Abstract
Hyperoxia can lead to a myriad of deleterious effects in the lung including epithelial damage and diffuse inflammation. The specific mechanisms by which hyperoxia promotes these pathological changes are not completely understood. Activation of ion channels has been proposed as one of the mechanisms required for cell activation and mediator secretion. The two-pore-domain K+ channel (K2P) Trek-1 has recently been described in lung epithelial cells, but its function remains elusive. In this study we hypothesized that hyperoxia affects expression of Trek-1 in alveolar epithelial cells and that Trek-1 is involved in regulation of cell proliferation and cytokine secretion. We found gene expression of several K2P channels in mouse alveolar epithelial cells (MLE-12), and expression of Trek-1 was significantly downregulated in cultured cells and lungs of mice exposed to hyperoxia. Similarly, proliferation cell nuclear antigen (PCNA) and Cyclin D1 expression were downregulated by exposure to hyperoxia. We developed an MLE-12 cell line deficient in Trek-1 expression using shRNA and found that Trek-1 deficiency resulted in increased cell proliferation and upregulation of PCNA but not Cyclin D1. Furthermore, IL-6 and regulated on activation normal T-expressed and presumably secreted (RANTES) secretion was decreased in Trek-1-deficient cells, whereas release of monocyte chemoattractant protein-1 was increased. Release of KC/IL-8 was not affected by Trek-1 deficiency. Overall, deficiency of Trek-1 had a more pronounced effect on mediator secretion than exposure to hyperoxia. This is the first report suggesting that the K+ channel Trek-1 could be involved in regulation of alveolar epithelial cell proliferation and cytokine secretion, but a direct association with hyperoxia-induced changes in Trek-1 levels remains elusive.
Keywords: hyperoxia, proliferation, cytokines
in both animals and humans, prolonged hyperoxia leads to histopathological and clinical changes in the lung characteristic of acute lung injury (ALI) and acute respiratory distress syndrome (31). However, the specific mechanisms by which hyperoxia causes ALI are poorly understood. Increased levels of oxygen free radicals are thought to play an important role in the pathophysiology of this disease (9). A second proposed mechanism by which hyperoxia could promote ALI is via regulation of cell proliferation. Interestingly, in vivo type I cells appear to undergo necrosis, whereas type II cells are thought to proliferate upon exposure to hyperoxia (6). A third mechanism by which hyperoxia may contribute to the development of lung inflammation is via stimulation of inflammatory mediator release. In animal models, exposure to hyperoxia increased cytokine and chemokine concentrations in bronchoalveolar lavage fluid (17, 39). Selective regulation of inflammatory mediators by hyperoxia has also been reported in isolated lung epithelial cells (40).
Cytokine secretion and cell proliferation, two characteristics of lung injury and repair, are commonly associated with changes in membrane potential (15, 37). In most biological systems, opening of Ca2+ channels is thought to cause membrane depolarization, cell activation, and mediator secretion (27, 45), whereas opening of K+ channels is generally associated with membrane repolarization and stabilization of the resting membrane potential (21, 32). In addition, cell proliferation is also known to be regulated by activation of both Ca2+ (11) and K+ channels (14). A relatively new family of K+ channels, the two-pore-domain K+ channels (K2P), is a strong candidate as important regulators of the cell membrane potential (3). Opening of K2P channels activates “background” or “leak” currents, which lead to membrane repolarization and reestablishment of the resting membrane potential (20). In brain tissue, activation of a particular member of this family, Trek-1, has been shown to decrease hypoxia-induced inflammation and to promote neuroprotection (29). Trek-1 has recently been reported to also be expressed in Calu-3 lung epithelial cells, where it is thought to control the rate of transepithelial anion secretion (7). The specific mechanisms of Trek-1 regulation in the lung and whether this channel fulfills functions other than regulation of anion transport are unknown.
In this study we hypothesized that Trek-1 plays a role in lung epithelial cytokine secretion and cell proliferation, two cornerstones of ALI and repair. We show that exposure to hyperoxia decreased Trek-1 expression in murine lungs and in cultured alveolar epithelial cells. In addition, downregulation of Trek-1 increased cell proliferation and modulated cytokine secretions. This is the first report proposing a role for Trek-1 in alveolar epithelial cell proliferation and cytokine secretion.
MATERIALS AND METHODS
Cell culture.
MLE-12 mouse alveolar epithelial cells (44) were purchased from the American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured in DMEM (Gibco, Carlsbad, CA) supplemented with 10% FBS (Gibco), 1% penicillin/streptomycin (Gibco), 20 mM HEPES (Sigma Aldrich, St. Louis, MO) and 2 mM l-glutamine (Gibco). Once cells were 80–90% confluent, they were exposed to either room air (with 5% CO2) or 90% hyperoxia (with 5% CO2) in a contained chamber. Cell viability was >90% under all conditions as assessed by Trypan blue staining and 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) proliferation assay (Invitrogen, Carlsbad, CA).
Gene expression by real-time PCR.
Total RNA was isolated from 2 × 106 MLE-12 cells using a High Pure RNA Isolation Kit (Roche Applied Science, Mannheim, Germany) according to the manufacturer's instructions. Single-stranded DNA was synthesized from 1 μg total RNA, and RT-PCR was performed using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Real-time PCR was performed using a TaqMan Gene Expression assay (Invitrogen). Primer sets for Trek-1, Trek-2, TRAAK, and transient receptor potential vanilloid (TRPV)-4 were purchased from Invitrogen (Trek-1: Mm01323942, Trek-2: Mm00504118, TRAAK: Mm00434626, TRPV-4: Mm00499025). Hypoxanthine-guanine phosphoribosyltransferase was used as a housekeeping gene. All experiments were repeated a minimum of three times, and each sample was run in triplicate. The ΔΔCt method was used to calculate fold changes in gene expression.
Western blot analysis.
Antibodies were purchased from the following companies and used in dilutions recommended by the manufacturers: Trek-1 (1:500; Alamone Laboratories, Jerusalem, Israel), proliferating cell nuclear antigen (PCNA) (1:1,000; Abcam, Cambridge, MA), Cyclin D1 (1:1,000; Cell Signaling, Billerica, MA), GAPDH (1:1,000; Cell Signaling), β-actin (1:1,000; Sigma Aldrich). Initially, 0.3 × 106 cells were seeded into six-well tissue culture plates (MidWest Science, St. Louis, MO). Once cells reached 80–90% confluence, they were lysed on ice in RIPA buffer (50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 1% Nonidet P-40, 0.1% SDS) with protease inhibitor cocktail (Roche, Burlington, NC). Lysates were centrifuged at 4°C and 17,000 g for 15 min, and total protein was measured (Bradford assay; Bio-Rad, Hercules, CA). A total of 30 μg protein of each sample was separated by SDS-PAGE on 4–12% NuPage Bis-Tris gradient gels (Invitrogen) and transferred onto nitrocellulose membranes at 35 mV for 2 h. All membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (Bio-Rad) containing 0.1% Tween-20 for 1 h at 37°C. The membranes were then incubated overnight with the indicated primary antibodies at 4°C. The following day, membranes were incubated for 1 h with the following secondary antibodies: for Trek-1, PCNA, GAPDH and β-actin, we used an anti-rabbit horseradish peroxidase (HRP)-conjugated IgG (1:50,000; GE Healthcare, Piscataway, NJ), and for Cyclin D1 we used an anti-mouse HRP-conjugated IgG1 (1:50,000; Abcam). Bands were visualized by enhanced chemiluminescence with ECL SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL). All experiments were repeated a minimum of three times. Densitometry of bands to determine relative quantities of protein was performed using Image J 1.42 software for Windows.
Animals.
Mice were exposed to hyperoxia as previously described (25). Briefly, male C57BL/6J mice aged 8–10 wk (26–30 g body wt) were obtained from Charles River Laboratories (Wilmington, MA). Mice were exposed to either room air (n = 3) or 90% hyperoxia (n = 3) for 24 h and then euthanized. All animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86–23). The animal care and use committees of the University of Tennessee Health Science Center (UTHSC) approved the study.
Mouse lung homogenates.
Mouse lungs were perfused with 10 ml of 1 M PBS containing 200 U of heparin to remove red blood cells. The left lung of each mouse was removed and homogenized (OMNI TH tissue homogenizer; OMNI International, Kennesaw, GA) with 3 ml of RIPA lysis buffer and sonicated for 10 s (Omni Sonic Ruptor 250 Watt Ultrasonic Homogenizer, OMNI International).
Development of a stable Trek-1 shRNA-transfected MLE-12 cell line.
A stable Trek-1 knockdown cell line was developed in our laboratory using commercially available Trek-1 shRNA Lentiviral particles (cat. no. SC-37181-V; control scrambled shRNA cat. no. 108080; Santa Cruz Biotechnology, Santa Cruz, CA) following the manufacturer's instructions. Details of the vector containing the control plasmid and Trek-1 shRNA can be obtained from the company website (www.scbt.com). Briefly, cells were cultured to 50% confluence before transfection using Polybrene (Santa Cruz Biotechnology). Stable clones containing Trek-1 shRNA were selected and propagated in puromycin-containing culture medium. Knockdown of Trek-1 was confirmed by Western Blotting and immunostaining using confocal microscopy.
Confocal microscopy.
Initially, 0.1 × 106 cells were seeded on sterile acid-treated glass cover slips (Fisher Scientific, Fair Lawn, NJ) and grown until 80–90% confluent. The cells were then fixed with 4% paraformaldehyde containing 0.2% Triton X-100 for 5 min at 4°C and blocked with 2% BSA containing 0.2% Triton X-100 for 30 min. The cover slips were incubated with antibodies against Trek-1 (Alamone Laboratories, 1:500) or PCNA (Abcam, 1:500) overnight at 4°C. The following day, the samples were incubated with an Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (Molecular Probes, 1:500) for 30 min at room temperature. Nuclear counterstaining was obtained using a DRAQ5 antibody (1:1,000, Cell Signaling). As a negative control, a species-specific IgG antibody was substituted for the primary antibody.
Frozen lung sections of 20-μm thickness were cut using a Leica CM3050 cryostat (Leica, Oxford, UK). Sections were fixed in 4% paraformaldehyde for 10 min at 4°C and blocked with 2% BSA containing 0.2% Triton X-100 for 1 h. Sections were incubated overnight at 4°C with Trek-1 (1:500), and nuclear counter-staining was obtained using a DRAQ5 antibody (1:1,000). Sections that were double-stained with Trek-1 and surfactant protein C (SPC, M20; Santa Cruz, CA, 1:200) were incubated the following day with a Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (Molecular Probes, 1:500) and a Alexa Fluor 594 donkey anti-goat IgG secondary antibody (Molecular Probes, 1:500).
Images were acquired with the MRC 1024 imaging system (Bio-Rad) attached to a BX50 microscope (Olympus, Downingtown, PA). Emitted fluorescence was collected using a ×100 magnification objective lens (NA 1.4 Oil), and the images were recorded using LaserSharp 2000 software (Bio-Rad).
MTT proliferation assay.
An MTT-based proliferation assay was used to determine cell growth rates in control and Trek-1 shRNA-transfected MLE-12 cells. Briefly, this assay measures cleavage of yellow tetrazolium salt MTT to purple formazan crystal by metabolically active cells (22). The formazan is then solubilized, and the concentration is determined by a standard microplate absorbance reader at 540 nm. The MTT reagent was purchased from Invitrogen, and the assay was performed according the instructions provided in the Vybrant MTT Cell Proliferation Kit (V-13154, Invitrogen). Briefly, 1.2 × 104 cells/well were seeded into a 96-well plate and grown to 80–90% confluence. Samples were exposed to either 90% hyperoxia (with 5% CO2) or room air (with 5% CO2) for 48 h at 37°C. Before the assay was performed, the medium was changed to phenol red-free DMEM supplemented with the MTT reagent to avoid interference of phenol red with the colorimetric analysis.
Luminex assay.
A total of 1.3 × 105 MLE-12 cells/well were seeded into 12-well tissue culture plates and cultured to 80–90% confluence. The culture medium was then replaced with 1 ml of fresh medium/well, and cells were exposed to 90% hyperoxia (with 5% CO2) or room air (with 5% CO2). Thereafter, cells were incubated for 24 h in the presence or absence of 5 ng/ml TNF-α (R&D Systems, Minneapolis, MN). Cytokine and chemokine concentrations for granulocyte/macrophage colony-stimulating factor (GM-CSF), IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12 (p70), IL-13, monocyte chemoattractant protein-1 (MCP-1), and TNF-α were measured in supernatants of control and Trek-1 shRNA-transfected cells using a 13-MilliplexMAP Mouse Cytokine/Chemokine kit (cat. no. MPXMCYTO; Millipore). The assay was performed according to the manufacturer's instructions using 25 μl of supernatant per well. All samples were run in triplicates, and samples from four different experiments were analyzed. Cytokine and chemokine concentrations in supernatants were normalized to micrograms of total protein from the cell pellet of each sample.
Statistical analysis.
All values are expressed as means ± SD. Student's t-test was used to compare means of two different groups. For real-time PCR assays, a change in gene expression of more than twofold was considered significant. In cytokine studies, ANOVA analysis was used to compare means of different groups. All statistical analyses were performed using SigmaStat 3.5 software, and a value of P < 0.05 was considered significant.
RESULTS
Hyperoxia decreased Trek-1 gene expression.
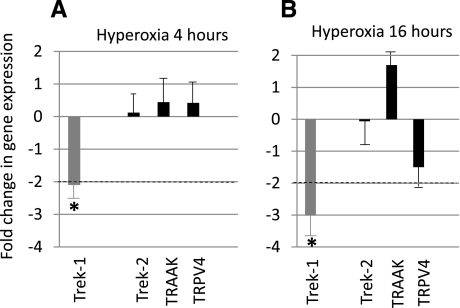
To examine the role of K2P channels in the response of epithelial cells to hyperoxia, we measured changes in gene expression in MLE-12 cells using real-time PCR (Fig. 1). We also examined the expression of the Ca2+-permeable channel TRPV-4 because previous studies have suggested a potential role of this channel in lung macrophages and pulmonary epithelial cells during the development of ALI (1, 12). However, presence of this channel in alveolar lung epithelial cells has never been documented. Figure 1, A–B, shows fold changes in gene expression after exposure of cells to 90% hyperoxia for 4 h and 16 h, respectively. A change in gene expression greater than twofold was considered significant (*P < 0.05). Hyperoxia decreased Trek-1 expression in a time-dependent fashion. After 4 h of hyperoxia, expression of Trek-1 decreased 2.2-fold and after 16 h 3.1-fold (n = 4). Gene expression of Trek-2, TRAAK, or TRPV-4 was not significantly affected by exposure to hyperoxia at either time point.
Fig. 1.
Hyperoxia decreased Trek-1 expression in MLE-12 cells. Treatment of cells with 90% hyperoxia for 4 h (A) showed a 2.2-fold decrease in Trek-1 expression but did not significantly affect expression of the other 2-pore-domain K+ channels Trek-2 and TRAAK, or expression of the Ca2+-permeable channel transient receptor potential vanilloid (TRPV)-4. Exposure of cells to 16 h of hyperoxia (B) further decreased Trek-1 gene expression but remained without effect on Trek-2, TRAAK, and TRPV-4. A change in gene expression >2-fold was considered significant (*P < 0.05, n = 3 or greater).
Hyperoxia decreases Trek-1 protein expression.
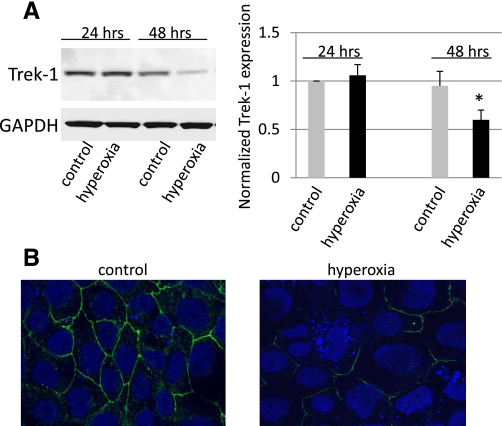
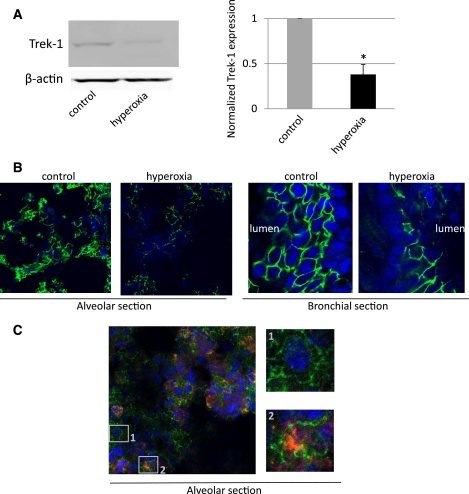
To determine whether hyperoxia affected Trek-1 protein expression, we examined both MLE-12 cells (Fig. 2, A–B) and mouse lung tissue (Fig. 3, A–B) after exposure to room air or hyperoxia for up to 48 h. Absolute cell numbers by Trypan blue exclusion did not change significantly during the 48-h period of exposure to hyperoxia. Figure 2A shows that Trek-1 expression was significantly decreased by 40% (P < 0.05) following 48 h of exposure to hyperoxia, but Trek-1 expression was not affected after 24 h of hyperoxia. When we examined Trek-1 expression by immunostaining in MLE-12 cells (Fig. 2B), we found that Trek-1 was uniformly expressed at the cell-to-cell junctions in control cells, but expression was sparse in cells following 48 h of hyperoxia. In addition, some of the cells exposed to hyperoxia showed signs of nuclear fragmentation, suggesting initiation of apoptotic processes. However, overall cell viability remained >90% in these experiments assessed by Trypan blue exclusion. To confirm that Trek-1 downregulation by hyperoxia was not a phenomenon limited to the MLE-12 cell line, we assayed lung homogenates of mice exposed to room air or hyperoxia for 24 h for Trek-1 protein (Fig. 3A). Samples from mice exposed to hyperoxia showed a 62% decrease in Trek-1 expression (n = 3, P < 0.05).
Fig. 2.
Hyperoxia decreased Trek-1 protein expression in MLE-12 cells. A: representative time course for Trek-1 expression using Western blotting. No change in Trek-1 expression was observed after exposure of cells to hyperoxia for 24 h, but after 48 h Trek-1 expression was decreased. Densitometry analysis shows a summary of 7 independent measurements. Trek-1 expression was first normalized to GAPDH expression, and treatment groups were then normalized to control expression at 24 h. *P < 0.05. B: immunostaining for Trek-1 (green) in MLE-12 cells by confocal microscopy (magnification ×100). Exposure of cells to hyperoxia for 48 h decreased Trek-1 immunoreactivity. Nuclei were counterstained with DRAQ5 (blue). Representative images from 3 or more fields from 3 different experiments are shown.
Fig. 3.
Hyperoxia decreased Trek-1 protein expression in mouse lungs. A: representative Western blot showing a decrease in normalized Trek-1 expression in lung homogenate after exposure of mice to hyperoxia for 24 h. β-Actin was used a loading control. Densitometry analysis shows a summary of 3 independent measurements (*P < 0.05). B: confocal images of decreased Trek-1 immunofluorescence (green) in alveolar and bronchial sections from mice exposed to hyperoxia for 24 h (magnification ×100). Again, representative images from 3 independent experiments are shown. Nuclei were counterstained with DRAQ5 (blue). C: coimmunostaining for Trek-1 (red) and surfactant protein C (SPC) (green) as a marker of type II alveolar epithelial cells in control cells. Trek-1 is expressed on both alveolar epithelial cell types. 1: Trek-1 staining on a type I cell and lack of SPC staining. 2: Trek-1 staining on a type II cell and intracellular SPC staining.
To obtain information about Trek-1 distribution in mouse lung tissue, we immunostained lung sections of mice exposed to room air or hyperoxia for 24 h (Fig. 3B). Trek-1 immunostaining decreased in both alveolar and bronchial tissue of mice exposed to hyperoxia compared with room air controls. To distinguish between type I and type II alveolar epithelial cells we coimmunostained alveolar sections for Trek-1 and SPC and found Trek-1 expression in both cell types (Fig. 3C, insets 1 and 2).
Effects of hyperoxia on PCNA and Cyclin D1.
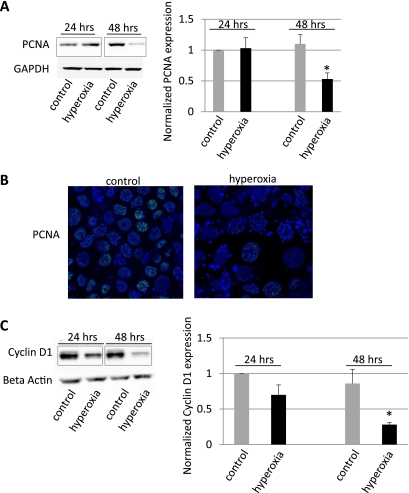
Epithelial cell proliferation is an important element of tissue regeneration following hyperoxia-induced ALI. We, therefore, studied the effects of hyperoxia on the expression levels of two markers of cell proliferation, PCNA and Cyclin D1 (Fig. 4). Exposure of MLE-12 cells to hyperoxia for 24 h did not affect PCNA expression, but after 48 h PCNA levels were significantly decreased by 47% (Fig. 4A, P < 0.05, n = 6). Downregulation of PCNA expression at 48 h of hyperoxia was also shown in MLE-12 cells by immunostaining (Fig. 4B). Another marker of cell proliferation, Cyclin D1, showed a similar decrease (72%) in protein expression after 48 h of hyperoxia (Fig. 4C) compared with cells grown at room air.
Fig. 4.
Hyperoxia downregulated proliferating cell nuclear antigen (PCNA) and Cyclin D1 expression. A: decrease in PCNA expression by Western blotting after exposure of MLE-12 cells to hyperoxia for 48 h, but not after 24 h. Densitometry analysis of 6 Western blot experiments showed a decrease of 47% in PCNA expression after 48 h hyperoxia exposure (*P < 0.05). GAPDH was used as a loading control. B: decrease in PCNA immunoreactivity (green) using confocal microscopy in MLE-12 cells exposed to hyperoxia for 48 h (magnification ×100). Nuclei were counterstained with DRAQ5 (blue). C: time-dependent decrease in Cyclin D1 expression by Western blotting and by densitometry analysis of 3 Western blot experiments (*P < 0.05). β-Actin was used a loading control. Black boxes indicate splicing of gels.
Development of a stable Trek-1 shRNA-transfected MLE-12 cell line.
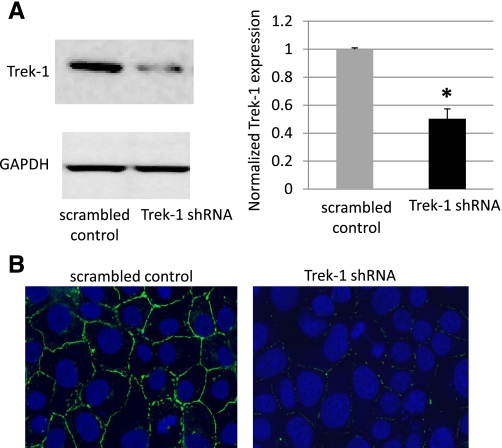
To study the functional relevance of Trek-1 downregulation by hyperoxia, we developed a stable Trek-1-deficient MLE-12 cell line using shRNA. Figure 5A shows that Trek-1 shRNA-transfected cells expressed 50% less Trek-1 protein than cells transfected with a scrambled control peptide (P < 0.05, n = 10). Immunostaining for Trek-1 (green) showed decreased immunoreactivity in the shRNA transfected cells (Fig. 5B).
Fig. 5.
Targeted knockdown of Trek-1 expression in shRNA-transfected MLE-12 cells. A: decreased Trek-1 expression in shRNA-treated cells in a representative Western blot and a 50% decrease in Trek-1 expression using densitometry analysis (n = 10, *P < 0.05). GAPDH was used a loading control. B: decreased Trek-1 immunofluorescence (green) using confocal microscopy in shRNA-transfected MLE-12 cells (magnification ×100). Nuclei were counterstained with DRAQ5 (blue).
Role of Trek-1 in epithelial cell proliferation.
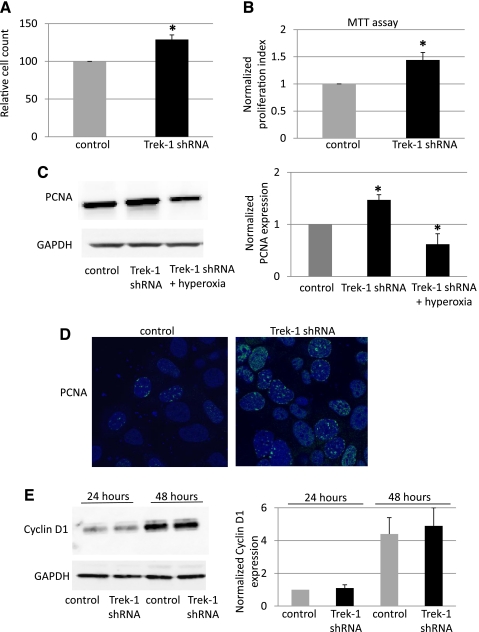
Because expression of both markers of cell proliferation, PCNA and Cyclin D1, was decreased by exposure to hyperoxia, we studied the role of Trek-1 in regulation of epithelial cell proliferation using Trek-1 shRNA-transfected MLE-12 cells (Fig. 6). Using Trypan blue staining to indentify nonviable cells, we counted the number of cells per wells 48 h after equivalent seeding of control and Trek-1 shRNA-transfected cells (Fig. 6A). Knockdown of Trek-1 caused a 30% increase in total cell numbers compared with control cells. As a further measure of proliferation, we used an MTT-based proliferation assay and found an increase in cell proliferation in Trek-1 shRNA-transfected cells of 44% after 48 h in culture (Fig. 6B). Cell viability was >90% in all experiments. When we studied the expression level of PCNA, we found a 47% increase in protein expression in Trek-1 shRNA-transfected cells compared with controls (Fig. 6C). When Trek-1 shRNA-transfected cells were exposed to hyperoxia for 48 h, we found a 37% decrease in PCNA expression compared with controls, an effect similar to the one described in Fig. 4 in nontransfected cells after exposure to hyperoxia. This suggests that the mechanisms responsible for downregulation of PCNA during hyperoxia may prevail over the mechanisms responsible for upregulation of PCNA induced by selective Trek-1 deficiency. Immunostaining for PCNA protein also showed an increase in PCNA immunoreactivity in Trek-1 shRNA-transfected MLE-12 cells compared with control cells (Fig. 6D).
Fig. 6.
Trek-1 deficiency increases MLE-12 cell proliferation. A: increase in absolute cell numbers in Trek-1 shRNA-transfected cells after 48 h of culture at room air (P < 0.05, n = 4). B: similar increase in cell proliferation at 48 h using a 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (n = 4, P < 0.05). C: increased expression of PCNA in shRNA-treated cells at room air and a decrease in PCNA expression in Trek-1-deficient cells after 48 h of hyperoxia exposure by Western blotting and by densitometry analysis of 4–6 independent experiments (*P < 0.05). GAPDH was used as a loading control. D: increased PCNA immunofluorescence by confocal microscopy in Trek-1 shRNA-transfected MLE-12 cells (magnification ×100, n = 3). E: representative Western blot and densitometry analysis of Cyclin D1 expression in control and Trek-1-deficient cells. Cyclin D1 expression increased over time but did not differ between control and Trek-1-deficient cells at either 24 h or 48 h.
Another marker of cell proliferation, Cyclin D1, was not affected by Trek-1 deficiency (Fig. 6E). We found that Cyclin D1 expression increased in a time-dependent fashion in proliferating MLE-12 cells but, in contrast to PCNA expression, did not change with selective Trek-1 downregulation. These data suggest that Trek-1 deficiency may affect regulation of MLE-12 cell proliferation via increased PCNA expression but not via changes in Cyclin D1 expression. However, these results do not imply that the PCNA and Cyclin downregulation by hyperoxia is mediated by decreased Trek-1 levels.
Trek-1 altered epithelial cell cytokine and chemokine secretion.
Inflammatory mediator release is a cornerstone in the development of ALI. Despite the well-known importance of ion channels in cytokine secretion in several cell types, a role for Trek-1 in mediator secretion from lung epithelial cells has never been described. We compared the release of IL-6, regulated on activation normal T-expressed and presumably secreted (RANTES), MCP-1, and the murine IL-8 homolog KC (KC/IL-8) from control MLE-12 cells and Trek-1 shRNA-transfected cells following exposure to room air or hyperoxia in the presence or absence of TNF-α stimulation (Fig. 7). A dose of 5 ng/ml TNF-α was chosen as a stimulus because in preliminary dose-response experiments higher doses of TNF-α did not show any significant further increase in mediator concentrations (data not shown). We did not detect significant concentrations of GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-5, IL-7, IL-10, IL-12 (p70), or IL-13, and, therefore, these results are not shown. All data were normalized to total amounts of protein in the corresponding cell pellets to account for different proliferation rates between control and Trek-1 shRNA-transfected cells.
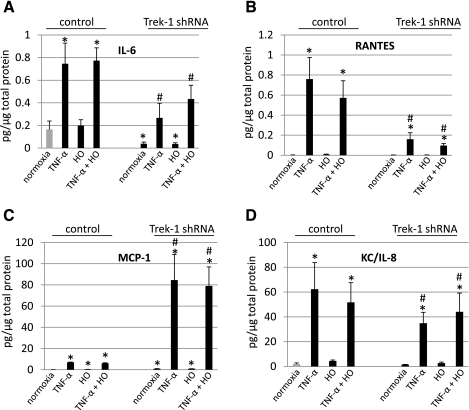
Fig. 7.
Trek-1 regulates mediator release from MLE-12 cells. Concentrations of IL-6, regulated on activation normal T-expressed and presumably secreted (RANTES), monocyte chemoattractant protein (MCP)-1, and KC/IL-8 were measured in supernatants from control and Trek-1 shRNA-transfected cells using the Luminex system. The amount of mediator release in pg/ml was normalized to μg/μl of total protein in cell pellet and is depicted as pg/μg total protein. Cells were exposed to room air or hyperoxia (HO) for 48 h. Cells stimulated with TNF-α (5 ng/ml) were incubated with this cytokine for 24 h (A–D). Secretion of IL-6 and RANTES was decreased in Trek-1 shRNA-deficient cells, whereas MCP-1 concentrations increased. Release of KC/IL-8 was not affected by Trek-1 deficiency (*P < 0.05, significantly different from control samples in normoxia, n = 4; #P < 0.05, significantly different from Trek-1 shRNA samples in normoxia, n = 4).
As depicted in Fig. 7A, unstimulated cells exposed to room air and hyperoxia showed similar baseline levels of IL-6 release. Stimulation of control cells with TNF-α increased IL-6 release fourfold regardless of exposure to room air or hyperoxia. Baseline release of IL-6 in Trek-1 shRNA-transfected cells was decreased compared with controls regardless of exposure to room air or hyperoxia. Similarly, TNF-α-induced IL-6 secretion was decreased in shRNA-transfected cells compared with controls regardless of exposure to room air or hyperoxia. Therefore, Trek-1 shRNA-transfected cells showed decreased amounts of IL-6 release at both baseline and after stimulation with TNF-α, and this phenomenon was unrelated to exposure of cells to hyperoxia.
As shown in Fig. 7B, unstimulated cells in both the control and the Trek-1 shRNA group showed minimal levels of RANTES release regardless of exposure to room air or hyperoxia. Stimulation of control cells with TNF-α induced a significant increase in RANTES release at room air and after hyperoxia exposure compared with unstimulated cells. Stimulation of shRNA transfected cells with TNF-α showed reduced levels of RANTES secretion in both room air- and hyperoxia-exposed samples. Therefore, whereas baseline release of RANTES was minimal in both control and Trek-1 shRNA transfected cells, RANTES release upon stimulation with TNF-α was reduced in shRNA-transfected cells compared with controls and was not significantly affected by exposure to hyperoxia.
Analysis of MCP-1 release (Fig. 7C) showed low but increased concentrations of this chemokine in unstimulated control cells exposed to hyperoxia (0.37 + 0.08 pg/μg total protein) compared with control cells exposed to room air (0.1 + 0.09 pg/μg total protein). Stimulation of control cells with TNF-α increased MCP-1 release to a similar degree in both room air- and hyperoxia-exposed cells. Baseline release of MCP-1 in Trek-1 shRNA-transfected cells was higher in both room air- (0.9 + 0.1 pg/μg total protein) and in hyperoxia-exposed cells (0.9 + 0.2 pg/μg total protein) than in control cells. Stimulation of shRNA-transfected cells with TNF-α increased MCP-1 secretion compared with control cells in both room air and hyperoxia conditions. Therefore, whereas hyperoxia itself caused a small increase in MCP-1 secretion in control cells, in Trek-1 shRNA-transfected cells MCP-1 release was increased in unstimulated cells and further increased after TNF-α stimulation compared with control cells in normoxia. This effect was unrelated to hyperoxia or room air exposure.
Figure 7D shows similar levels of baseline KC/IL-8 secretion at room air and after hyperoxia exposure in both control and Trek-1 shRNA-transfected cells. Stimulation with TNF-α increased KC/IL-8 secretion to a similar degree in cells exposed to room air and to hyperoxia in both the control and the shRNA-transfected group. Therefore, whereas MLE-12 cells secrete KC/IL-8 at baseline and show increased KC/IL-8 levels after TNF-α stimulation, there was no significant difference in release of this chemokine between control and Trek-1 shRNA-transfected cells.
DISCUSSION
In this study we suggest for the first time that the K2P channel Trek-1 is regulated by the effects of hyperoxia and may play a role in epithelial cell mediator secretion and cell proliferation. Oxygen supplementation, often at high concentrations, is one of the most commonly utilized supportive measures in treatment of ALI (2). Inflammatory cytokine and chemokine release, epithelial cell proliferation, and wound healing are key processes in ALI (23, 28). However, the specific pathways leading to these findings are poorly understood. We propose that Trek-1 may be an important regulator of alveolar epithelial cell proliferation and cytokine secretion.
K+ channels such as KCa2+ channels and Kv+ channels are known regulators of cell proliferation (13, 33). In our hyperoxia model we found downregulation of both Trek-1 and the proliferation markers PCNA and Cyclin D1 at 48 h. Similarly, another marker of proliferation, survivin, has recently been shown to decrease in human bronchial epithelial cells after 48 h of exposure to hyperoxia (47). To further study these effects, we created a shRNA-transfected MLE-12 cell line and hypothesized that expression of PCNA and Cyclin D1 in Trek-1-deficient cells will be downregulated similar to the findings observed during hyperoxia. Surprisingly, we found increased levels of PCNA expression and no effect on Cyclin D1 expression in shRNA-transfected cells despite Trek-1 levels being decreased. It is possible that during the 48-h time period of hyperoxia cells acutely downregulated not only Trek-1 but also PCNA and Cyclin D1 expression, whereas over weeks in culture our shRNA-transfected cells developed compensatory mechanisms to adjust for decreased Trek-1 levels, resulting in an increase in PCNA protein. To rule out nonspecific effects of Trek-1 shRNA treatment, we studied gene expression of another K2P channel, Trek-2, and of the non-K2P channel TRPV-4 in Trek-1 shRNA-transfected cells using real-time PCR. Expression of both channels was unchanged in Trek-1 shRNA-transfected cells compared with control cells (0.6-fold and 0.2-fold change, respectively), making nonspecific effects of shRNA treatment unlikely. Downregulation of Cyclin D1-dependent kinase activity by hyperoxia has also been reported in human A549 cells (16) and ovarian tumor cells (38), possibly via inhibition of STAT3. Further studies are necessary to evaluate whether in our lung injury model downregulation of STAT3 is responsible for the hyperoxia-induced changes in Cyclin D1 expression. The downregulatory effect of hyperoxia on PCNA expression is supported by similar findings in rat type II alveolar epithelial cells (48) and in the human lung adenocarcinoma cell line H1299 (42). The exact mechanism by which hyperoxia downregulates PCNA expression during ALI remains unknown, but over 22 molecules have been described to interact with PCNA during DNA replication (30). This is the first report suggesting that the K+ channel Trek-1 could be involved in regulation of alveolar epithelial cell proliferation, but a direct association with hyperoxia-induced changes in Trek-1 levels remains elusive. Interestingly, Lauritzen et al. (19) suggested a cross-talk between Trek-1 and the actin cytoskeleton in neuronal cells where expression of Trek-1 altered the cytoskeletal network and, conversely, the actin cytoskeleton tonically repressed Trek-1 opening (19). It is intriguing to speculate that a similar cross-talk could exist in the lung and may affect regulation of epithelial cell proliferation and epithelial repair mechanisms in ALI. It has been reported that in vivo alveolar type II cells proliferate upon exposure to hyperoxia, whereas type I cell numbers decrease (6). Cultured alveolar type II cells may show a different behavior attributable to the confluent nature of the cell culture and the absence of interactions with neighboring type I cells. Alternatively, the increased type II cell proliferation in vivo may be due to the hyperproliferation of cells that survived the exposure to hyperoxia. In fact, primary rat alveolar type II epithelial cells in culture show decreased proliferation upon exposure to hyperoxia (50), similar to our results. These results do not suggest that downregulation of PCNA and Cyclin D1 during hyperoxia was Trek-1 dependent but that in Trek-1 deficient cells PCNA-mediated cell proliferation appears upregulated, whereas Cyclin D1-mediated proliferation seems unaffected. The regulation of PCNA and Cyclin D1 function during the cell cycle is complex and modulated at multiple levels, and a discussion of these interactions would be beyond the scope of this manuscript. Undoubtedly, further studies are necessary to elucidate the mechanism of PCNA upregulation by Trek-1 deficiency.
To the best of our knowledge, to date only one study has suggested expression of K2P channels in airway epithelial cells (7). Aside from participating in anion secretion, no function has been assigned to these channels in lung epithelium. In other tissues that express K2P channels, the main function of these channels is thought to revolve around maintenance of the resting cell membrane potential and cell repolarization (3). The results of this study suggest that hyperoxia may be a potential regulator of at least one K2P channel, namely Trek-1. Whereas Trek-1 expression was downregulated by hyperoxia, expression of other K2P channels such as Trek-2 and TRAAK was not affected, and these effects did not appear to be caused by hyperoxia-induced cell death. Similar data on cell viability after hyperoxia exposure were recently reported in primary alveolar epithelial cells (40).
Investigating the functional consequences of Trek-1 downregulation by hyperoxia is complicated by a lack of universally accepted selective pharmacological inhibitors or activators of this particular channel (4). This challenge stems from the fact that Trek-1 is known to be insensitive to classical K+ channel inhibitors including glibenclamide, 4-aminopyridine, and tetraethylammonium (35, 44). Other pharmacological approaches including amiloride, gadolininum, and riluzuole have been criticized as nonspecific (5, 18, 24, 34, 43, 46). Therefore, to selectively inhibit Trek-1, we created a stable Trek-1 shRNA-transfected MLE-12 cell line. Because a unique feature of K2P channels is to be constitutively open producing so-called background or leak currents (10), it is reasonable to assume that expression levels correlate closely with channel function.
In addition to regulation of epithelial cell proliferation, our data suggest that Trek-1 may also be involved in regulation of cytokine and chemokine secretion from lung epithelial cells. Although the role of ion channels in stimulus-secretion coupling has gained particular interest in inflammatory cells (15, 35), little is known about this process in lung epithelial cells. Most of the literature revolves around the requirement of increased intracellular Ca2+ concentrations before initiation of mediator secretion (36, 49). For instance, mice deficient in the Ca2+-permeable channel TRPV-4 show impaired mediator release (41). The exact mechanisms of how Ca2+ and other conductances such as K+ and Cl− currents (8, 9a) affect mediator release are poorly understood. Most authors would agree that an increase in intracellular Ca2+ remains the final common pathway leading to mediator secretion and that K+ and Cl− currents are likely to affect Ca2+ entry via alteration of the resting membrane potential, thus increasing or decreasing the electrical driving forces for Ca2+ to enter the cell. Trek-1 may be particularly important in preservation of the resting membrane potential and in preventing cell depolarization. We speculate that downregulation of Trek-1, as observed by exposure to hyperoxia and mimicked by shRNA transfection, decreased Trek-1 leak currents, leading to an accumulation of positive charges within the cell and lowering the resting membrane potential toward more positive values. This sequence of events would reduce the driving force for Ca2+ entry and, as a consequence, impair the mechanisms leading to mediator secretion. In fact, both IL-6 and RANTES secretions were decreased in Trek-1 shRNA-transfected cells. However, KC/IL-8 and MCP-1 secretion was not inhibited in Trek-1-deficient cells, and the specific mechanisms accounting for this difference in cytokine secretion is presently unclear. A recently published study showed a hyperoxia-induced increase in MCP-1 release in primary alveolar epithelial cells (40). The reason for the absolute amounts of MCP-1 released in our study being lower (17 ± 12 pg/ml in the room air group and 58 ± 10 pg/ml in the hyperoxia group) was likely related to the different epithelial cell types used. In summary, the changes in cytokine secretion observed in Trek-1-deficient cells suggest a potential role for this ion channel in mediator release from MLE-12 cells. However, we do not propose that these alterations in cytokine release were directly mediated by hyperoxia-induced downregulation of Trek-1.
Limitations of this study include the fact that both the proliferation data and cytokine data were obtained from in vitro studies using cultured MLE-12 cells. Establishing a Trek-1 knockdown system in primary epithelial cells would be challenging given the limited number of type II alveolar cells that can be obtained from mice or rats and the difficulties associated with phenotypic changes in culture. We are, nevertheless, aware of the fact that the secretory pattern of cultured cells could be different from primary cells or in vivo mediator release. In fact, a recent report showed differences in GM-CSF secretion in primary airway epithelial cells compared with cultured MLE-12 cells (40). In addition, to assure that the effects of hyperoxia on Trek-1 expression were not limited to MLE-12 cells, we repeated key experiments in mouse lung samples and found similar results.
In summary, our results show for the first time expression of Trek-1 in cultured alveolar MLE-12 cells and mouse lung tissue. Furthermore, we propose two new potential functions for this channel in alveolar epithelial cells, namely regulation of cell proliferation and mediator secretion. These new findings may lead to a better understanding of the pathogenesis of lung inflammation and may contribute to the development of novel pharmacological strategies in the treatment of ALI.
GRANTS
This study was supported by a grant from the Le Bonheur Children's Medical Center Research Foundation of the University of Tennessee Health Science Center, and the National Institutes of Health Grant HL094366 (C. Waters).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.S., B.T., M.C.G., A.N.W., P.S.M., S.E.S., and C.M.W. conception and design of research; A.S., B.T., M.C.G., A.N.W., P.S.M., and V.K.G. performed experiments; A.S., B.T., M.C.G., A.N.W., and P.S.M. analyzed data; A.S., M.C.G., A.N.W., P.S.M., S.E.S., and C.M.W. interpreted results of experiments; A.S. prepared figures; A.S. drafted manuscript; A.S. and C.M.W. edited and revised manuscript; A.S., B.T., M.C.G., A.N.W., P.S.M., V.K.G., S.E.S., and C.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Ryan Yates for support with the Luminex system.
REFERENCES
- 1. Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res 99: 988–995, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Antonelli M, Azoulay E, Bonten M, Chastre J, Citerio G, Conti G, De Backer D, Gerlach H, Hedenstierna G, Joannidis M, Macrae D, Mancebo J, Maggiore SM, Mebazaa A, Preiser JC, Pugin J, Wernerman J, Zhang H. Year in review in Intensive Care Medicine 2010: III. ARDS and ALI, mechanical ventilation, noninvasive ventilation, weaning, endotracheal intubation, lung ultrasound and paediatrics. Intensive Care Med 37: 394–410, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bayliss DA, Barrett PQ. Emerging roles for two-pore-domain potassium channels and their potential therapeutic impact. Trends Pharmacol Sci 29: 566–575, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bryan RM, Jr, Joseph BK, Lloyd E, Rusch NJ. Starring TREK-1: the next generation of vascular K+ channels. Circ Res 101: 119–121, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Chow GE, Muller CH, Curnow EC, Hayes ES. Expression of two-pore domain potassium channels in nonhuman primate sperm. Fertil Steril 87: 397–404, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crapo JD, Barry BE, Foscue HA, Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis 122: 123–143, 1980 [DOI] [PubMed] [Google Scholar]
- 7. Davis KA, Cowley EA. Two-pore-domain potassium channels support anion secretion from human airway Calu-3 epithelial cells. Pflügers Arch 451: 631–641, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dietrich J, Lindau M. Chloride channels in mast cells: block by DIDS and role in exocytosis. J Gen Physiol 104: 1099–1111, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dos Santos CC. Hyperoxic acute lung injury and ventilator-induced/associated lung injury: new insights into intracellular signaling pathways (Abstract). Crit Care 11: 126, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a. Duffy MS, Berger P, Cruse G, Yang W, Bolton SJ, Bradding P. The K+ channel iKCA1 potentiates Ca2+ influx and degranulation in human lung mast cells. J Allergy Clin Immunol 114: 66–72, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci 2: 175–184, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Graziani A, Poteser M, Heupel WM, Schleifer H, Krenn M, Drenckhahn D, Romanin C, Baumgartner W, Groschner K. Cell-cell contact formation governs Ca2+ signaling by TRPC4 in the vascular endothelium: evidence for a regulatory TRPC4-beta-catenin interaction. J Biol Chem 285: 4213–4223, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, Eyal FG, Clapp MM, Parker JC. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 299: L353–L362, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ivanov A, Gerzanich V, Ivanova S, Denhaese R, Tsymbalyuk O, Simard JM. Adenylate cyclase 5 and KCa1.1 channel are required for EGFR up-regulation of PCNA in native contractile rat basilar artery smooth muscle. J Physiol 570: 73–84, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jang SH, Choi SY, Ryu PD, Lee SY. Anti-proliferative effect of Kv1.3 blockers in A549 human lung adenocarcinoma in vitro and in vivo. Eur J Pharmacol 651: 26–32, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Janiszewski J, Huizinga JD, Blennerhassett MG. Mast cell ionic channels: significance for stimulus-secretion coupling. Can J Physiol Pharmacol 70: 1–7, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Ko JC, Wang YT, Yang JL. Dual and opposing roles of ERK in regulating G (1) and S-G (2)/M delays in A549 cells caused by hyperoxia. Exp Cell Res 297: 472–483, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Kroon AA, Wang J, Huang Z, Cao L, Kuliszewski M, Post M. Inflammatory response to oxygen and endotoxin in newborn rat lung ventilated with low tidal volume. Pediatr Res 68: 63–69, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Kusche-Vihrog K, Callies C, Fels J, Oberleithner H. The epithelial sodium channel (ENaC): mediator of the aldosterone response in the vascular endothelium? Steroids 75: 544–549, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Lauritzen I, Chemin J, Honore E, Jodar M, Guy N, Lazdunski M, Patel JA. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep 6: 642–648, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol 279: F793–F801, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Liao Y, Kristiansen AM, Oksvold CP, Tuvnes FA, Gu N, Runden-Pran E, Ruth P, Sausbier M, Storm JF. Neuronal Ca2+-activated K+ channels limit brain infarction and promote survival. PLos One 5: e15601, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69: 581–593, 1997 [DOI] [PubMed] [Google Scholar]
- 23. Lu HY, Shao GB, Li WB, Wang H. Effects of hyperoxia on transdifferentiation of primary cultured type II alveolar epithelial cells from premature rats. In Vitro Cell Dev Biol Anim 47: 64–72, 2011 [DOI] [PubMed] [Google Scholar]
- 24. Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K (+) channels TREK-1 and TRAAK. J Biol Chem 275: 10128–10133, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Makena PS, Luellen CL, Balazs L, Ghosh MC, Parthasarathi K, Waters CM, Sinclair SE. Preexposure to hyperoxia causes increased lung injury and epithelial apoptosis in mice ventilated with high tidal volumes. Am J Physiol Lung Cell Mol Physiol 299: L711–L719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, Lieben L, Torrekens S, Moermans K, Vanden Bosch A, Bouillon R, Nilius B, Carmeliet G. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab 8: 257–265, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, Leeper K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 107: 1062–1073, 1995 [DOI] [PubMed] [Google Scholar]
- 29. Miller P, Kemp PJ, Lewis A, Chapman CG, Meadows HJ, Peers C. Acute hypoxia occludes hTREK-1 modulation: re-evaluation of the potential role of tandem P domain K+ channels in central neuroprotection. J Physiol 548: 31–37, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naryzhny SN. Proliferating cell nuclear antigen: a proteomics view. Cell Mol Life Sci 65: 3789–3808, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nash G, Blennerhassett J, Pontoppidan H. Pulmonary lesions associated with oxygen therapy and artificial ventilation. Laval Med 39: 59–64, 1968 [PubMed] [Google Scholar]
- 32. Nerbonne JM. Repolarizing cardiac potassium channels: multiple sites and mechanisms for CAMK II-mediated regulation. Heart Rhythm 8: 938–941, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Grady SM, Lee SY. Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol 37: 1578–1594, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K (+) channels. Curr Opin Cell Biol 13: 422–428, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Qiu MR, Campbell TJ, Breit SN. A potassium ion channel is involved in cytokine production by activated human macrophages. Clin Exp Immunol 130: 67–74, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reinsprecht M, Rohn MH, Spadinger RJ, Pecht I, Schindler H, Romanin C. Blockade of capacitive Ca2+ influx by Cl- channel blockers inhibits secretion from rat mucosal-type mast cells. Mol Pharmacol 47: 1014–1020, 1995 [PubMed] [Google Scholar]
- 37. Sel S, Rost BR, Yildirim AO, Sel B, Kalwa H, Fehrenbach H, Renz H, Gudermann T, Dietrich A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin Exp Allergy 38: 1548–1558, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Selvendiran K, Kuppusamy ML, Ahmed S, Bratasz A, Meenakshisundaram G, Rivera BK, Khan M, Kuppusamy P. Oxygenation inhibits ovarian tumor growth by downregulating STAT3 and cyclin-D1 expressions. Cancer Biol Ther 10: 386–390, 2010 [DOI] [PubMed] [Google Scholar]
- 39. Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med 32: 2496–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 40. Sturrock A, Vollbrecht T, Mir-Kasimov M, McManus M, Wilcoxen SE, Paine R., 3rd Mechanisms of suppression of alveolar epithelial cell GM-CSF expression in the setting of hyperoxic stress. Am J Physiol Lung Cell Mol Physiol 298: L446–L453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8: 312–320, 2007 [DOI] [PubMed] [Google Scholar]
- 42. Vitiello PF, Staversky RJ, Gehen SC, Johnston CJ, Finkelstein JN, Wright TW, O'Reilly MA. p21Cip1 protection against hyperoxia requires Bcl-XL and is uncoupled from its ability to suppress growth. Am J Pathol 168: 1838–1847, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weiss S, Benoist D, White E, Teng W, Saint DA. Riluzole protects against cardiac ischaemia and reperfusion damage via block of the persistent sodium current. Br J Pharmacol 160: 1072–1082, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, Whitsett JA. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 90: 11029–11033, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther 326: 443–452, 2008 [DOI] [PubMed] [Google Scholar]
- 46. Wolfle SE, Navarro-Gonzalez MF, Grayson TH, Stricker C, Hill CE. Involvement of nonselective cation channels in the depolarisation initiating vasomotion. Clin Exp Pharmacol Physiol 37: 536–543, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Zhang M, Lin L, Lee SJ, Mo L, Cao J, Ifedigbo E, Jin Y. Deletion of caveolin-1 protects hyperoxia-induced apoptosis via survivin-mediated pathways. Am J Physiol Lung Cell Mol Physiol 297: L945–L953, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhong LL, Yang YJ, Liu CT, Xie M. Effect of hepatocyte growth factor on proliferation and apoptosis of hyperoxia exposed type II alveolar epithelial cells isolated from premature rat lungs. Zhong Nan Da Xue Xue Bao Yi Xue Ban 32: 1051–1057, 2007 [PubMed] [Google Scholar]
- 49. Zhou X, Yang W, Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-kappaB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-alpha production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem 281: 31337–31347, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Zhu H, Chang L, Li W, Liu H. Effect of amygdalin on the proliferation of hyperoxia-exposed type II alveolar epithelial cells isolated from premature rat. J Huazhong Univ Sci Technolog Med Sci 24: 223–225, 2004 [DOI] [PubMed] [Google Scholar]