Abstract
We hypothesized that vascular nitric oxide synthase (NOS) function and expression is differentially regulated in adult Dahl salt-sensitive rats maintained on Teklad or American Institutes of Nutrition (AIN)-76A standard chow diets from 3 to 16 wk old. At 16 wk old, acetylcholine (ACh)-mediated vasorelaxation and phenylephrine (PE)-mediated vasoconstriction in the presence and absence of NOS inhibitor, Nω-nitro-l-arginine methyl ester (l-NAME), was assessed in small-resistance mesenteric arteries and aortas. Rats maintained on either diet throughout the study had similar responses to ACh and PE in the presence or absence of l-NAME in both vascular preparations. We reasoned that changing from one diet to another as adults may induce vascular NOS dysfunction. In the absence of l-NAME, small arteries from Teklad-fed rats switched to AIN-76 diet and vice versa had similar responses to ACh and PE. Small-arterial NOS function was maintained in rats switched to AIN-76A from Teklad diet, whereas NOS function in response to ACh and PE was lost in the small arteries from rats changed to Teklad from AIN-76A diet. This loss of NOS function was echoed by reduced expression of NOS3, as well as phosphorylated NOS3. The change in NOS phenotype in the small arteries was observed without changes in blood pressure. Aortic responses to ACh or PE in the presence or absence of l-NAME were similar in all diet groups. These data indicate that changing standard chow diets leads to small arterial NOS dysfunction and reduced NOS signaling, predisposing Dahl salt-sensitive rats to vascular disease.
Keywords: standard diet, Dahl rat, Nω-nitro-l-arginine methyl ester, nitric oxide synthase, vascular reactivity
the nitric oxide synthase (NOS) pathway plays an obligatory role in vascular tone homeostasis (1). Loss of NOS signaling results in a loss of tonic vasorelaxation, favoring greater vasoconstriction (11, 26, 42), and is linked to increased risk of vascular disease (3, 20, 43). Humans that present increased blood pressure to salt have a greater disposition to vascular disease risk even under normotensive conditions (44, 45). A prototypical model to study vascular dysfunction is the Dahl salt-sensitive (Dahl S) rat. In this rat strain, a high-salt diet induces robust hypertension and vascular dysfunction (5, 6, 14, 25, 28, 32, 37). Intriguingly, under normotensive conditions without high-salt diet, Dahl S rats have lower vascular NOS expression (30) and greater blood pressure responsiveness to bolus doses of phenylephrine (PE; α1-specific agonist) compared with the genetic salt-resistant control strain (10). These data suggest that a genetic predisposition to salt sensitivity per se, not frank hypertension, enhances vascular disease risk.
Our laboratory is interested in understanding mechanisms that predispose salt-sensitive individuals to vascular disease using the Dahl S model. Previous investigations showed that promotion of salt-sensitive hypertension in adult Dahl S rats is dependent on the type of weaning diet (27). This led us to investigate whether vascular NOS function and expression are sensitive to the choice of standard chow diets. The two popular commercial suppliers of Dahl S rats, Harlan Laboratories (www.harlan.com) and Charles River Laboratories (www.criver.com), each maintains their Dahl S colonies on different commercially available standard chows. Harlan uses Teklad diet, and Charles River utilizes American Institutes of Nutrition (AIN) purified diet. The standard chow at many institutions is Teklad diet; therefore, some experimental designs would incorporate changing the standard chow diet during adulthood once the rats are housed at the investigator's institution. We reasoned that changing between standard chow diets may differentially regulate vascular NOS function. Thus we designed experiments to test the hypothesis that vascular NOS function and expression are differentially regulated in adult Dahl S rats on Teklad 8604 or AIN-76A standard chow diets. For this purpose, a colony of Dahl S rats was generated at Georgia Health Sciences University to maintain a comparable stable environment. Male offspring were weaned on Teklad 8604 or AIN-76A standard chow diet and were either maintained on the same weaning diet or switched to the other standard chow diet at 12 wk old. Mesenteric arterial and aortic reactivity, as well as NOS function and expression were assessed at 16 wk old.
MATERIALS AND METHODS
Animal model.
Dahl S rat breeders were purchased from Charles River Laboratories (Wilmington, MA) and placed on Teklad 8604 diet on arrival at Georgia Health Sciences University (GHSU). First-generation Dahl S rats from six breeding pairs were used in this study. A genomewide scan using microsatellite primers, as detailed by Moreno et al. (29), that were specific to Dahl DNA confirmed the genetic background of the breeders. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animal use protocols were approved by the Institutional Animal Care and Use Committee at GHSU.
At weaning (3 wk old), a subset of male pups from each breeding pair were fed Teklad 8604 rodent diet (Teklad, Madison, WI) or AIN-76A purified diet (TestDiet, Richmond, IN) ad libitum. The Teklad diet consisted of calories from 33% protein, 53% carbohydrates, and 14% fat and 3.93 kcal/g gross energy. The AIN-76A diet consisted of calories from 19% protein, 69% carbohydrates, and 12% fat and 3.84 kcal/g gross energy. Both the Teklad and AIN-76A diets contained 0.4% NaCl with similar vitamin compositions. All rats were given tap water ad libitum.
At 12 wk old, Teklad-weaned or AIN-weaned rats underwent a diet-switch protocol, where a subset of Teklad rats was switched to AIN and vice versa. Importantly, rats generated from each breeding pair were included in each of the four diet groups (Fig. 1A). Weekly body weights were assessed on all rats. At 16 wk old, rats were euthanized (Nembutal, Abbott Laboratories, Abbott Park, IL; 0.5 mg/kg). Kidney, heart, epididymal adipose tissue weights, and tibia lengths were assessed. Blood was collected in EDTA (Sigma, St. Louis, MO)-primed syringes, spun at 3,000 g for 10 min, and snap-frozen in liquid N2. Mesenteric arteries and aortas were isolated, cleaned for ex vivo vascular reactivity analysis, or snap-frozen in liquid N2 for Western blotting, as described below.
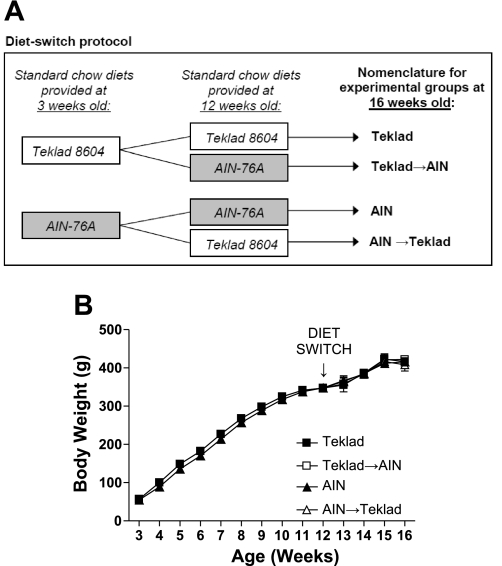
Fig. 1.
A: diagram of diet-switch protocol performed in Dahl salt-sensitive (S) rats. Rats were fed Teklad diet or American Institutes of Nutrition (AIN) diet at weaning (3 wk old). Rats either remained on respective diet until 16 wk old, or, at 12 wk old, diets were switched, with Teklad-fed rats changed to AIN diet (Teklad→AIN), or AIN-fed rats changed to Teklad diet (AIN→Teklad). B: weekly body weights for Dahl S rats for Teklad (N = 9), AIN (N = 9), Teklad→AIN (N = 10), and AIN→Teklad (N = 9). Data were analyzed by two-way ANOVA.
Telemetry hemodynamic and activity measurements.
Rats were implanted with telemetry transmitters (Data Sciences International, St. Paul, MN) at 11 wk old, as described previously (16). Rats recovered from surgery for ∼1 wk, while having free access to tap water and their respective diet. From 12–16 wk old, the diet-switch protocol was performed (Fig. 1A), while mean arterial blood pressure measurements were collected every 10th min. Blood pressure is reported as a 24-h average or 12-h average.
Urine collection.
At 16 wk old, rats were placed in metabolic cages to collect 24-h urine volumes. Urines were snap frozen in liquid N2 and stored at −80°C until analyzed. Urinary Na excretion was determined (EasyLite; Medica, Bedford, MA) with data expressed as milliequivalent per 24 h. Urinary total protein excretion was quantified using standard protein assay (BCA assay, Bio-Rad, Hercules, CA).
Vascular reactivity.
Thoracic aortas and third-order mesenteric arteries were cleaned of adherent fat, cut into concentric rings, and mounted on pins and chucks, respectively, for wire myography (Danish Myo Technology A/S, Aarhus, Denmark), as previously described (24). Aortic and small mesenteric artery segments were constricted with 1 μM and 2 μM PE, respectively, followed by evaluation of vasorelaxation with cumulative-concentration response curves to acetylcholine (ACh; 1 × 10−9 M to 3 × 10−5.5 M for mesenteric arteries and 1 × 10−9 M to 3 × 10−4.5 M for aortas) and then to sodium nitroprusside (SNP; 1 × 10−10 M to 3 × 10−6.5 M for mesenteric arteries and 1 × 10−10 M to 3 × 10−5.5 M for aortas) in the same artery segment. Vasorelaxation data are presented as relaxation (%PE constriction), as analyzed by the equation [(maximum PE response − ACh response)/(maximum PE response − baseline before PE constriction)] × 100. Vasoconstriction was assessed with PE (1 × 10−9 M to 3 × 10−5 M), followed by KCl concentration-response curve (8 × 10−3 M to 100 × 10−3 M). Constriction responses are presented as percent increase in force, as analyzed by the equation [(response to vasoconstrictor − baseline before constriction)/baseline before constriction] × 100. Rings were incubated in the presence or absence of the nonspecific NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME; 100 μM; Sigma) for 15 min before construction of response curves. Maximum response and sensitivity to the vasoactive agonists are expressed as Emax and logEC50, respectively. LogEC50 was determined with GraphPad Prism software (La Jolla, CA).
Western blotting.
In a subset of rats from each diet group, whole mesenteric arterial beds were homogenized in 400-μl ice-cold lysis buffer (50 mM Tris, 0.1 mM EDTA disodium salt, 0.1 mM EGTA, 0.1 mM sucrose, 0.1% 2-mercaptoethanol, 10% glycerol, 2 μM leupeptin, 2 μM pepstatin A, 1 mM phenylmethylsulfonyl fluoride, 0.1% aprotinin, 20 mM NaVO3; pH 7.4); PhosSTOP tablets were used according to manufacturer's instructions (Roche Diagnostics; Indianapolis, IN). Homogenates were spun at 10,000 g at 4°C for 5 min, and supernatants were isolated. Protein concentrations of supernatants were determined (BCA assay, Bio-Rad). Thirty micrograms of protein were separated via 8% SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed using anti-NOS1, anti-NOS3, anti-NOS3-phosphoserine-1177 (p1177; all NOS antibodies at 1:500; BD Biosciences, San Jose, CA) and β-actin (1:10,000; Sigma). NOS antibodies were visualized with goat anti-mouse (1:1,000; Invitrogen, Carlsbad, CA), and β-actin was detected with goat anti-rabbit (1:10,000; Invitrogen) secondary antibodies using the Odyssey Infrared Imaging System (LI-COR Biosciences; Lincoln, NE). Analysis of NOS expression was normalized to β-actin. Further analysis of NOS3-p1177 was normalized to NOS3 expression.
Plasma nitrite/nitrate measurement.
Plasma was extracted using 1:1 (vol/vol) HPLC-grade methanol (Fisher Scientific, Fair Lawn, NJ), followed by centrifugation at 10,000 g for 5 min at 4°C to evaluate nitrite and nitrate levels by HPLC (ENO-20; EiCom, Kyoto, Japan), as previously described (17).
Statistical analyses.
All data are expressed as means ± SE. Statistical significance was defined as P < 0.05, as determined by Student's t-test or two-way ANOVA, where indicated (GraphPad Prism).
RESULTS
Metabolic parameters.
Figure 1A shows the experimental design and nomenclature utilized for the study. Dahl S rats, fed either Teklad or AIN standard chow diets from 3 wk until 16 wk old, gained weight similarly and had comparable tibia lengths, demonstrating that neither standard diet differentially affected rat growth (Fig. 1B; Table 1). Food and water intakes were also similar at 16 wk old (Table 1). Heart weight and epididymal fat mass were similar in all diet groups (Table 1); however, kidney weights were significantly smaller in the groups raised on the AIN diet vs. the Teklad diet, with no difference between the other groups (Table 1).
Table 1.
Body and tissue weights and metabolic parameters in Dahl S rats at 16 wk old
| Parameter | Teklad | Teklad→AIN | AIN | AIN→Teklad |
|---|---|---|---|---|
| Tibia length, cm | 4.24 ± 0.01 (7) | 4.26 ± 0.02 (9) | 4.30 ± 0.03 (6) | 4.34 ± 0.03 (8) |
| Body weight, g/cm tibia | 100.21 ± 1.76 (7) | 98.04 ± 2.35 (9) | 96.12 ± 1.28 (6) | 97.84 ± 2.55 (8) |
| Heart, g/cm tibia | 0.37 ± 0.02 (7) | 0.34 ± 0.01 (9) | 0.31 ± 0.01 (6) | 0.32 ± 0.01 (8) |
| Kidney, g/cm tibia | 0.81 ± 0.03 (7) | 0.73 ± 0.01 (9) | 0.71 ± 0.03* (6) | 0.75 ± 0.03 (8) |
| Epididymal fat, g/cm tibia | 1.17 ± 0.05 (7) | 1.33 ± 0.07 (9) | 1.21 ± 0.09 (6) | 1.21 ± 0.12 (8) |
| Food intake, g/24 h | 25.2 ± 1.3 (8) | 21.4 ± 1.1 (9) | 20.9 ± 1.4 (7) | 25.4 ± 1.2 (5) |
| Water intake, ml/24 h | 30.1 ± 1.8 (8) | 21.8 ± 1.6 (9) | 18.4 ± 2.4 (7) | 30.1 ± 0.8 (5) |
| Na excretion, meq/24 h | 6.0 ± 0.9 (7) | 6.4 ± 0.4 (9) | 9.0 ± 1.3 (7) | 5.9 ± 0.4 (4) |
| Proteinuria, mg/24 h | 0.6 ± 0.1 (6) | 1.0 ± 0.2 (9) | 0.7 ± 0.07 (6) | 0.8 ± 0.06 (6) |
Values are means ± SE; no. of rats are in parentheses. Dahl salt-sensitive (S) rats were given Teklad or American Institutes of Nutrition (AIN) standard chow diets at weaning (3 wk old). At 12 wk, weaning-diet groups were divided, and a subset of rats underwent a diet switch, generating two additional diet groups: Teklad→AIN and AIN→Teklad.
P < 0.05 vs. Teklad. Data were analyzed by two-way ANOVA.
A subset of each weaning-diet group underwent a diet switch protocol from 12 to 16 wk old (i.e., Teklad diet-fed rodents switched to AIN diet at 12 wk old, referred to as Teklad→AIN, and vice versa). Body and organ weights were similar to respective weaning diet counterparts at 16 wk old (Table 1). No statistically significant difference in food or water intake was observed at 16 wk old between the four diet groups (Table 1).
Hemodynamic and activity measurements.
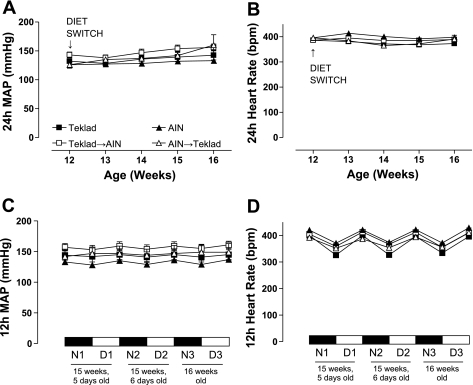
At 16 wk old, 24-h mean arterial pressure (MAP) and heart rate were similar in the nonswitched weaning diet groups (Teklad or AIN); the trend for increased 24-h MAP in the diet-switch groups (Teklad→AIN or AIN→Teklad) was not significant (Fig. 2A). Additionally, the tendency for 24-h MAP to increase from 12 to 16 wk old was not statistically different between the four diet groups (Fig. 2A). At 16 wk old, 24-h heart rate was similar in all diet groups (Fig. 2B). Circadian rhythms of 12-h MAP (Fig. 2C) and heart rate (Fig. 2D) were similar in all groups during the last 3 days of the diet-switch protocol (at 15 wk, 5 and 6 days old and at 16 wk old).
Fig. 2.
Twenty-four-hour mean arterial blood pressure (MAP) (A) and heart rate (B) tracings in Dahl S rats fed Teklad (N = 6) or AIN (N = 4) standard chow diets since weaning (3 wk old). Twelve-hour MAP (C) and heart rate (D) (at 15 wk, 5 and 6 days old, and at 16 wk old) are shown. At 12 wk old, weaning-diet groups were divided, with a subset of rats undergoing a diet switch, thereby generating two additional diet groups: Teklad→AIN (N = 6) and AIN→Teklad (N = 3). Values are means ± SE. Data were analyzed by two-way ANOVA. N, night; D, day; bpm, beats per minute.
Vasorelaxation.
Cumulative concentration-response curves to ACh were generated to assess endothelial function in third-order small-resistance mesenteric arteries and thoracic aortas. No difference in maximum relaxation (Emax, Table 2) or sensitivity (logEC50, Table 2) was detected between weaning diet groups or diet switch groups in small mesenteric arteries, as well as the response to the exogenous nitric oxide (NO) donor, SNP, between all four groups of Dahl S rats (Table 2).
Table 2.
Maximum response (Emax) and sensitivity (logEC50) to ACh or SNP in small mesenteric arteries from Dahl S rats at 16 wk old
| Teklad | Teklad→AIN | AIN | AIN→Teklad | |
|---|---|---|---|---|
| Emax to [ACh, 10−5.5 M] and [SNP, 10−6.5 M] | ||||
| ACh, %PE | 99.71 ± 0.27 (6) | 99.53 ± 0.41 (10) | 100.24 ± 0.28 (6) | 100.14 ± 0.37 (6) |
| ACh + l-NAME, %PE | 94.99 ± 0.39* (5) | 64.50 ± 15.34* (10) | 91.96 ± 2.76* (6) | 92.95 ± 1.46* (6) |
| SNP, %PE | 97.36 ± 0.65 (7) | 96.65 ± 1.91 (10) | 98.24 ± 0.82 (7) | 98.54 ± 0.51 (6) |
| logEC50 | ||||
| ACh, M concn. | −7.1 ± 0.10 (6) | −7.4 ± 0.10 (10) | −7.2 ± 0.10 (6) | −7.2 ± 0.10 (6) |
| ACh + l-NAME, M concn. | −6.2 ± 0.10* (5) | −6.3 ± 0.20* (8) | −6.6 ± 0.10* (6) | −6.7 ± 0.20 (5) |
| SNP, M concn. | −7.9 ± 0.12 (7) | −8.0 ± 0.09 (10) | −7.9 ± 0.17 (6) | −8.4 ± 0.21 (5) |
Values are means ± SE; no. of rats are in parentheses. Dahl S rats were given Teklad or AIN standard chow diets at weaning (3 wk old). At 12 wk, weaning-diet groups were divided, and a subset of rats underwent a diet switch, generating two additional diet groups: Teklad→AIN and AIN→Teklad. Nω-nitro-l-arginine methyl ester (l-NAME) was used at 100-μM concentration to nonselectively inhibit nitric oxide synthase (NOS). ACh, acetylcholine; SNP, sodium nitroprusside; PE, phenylephrine. Brackets denote concentration.
P < 0.05 vs. corresponding untreated mesenteric artery segment. Data were analyzed by two-way ANOVA.
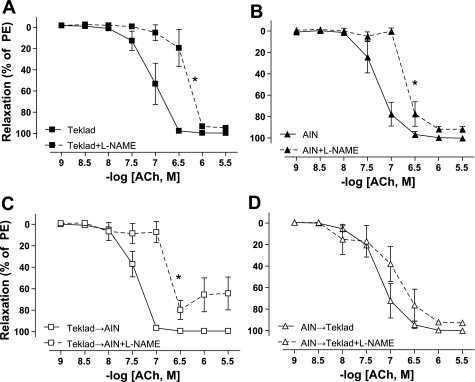
To assess NOS function, ACh-mediated relaxation curves were generated in the presence of the nonspecific NOS inhibitor, l-NAME. l-NAME significantly reduced sensitivity to ACh in Teklad (Fig. 3A; Table 2), AIN (Fig. 3B; Table 2), and Teklad→AIN (Fig. 3C; Table 2) diet groups; however, the trend for l-NAME pretreatment to reduce ACh sensitivity in mesenteric artery segments from AIN→Teklad Dahl S rats was not statistically significant (Fig. 3D; Table 2).
Fig. 3.
Acetylcholine (ACh)-mediated relaxation in the presence or absence of Nω-nitro-l-arginine methyl ester (l-NAME) in small mesenteric arteries from adult (16 wk old) Dahl S rats fed Teklad (N = 6; A) or AIN (N = 6; B) since weaning (3 wk old). At 12 wk old, weaning-diet groups were divided, and a subset of rats underwent a diet switch, generating two additional diet groups: Teklad→AIN (N = 10; C) and AIN→Teklad (N = 6; D). Nω-nitro-l-arginine methyl ester (l-NAME) was used at 100 μM to nonselectively inhibit nitric oxide synthese(NOS). Values are means ± SE. *P < 0.05 for maximum sensitivity to the vasoactive agonists (logEC50) of l-NAME-treated vs. untreated mesenteric artery segments. Data were analyzed by t-test. PE, phenylephrine.
Aortic vasorelaxation to ACh and SNP was similar in all four diet groups, and l-NAME totally blocked the ACh response (Table 3).
Table 3.
Maximum response (Emax) and sensitivity (logEC50) to ACh or SNP in aortas from Dahl S rats at 16 wk old
| Teklad | Teklad→AIN | AIN | AIN→Teklad | |
|---|---|---|---|---|
| Emax to [ACh, 10−4.5 M] or [SNP, 10−5.5 M] | ||||
| ACh, %PE | 70.84 ± 4.18 (4) | 73.42 ± 5.62 (7) | 80.04 ± 2.63 (4) | 78.37 ± 1.30 (5) |
| ACh + l-NAME, %PE | −19.03 ± 4.01* (6) | −29.33 ± 8.13* (10) | −12.94 ± 4.38* (7) | −16.62 ± 7.27* (6) |
| SNP, %PE | 98.76 ± 0.68 (6) | 96.25 ± 1.6 (10) | 95.47 ± 2.53 | 99.39 ± 0.74 (6) |
| logEC50 | ||||
| ACh, M concn. | −6.31 ± 0.23 (4) | −6.33 ± 0.25 (7) | −6.49 ± 0.13 (4) | −6.49 ± 0.09 (5) |
| SNP, M concn. | −8.08 ± 0.11 (6) | −7.93 ± 0.15 (10) | −8.37 ± 0.13 (7) | −8.24 ± 0.13 (6) |
Values are means ± SE; no. of rats are in parentheses. Dahl S rats were given Teklad or AIN standard chow diets at weaning (3 wk old). At 12 wk, weaning-diet groups were divided, and a subset of rats underwent a diet switch, generating two additional diet groups: Teklad→AIN and AIN→Teklad. l-NAME was used at 100-μM concentration to nonselectively inhibit NOS.
P < 0.05 vs. corresponding untreated aortic artery segment. Data were analyzed by two-way ANOVA.
Vasoconstriction.
Cumulative concentration-response curves to PE were used to assess vasoconstriction in third-order mesenteric arteries. PE-induced vasoconstriction was similar in all four diet groups (Table 4). In addition, KCl-induced vasoconstriction was similar in mesenteric arteries isolated from Teklad-fed, AIN-fed, and Teklad→AIN Dahl S rat diet groups (Table 4). However, the KCl response was significantly less in AIN vs. all other diet groups (Table 4).
Table 4.
Maximum response (Emax) and sensitivity (logEC50) to PE or KCl in small mesenteric arteries from Dahl S rats at 16 wk old
| Teklad | Teklad→AIN | AIN | AIN→Teklad | |
|---|---|---|---|---|
| Emaxto [PE, 10−4.5M] and [KCl, 100 mM] | ||||
| PE, %increase in force | 694.94 ± 34.53 (7) | 607.58 ± 51.55 (10) | 542.39 ± 49.85 (6) | 641.52 ± 62.37 (6) |
| PE + l-NAME, %increase in force | 756.89 ± 61.56 (7) | 626.89 ± 61.39 (9) | 564.27 ± 57.82 (6) | 665.33 ± 79.91 (5) |
| KCl, %increase in force | 312.35 ± 20.21(7) | 301.44 ± 27.24 (10) | 204.02 ± 27.88* (7) | 304.41 ± 50.57 (6) |
| logEC50 | ||||
| PE, M concn. | −5.9 ± 0.07 (7) | −5.9 ± 0.06 (10) | −5.9 ± 0.07 (6) | −5.9 ± 0.1 (6) |
| PE + l-NAME, M concn. | −6.1 ± 0.02† (7) | −6.2 ± 0.09† (9) | −6.3 ± 0.1† (6) | −6.0 ± 0.08 (5) |
| KCl, mM concn. | 52.4 ± 1.48 (7) | 50.2 ± 1.28 (10) | 49.5 ± 1.34 (7) | 47.9 ± 1.67 (6) |
Values are means ± SE; no. of rats are in parentheses. Dahl S rats were given Teklad or AIN standard chow diets at weaning (3 wk old). At 12 wk, weaning-diet groups were divided, and a subset of rats underwent a diet switch, generating two additional diet groups: Teklad→AIN and AIN→Teklad. l-NAME was used at 100-μM concentration to nonselectively inhibit NOS.
P < 0.05 for Emax vs. Teklad.
P < 0.05 vs. corresponding untreated mesenteric artery segment. Data were analyzed by two-way ANOVA.
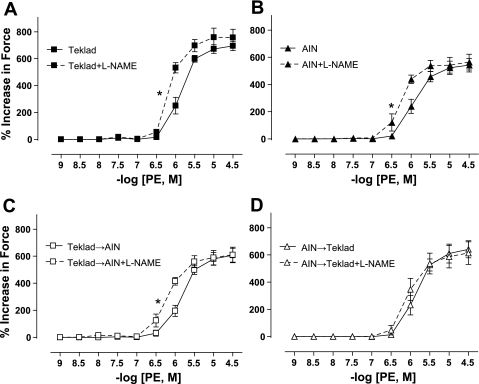
l-NAME pretreatment significantly increased sensitivity to PE-induced constriction in mesenteric arteries isolated from Teklad-fed (Fig. 4A; Table 4), AIN-fed (Fig. 4B; Table 4), and Teklad→AIN (Fig. 4C; Table 4) Dahl S rats, whereas l-NAME had no significant effect on the PE-induced vasoconstriction of mesenteric arteries from AIN→Teklad Dahl S rats (Fig. 4D; Table 4).
Fig. 4.
Phenylephrine (PE)-induced concentration in the presence or absence of l-NAME in small mesenteric arteries from adult (16 wk old) Dahl S rats fed Teklad (N = 7; A) or AIN (N = 6; B) since weaning (3 wk old). At 12 wk old, weaning-diet groups were divided, with a subset of rats undergoing a diet switch, generating two additional diet groups: Teklad→AIN (N = 9; C) and AIN→Teklad (N = 5; D). l-NAME was used at 100 μM concentration to nonselectively inhibit NOS. Values are means ± SE. *P < 0.05 for logEC50 in l-NAME-treated vs. untreated mesenteric artery segments. Data were analyzed by t-test.
Aortic response to PE and KCl was similar in all four diet groups (Table 5). l-NAME had no significant effect on aortic response to PE in any of the four diet groups (Table 5).
Table 5.
Maximum response (Emax) and sensitivity (logEC50) to PE or KCl in aortas from Dahl S rats at 16 wk old
| Teklad | Teklad→AIN | AIN | AIN→Teklad | |
|---|---|---|---|---|
| Emaxto [PE, 10−4.5M] or [KCl, 100 mM] | ||||
| PE, %increase in force | 137.93 ± 14.39 (6) | 152.16 ± 9.93 (9) | 127.51 ± 11.16 (6) | 116.04 ± 14.74 (6) |
| PE + l-NAME, %increase in force | 166.82 ± 18.96 (7) | 172.94 ± 8.77 (9) | 122.48 ± 11.67 (6) | 130.79 ± 11.69 (6) |
| KCl, %increase in force | 125.48 ± 9.62 (7) | 123.82 ± 3.13 (10) | 107.42 ± 9.28 (7) | 108.88 ± 14.28 (5) |
| logEC50 | ||||
| PE, M concn. | −7.3 ± 0.10 (6) | −7.3 ± 0.13 (9) | −7.3 ± 0.16 (6) | −7.2 ± 0.14 (5) |
| PE + l-NAME, M concn. | −7.6 ± 0.16 (7) | −7.6 ± 0.09 (9) | −7.5 ± 0.15 (6) | −7.3 ± 0.15 (5) |
| KCl, mM concn. | 24.6 ± 3.21 (7) | 25.0 ± 0.75 (10) | 25.1 ± 2.27 (7) | 22.9 ± 4.34 (4) |
Values are means ± SE; no. of rats are in parentheses. Dahl S rats were given Teklad or AIN standard chow diets at weaning (3 wk old). At 12 wk, weaning-diet groups were divided, and a subset of rats underwent a diet switch, generating two additional diet groups: Teklad→AIN and AIN→Teklad. l-NAME was used at 100-μM concentration to nonselectively inhibit NOS. Data were analyzed by two-way ANOVA.
NOS protein expression.
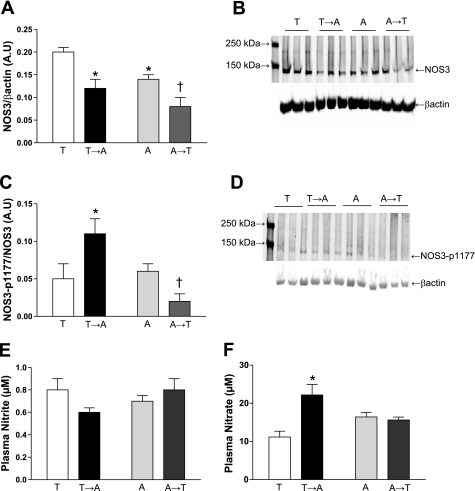
NOS3 protein expression was significantly reduced in small mesenteric arteries from AIN and Teklad→AIN compared with Teklad (Fig. 5, A and B). Moreover, NOS3 protein expression was further reduced in small mesenteric arteries from AIN→Teklad compared with AIN (Fig. 5, A and B). The NOS3-p1177 expression (Fig. 5, C and D) was similar in small mesenteric arteries from Teklad and AIN. NOS3-p1177 expression in arteries from Teklad→AIN was significantly higher compared with arteries from Teklad (Fig. 5, C and D), while the arteries from AIN→Teklad demonstrated a significantly reduced NOS3-p1177 expression compared with AIN (Fig. 5, C and D). NOS1 expression was not significantly different between the four diet groups (data not shown).
Fig. 5.
Densitometric analysis and representative Western blots of nitric oxide synthase (NOS) 3/β-actin (A and B), NOS3-phosphoserine-1177 (p1177)/NOS3 (C and D) protein expression in homogenates of mesenteric arteries and plasma nitrite (E) and nitrate (F) levels from Teklad-fed (T; N = 5) and AIN-fed (A; N = 4) adult (16-wk-old) Dahl S rats. At 12 wk old, weaning-diet groups were divided, with a subset of rats undergoing a diet switch, thereby generating two additional diet groups: Teklad→AIN (T→A; N = 7) and AIN→Teklad (A→T; N = 6). Values are means ± SE. *P < 0.05 vs. T. †P < 0.05 vs. corresponding weaning group (T or A). Data were analyzed by two-way ANOVA. AU, arbitrary units.
Plasma nitrite/nitrate.
Nitrite levels were similar in all four diet groups (Fig. 5E). Moreover, nitrate levels were similar in nonswitched weaning diet groups (Fig. 5F). Interestingly, nitrate levels were increased following the Teklad→AIN diet switch, whereas no change was detected in the AIN→Teklad diet-switch group (Fig. 5F).
DISCUSSION
The principal finding of this study is that changing standard chow diets in adult Dahl S rats can lead to alterations in the NOS phenotype of small-resistance arteries. Specifically, adult rats weaned on AIN-76A diet and switched to Teklad 8604 diet as adults had a loss of NOS-mediated vasorelaxation and NOS buffered PE-induced vasoconstriction in third-order mesenteric arteries. Interestingly, this loss of vascular NOS function was echoed by reduced expression of NOS3, as well as reduced expression of phosphorylated NOS3. In contrast, the group of Dahl S rats switched to AIN from the weaning Teklad diet demonstrated significantly enhanced phosphorylated NOS3 expression and maintenance of vascular NOS function. Vascular NOS function was intact in Dahl S rats weaned and maintained on Teklad or AIN standard diets with similar expression of phosphorylated NOS3.
Our laboratory is interested in determining the mechanisms that predispose salt-sensitive humans to vascular risk by utilizing the Dahl S rat model under normotensive (normal-salt diet) conditions. The major driving force behind the present study stems from the work in Dr. Mattson's laboratory, where they reported that maintaining Dahl S rats at weaning on two different standard chow/normal salt diets differentially affected salt-dependent blood pressure and renal injury phenotypes as adults (27). Specifically, their study demonstrated that the response to a high-salt diet in adult Dahl S rats maintained on AIN-76A diet developed salt-dependent hypertension and greater renal injury compared with counterparts weaned on Teklad diet. We did not observe a difference in blood pressure between our Teklad diet and AIN-76A-diet groups or a difference in proteinuria. However, it is important to mention that the Teklad diet used in Mattson's study (27) was Teklad 3075S diet; this diet was custom-made for the Mattson study, whereas our study exploited the widely available Teklad 8604 diet that many institutions, including our own, use as normal rat chow. Our laboratory has a long-standing interest in examining vascular NOS function in cardiovascular disease states, thus prompting us to study arterial NOS function in Dahl S rats.
Using the nonspecific NOS inhibitor, l-NAME, we showed a reduced sensitivity to ACh and increased sensitivity to PE in third-order mesenteric arteries from Teklad, AIN, and Teklad→AIN groups. These data indicate that NOS function is intact in these three diet groups; however, NOS function was lost in the AIN→Teklad group. Importantly, the vasorelaxation response to the exogenous NO donor SNP was similar in all four diet groups, indicating the reduced NOS-mediated vasorelaxation in the AIN→Teklad group is not dependent on reduced vascular smooth muscle response to NO. In probing a mechanism for this loss in NOS function, it was observed that both total NOS3 and NOS3-p1177 expression were reduced compared with AIN-fed rats. It has been demonstrated that reduced NOS3-p1177 expression is associated with reduced NOS3 enzyme activity (1). These data indicate that adaptation to the Teklad diet in adulthood from the AIN weaning diet results in dysfunctional vascular NOS signaling most likely due to reduced NOS3 and NOS3-p1177. Protein kinase B/Akt kinase phosphorylates NOS3 at Ser1177 to increase NOS3 activity (38). We propose that switching adult Dahl S rats to Teklad diet reduces small-arterial Akt kinase function. Interestingly, the Teklad→AIN Dahl S rat group demonstrated reduced NOS3 expression, whereas phosphorylated NOS3 was greatly enhanced, suggesting that switching to AIN diet activates small-arterial Akt kinase function. We probed the possible activation status of NOS in the Teklad→AIN by measuring metabolites of bioavailable NO; whereas plasma nitrite was similar in all diet groups, plasma nitrate was increased in the Teklad→AIN diet-switch group with no change in the AIN→Teklad diet-switch group. These data suggest that the Teklad→AIN diet switch, but not the AIN→Teklad diet switch, may influence bioavailable NO in Dahl S rats.
In adult Dahl S rats, basal ACh-mediated vasorelaxation and PE-mediated vasoconstriction in small mesenteric artery segments was not altered by placing rats on different standard chow diets (AIN or Teklad) at weaning or following a diet-switch protocol, whereby the “weaning diets” were switched as adults. Intriguingly, this was evident, regardless of the loss of NOS function in the AIN→Teklad diet-switch group. Importantly, small-artery vascular reactivity is modulated by NOS-independent mechanisms, including endothelium-derived hyperpolarizing factors (EDHF) (8, 13, 40). Scotland et al. (39) demonstrated in small mesenteric arteries that EDHF functions to maintain normal vascular reactivity in NOS3 knockout mice. Future experiments will examine the degree of EDHF function in our Dahl S rat diet-switch groups.
Diet switch-induced changes in vascular NOS function and signaling in small arteries from adult Dahl S rats occurred without changes in blood pressure and heart rate or the circadian rhythm of these parameters, rat growth, or body weight. These data suggest that the changes in vascular NOS phenotype detected in our study are specific to the macronutrient composition and/or the source of each macronutrient in the Teklad vs. AIN diet. Teklad 8604 is a proprietary diet containing 33% protein from soy, fish meal, wheat, corn, yeast, molasses, and whey; 53% carbohydrates from corn, wheat, soy molasses, whey, and yeast; and 14% fat derived from soy, corn, wheat, and fish, whereas AIN-76A is a purified diet composed of 19% protein derived from casein; 69% carbohydrates from corn starch and sucrose; and 12% fat from corn oil and trace amounts from casein. Currently, it is unknown which component in the diet is responsible for the observed change in vascular NOS phenotype; however, we speculate that, although the protein in the Teklad diet comes from many sources, the total protein content is higher than that in the AIN diet. In Dahl S rats, the consumption of a high-protein diet consisting of 33% protein and normal salt for 8 wk induces greater vascular injury in the kidney compared with rats on a diet with normal (18%) protein content (12); however, vascular NOS function or expression was not examined in that study. Our present investigation revealed that switching rats weaned on AIN diet, which has a normal protein composition compared with the Teklad diet, to the Teklad diet resulted in loss of NOS function and loss of NOS3 expression and signaling.
Perspectives
Collectively, our present study demonstrates that manipulating the standard chow/normal-salt diet in adult Dahl S rats, which mimic cardiovascular disease progression in salt-sensitive humans, differentially affects small-artery NOS phenotype. Although the Dahl S rat has been widely used to study mechanisms of high-salt, diet-induced cardiovascular disease and NOS dysfunction (2, 4–7, 9, 10, 12, 14, 15, 18, 19, 21–23, 25, 27, 28, 30–37, 41, 46), far fewer studies have examined vascular NOS function or signaling in Dahl S rats maintained on normal-salt diet. Our present data suggest that switching standard chow diets alone may result in enhanced sensitivity to additional cardiovascular insults, such as behavioral stressors or a high-salt diet, in this rat strain. Our study should encourage investigators to more carefully consider that additional environmental stressors from dietary paradigms may influence the vascular NOS phenotype and, therefore, the vascular injury risk.
GRANTS
This study was supported by National Institutes of Health (NIH) P01 HL69999 (J. S. Pollock and D. M. Pollock), NIH R01 HL60653 (J. S. Pollock), T32 National Heart, Lung, and Blood Institute (NHLBI) Postdoctoral Training Fellowship (D. H. Ho), T32 NHLBI Predoctoral Training Fellowship (F. T. Spradley), and American Heart Association Predoctoral Fellowship (F. T. Spradley).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.T.S. and J.S.P. conception and design of research; F.T.S., D.H.H., and K.-T.K. performed experiments; F.T.S., D.H.H., K.-T.K., and J.S.P. analyzed data; F.T.S., D.H.H., D.M.P., and J.S.P. interpreted results of experiments; F.T.S. prepared figures; F.T.S. drafted manuscript; F.T.S., D.H.H., K.-T.K., D.M.P., and J.S.P. edited and revised manuscript; F.T.S., D.H.H., K.-T.K., D.M.P., and J.S.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors greatly thank Hiram Ocasio for technical expertise in biotelemetry. We greatly appreciate the efforts of Dr. Carol Moreno-Quinn at the Human and Molecular Genetics Center for genotyping our Dahl S rat breeders.
Current address of K.-T. Kang: Vascular Biology Program, Karp Family Research Building (#12.004A), Children's Hospital Boston, 300 Longwood Ave., Boston, MA 02115.
REFERENCES
- 1. Atochin DN, Huang PL. Endothelial nitric oxide synthase transgenic models of endothelial dysfunction. Pflügers Arch 460: 965–974, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barton M, Vos I, Shaw S, Boer P, D'Uscio LV, Grone HJ, Rabelink TJ, Lattmann T, Moreau P, Luscher TF. Dysfunctional renal nitric oxide synthase as a determinant of salt-sensitive hypertension: mechanisms of renal artery endothelial dysfunction and role of endothelin for vascular hypertrophy and Glomerulosclerosis. J Am Soc Nephrol 11: 835–845, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Black SM, Mata-Greenwood E, Dettman RW, Ovadia B, Fitzgerald RK, Reinhartz O, Thelitz S, Steinhorn RH, Gerrets R, Hendricks-Munoz K, Ross GA, Bekker JM, Johengen MJ, Fineman JR. Emergence of smooth muscle cell endothelin B-mediated vasoconstriction in lambs with experimental congenital heart disease and increased pulmonary blood flow. Circulation 108: 1646–1654, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Boegehold MA. Enhanced arteriolar vasomotion in rats with chronic salt-induced hypertension. Microvasc Res 45: 83–94, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Boegehold MA. Microvascular structure and function in salt-sensitive hypertension. Microcirculation 9: 225–241, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Boegehold MA. Reduced influence of nitric oxide on arteriolar tone in hypertensive Dahl rats. Hypertension 19: 290–295, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Boulanger CM, Desta B, Clozel JP, Vanhoutte PM. Chronic treatment with the Ca2+ channel inhibitor RO 40–5967 potentiates endothelium-dependent relaxations in the aorta of the hypertensive salt sensitive Dahl rat. Blood Press 3: 193–196, 1994 [DOI] [PubMed] [Google Scholar]
- 8. Brandes RP, Schmitz-Winnenthal FH, Feletou M, Godecke A, Huang PL, Vanhoutte PM, Fleming I, Busse R. An endothelium-derived hyperpolarizing factor distinct from NO and prostacyclin is a major endothelium-dependent vasodilator in resistance vessels of wild-type and endothelial NO synthase knockout mice. Proc Natl Acad Sci U S A 97: 9747–9752, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cosentino F, Bonetti S, Rehorik R, Eto M, Werner-Felmayer G, Volpe M, Luscher TF. Nitric-oxide-mediated relaxations in salt-induced hypertension: effect of chronic beta1-selective receptor blockade. J Hypertens 20: 421–428, 2002 [DOI] [PubMed] [Google Scholar]
- 10. D'Angelo G, Pollock JS, Pollock DM. In vivo evidence for endothelin-1-mediated attenuation of alpha1-adrenergic stimulation. Am J Physiol Heart Circ Physiol 290: H1251–H1258, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Davidge ST. Oxidative stress and altered endothelial cell function in preeclampsia. Semin Reprod Endocrinol 16: 65–73, 1998 [DOI] [PubMed] [Google Scholar]
- 12. De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension 57: 269–274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doughty JM, Plane F, Langton PD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am J Physiol Heart Circ Physiol 276: H1107–H1112, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Drenjancevic-Peric I, Frisbee JC, Lombard JH. Skeletal muscle arteriolar reactivity in SS. BN13 consomic rats and Dahl salt-sensitive rats. Hypertension 41: 1012–1015, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Duggan JA, Tabrizchi R. Effect of nitric oxide synthase inhibitor N(omega) nitro-l-arginine methyl ester on relaxant responses to calcium channel antagonists in isolated aortic rings from Dahl normotensive and hypertensive rats. J Cardiovasc Pharmacol 39: 354–362, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension 45: 283–287, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol 297: F471–F480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hayakawa H, Coffee K, Raij L. Endothelial dysfunction and cardiorenal injury in experimental salt-sensitive hypertension: effects of antihypertensive therapy. Circulation 96: 2407–2413, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Hayakawa H, Raij L. Relationship between hypercholesterolaemia, endothelial dysfunction and hypertension. J Hypertens 17: 611–619, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673–2678, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Hirawa N, Uehara Y, Kawabata Y, Akie Y, Ichikawa A, Funahashi N, Goto A, Omata M. Restoration of endothelial cell function by chronic cicletanine treatment in Dahl salt-sensitive rats with salt-induced hypertension. Hypertens Res 19: 263–270, 1996 [DOI] [PubMed] [Google Scholar]
- 22. Johnson FK, Durante W, Peyton KJ, Johnson RA. Heme oxygenase inhibitor restores arteriolar nitric oxide function in Dahl rats. Hypertension 41: 149–155, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol 288: R1057–R1062, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kang KT, Sullivan JC, Sasser JM, Imig JD, Pollock JS. Novel nitric oxide synthase–dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49: 893–901, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Kido M, Ando K, Onozato ML, Tojo A, Yoshikawa M, Ogita T, Fujita T. Protective effect of dietary potassium against vascular injury in salt-sensitive hypertension. Hypertension 51: 225–231, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kung CF, Moreau P, Takase H, Luscher TF. l-NAME hypertension alters endothelial and smooth muscle function in rat aorta. Prevention by trandolapril and verapamil. Hypertension 26: 744–751, 1995 [DOI] [PubMed] [Google Scholar]
- 27. Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW., Jr Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Physiological genomics 16: 194–203, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Maude DL, Kao-Lo G. Salt excretion and vascular resistance of perfused kidneys of Dahl rats. Hypertension 4: 532–537, 1982 [DOI] [PubMed] [Google Scholar]
- 29. Moreno C, Kennedy K, Andrae JW, Jacob HJ. Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Ni Z, Oveisi F, Vaziri ND. Nitric oxide synthase isotype expression in salt-sensitive and salt-resistant Dahl rats. Hypertension 34: 552–557, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Nishida Y, Ding J, Zhou MS, Chen QH, Murakami H, Wu XZ, Kosaka H. Role of nitric oxide in vascular hyper-responsiveness to norepinephrine in hypertensive Dahl rats. J Hypertens 16: 1611–1618, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Ozawa Y, Hayashi K, Kanda T, Homma K, Takamatsu I, Tatematsu S, Yoshioka K, Kumagai H, Wakino S, Saruta T. Impaired nitric oxide- and endothelium-derived hyperpolarizing factor-dependent dilation of renal afferent arteriole in Dahl salt-sensitive rats. Nephrology (Carlton) 9: 272–277, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Parai K, Tabrizchi R. Effects of chloride substitution in isolated mesenteric blood vessels from Dahl normotensive and hypertensive rats. J Cardiovasc Pharmacol 46: 105–114, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Parai K, Tabrizchi R. Impact of nitric oxide synthase inhibitor and chloride channel antagonist on mesenteric vascular conductance in anesthetized Dahl normotensive and hypertensive rats. J Cardiovasc Pharmacol 45: 569–579, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Quaschning T, d'Uscio LV, Shaw S, Luscher TF. Vasopeptidase inhibition exhibits endothelial protection in salt-induced hypertension. Hypertension 37: 1108–1113, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Raffai G, Wang J, Roman RJ, Anjaiah S, Weinberg B, Falck JR, Lombard JH. Modulation by cytochrome P450–4A omega-hydroxylase enzymes of adrenergic vasoconstriction and response to reduced PO in mesenteric resistance arteries of Dahl salt-sensitive rats. Microcirculation 17: 525–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rafi JA, Boegehold MA. Microvascular responses to oxygen and muscle contraction in hypertensive Dahl rats. Int J Microcirc Clin Exp 13: 83–97, 1993 [PubMed] [Google Scholar]
- 38. Schleicher M, Yu J, Murata T, Derakhshan B, Atochin D, Qian L, Kashiwagi S, DiLorenzo A, Harrison KD, Huang PL, Sessa WC. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal 2: ra41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scotland RS, Chauhan S, Vallance PJ, Ahluwalia A. An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide. Hypertension 38: 833–839, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation 111: 796–803, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Tabrizchi R, Duggan JA. The interrelationship between chloride ions and endothelium on alpha(1)-adrenoceptor-mediated contractions in aortic rings from Dahl normotensive and hypertensive rats. Cardiovasc Res 48: 393–401, 2000 [DOI] [PubMed] [Google Scholar]
- 42. Vita JA, Treasure CB, Yeung AC, Vekshtein VI, Fantasia GM, Fish RD, Ganz P, Selwyn AP. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation 85: 1390–1397, 1992 [DOI] [PubMed] [Google Scholar]
- 43. Vrints CJ, Bult H, Bosmans J, Herman AG, Snoeck JP. Paradoxical vasoconstriction as result of acetylcholine and serotonin in diseased human coronary arteries. Eur Heart J 13: 824–831, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Weinberger MH. Salt sensitivity is associated with an increased mortality in both normal and hypertensive humans. J Clin Hypertens (Greenwich) 4: 274–276, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Zhou MS, Jaimes EA, Raij L. Atorvastatin prevents end-organ injury in salt-sensitive hypertension: role of eNOS and oxidant stress. Hypertension 44: 186–190, 2004 [DOI] [PubMed] [Google Scholar]