Abstract
Our recent study showed that prenatal and early postnatal exposure of mice to side-steam tobacco smoke (SS), a surrogate to environmental tobacco smoke (ETS), leads to increased airway responsiveness and sensory innervation later in life. However, the underlying mechanism initiated in early life that affects airway responses later in life remains undefined. The concomitant increase in nerve growth factor (NGF) after exposures suggests that NGF may be involved the regulation of airway innervation. Since NGF regulates sympathetic nerve responses, as well as sensory nerves, we extended previous studies by examining neuropeptide Y (NPY), a neuropeptide associated with sympathetic nerves. Different age groups of mice, postnatal day (PD) 2 and PD21, were exposed to either SS or filtered air (FA) for 10 consecutive days. The level of NPY protein in lung and the density of NPY nerve fibers in tracheal smooth muscle were significantly increased in the PD2–11SS exposure group compared with PD2–11FA exposure. At the same time, the level of NGF in lung tissue was significantly elevated in the PD2–11SS exposure groups. However, neither NPY (protein or nerves) nor NGF levels were significantly altered in PD21–30SS exposure group compared with the PD21–30FA exposure group. Furthermore, pretreatment with NGF antibody or K252a, which inhibits a key enzyme (tyrosine kinase) in the transduction pathway for NGF receptor binding, significantly diminished SS-enhanced NPY tracheal smooth muscle innervation and the increase in methacholine-induced airway resistance. These findings show that SS exposure in early life increases NPY tracheal innervation and alters pulmonary function and that these changes are mediated through the NGF.
Keywords: airway innervation, asthma, secondhand smoke, neurotrophic factor
environmental tobacco smoke (ETS) is an environmental trigger factor that leads to airway inflammation and asthma symptoms in susceptible individuals and animals (25, 33, 34). Exposure to ETS in utero or during early postnatal development increases the incidence of respiratory illnesses (11, 13, 28, 31, 35) later in life. Epidemiological studies (11, 13, 25, 28, 32–35) show that the probability of developing or exacerbating childhood asthma increases in children of mothers who smoke cigarettes. These results suggest that the prenatal and early postnatal periods are critical periods of developmental sensitivity to cigarette smoke exposure. Indeed, our recent study (40) showed that exposure to side-steam tobacco smoke (SS) during prenatal and early postnatal life produces changes in lung function and airway innervation later in life, supporting the concept of an early life critical period of susceptibility. However, the underlying mechanism initiated by early life exposures that affect lung structure and function later in life remains undefined.
The nervous system, including the nerves supplying the airways, is highly susceptible to environmental influences during development (9). Our previous studies (40) have shown that SS exposure in early life alters substance P (SP) airway innervation later in life, suggesting that airway sensory nerves are involved in a putative critical period of developmental sensitivity to cigarette smoke exposure. Neuropeptide Y (NPY), a 36-amino acid peptide, is a cotransmitter and neuromodulator in the peripheral sympathetic nerves (1, 24). Recent studies (12) showed that NPY not only produces vasoconstriction of airway vasculature, but it also plays an important role in the regulation of a large number of physiological and pathophysiological processes in the respiratory system. Clinical studies (6) have found that higher NPY concentrations have been found in patients with bronchial asthma compared with normal subjects, indicating elevated NPY may be involved in asthma.
Nerve growth factor (NGF) is a neurotrophic factor that promotes and maintains growth of the central and peripheral nervous systems (22). NGF in the lung increases during gestation and decreases progressively with postnatal age (16). Disruption of normal synthesis and release of NGF result in changes in airway innervation, which lead to disease-related abnormalities in the respiratory system (16, 30, 36, 37). Our recent studies (36, 37) showed that NGF was produced by irritant exposures and mediated changes in the distribution of airway nerves. Thus we hypothesized that SS exposure during the early postnatal period alters NPY airway innervation, which is possibly mediated by NGF. These neural changes may contribute to increased susceptibility to asthma in later life. The present experiments characterize changes in NPY innervation and NGF expression during early postnatal exposure to SS and demonstrate attenuation of the neural response when NGF actions are inhibited.
METHODS
ICR mice (Harlan, Indianapolis, IN) were housed with access to food and water ad libitum in an Food and Drug Administration-approved facility. All procedures were performed in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health, and the protocols were approved by the West Virginia University Animal Care and Use Committee (#05–0503). The animals were treated humanely and with regard for alleviation of suffering.
SS exposure.
By classical definition, environmental tobacco smoke (ETS) is a diluted mixture of the smoke given off by the burning end of a tobacco product (SS, ∼85%) and the smoke exhaled by smokers (mainstream smoke, ∼15%). Based on previous ETS exposure studies (27, 40, 43), we used SS as a surrogate to ETS to identify critical developmental periods of susceptibility.
Initial experiments were conducted to determine an exposure time course for neural responses to SS. For these initial studies, mice were exposed to either SS or filtered air (FA) 6 h per day for 1 day [beginning on postnatal day (PD) 11 or PD30], 5 days (beginning on PD7 or PD26), or 10 days (beginning on PD2 or PD21) to test SS effect of exposure dose in early and later postnatal period, respectively. In each of these protocols, the final exposure day was PD11 (within the proposed critical period) or PD30 (beyond the critical period). These groups are designated as PD2, PD7, PD11, PD21 et al. Based on our results (Fig. 1) and recent publication (40), a 10-day exposure protocol beginning on PD2 (designated PD2–11) or PD21 (designated PD21–30) was chosen for more extensive investigation. In all studies, lung tissues were collected 16 h after the last SS exposure. A major goal of the current study was to understand possible neuronal mechanisms initiated by SS in early life that affects lung function later in life. Our previous study (40) showed that SS exposure in the PD21–30 group did not alter airway responsiveness to reexposure on PD59 Therefore, in the current study, mice in the PD2–11 group were allowed to mature to PD59 and pulmonary function testing was conducted on PD60, 16 h after a SS reexposure on PD59. Rough comparisons of approximate mouse and human postnatal lung development are based primarily on age relative to puberty (about PD28). The PD2 mouse is similar to a human neonate, and PD21 is similar to a prepubertal child (27, 32).
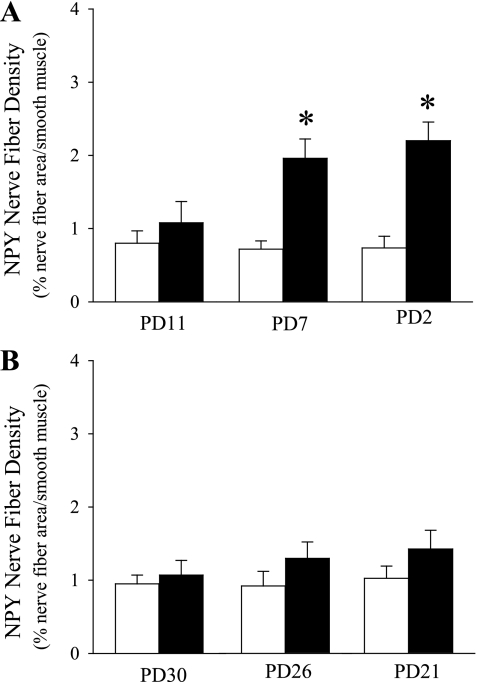
Fig. 1.
Exposure time course of side-steam tobacco smoke (SS; closed bar) or filtered air (FA; opened bar) during early (A) and late (B) postnatal periods on the change of neuropeptide Y (NPY) nerves in tracheal smooth muscle. Values are means ± SE; n = 6 in each group. *P ≤ 0.05, significant difference between FA- and SS-exposed mice.
The SS exposure protocol and methods used in this study have been described in our recent publication (40). Briefly, mice were randomly placed in an exposure chamber (BioClean, DuoFlo, model H 5500; Lab Products) that measured 1.92 × 1.92 × 0.97 m (3.58 m3). The mice were housed in separate cages located in the exposure chamber. SS from Marlboro filter cigarettes (Phillip Morris, Richmond, VA) was introduced into the exposure chamber at a rate of four cigarettes every 15 min for 6 h per day for 10 days using a smoking machine (RM 1/G; Heinr Borgwald, Hamburg, Germany). At the end of the 6-h exposure period, the exhaust fan on the BioClean unit was turned on to rapidly lower the level of smoke in the exposure chamber. The mice were then transported to the animal facilities overnight. The concentrations of carbon monoxide in the exposure chamber were monitored and kept to an average of ∼50 parts per million, relative humidity was ∼50%, and temperature was ∼23°C. Total suspended particulate concentration was ∼1.1 mg/m3, similar to exposure levels used by others to approximate the cloud of particulates surrounding a person during active smoking (43). The level of nicotine in blood was also measured in some experiments. In FA-exposed animals, the nicotine level in blood was ∼0 ng/ml. After 10 days of SS exposure, the nicotine level in blood was ∼20 ng/ml, which was similar with the nicotine levels typically found in human smokers (10–50 ng/ml; Refs. 3, 38).
After daily exposure to SS or FA for 10 days, the average weights of the SS exposure in PD2–11 (10.5 ± 1.8 g; n = 12) and PD21–30 (21.4 ± 2.3 g; n = 12) were not significantly different from the PD2–11 (11.5 ± 2.2 g; n = 12) and PD21–30 (23.1 ± 2.8 g; n = 12) FA exposure groups, respectively.
To test the role of NGF on SS-altered NPY and lung function in the PD2–11 group, the tyrosine kinase antagonist K252a or a specific NGF antibody was used to block NGF effects. K252a has been shown to inhibit NGF receptor trkA, trkB, and trkC phosphorylation (26) and block NGF-induced neuropeptide production (7, 36). Also, NGF antibody has been shown to inhibit enhanced airway innervation after irritant exposures (5, 16). To ensure sufficient reduction of NGF effects, the mice were treated using both aerosol exposure and subcutaneous injection. The final dosages of K252a and NGF antibody were based on previous studies (4, 5, 7, 36) and our preliminary data. The final concentration of K252a for the aerosol exposure (Sigma-Aldrich, St. Louis, MO) was 100 nM, and 100 μg/kg of K252a were subcutaneously injected. The rabbit anti-NGF antibody and IgG (Sigma-Aldrich) were diluted 1:2,000 (3 ug of total protein/ml) for the aerosol exposure and subcutaneous injection. For the aerosol exposure, mice were placed in a Plexiglas chamber (15× 15 × 10 cm), which was connected to a mini-ultrasonic nebulizer and placed under a negative-pressure exhaust hood for 10 min 1 h before SS exposure on each day. Two milliliters of K252a, or K252a vehicle (2% DMSO in normal saline solution), or rabbit anti-NGF antibody or IgG control were nebulized with an output rate of 0.1 ml/min. Before first SS exposure and after the last SS exposure, mice were injected subcutaneously with K252a (100 μg/kg), or equal volume K252a vehicle, or NGF rabbit anti-mouse antibody (1:2,000 dilution, 4 ml/kg,) or IgG (1:2,000 dilution, 4 ml/kg).
NGF ELISA.
NGF ELISA assay in lung tissue was conducted as previously described (40). Lungs were obtained from each animal 16 h after the last SS exposure. The specimens were weighed, homogenized, and centrifuged (40,000 g). Supernatant fractions were collected, filtered, and frozen at −80°C until assay. The concentration of NGF (7.8–500 pg/ml) in each sample was assayed using the NGF Emax immunoassay system (Promega, Madison, WI) according to the manufacturer's instructions. All samples were run in duplicate.
Immunoblotting analyses.
Lung tissue obtained 16 h after the last SS exposure was homogenized in ice-cold RIPA lysis buffer (Upstate, Temecula, CA) and centrifuged at 16,000 g for 30 min. Supernatant was mixed with an equal volume of Laemmli sample buffer and heated to 100°C for 2 min. Variable volumes of sample containing equal amounts of protein were loaded onto gels for SDS-PAGE. Following separation by electrophoresis, proteins in the gels were transferred to nitrocellulose membranes, which were incubated with rabbit anti-NPY or anti-β-actin antibodies (1:1,000; Chemicon, Temecula, CA) overnight at 4°C and then with Alexa Fluor 680-conjugated secondary antibody (1:20,000; Invitrogen, Carlsbad, CA) for 30 min at room temperature (Invitrogen). The detection and quantification of specific bands were carried out using a fluorescence scanner (Odyssey Infrared Imaging System; LI-COR Biotechnology, Lincoln, NE).
Real-time PCR analysis.
Lungs obtained 16 h after the last SS exposure were immediately flash frozen in liquid nitrogen and stored in a freezer at −80°C. The frozen specimens were placed in lysis buffer and homogenized using a conventional rotor-stator homogenizer. Total RNA was extracted from lung homogenates using TRIzol reagent (Invitrogen), according to the manufacturers' protocol. Purity and quantity of total RNA were determined using the Nanodrop ultraviolet-visible spectrophotometer (NanoDrop Technologies, Wilmington, DE). The reverse-transcribed cDNA was generated using SuperScript II kit (Invitrogen) following the manufacturer's instructions. The cDNAs were amplified with TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) in a 7500 Real Time PCR System (Applied Biosystems) according to the manufacturers' instructions and protocols. Primers for the cDNA amplification were designed using Primer Express software (Applied Biosystems): NGF forward primer, 5′-AGCAAGCGGTCATCATCC-3′; and NGF reverse primer, 5′-GTGGCGGTGGTCTTATCC-3′. Thermal cycling was performed as follows: initial denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The expression of a housekeeping gene encoding β-actin was used as the endogenous control for transcripts normalization and was amplified with primers. The relative change in gene expression was calculated with the following formula: fold change = 2−(ddCt) = 2-dCt (treated samples) − dCt (control samples), where dCt = Ct (detected gene) − Ct (HPRT1) and Ct is the threshold number. Fold changes were calculated using the system software.
Immunocytochemistry.
The procedures for immunocytochemical quantification of airway nerves have been described previously (8, 39, 40). Briefly, tracheal segments were removed 16 h after the last smoke exposure and fixed in picric acid-formaldehyde fixative for 3 h, rinsed three times with 0.1 M PBS containing 0.3% Triton-X-100 (PBS-Tx), frozen in isopentane, cooled with liquid nitrogen, and stored at −80°C. Cryostat sections (12 μm thickness) were collected on gelatin-coated coverslips and dried briefly at room temperature. Then, cryostat sections were covered with rabbit NPY antibody (Peninsula, Belmont, CA) diluted 1:100, incubated in a humid chamber at 37°C for 30 min and rinsed with a 1% BSA-PBS containing Triton X-100 solution (PBS-Tx plus BSA) three times allowing 5 min for each rinse. The sections were then covered with fluorescein isothiocyanate-labeled goat anti-rabbit antibody (Invitrogen) diluted 1:100, incubated at 37°C for 30 min, and rinsed. Then, some sections were processed for protein gene product (PGP) 9.5 immunoreactivity using guinea pig anti-PGP 9.5 (1:100; Millpore, Billerica, MA) and donkey anti-guinea pig labeled with rhodamine (1:100; Jackson Laboratory, Bar Harbor, Maine). After all immunocytochemical procedures were conducted, the coverslips were mounted with fluoromount and observed under a fluorescence microscope equipped with fluorescein (excitation wavelengths from 455 to 500 nm and emission wavelengths >510 nm) and rhodamine (excitation 540–504 nm and emission >580) filters. Controls consisted of testing the NPY specificity of the primary antiserum by absorption with 100 ng/ml of NPY. Nonspecific background labeling was determined by omission of primary antiserum.
For measuring nerve fiber density in tracheal smooth muscle, we collected images of control, NPY, and PGP 9.5 nerve fibers in series under the Zeiss LSM 510 confocal microscope. A series of images representing all of the tracheal smooth muscle in a section was collected in digital files and saved to an internal database and measured using Optimas software. We selected regions of smooth muscle using the rhodamine channel to avoid possible bias created by the presence or absence of nerve fibers. The smooth muscle regions were outlined to measure total cross-sectional area of smooth muscle. The microscope was then switched to reveal nerve fibers in the fluorescein channel, and the image was digitally captured. The thresholding levels were manually adjusted to subjectively optimize the appearance of fluorescent nerves. The area of nerve fibers was determined by segmentation with the Optimas software. Then, nerve fiber area was standardized to the total cross-sectional area of smooth muscle. The final value of nerve fiber density is expressed as percentage of dividing the SP or PGP 9.5 nerve fiber area by the total area of smooth muscle. At least 10 measurements were made for each section, and 15 sections were measured in each animal.
Measurements of lung function.
Lung function was determined by measuring changes of pulmonary resistance (RL) and dynamic compliance (Cdyn) after aerosolized methacholine (MCh) challenge using a modification of our previously described technique (40, 41). Briefly, mice were anesthetized with pentobarbital (70 mg/kg ip) before or 16 h after reexposure to SS on PD59. Pulmonary function testing was conducted at PD60 to determine if the critical period exposure produced airway hyperresponsiveness in later life. Testing could not be conducted at PD11 or PD30 due to the small size of the mice. For the pulmonary function measurements, the trachea was cannulated just below the larynx via a tracheotomy and a four-way connector was attached to the tracheotomy tube. Two ports were connected to the inspiratory and expiratory tubes of a respirator (Harvard model 683; South Natick, MA). The mice were ventilated at a constant rate of 200 breaths/min and a tidal volume of ∼0.2 ml. Aerosolized MCh chloride (Sigma) was administered for 30 s in increasing concentrations (0, 6.25, 12.5, 25, and 50 mg/ml). For 5 min before and after each MCh challenge, total RL and Cdyn were analyzed by computer on a breath-by-breath basis (Biosystem; Buxco, Wilmington, NC).
Data analysis.
Unless otherwise stated, results are expressed as means ± SE. The RL and Cdyn elicited by MCh were expressed as a percentage of the baseline. Nerve fiber density was expressed as percent area of NPY-immunoreactive nerve fibers in the total area of the smooth muscle. Statistical analysis of the Fig. 1, 2, 3, and 4 was performed using one-way ANOVA with multiple comparisons and in Figs. 5 and 6 was performed by two-way repeated-measures ANOVA. When the main effect was considered significant at P < 0.05, pair-wise comparisons were made with a post hoc analysis (Fisher's least significant difference). A value of P ≤ 0.05 was considered significant, and n represents the number of animals studied.
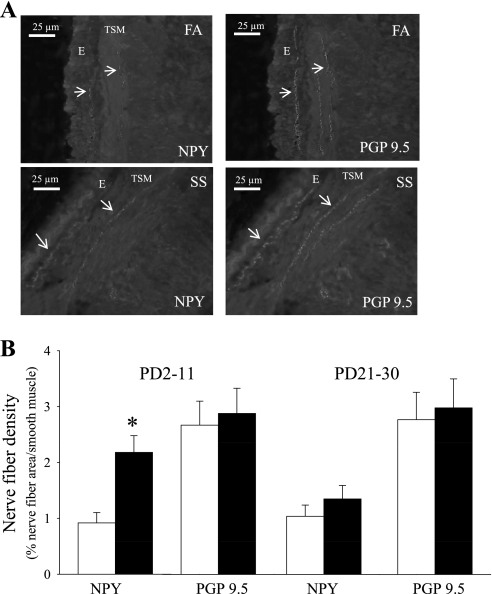
Fig. 2.
Effect of SS on NPY and protein gene product (PGP) 9.5 nerve fiber in trachea of mice. A: fluorescence and rhodamine photomicrographs of NPY and PGP 9.5 nerve fibers in tracheal epithelium (E) and smooth muscle (TSM) in postnatal day (PD)2–11 of FA- or SS-exposed mice. B: changes of NPY and PGP 9.5 nerve fiber density in tracheal smooth muscle after 10 days FA (opened bar) or SS (closed bar) exposure in PD2–11 and PD21–30 group. Arrows: the colocalization of NPY-immunoreactive nerve fibers and PGP 9.5 nerve fibers. *P ≤ 0.05, significant difference between FA- and SS-exposed mice.
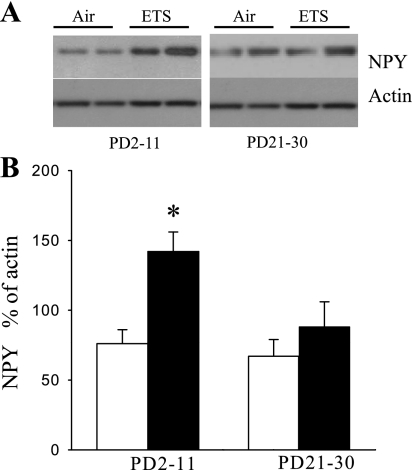
Fig. 3.
Effect of SS during early and late postnatal periods on NPY in lung. A: representative Western blot for NPY and actin. B: changes of NPY in lung in PD2–11 and PD21–30 of FA (opened bar)- or SS (closed bar)-exposed mice determined by Western blotting. Values are means ± SE; n = 6 in each group. *P ≤ 0.05, significant difference comparing corresponding data between FA and SS animals.
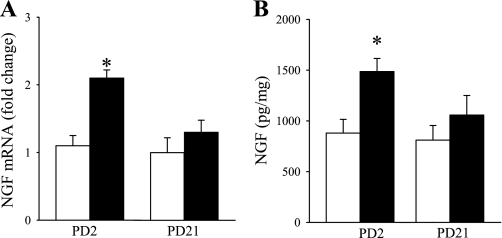
Fig. 4.
Effect of SS during early and late postnatal periods on nerve growth factor (NGF) in lung. A: change of mRNA NGF in PD2–11 and PD21–30 of FA (opened bar)- or SS (closed bar)-exposed mice determined by RT-PCR. B: change of NGF protein in PD2–11 and PD21–30 FA (opened bar)- or SS (closed bar)-exposed mice measured by ELISA. Values are means ± SE; n = 6 in each group. *P ≤ 0.05, significant difference comparing corresponding data between FA and SS animals.
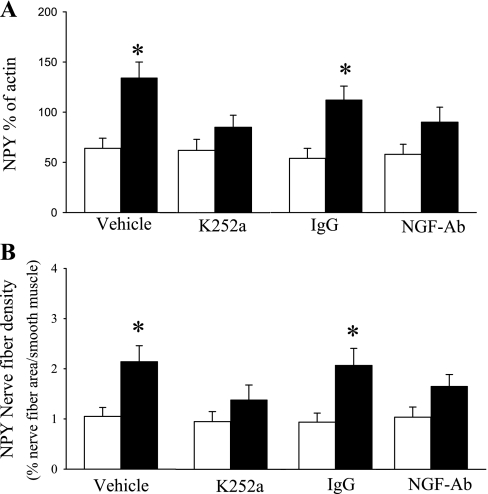
Fig. 5.
Effects of K252a on the change of NPY in PD2–11 of FA (opened bar)- or SS (closed bar)-exposed lungs of the mice. A: change of NGF protein determined by Western blotting. B: change of NPY nerves fiber density within tracheal smooth muscle determined by immunocytochemistry. Values are means ± SE; n = 6. *P ≤ 0.05, significant difference between FA- and SS-exposed mice.
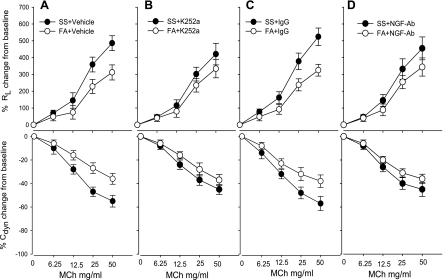
Fig. 6.
Effects of K252a on the change of pulmonary resistance (RL) and dynamic compliance (Cdyn) to methacholine (MCh) in PD2–11 of FA (opened bar)- or SS (closed bar)-exposed mice. A: pretreatment with vehicle (2% DMSO in normal saline solution). B: pretreatment with K252a. C: pretreatment with IgG. D: pretreatment with NGF antibody. Values are means ± SE; n = 5. *P ≤ 0.05, significant difference between FA- and SS-exposed mice.
RESULTS
Exposure time course of SS during early and late postnatal periods on the change of NPY nerves.
To determine the initial time course effect of SS on NPY innervation, the density of NPY nerve fibers in the airway smooth muscle was examined after 1 day, 5 days, and 10 days of SS exposure during both the early (PD11, 7, and 2) or late (PD30, 26 or 21) postnatal periods. In the PD2 and PD7 exposure groups, NPY nerve fiber density was significantly increased (Fig. 1). However, NPY nerve fiber density did not change significantly in any of the late postnatal groups (Fig. 1). Based on these findings, an exposure protocol of 10 days, beginning on either PD2 (PD2–11 group) or PD21 (PD21–30 group) was used in subsequent experiments.
Effects of early and late 10 day SS exposure on NPY.
NPY and PGP 9.5 nerves in the tracheal smooth muscle of mice were analyzed based on the immunocytochemistical localization by fluorescein and rhodamine (Fig. 2A). The NPY immunoreactive nerve fibers were colocalized with PGP 9.5, confirming their identity as nerve fibers. The density of PGP 9.5 nerve fibers in airway smooth muscle did not significantly change in SS exposed animals compared with FA exposed animals of either age group (Fig. 2B). However, the density of NPY nerve fibers in the airway smooth muscle was significantly increased after 10 days of SS exposure in the PD2–11 group (Fig. 2). These findings suggest that SS exposure increases NPY content in existing nerves in PD2–11 group, but total airway innervation was unaltered.
Western blots indicated that NPY levels were elevated in lung in PD2–11SS group compared with PD2–11FA exposure group (Fig. 3). However, the level of NPY was not significantly altered in PD21–30 SS exposure group compared with PD21–30 FA exposure group (Fig. 3).
Effect of SS during early and late postnatal periods on change of NGF.
Based on our previous studies (36, 37) that NGF production was stimulated by irritant exposures and mediated the distribution of airway innervation, our next experiment focused on the effects of SS on NGF. NGF mRNA and protein in lung tissue were measured by RT-PCR and ELISA (Fig. 4). After 10 days of SS exposure, RT-PCR data showed that NGF mRNA expression was significantly increased in the PD2–11SS group compared with FA exposure (Fig. 4A). However, there was no significant difference between the PD21–30 SS or FA exposure group. Similarly, ELISA data showed that NGF protein was increased in the PD2–11 group after SS exposure but not in the PD21–30 group (Fig. 4B).
Effect of NGF inhibition on SS-altered NPY in lung during early postnatal periods.
The next set of studies examined the role of NGF in SS-altered NPY nerves and lung function by blocking the NGF in PD2–11 mice. These studies were conducted only in the PD2–11 group because the PD21–20 group did not show altered NGF after SS exposure. Pretreatment with K252a vehicle or IgG did not affect the enhanced NPY innervation or protein levels in lung after SS exposure (Fig. 5, A and B). However, pretreatment with the tyrosine kinase inhibitor K252a or NGF antibody attenuated the SS-enhanced NPY protein levels in lung (Fig. 5A). Similar changes were observed for NPY innervation in tracheal smooth muscle (Fig. 5B). There was no significant difference in NPY innervation between PD2–11SS or FA exposure group after pretreatment with K252a or NGF antibody.
A major goal of the current study was to understand possible neuronal mechanisms initiated by SS in early life that affects lung function later in life. Our previous study (40) showed that SS exposure in the PD21–30 group did not alter airway responsiveness to reexposure on PD59. Therefore, in the current study, mice in the PD2–11 group were allowed to mature to PD59. Pulmonary function testing was measured on PD60, 16 h after a 6-h reexposure to SS on PD59. The MCh dose-response curves for RL were significantly elevated, and Cdyn was significantly decreased in the PD2–11SS exposure group treated with K252a vehicle or IgG (Fig. 6, A and C) compared FA exposure groups with vehicle or IgG. However, there was no significant difference in the in RL or Cdyn to MCh between FA exposure and SS exposure after K252a or NGF antibody treatment (Fig. 6, B and D). Table 1 shows the baseline RL and Cdyn in different groups before MCh challenge 16 h after the SS reexposure on PD59.
Table 1.
Baseline of RL and Cdyn in different groups
| Groups | RL, cm H2O·ml−1·s−1 | Cdyn, ml/cm H2O |
|---|---|---|
| SS + vehicle | 1.08 ± 0.14 | 0.039 ± 0.008 |
| FA + vehicle | 1.01 ± 0.12 | 0.044 ± 0.011 |
| SS + K252a | 1.19 ± 0.18 | 0.061 ± 0.015 |
| FA + K252a | 1.06 ± 0.13 | 0.062 ± 0.012 |
| SS + IgG | 1.11 ± 0.21 | 0.092 ± 0.031 |
| FA + IgG | 1.23 ± 0.19 | 0.098 ± 0.035 |
| SS + NGF-Ab | 1.09 ± 0.16 | 0.089 ± 0.020 |
| FA + NGF-Ab | 1.16 ± 0.15 | 0.092 ± 0.024 |
Data are means ± SE; n = 4 mice in each group. RL, pulmonary resistance; Cdyn, dynamic compliance; SS, side-steam tobacco smoke; FA, filtered air; NGF, nerve growth factor; Ab, anitbody.
DISCUSSION
Our recent study (40) showed that exposure to SS during prenatal and early postnatal periods produces changes in airway innervation and lung function that occur later in life, indicating that a critical period of susceptibility to SS exposure exists during these early periods of development. The results obtained from the current study show that exposure to SS during early postnatal periods significantly changed NPY tracheal innervation and the levels of NPY in lung with a parallel change in pulmonary function in later life. These findings suggest that NPY, a neuropeptide associated with sympathetic nerves, may be associated with the functional changes. Since the neurotrophin NGF influences growth of the sympathetic nervous system, including innervation of the airways (15), experiments were conducted to demonstrate a possible role for NGF in mediating the SS-induced increase in NPY innervation during the PD2–11 exposure period. We found that exposure to SS at this age significantly increased the level of NGF in lung, but the NGF level in SS exposure at an older age, i.e., PD21–30, was not increased. The altered NPY and lung function by SS exposure in the PD2–11 group were attenuated by pretreatment with the tyrosine kinase inhibitor K252a, a key enzyme of the NGF receptor transduction pathway or by NGF antibody, indicating that increased levels of NPY in the lung and altered lung function are regulated by elevated NGF. These findings support the conclusion that the disruption of normal synthesis and release of NGF after SS exposure during early postnatal life contribute to the changes in airway innervation and lung function, which may lead to disease-related abnormalities in the respiratory system.
Tracheal and bronchial innervation consists of sensory, parasympathetic, and sympathetic nerves. Although airway sensory nerves, such as C fibers (21) and HTA-delta fibers (42), play a central role in airway regulation, recent studies (12) show that NPY, a cotransmitter and neuromodulator in sympathetic nerves, also plays an important role in the regulation of physiological and pathophysiological processes in the respiratory system. However, previous studies examining age-related changes in airway innervation and neurotransmitter expression in young animals and children are very limited. One of the intriguing findings of this study is that NPY protein and NPY innervation were elevated by PD2–11SS exposure, but they were not increased in the PD21–30 exposure, suggesting a critical period in early postnatal life when normal NPY innervation may be adversely affected. In an earlier report (40), we showed that airway methacholine responsiveness and SP innervation were increased on PD59 after SS exposure during the early postnatal period but not in the later period. In an entirely different model, Hunter et al. (17) showed that ozone exposure in rat pups before PD15 resulted in enhanced SP innervation, but exposures at PD21 and PD28 did not increase SP innervation. Early life exposures to ozone and house dust mite antigen in monkeys also lead to long-lasting effects on airway innervation (19). These findings are consistent with the concept in animal models that early postnatal life is a particularly vulnerable developmental period when acute or extended exposure to pollutants such as cigarette smoke (40) or ozone (18) may produce prolonged effects on airway innervation. These findings parallel epidemiological studies indicating that children exposed to cigarette smoke in early postnatal life have a higher incidence of asthma (10). Although many factors may contribute to early life susceptibility, our studies here, as well as those of others, suggest that detrimental effects of early SS exposures may result in part from altered innervation affecting sensory or autonomic pathways regulating smooth muscle reactivity, bronchial vascular permeability, or mucous secretion. The current study is unique in showing that NPY, a putative neural mediator associated with sympathetic pathways, is increased by early life exposure to SS.
Clinic data demonstrate higher NPY concentrations in the blood of bronchial asthma patients (6), indicating NPY is associated with the pathophysiological processes of asthma. However, the role of NPY in asthma is not well established. NPY may regulate airway smooth muscle tone. In isolated airway, low concentrations of NPY induced a significant airway contractile response (2). Although this response was weak and accounted for <6% of response elicited by maximal contraction (2), NPY also reduced the relaxation response to vasoactive intestinal peptide and norepinephrine. Thus NPY may serve as a neuromodulator in the airway. The enhanced NPY innervation may affect airway smooth muscle tone and inhibit norepinephrine- or vasoactive intestinal peptide-evoked airway relaxation. Another possible pathophysiological mechanism for exacerbation of asthma is that the alteration of NPY levels in the lungs may affect the normal immune system. It was found that direct or indirect stimulation by NPY in vitro leads to increased IL-4 release in Th2 cells, decreased IFN-γ production in Th1 cells, and induced Th2 shift (12). One of the most important steps in the development of asthma is the Th2 cell shift (20). Thus the change of NPY level in lung and airway may alter susceptibility to asthma through Th2 cell shifts after smoke exposure.
Our study demonstrated significant increases in NGF protein and mRNA in lung after SS exposure during the early postnatal period. NGF is well known to promote survival and axon outgrowth of sympathetic nerves (23), and there is strong evidence that NGF maintains and even enhances the expression of NPY in sympathetic neurons (29). In mice overexpressing NGF in bronchiolar Clara cells, sympathetic airway innervation is enhanced (14). These studies suggest that the SS-induced increase in NPY level and tracheal innervation may be stimulated by NGF. Inhibitors of NGF action (i.e., NGF antibody and K252a) directly attenuated NPY responses to SS and attenuated the increase in pulmonary resistance and decrease in dynamic compliance. Although K252a is not specific for trkA signaling, a number of studies (7, 36) have used it as supportive evidence of NGF actions in the airways. These data indicate that SS exposure during early postnatal life increases the level of NGF in lung. We reported recently that NGF was involved in mediating increased substance P expression in ferret airways (39) and that tracheal instillation of NGF during the early postnatal period enhances substance P innervation in rat airway (17). These findings, coupled with the current study, support the possibility that NGF serves as a signaling molecule during inflammatory events in the airways by regulating neuropeptide production. Another possible way that NPY innervation of the airway wall may increase is through NGF-stimulated axonal branching. However, the current study found that while SS exposed to PD2–11 mice had increased NPY innervation in airway, the density of PGP 9.5 nerves was not significantly altered, suggesting that SS exposure does not affect total airway innervation. Thus the observed increase in NPY innervation occurs in nerves that initially did not contain detectable NPY but that later acquired NPY immunoreactivity. This is consistent with an increase in NPY synthesis in sympathetic neurons. However, additional studies are needed to establish the validity of this idea.
In conclusion, the findings of this study show that exposure to SS during early postnatal life (PD2–11) increases the level of NPY protein in the lungs and NPY nerve fibers density in trachea. At the same time, the level of NGF is significantly elevated. Interestingly, these responses were not observed when SS exposure occurred in later postnatal life (PD21–30), indicating that SS exposure stimulates NPY innervation in the airways during early life but not later. The enhanced level of NPY and the airway hyperresponsiveness induced by SS during early postnatal life exposure are modulated by NGF. Overall, these findings suggest that early life susceptibility to SS may have a neural component regulated by neurotrophins. Table 1
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-35812 and HL-80566 and American Lung Association Grant BRG 71670136.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.-X.W. and R.D.D. conception and design of research; Z.-X.W., K.B.B., and D.D.H. analyzed data; Z.-X.W. interpreted results of experiments; Z.-X.W. prepared figures; Z.-X.W. drafted manuscript; Z.-X.W. and R.D.D. edited and revised manuscript; K.B.B. and D.D.H. performed experiments; R.D.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to Dr. Chunlin Dong in the Department of Statistics, West Virginia University, for statistical analysis.
REFERENCES
- 1. Barnes PJ, Baraniuk JN, Belvisi MG. Neuropeptides in the respiratory tract. Am Rev Respir Dis 144: 1391–1399, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Benchekroun T, Fournier A, St-Pierre S, Cadieux A. Inhibitory action of neuropeptide Y on agonist-induced responses in isolated guinea pig trachea. Eur J Pharmacol 216: 421–428, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Benowitz NL, Jacob P., III Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther 35: 499–504, 1984 [DOI] [PubMed] [Google Scholar]
- 4. Buck H, Winter J. K252a modulates the expression of nerve growth factor-dependent capsaicin sensitivity and substance P levels in cultured adult rat dorsal root ganglion neurones. J Neurochem 67: 345–351, 1996 [DOI] [PubMed] [Google Scholar]
- 5. Cardenas S, Scuri M, Samsell L, Ducatman B, Bejarano P, Auais A, Doud M, Mathee K, Piedimonte G. Neurotrophic and neuroimmune responses to early-life Pseudomonas aeruginosa infection in rat lungs. Am J Physiol Lung Cell Mol Physiol 299: L334–L344, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dahlof C, Dahlof P, Lundberg JM, Strombom U. Elevated plasma concentration of neuropeptide Y and low level of circulating adrenaline in elderly asthmatics during rest and acute severe asthma. Pulm Pharmacol 1: 3–6, 1988 [DOI] [PubMed] [Google Scholar]
- 7. de Vries A, Engels F, Henricks PA, Leusink-Muis T, McGregor GP, Braun A, Groneberg DA, Dessing MC, Nijkamp FP, Fischer A. Airway hyper-responsiveness in allergic asthma in guinea-pigs is mediated by nerve growth factor via the induction of substance P: a potential role for trkA. Clin Exp Allergy 36: 1192–1200, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Dey RD, Satterfield B, Altemus JB. Innervation of tracheal epithelium and smooth muscle by neurons in airway ganglia. Anat Rec 254: 166–172, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, Landreth K, Peden DB, Pinkerton K, Smialowicz RJ, Zoetis T. Workshop to identify critical windows of exposure for children's health: immune and respiratory systems work group summary. Environ Health Perspect 108, Suppl 3: 483–490, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics 113: 1007–1015, 2004 [PubMed] [Google Scholar]
- 11. Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med 167: 917–924, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Groneberg DA, Folkerts G, Peiser C, Chung KF, Fischer A. Neuropeptide Y (NPY). Pulm Pharmacol Ther 17: 173–180, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Haberg SE, Stigum H, Nystad W, Nafstad P. Effects of pre- and postnatal exposure to parental smoking on early childhood respiratory health. Am J Epidemiol 166: 679–686, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Hoyle GW, Graham RM, Finkelstein JB, Nguyen KPT, Gozal D, Friedman B. Hyperinnervation of the airways in transgenic mice overexpressing nerve growth factor. Am J Respir Cell Mol Biol 18: 149–157, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Hoyle GW, Mercer EH, Palmiter RD, Brinster RL. Expression of NGF in sympathetic neurons leads to excessive axon outgrowth from ganglia but decreased terminal innervation within tissues. Neuron 10: 1019–1034, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Hu C, Wedde-Beer K, Auais A, Rodriguez MM, Piedimonte G. Nerve growth factor and nerve growth factor receptors in respiratory syncytial virus-infected lungs. Am J Physiol Lung Cell Mol Physiol 283: L494–L502, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Hunter DD, Carrell-Jacks LA, Batchelor TP, Dey RD. Role of nerve growth factor in ozone-induced neural responses in early postnatal airway development. Am J Respir Cell Mol Biol 45: 359–365, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hunter DD, Wu Z, Dey RD. Sensory neural responses to ozone exposure during early postnatal development in rat airways. Am J Respir Cell Mol Biol 43: 750–757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir Physiol Neurobiol 155: 55–63, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Krug N, Frew AJ. The Th2 cell in asthma: Initial expectations yet to be realised. Clin Exp Allergy 27: 142–150, 1997 [PubMed] [Google Scholar]
- 21. Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol 125: 47–65, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Levi-Montalcini R. The nerve growth factor: thirty-five years later. Biosci Rep 7: 681–699, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Levi-Montalcini R, Booker B. Excessive growth of the sympathetic ganglia evoked by a protein isolated from mouse salivary glands. Proc Natl Acad Sci USA 46: 373–384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundberg JM, Terenius L, Hokfelt T, Martling CR, Tatemoto K, Mutt V, Polak J, Bloom S, Goldstein M. Neuropeptide Y (NPY)-like immunoreactivity in peripheral noradrenergic neurons and effects of NPY on sympathetic function. Acta Physiol Scand 116: 477–480, 1982 [DOI] [PubMed] [Google Scholar]
- 25. Martinez FD, Cline M, Burrows B. Increased incidence of asthma in children of smoking mothers. Pediatrics 89: 21–26, 1992 [PubMed] [Google Scholar]
- 26. Ohmichi M, Decker SJ, Pang L, Saltiel AR. Inhibition of the cellular actions of nerve growth factor by staurosporine and K252A results from the attenuation of the activity of the trk tyrosine kinase. Biochemistry 31: 4034–4039, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Pinkerton KE, Joad JP. The mammalian respiratory system and critical windows of exposure for children's health. Environ Health Perspect 108, Suppl 3: 457–462, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raherison C, Penard-Morand C, Moreau D, Caillaud D, Charpin D, Kopfersmitt C, Lavaud F, Taytard A, Annesi-maesano IA. In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir Med 101: 107–117, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Shadiack AM, Sun Y, Zigmond RE. Nerve growth factor antiserum induces axotomy-like changes in neuropeptide expression in intact sympathetic and sensory neurons. J Neurosci 21: 363–371, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med 172: 233–237, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Wang C, Salam MT, Islam T, Wenten M, Gauderman WJ, Gilliland FD. Effects of in utero and childhood tobacco smoke exposure and beta2-adrenergic receptor genotype on childhood asthma and wheezing. Pediatrics 122: e107–e114, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang L, Pinkerton KE. Detrimental effects of tobacco smoke exposure during development on postnatal lung function and asthma. Birth Defects Res C Embryo Today 84: 54–60, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Weiss ST. The origins of childhood asthma. Monaldi Arch Chest Dis 49: 154–158, 1994 [PubMed] [Google Scholar]
- 34. Weiss ST, Utell MJ, Samet JM. Environmental tobacco smoke exposure and asthma in adults. Environ Health Perspect 107: 891–895, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weitzman M, Gortmaker S, Walker DK, Sobol A. Maternal smoking and childhood asthma. Pediatrics 85: 505–511, 1990 [PubMed] [Google Scholar]
- 36. Wilfong ER, Dey RD. Nerve growth factor and substance P regulation in nasal sensory neurons after toluene diisocyanate exposure. Am J Respir Cell Mol Biol 30 793–800, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Wilfong ER, Dey RD. The release of nerve growth factor from the nasal mucosa following toluene diisocyanate. J Toxicol Environ Health A 68: 1337–1348, 2005 [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization IPCS: Environmental Health Criteria Series. Carbon Monoxide. Geneva, Switzerland: World HEalth Organization, 2003 [Google Scholar]
- 39. Wu ZX, Dey RD. Nerve growth factor-enhanced airway responsiveness involves substance P in ferret intrinsic airway neurons. Am J Physiol Lung Cell Mol Physiol 291: L111–L118, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Wu ZX, Hunter DD, Kish VL, Benders KM, Batchelor TP, Dey RD. Prenatal and early, but not late, postnatal exposure of mice to sidestream tobacco smoke increases airway hyperresponsiveness later in life. Environ Health Perspect 117: 1434–1440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu ZX, Lee LY. Airway hyperresponsiveness induced by chronic exposure to cigarette smoke in guinea pigs: role of tachykinins. J Appl Physiol 87: 1621–1628, 1999 [DOI] [PubMed] [Google Scholar]
- 42. Yu J, Lin S, Zhang J, Otmishi P, Guardiola JJ. Airway nociceptors activated by pro-inflammatory cytokines. Respir Physiol Neurobiol 156: 116–119, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Yu M, Zheng X, Peake J, Joad JP, Pinkerton KE. Perinatal environmental tobacco smoke exposure alters the immune response and airway innervation in infant primates. J Allergy Clin Immunol 122: 640–647, 2008 [DOI] [PubMed] [Google Scholar]