc of zero.
Another possible explanation of our data is that our inhibitors are acting directly on ENaC or CFTR to inhibit resting and forskolin-stimulated Isc, respectively. We attempted to address this issue using permeabilization experiments. These data suggest that bupivacaine has little effect on isolated CFTR conductance even at concentrations that inhibited all forskolin-stimulated Isc (Fig. 4A). Data regarding the effects of ENaC were inconclusive because neither bupivacaine nor amiloride inhibited diffusive Na+ currents in permeabilized cells (Fig. 4B) and, therefore, we cannot completely exclude the possibility that bupivacaine acts directly on ENaC.
Inhibition of amiloride-sensitive Isc and prevention of forskolin-stimulated Isc by K2P inhibitors.
We next performed experiments in which we altered the order of inhibitor addition such that bupivacaine or quinidine was added before amiloride. We demonstrate that both bupivacaine and quinidine inhibited basal, electrogenic Na+ absorption, which represents ∼75% of basal Isc in HBE cells in our experience (19–20). Similar to our results with inhibition of forskolin-stimulated Isc, we found membrane-dependent differences in the sensitivity of basal, amiloride-sensitive Isc to bupivacaine and quinidine. This observation extends those of Davis and Cowley (8), who studied non-Na+ absorbing cells, and those of Inglis et al. (15), who studied the effects of basolateral membrane K2P K+ channels in NCI-H441 cells. These experiments also demonstrate that bupivacaine and quinidine can prevent cAMP-mediated, CFTR-dependent Isc when the inhibitor is added before forskolin. The observation that either bupivacaine or quinidine applied to the mucosal surface can inhibit basal Isc in HBE cells suggests that K2P channels provide a constitutively active K+ conductance at the apical membrane. This conclusion is also supported by the observation of a small, but appreciable, K+ conductance at the apical membrane of HBE cells with the basolateral membrane rendered permeable to cations. Other investigators have also found constitutively active K+ conductances in airway epithelial cells (10). Furthermore, one might expect K2P channels to be constitutively active given their well-described role as K+ leak channels (18).
Multiple KCNK channels are expressed in normal HBE cells.
There are ≥15 different proteins encoded by KCNK genes. To identify which of these genes were transcribed in our model system, we performed RT-PCR. In agreement but not identical to those transcripts found by Davis and Cowley (8), we found transcripts for KCNK1, KCNK2, KCNK5, and KCNK6 (Fig. 4), suggesting that these genes are important in the physiology of both surface epithelial cells and submucosal gland serous cells. Immunofluorescence staining for the proteins encoded for by these genes demonstrated predominantly apical staining. Each protein appeared to be expressed in both ciliated and nonciliated cells and somewhat diffusely across the mucosal surface of the culture. Notably, KCNK6 appeared to be expressed in cilia, where it could serve as a chemical sensor. This novel function for motile cilia has recently been postulated because of the presence of taste receptors on airway cilia (29). Nonetheless, we cannot speculate as to the specific channel(s) that is responsible for the observed effects on ion transport. Ideally, to define which channel is responsible or to determine if there is redundancy among the expressed channels, studies would be performed on cells that lack protein expression of one or more of the expressed KCNK channels. Inhibiting expression of even a single protein in primary, normal HBE cells has proven to be difficult, and future studies will need to be undertaken to determine if multiple channels can be eliminated simultaneously to isolate the function of single channels in this model system.
The hypothesis that functional K+ channels reside in the apical membrane of airway epithelial cells has gained increasing support in the literature. Evidence for apical membrane expression exists for all three major topological classes of K+ channels: six-transmembrane domain, four-transmembrane domain, and two-transmembrane domain. Of the previously published studies reviewed by Bardou et al. (2), those dealing two- and four-transmembrane domain channels were less numerous compared with those describing the role of six-transmembrane domain channels, which include the voltage-gated and Ca2+-activated channels. For example, there is pharmacological, immunohistochemical, and function evidence for KCNQ family members in the apical membrane of bronchial epithelial cells and Calu-3 cells (24, 26), and there is evidence for calcium-activated K+ channels in the apical membrane of distal lung epithelial cells (14). With respect to the model systems in which these channels have been identified, to our knowledge this is the first time that K2P channels have been studied in primary or normal HBE cells, but not the first time that K2P or other K+ channels have been studied in the apical membrane of lung epithelial cells. In addition to the work of Davis and Cowley (8), work by Wu et al. (34) demonstrated evidence for expression of inwardly rectified K+ channels at the apical membrane of Calu-3 cells, and the work by Han et al. (14) suggests a role for apical membrane K+ conductance in regard to clearance of alveolar clearance in vivo. Moreover, work by Namkung et al. (26) demonstrates that airway epithelial cells express KCNQ family members in the apical membrane and are capable of secreting K+ to regulate airway surface liquid K+ concentration, which as would be expected for a cell with parallel K+ and Na+ conductances is above that of extracellular K+.
Taken together, there is now strong evidence not only that K+ channels are expressed at the apical membrane of airway and alveolar epithelial cells. The diversity of K+ channels found at the apical membrane of lung epithelial cells may indicate the importance of K+ channels at the apical membrane as well as diverse and potentially redundant functionality. This redundancy is likely to be a significant challenge if these channels are targeted for therapeutic manipulation. Additionally, because many of the K+ channels found at the apical membrane of airway epithelial cells are also expressed in the heart, nervous system, kidneys, and elsewhere (18), therapy aimed at modulating these channels in the lung could potentially have unwanted side effects on other organ systems. For example, administration of bupivacaine has been associated with seizure activity. Therefore, bupivacaine itself might not be an optimal choice for an inhaled therapeutic compared with amiloride, which appears to be well tolerated when used as an inhaled medication (32). Nonetheless, the finding that K2P and other K+ channels are expressed and functional at the apical membrane of airway and alveolar epithelial cells raises the possibility that they are potential targets of inhaled therapeutics or topical therapeutics in the case of sinonasal disease. Our data suggest that highly selective inhibitors of these channels might serve as therapeutic agents capable of reducing Na+ absorption in the airways, which has been shown to improve mucociliary clearance, for example, with the use of inhaled amiloride (32). Alternatively, in the presence of a drug to inhibit ENaC, highly selective activators of these channels might serve as therapeutic agents capable of maximizing Cl− secretion in the airways.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant K08-HL-081080 (to J. L. Kreindler) and by institutional funds from the Children's Hospital of Philadelphia.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
K.-Q.Z., G.X., M.W., and J.L.K. performed experiments; K.-Q.Z. and J.L.K. interpreted results of experiments; K.-Q.Z., G.X., M.W., N.A.C., and J.L.K. edited and revised manuscript; K.-Q.Z., G.X., M.W., N.A.C., and J.L.K. approved final version of manuscript; N.A.C. and J.L.K. conception and design of research; N.A.C. and J.L.K. analyzed data; J.L.K. prepared figures; J.L.K. drafted manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge Adam J. Ratner and Ronald C. Rubenstein for helpful discussion and assistance in preparation of the manuscript.
REFERENCES
- 1. Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science 253: 202–205, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Bardou O, Trinh NT, Brochiero E. Molecular diversity and function of K+ channels in airway and alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L145–L155, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Bernard K, Bogliolo S, Soriani O, Ehrenfeld J. Modulation of calcium-dependent chloride secretion by basolateral SK4-like channels in a human bronchial cell line. J Membr Biol 196: 15, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23: 146–158, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143: 911–923, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook DI, Young JA. Effect of K+ channels in the apical plasma membrane on epithelial secretion based on secondary active Cl− transport. J Membr Biol 110: 139–146, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Cowley EA, Linsdell P. Characterization of basolateral K+ channels underlying anion secretion in the human airway cell line Calu-3. J Physiol 538: 747–757, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis KA, Cowley EA. Two-pore-domain potassium channels support anion secretion from human airway Calu-3 epithelial cells. Pflügers Arch 451: 631–641, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Dawson DC, Richards NW. Basolateral K conductance: role in regulation of NaCl absorption and secretion. Am J Physiol Cell Physiol 259: C181–C195, 1990 [DOI] [PubMed] [Google Scholar]
- 10. Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and ΔF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol 279: C461–C479, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Devor DC, Singh AK, Bridges RJ, Frizzell RA. Modulation of Cl− secretion by benzimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. Am J Physiol Lung Cell Mol Physiol 271: L785–L795, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Gray TE, Guzman K, Davis CW, Abdullah LH, Nettesheim P. Mucociliary differentiation of serially passaged normal human tracheobronchial epithelial cells. Am J Respir Cell Mol Biol 14: 104–112, 1996 [DOI] [PubMed] [Google Scholar]
- 13. Halm DR, Frizzell RA. Intestinal chloride secretion. In: Textbook of Secretory Diarrhea, edited by Lebenthal E, Duffey M. New York: Raven, 1990, p. 47–58 [Google Scholar]
- 14. Han DY, Nie HG, Gu X, Nayak RC, Su XF, Fu J, Chang Y, Rao V, Ji HL. K+ channel openers restore verapamil-inhibited lung fluid resolution and transepithelial ion transport. Respir Res 11: 65, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Inglis SK, Brown SG, Constable MJ, McTavish N, Olver RE, Wilson SM. A Ba2+-resistant, acid-sensitive K+ conductance in Na+-absorbing H441 human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1304–L1312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem 281: 7392–7398, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kerem E, Bistritzer T, Hanukoglu A, Hofmann T, Zhou Z, Bennett W, MacLaughlin E, Barker P, Nash M, Quittell L, Boucher R, Knowles MR. Pulmonary epithelial sodium-channel dysfunction and excess airway liquid in pseudohypoaldosteronism. N Engl J Med 341: 156–162, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des 11: 2717–2736, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Kreindler JL, Bertrand CA, Lee RJ, Karasic T, Aujla S, Pilewski JM, Frizzell RA, Kolls JK. Interleukin-17A induces bicarbonate secretion in normal human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 296: L257–L266, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol 288: L894–L902, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Lotshaw D. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys 47: 209–256, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 10: 487–493, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell 95: 1005–1015, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Moser SL, Harron SA, Crack J, Fawcett JP, Cowley EA. Multiple KCNQ potassium channel subtypes mediate basal anion secretion from the human airway epithelial cell line Calu-3. J Membr Biol 221: 153–163, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Namkung W, Song Y, Mills AD, Padmawar P, Finkbeiner WE, Verkman AS. In situ measurement of airway surface liquid [K+] using a ratioable K+-sensitive fluorescent dye. J Biol Chem 284: 15916–15926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Namkung W, Song Y, Mills AD, Padmawar P, Finkbeiner WE, Verkman AS. In situ measurement of airway surface liquid [K+] using a ratioable K+-sensitive fluorescent dye. J Biol Chem 284: 15916–15926, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rich DP, Anderson MP, Gregory RJ, Cheng SH, Paul S, Jefferson DM, McCann JD, Klinger KW, Smith AE, Welsh MJ. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 347: 358–363, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Savitski AN, Mesaros C, Blair IA, Cohen NA, Kreindler JL. Secondhand smoke inhibits both Cl− and K+ conductances in normal human bronchial epithelial cells. Respir Res 10: 120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen BQ, Finkbeiner WE, Wine JJ, Mrsny RJ, Widdicombe JH. Calu-3: a human airway epithelial cell line that shows cAMP-dependent Cl− secretion. Am J Physiol Lung Cell Mol Physiol 266: L493–L501, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Smith PL, Frizzell RA. Chloride secretion by canine tracheal epithelium: IV. Basolateral membrane K permeability parallels secretion rate. J Membr Biol 77: 187–199, 1984 [DOI] [PubMed] [Google Scholar]
- 32. Sood N, Bennett WD, Zeman K, Brown J, Foy C, Boucher RC, Knowles MR. Increasing concentration of inhaled saline with or without amiloride: effect on mucociliary clearance in normal subjects. Am J Respir Crit Care Med 167: 158–163, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Willumsen NJ, Davis CW, Boucher RC. Intracellular Cl− activity and cellular Cl− pathways in cultured human airway epithelium. Am J Physiol Cell Physiol 256: C1033–C1044, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Wu JV, Krouse ME, Rustagi A, Joo NS, Wine JJ. An inwardly rectifying potassium channel in apical membrane of Calu-3 cells. J Biol Chem 279: 46558–46565, 2004 [DOI] [PubMed] [Google Scholar]