Abstract
The intact ovine uterine vascular bed (UVB) is sensitive to α-agonists and refractory to angiotensin II (ANG II) during pregnancy; the converse occurs in the systemic circulation. The mechanism(s) responsible for these differences in uterine sensitivity are unclear and may reflect predominance of nonconstricting AT2 receptors (AT2R) in uterine vascular smooth muscle (UVSM). The contribution of the placental vasculature also is unclear. Third generation and precaruncular/placental arteries from nonpregnant (n = 16) and term pregnant (n = 23) sheep were used to study contraction responses to KCl, norepinephrine (NE), and ANG II (with/without ATR specific inhibitors) and determine UVSM ATR subtype expression and contractile protein content. KCl and NE increased third generation and precaruncular/placental UVSM contractions in a dose- and pregnancy-dependent manner (P ≤ 0.001). ANG II only elicited modest contractions in third generation pregnant UVSM (P = 0.04) and none in precaruncular/placental UVSM. Moreover, compared with KCl and NE, ANG II contractions were diminished ≥ 5-fold. Whereas KCl and ANG II contracted third generation>>precaruncular/placental UVSM, NE-induced contractions were similar throughout the UVB. However, each agonist increased third generation contractions ≥ 2-fold at term, paralleling increased actin/myosin and cellular protein content (P ≤ 0.01). UVSM AT1R and AT2R expression was similar throughout the UVB and unchanged during pregnancy (P > 0.1). AT1R inhibition blocked ANG II-mediated contractions; AT2R blockade, however, did not enhance contractions. AT2R predominate throughout the UVB of nonpregnant and pregnant sheep, contributing to an inherent refractoriness to ANG II. In contrast, NE elicits enhanced contractility throughout the ovine UVB that exceeds ANG II and increases further at term pregnancy.
Keywords: placental artery, systemic artery, actin/myosin, angiotensin II receptors, α-agonists
ovine pregnancy is associated with numerous cardiovascular changes (43, 48), including a >30-fold rise in uterine blood flow (UBF) and >40% increase in cardiac output. Additionally, there is development of refractoriness to the pressor effects of infused norepinephrine (NE) and angiotensin II (ANG II) in women and sheep (44, 49, 53). Although the ovine uterine vascular bed (UVB) also develops refractoriness to these agonists during pregnancy (32, 49), Naden and Rosenfeld (36) reported that the intact pregnant ovine UVB was more refractory to the vasoconstricting effects of ANG II than the systemic vasculature. Thus, blood pressure increased in the absence of significant decreases in uteroplacental blood flow (UPBF). In contrast, the UVB was quite sensitive to α-agonists, which decreased ovine UPBF at nonpressor doses, demonstrating differences in ovine uterine and systemic sensitivity to NE and ANG II (32, 40, 47). Similar observations have been reported in women (19–21). The mechanisms responsible for the differences within the UVB and between the uterine and systemic vasculature remain unclear.
Magness et al. (30, 31) suggested that increases in basal and ANG II-mediated synthesis of endothelium-derived prostacyclin (PGI2) and/or nitric oxide (NO) contribute to the attenuated ANG II contractions in the pregnant ovine UVB. Alternatively, the pregnancy-related rise in plasma ANG II levels could decrease ANG II receptor (ATR) expression and/or binding and alter vascular responsiveness (29, 32). However, total or nonspecific ATR binding density is unchanged in uterine and systemic vascular smooth muscle (VSM) during ovine pregnancy (28, 41).
Two major ATRs exist in large mammals. AT1R predominate in adult VSM and mediate ANG II-induced contractions (7, 25). AT2Rs (derived from a separate gene product encoded on the X-chromosome) do not mediate VSM contractions, are predominantly expressed in fetal tissues, including VSM, and are downregulated after birth (14, 22, 25). However, AT2R remain the predominant receptor in reproductive tissues in women and ewes, accounting for >85% of binding in proximal nonpregnant and pregnant human and ovine uterine VSM (UVSM; 13, 15, 18). Cox et al. (16, 17) suggested AT2R predominance in UVSM resulted in the attenuated contractile responses to infused ANG II, and ANG II-mediated release of α-agonists accounted for the UVSM contractions during systemic ANG II infusions. To date, there are few studies comparing UVSM ATR subtype expression and ANG II-mediated contractions across gestation and within the UVB, especially in the maternal placental circulation (8, 46). Thus, the distribution of UVSM ATR subtype expression within the UVB is unclear, and the role of AT2R activation in the attenuated uterine responses to infused ANG II in large mammals is controversial (5, 8, 10, 16, 27).
The purpose of the present study was to quantify and compare VSM function, in particular, responses to the α-agonist NE and ANG II, in proximal uterine arteries from nonpregnant and term pregnant sheep and in precaruncular (nonpregnant) and placental (pregnant) VSM, and determine VSM ATR subtype expression during pregnancy and within the UVB. We postulated UVSM contractile responses to ANG II would be attenuated due to the predominance of AT2R expression, while responses to NE would be greater in the proximal UVB and decreased in the placental vasculature.
MATERIALS AND METHODS
Tissue preparation.
Third generation uterine and precaruncular/placental arteries were collected from 16 nonpregnant and 23 term pregnant ewes (142–149 days; term ∼150 days). The nonpregnant animals were randomly selected; 10 were in the luteal phase of the ovarian cycle at the time of tissue collection (26). Four additional nonpregnant ewes were ovariectomized under general anesthesia, allowed to recover without estrogen replacement, and third generation uterine and posterior popliteal arteries were collected at 7 days. Animals were euthanized with intravenous pentobarbital sodium (100 mg/kg). In pregnant animals, the euthanized fetuses were quickly delivered, and the intact uteri removed. The uterine arterial tree was carefully dissected starting with the main uterine artery, i.e., first generation. A 2.5- to 3.0-cm segment of third generation uterine artery, which served as a proximal artery, was dissected from nonpregnant and pregnant uteri, placed in physiological salt solution (PSS; in mM: 120.5 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 20.4 NaHCO3, 1.6 CaCl2, 10 dextrose, 1.0 pyruvate) at room temperature. The uterine arteries distal to the third generation were carefully dissected to the fifth and sixth generation to identify the precaruncular (≤1 mm diameter) and placental (∼1 mm diameter) arteries proximal to their insertion into caruncles and placentomes in nonpregnant and pregnant ewes, respectively. Segments 2- to 2.5-cm long were placed in PSS at room temperature. Carotid VSM expresses > 96% AT1R; thus, carotid arteries were collected from four pregnant animals in the last third of pregnancy to compare ANG II-induced contractions by peripheral VSM and UVSM (8, 15, 33). The remaining third generation and precaruncular/placental arteries were placed in iced PBS (8 mM Na2HPO4, 137 mM NaCl, pH 7.5), opened along the long axis, and the endothelium removed with a soft cotton swab, blotted dry to remove excess fluid, frozen in liquid nitrogen, and stored at −80°C (3, 24). These studies were approved by the Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center.
Force measurements.
Samples of uterine (third generation and precaruncular/placental) and peripheral arteries (carotid and popliteal) were transported to the laboratory to study VSM function. Consecutive 3- to 4-mm rings were cut from a single segment. Since we were primarily interested in studying differences in VSM force generation, and in particular, the effects of NE and ANG II, the endothelium was removed by rotating the end of ophthalmologic forceps in the lumen. This was verified histologically in random samples (24). It should be noted that AT1R are expressed in ovine uterine artery endothelium, upregulated in pregnancy, and mediate endothelial PGI2 and NO synthesis in uterine arteries from pregnant sheep (6, 31, 52). Thus, leaving the endothelium intact would have complicated any assessment of VSM function. Each ring was placed on a stirrup attached to a transducer to measure force generation in a 25-ml chamber. After a 30-min equilibration period, rings were progressively stretched to obtain optimal length (Lo), determining forces with 65 mM KCl (24). Dose-response curves were constructed at Lo for each ring using cumulative doses of KCl (10–120 mM) to determine nonreceptor-mediated responses and NE (10−8 to 10−4 M; Sigma-Aldrich, St. Louis, MO) to examine α-receptor-mediated contractions. ANG II dose-responses were generated using four doses (10−8 to 10−5 M; Sigma-Aldrich) with eight rings, but only one dose per ring performed in duplicate to remove the effects of tachyphylaxis seen in preliminary studies. Since there may be an interaction between simultaneous AT1R and AT2R activation in VSM (5, 10, 33), we also determined whether AT2R inhibition enhanced AT1R-mediated contractions in denuded uterine and carotid arteries from pregnant sheep and endothelium-intact uterine and popliteal arteries from nonpregnant sheep. The latter allowed us to determine whether the endothelium contributed to the regulation of AT1R-induced contractions. We used the AT1R and AT2R inhibitors losartan (10−5 M; Sigma-Aldrich) and PD123,319 (10−5 M; Sigma-Aldrich), respectively, in the presence of ANG II (10−8 to 10−5 M). ATR inhibitors were added to the water bath, incubated for 30 min, and a dose of ANG II was added. Data were recorded on an electronic data acquisition system (ACQuire; Gould Systems, Valley View, OH). At completion of studies, vessels were fixed in formalin, and length and medial width were measured. Data are expressed in Newtons/m2 generated at Lo, which permits a comparison between arteries, across pregnancy, and between agonists.
Protein analysis.
Annibale et al. (1, 2) reported that ovine pregnancy is associated with proximal uterine artery remodeling, increased myosin and enhanced contractility with KCl and NE, which is also seen in pregnant rats (38). However, ANG II was not examined. Samples of frozen endothelium-denuded arteries (50 mg) were weighed and homogenized in 40× volumes of SDS buffer containing 2% SDS, 20% sucrose, and 0.4 M Tris (pH 6.8) as previously reported (3, 24). Homogenates were divided into two aliquots. The first was used to measure the total homogenate protein content, which includes cellular and noncellular components. The second was centrifuged at 10,000 g for 2 min, and the supernatant was removed to measure soluble or cellular protein. Total and soluble protein concentrations were measured using a BCA Protein Assay Reagent Kit (Pierce, Rockfort, IL). To directly measure actin and myosin contents, bromophenol blue and 2-mercaptoethanol were added to aliquots of supernatant from each artery, and 20 μg of soluble protein was subjected to SDS-PAGE using 4–20% polyacrylamide minigels as described (3, 24). Gels containing molecular mass standards to confirm relative mobility were subjected to electrophoresis at 150 V until the dye front had run off the gel for 10 min and were stained overnight with Coomassie brilliant blue and destained to remove background staining. The fractions of Coomassie blue-stained protein accounted for by actin (42 kDa) and total myosin (200–204 kDa) were scanned and analyzed with TotalLab software package (Biosystematica; Sarnau, Wales, UK). Protein fractions are expressed as μg/mg of wet weight.
Immunoblot analysis.
ATR subtype expression has not been examined in maternal precaruncular or placental VSM and compared with proximal UVSM or with each other. There also is debate about ATR subtype regulation in UVSM in pregnancy (8, 15). Since AT1R are expressed in ovine uterine artery endothelium and UVSM (6, 52), we used denuded arteries to determine VSM expression and examine its relationship to contractile responses. We also did side-by-side comparisons of subtype expression in carotid and third generation uterine VSM to show differences (15). At the time of assay, SDS homogenates were prepared from 15–20 mg samples of the arteries of interest as described above, and 20 μg of soluble protein was loaded for all samples and subjected to electrophoresis in 10% polyacrylamide gels and transferred to nitrocellulose paper (Amersham Pharmacia Biotech, Piscataway, NJ) (14). Immunoblots were blocked in buffer containing powdered milk (5% wt/vol) and incubated overnight at 4°C with antiserum against AT1R (1:500; N-10, Santa Cruz Biotechnology, Santa Cruz, CA) or AT2R (1:500; ab 19134; Abcam, Cambridge, MA or a gift from S. J. Fluharty, Univ. of Pennsylvania School of Veterinary Medicine, Philadelphia, PA). The nitrocellulose paper was incubated with donkey anti-rabbit IgG conjugated with affinity purified horseradish peroxidase diluted at 1:5,000 with TTBS. Regions containing receptor proteins were visualized by enhanced chemiluminescence. Densitometry was performed, and values are expressed as arbitrary units. Ovine umbilical artery smooth muscle obtained at 145 and 116 days of gestation served as positive controls for AT1R and AT2R, respectively (14). We examined α-actin, myosin, tubulin, and GAPDH as loading proteins; however, each was modified by pregnancy (data not shown). We were unable to identify another VSM protein unaltered by pregnancy; thus, we loaded the same quantity of soluble protein from all samples and only made comparisons within a single gel (14). Thus, comparisons in subtype expression are always made on a single immunoblot to decrease variation in protein transfer or antibody affinity between immunoblots that might alter densitometry measurements.
Statistics.
Two-way ANOVA for multiple groups (ANG II) or repeated measures (KCl and NE) was used to construct and compare dose-response curves for KCl, NE, and ANG II in nonpregnant and pregnant arteries and between third generation and precaruncular/placental arteries. When significance was observed by ANOVA at P < 0.05, multiple comparison procedure was used to isolate groups and doses and to determine differences between groups. Data obtained for protein contents were analyzed by Student's t-test. Data are presented as means ± SE.
RESULTS
UVSM contractile responses.
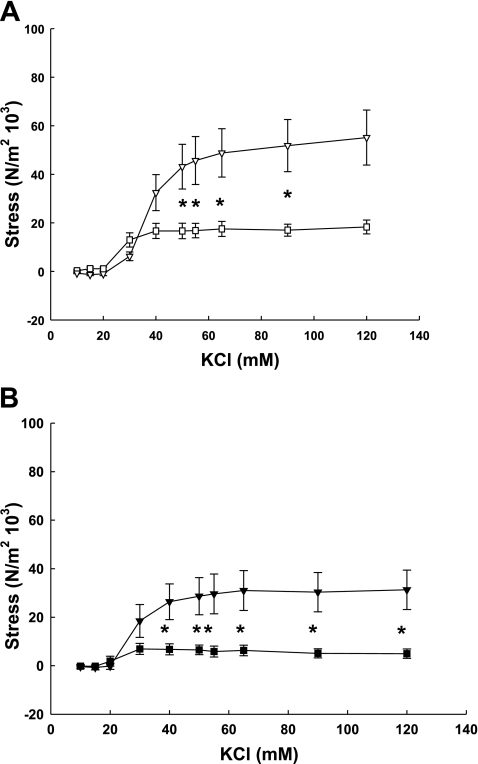
Contractile responses to KCl, NE, and ANG II were quantified in endothelium-denuded third generation uterine artery rings from nonpregnant and term pregnant sheep and in precaruncular (nonpregnant) and placental (pregnant) artery rings. KCl elicited dose-dependent contractions in third generation uterine (Fig. 1A) and precaruncular/placental (Fig. 1B) arteries from nonpregnant and term pregnant sheep (P < 0.001, 2-way ANOVA). However, contractile force generation by third generation and placental UVSM from term pregnant animals were threefold greater than nonpregnant proximal and precaruncular UVSM (P ≤ 0.036). We then compared differences in contractility within the UVB of nonpregnant and pregnant animals. KCl-induced stresses in third generation UVSM rings were greater than either precaruncular or placental responses (P ≤ 0.008, 2-way ANOVA).
Fig. 1.
KCl dose-response curves generated by endothelium-denuded third generation (A) and precaruncular/placental (B) arteries from nonpregnant (□, ■) and term pregnant (▿, ▾) sheep. Responses by nonpregnant and term pregnant arteries are dose- and pregnancy-dependent, pregnant stresses exceeding nonpregnant (P < 0.04, 2-way ANOVA, n = 5–7/group). *Significant dose differences between nonpregnant and term pregnant arteries (P < 0.05). When contractile responses within the uterus of nonpregnant and term pregnant sheep were assessed, third generation vascular rings exhibited greater responses to KCl than either precaruncular or placental rings, respectively (P < 0.008, 2-way ANOVA). Data are means ± SE.
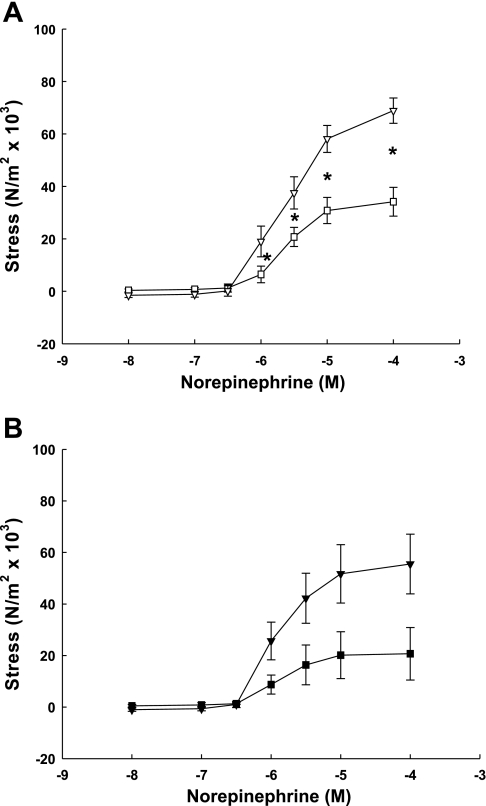
NE also caused dose- and pregnancy-related increases in VSM contractions in third generation (Fig. 2A) and precaruncular/placental (Fig. 2B) artery rings from nonpregnant and term pregnant animals (P < 0.001, 2-way ANOVA). Notably, responses by pregnant third generation UVSM were more than twofold greater than nonpregnant. Furthermore, NE-induced contractions within the nonpregnant and pregnant UVB, e.g., third vs. placental, did not differ (P > 0.1, 2-way ANOVA).
Fig. 2.
Norepinephrine dose-response curves generated by endothelium-denuded third generation (A) and precaruncular/placental (B) arteries from nonpregnant (□, ■) and term pregnant (▿, ▾) sheep. Responses by nonpregnant and term pregnant arteries are dose- and pregnancy-dependent, pregnant exceeding nonpregnant (P < 0.001, 2-way ANOVA, n = 4–8/group). *Significant dose differences between nonpregnant and term pregnant arteries (P < 0.05). When contractile responses within the uterus of nonpregnant and term pregnant sheep were assessed, there was no difference in NE responses between third generation vascular rings and their respective precaruncular or placental rings (P = 0.1, ANOVA). Data are means ± SE.
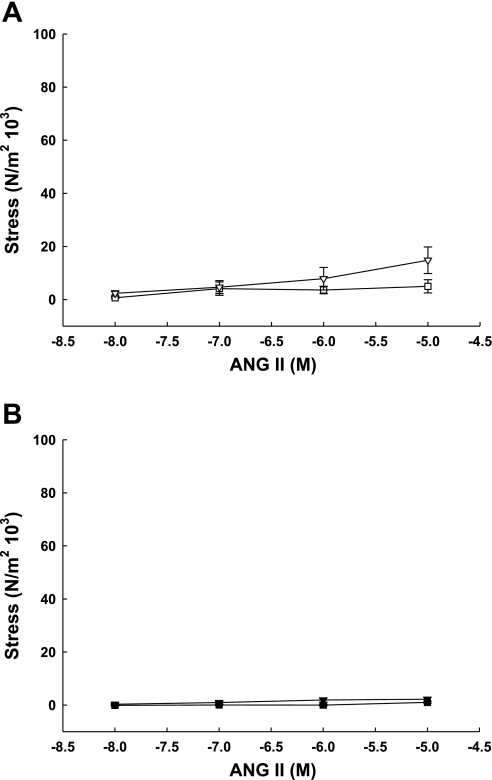
Although ANG II elicited a modest dose-effect in third generation UVSM rings from pregnant sheep (Fig. 3A; P < 0.02, 2-way ANOVA for multiple groups), contractile responses were only significant at 10−5 M, which was threefold greater than responses by nonpregnant third generation UVSM. There was no dose- or pregnancy-effect of ANG II in precaruncular or placental VSM from nonpregnant and term pregnant animals, respectively (Fig. 3B; P > 0.1, 2-way ANOVA for multiple groups). Within the nonpregnant and pregnant UVB, third generation responses exceeded those in precaruncular and placental VSM rings (P < 0.04, 2-way ANOVA for multiple groups). The maximum response to ANG II by either artery was < 20% of maximum KCl responses.
Fig. 3.
ANG II dose-response curves generated by endothelium-denuded third generation (A) and precaruncular/placental (B) arteries from nonpregnant (□, ■) and term pregnant (▿, ▾) sheep. Only third generation pregnant arteries demonstrated a dose effect (P = 0.02, 2-way ANOVA, n = 6) that differed significantly from nonpregnant (P = 0.03, ANOVA). There was no dose- or artery effect in either precaruncular or placental arteries, which did not differ. When contractile responses to ANG II were assessed within the uterus of nonpregnant and term pregnant sheep, responses by term and nonpregnant third generation arteries exceeded placental and precaruncular artery responses (P ≤ 0.04, 2-way ANOVA). Data are means ± SE.
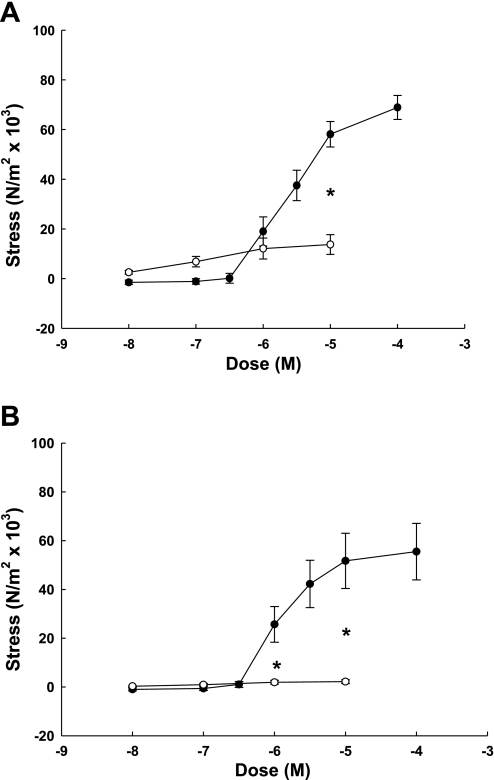
We were especially interested in the differences in UVSM sensitivity to NE and ANG II within the pregnant UVB (32, 36, 40, 47); thus, we compared contraction responses in third generation and placental UVSM rings (Fig. 4). NE elicited dose-dependent contractions in third generation UVSM that were more than sixfold greater than responses to ANG II (Fig. 4A; P < 0.001, 2-way ANOVA for multiple groups), which were not dose dependent. When placental VSM rings were examined, NE-induced contractions were 20-fold greater than ANG II (Fig. 4B; P < 0.001, 2-way ANOVA for multiple groups), which again did not elicit a dose effect.
Fig. 4.
Comparison of dose-response curves by endothelium-denuded (A) third generation and (B) placental arteries from term pregnant sheep to norepinephrine (●, n = 6) and angiotensin II (○, n = 8–9/dose). Norepinephrine elicited a dose-effect in third generation and placental arteries that did not differ (P = 0.9), but was greater than ANG II responses (P ≤ 0.008, 2-way ANOVA). *Significant dose differences between norepinephrine and angiotensin II (*P < 0.001). Data are means ± SE.
ATR subtype expression and function.
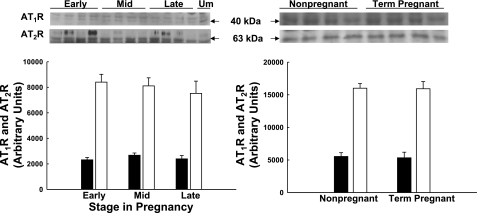
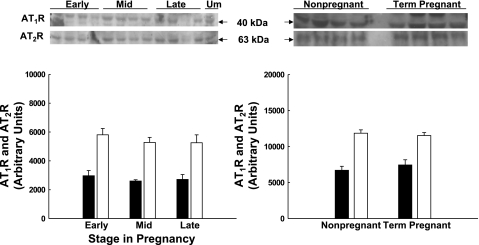
Few investigators have examined ATR subtype expression across gestation (8), and none have looked within the maternal UVB. We studied UVSM from nonpregnant, early pregnant at 55 days gestation, midpregnant at 85–90 days, and term pregnant at ∼145 days. There was no difference in ATR subtype expression in third generation UVSM (Fig. 5; P > 0.1) or precaruncular/placental VSM (Fig. 6; P > 0.1) across pregnancy. Additionally, ATR subtype expression in third generation and placental artery VSM did not differ at any time (P > 0.1). Although the densitometry suggests AT2R exceeds AT1R greater than threefold within each group, this cannot be accurately assessed by immunoblot analyses (see discussion).
Fig. 5.
Immunoblot analysis for AT2 receptors (AT2R; ■) and AT2R (□) expression in third generation uterine artery smooth muscle during ovine pregnancy. Immunoblots and summary densitometry are illustrated. There is no significant difference in AT1R or AT2R subtype expression across pregnancy or between nonpregnant and term pregnant vascular smooth muscle (P > 0.1, n = 4 in each group). Data are means ± SE.
Fig. 6.
Immunoblot analysis for AT1R (■) and AT2R (□) expression in precaruncular and placental artery smooth muscle during ovine pregnancy. Immunoblots and summary densitometry results are illustrated. There is no significant difference in AT1R or AT2R subtype expression across pregnancy or between nonpregnant precaruncular and term pregnant placental arteries (P > 0.1, n = 3–4 in each group). Data are means ± SE.
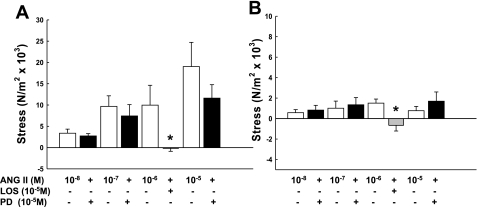
Both ATR subtypes are expressed in UVSM throughout reproduction and pregnancy; thus, we determined whether there was a functional interaction between subtypes during ANG II exposure as reported in small mammals (5, 10, 56). We first examined the effects of subtype inhibition on ANG II-mediated contractions in third generation (Fig. 7A) and placental (Fig. 7B) arteries from term pregnant sheep. Losartan, the AT1R specific inhibitor, blocked ANG II-induced contractions in endothelium-denuded third generation and placental artery rings (Fig. 7; note the 3-fold difference in the y-axis). AT2R inhibition with PD123,319 did not increase ANG II-mediated contractions in either artery at varying doses of ANG II. Neither inhibitor altered basal tension, P > 0.1.
Fig. 7.
Effects of ANG II subtype receptor blockade with losartan (LOS; AT1R, gray bar) and PD123,319 (PD; AT2R, black bar) on angiotensin II-mediated contractions by denuded third generation uterine (A) and placental arteries (B) from term pregnant sheep. Note the 3-fold difference in the y-axis. Each dose includes 4–6 animals; *P ≤ 0.03 by t-test vs. ANG II alone. AT2R inhibition with PD did not enhance ANG II-mediated contractions, P > 0.1. Data are means ± SE.
Peripheral vs. uterine artery contractile responses to ANG II.
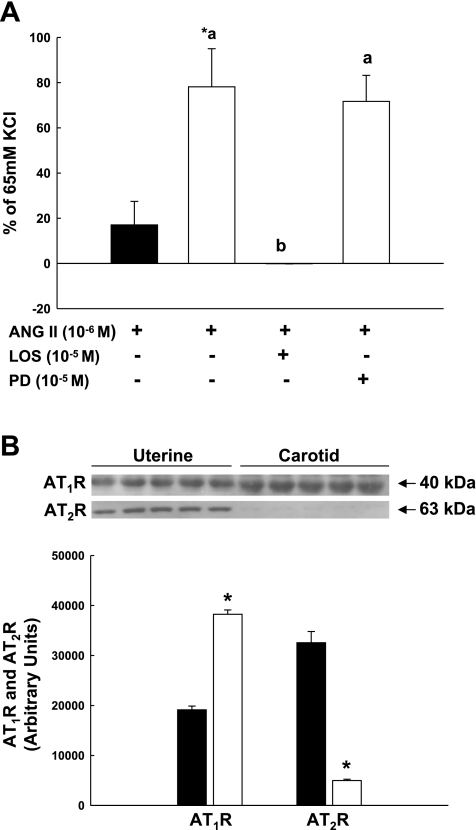
Systemic responses to infused ANG II in pregnant women and sheep exceed responses in the UVB, suggesting differences in vascular sensitivity due to greater AT1R expression in systemic vs. UVSM (8, 15, 20, 33, 36). Few investigators, however, have directly compared uterine and systemic VSM sensitivity to ANG II. We, therefore, studied carotid and posterior popliteal arteries from pregnant and nonpregnant animals, respectively. ANG II-mediated contractions were more than fourfold greater in carotid vs. UVSM rings (Fig. 8A; P = 0.01) and were abolished after preincubation with losartan. AT2R blockade did not enhance contraction responses to ANG II. Immunoblot analysis demonstrated a twofold greater expression of AT1R in carotid VSM vs. a 6.5-fold greater expression of AT2R in UVSM (Fig. 8B; P < 0.001). Since interactions might occur between the endothelium and VSM in response to ANG II (5, 10), we examined ANG II contraction responses in endothelium-intact popliteal and third generation uterine arteries (Fig. 9). ANG II-induced contractions in popliteal arteries were 3- to 4.5-fold greater than UVSM (P < 0.05). Losartan blocked ANG II contractions in both arteries; AT2R blockade, however, had no visible effects on ANG II-mediated contractions, i.e., they did not increase.
Fig. 8.
Comparison of (A) ANG II-induced contractions by denuded third generation uterine (■, n = 6) and carotid (□, n = 4) arteries from term pregnant sheep and the effects of receptor subtype inhibition with losartan (LOS) or PD123,319 (PD) on carotid responses to ANG II and (B) AT1R and AT2R subtype expression in uterine (n = 5, ■) and carotid (n = 5, □) vascular smooth muscle. Immunoblots and summary data for densitometry are presented. *P = 0.01 compared with uterine artery. Different letters represent significant differences by ANOVA, P = 0.002, for comparison of responses to ANG II after ATR subtype inhibition. Data are means ± SE.
Fig. 9.
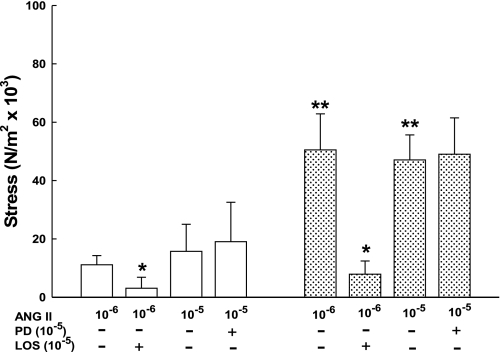
Effects of ANG II receptor subtype blockade in endothelium-intact third generation uterine (□) and posterior popliteal (stippled bars) arteries from nonpregnant sheep after treatment with losartan (LOS; AT1R) or PD123,319 (PD; AT2R). Each group includes 3–4 animals; *P ≤ 0.05 compared with ANG II alone, **P < 0.05 compared with uterine arteries at the same ANG II dose. AT2R inhibition did not enhance ANG II-induced contractions, P > 0.1. Data are means ± SE.
Smooth muscle protein.
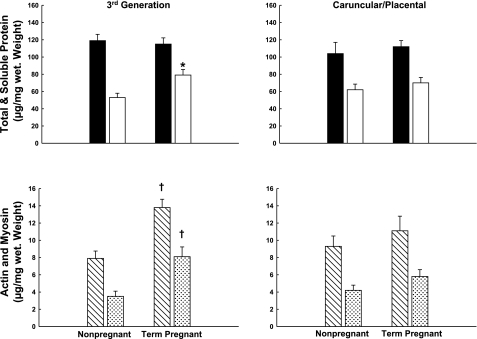
Contractile responses to KCl, NE, and ANG II by third generation UVSM increased ∼2.5-fold at term gestation. Thus, we examined two aspects of vascular remodeling, i.e., the content of total and soluble protein and actin/myosin (1, 2) (Fig. 10). Soluble protein content was ≥ 1.5-fold greater in third generation UVSM from term pregnant vs. nonpregnant sheep (P ≤ 0.01). This was paralleled by approximately twofold increases in actin and total myosin content in UVSM from term pregnant sheep (P ≤ 0.006). There were no changes in the placental artery VSM (P > 0.4).
Fig. 10.
Measurements of total/soluble and contractile proteins in third generation uterine and precaruncular/placental artery smooth muscle from nonpregnant and term pregnant sheep. Soluble protein (□; *P = 0.01), actin (slashed bars), and myosin (stippled bars) increased significantly at term pregnancy (†P ≤ 0.006). There are no significant changes in precaruncular/placental artery smooth muscle (P > 0.1). Data are means ± SE.
DISCUSSION
Uterine vascular responses to ANG II are attenuated in pregnant women and sheep compared with the peripheral vasculature (20, 36). In contrast, the pregnant UVB, is more sensitive to α-agonists than the systemic circulation (32, 40, 43, 47). The mechanisms responsible for these differences in uterine and systemic vascular sensitivity to ANG II and α-agonists remain unclear. Using binding assays to assess ATR subtype expression, Cox et al. (15, 17, 18) suggested this was due to predominance of AT2R in UVSM (>85%) vs. AT1R in peripheral VSM (>95%) and thus, the inability of AT2R to mediate ANG II contractions. No one, however, has examined AT2R expression within the UVB or across pregnancy, and few have determined whether differences exist in ANG II- and/or NE-mediated responses by proximal uterine and placental arteries. In the present study, UVSM ATR subtype expression was similar throughout the UVB of nonpregnant and pregnant sheep and unchanged during pregnancy. Moreover, attenuated ANG II-induced contractions by UVSM were inherent within the UVB, associated with apparent AT2R predominance, and less than responses to KCl and NE and peripheral VSM responses to ANG II. Unlike ANG II and KCl, NE-induced contractions were similar in proximal uterine and placental VSM. There also was a pregnancy effect in third generation responses to each agonist that paralleled increases in VSM actin/myosin and soluble protein, suggesting arterial remodeling. Thus, AT2R predominance throughout the ovine UVB contributes to inherent ANG II refractoriness, differences between uterine and systemic ANG II sensitivity, and greater NE- vs. ANG II-mediated responses within the UVB.
Plasma ANG II levels double in pregnancy (4, 28, 29, 35, 43); thus, ATR downregulation could explain the attenuated systemic and UVB responses to infused ANG II in women and sheep. Baker et al. (4) observed downregulation of platelet ATR binding in pregnant women, but were unable to study blood vessels. We (28, 41) did not find changes in ATR binding density in uterine or systemic VSM during ovine pregnancy; but affinity was less in uterine vs. mesenteric VSM, which could contribute to differences between the UVB and systemic vasculature. Cox et al. (15, 18) also found no differences in total ATR binding in UVSM from nonpregnant and term pregnant women and sheep. Burrell and Lumbers (8), however, suggested that binding density increased in endothelium-intact ovine uterine arteries but not systemic arteries. They later reported that UVSM binding density was unchanged in ovine pregnancy (33). If ATR expression by immunoblots reflects binding density, we provide further evidence that ATR expression is unchanged not only in third generation UVSM, but also precaruncular/placental VSM during ovine pregnancy. Similar findings have been observed in human UVSM (42).
If total ATR binding is unchanged in pregnancy, there may be changes in the relative expression of AT1R and AT2R. We cannot measure the relative subtype expression with immunoblots due to differences in antibody affinity and protein transfer; nonetheless, it is notable that with similar protein loads, AT2R density in UVSM is consistently more than threefold greater than AT1R. The converse was seen in carotid VSM and is consistent with binding studies (15). If subtype protein is unchanged across pregnancy and within the UVB, the relative amount of ATR subtype is unlikely to change, i.e., AT2R will account for ≥80% of UVSM ATR expression at all times. This is consistent with other reports (6, 52) but differs from Burrell and Lumbers (8) who reported increases in intact uterine artery AT2R expression in pregnant ewes. If AT2R cannot mediate contractions, are predominant throughout the UVB, and are unchanged in pregnancy, then the attenuated ANG II-induced contractions in nonpregnant and pregnant UVSM are likely due to the paucity of AT1R, confirming in vivo observations (36, 46). This is supported by studies of ovine and human myometrium in which increases in AT1R binding from 15 to 80% during pregnancy parallel increases in ANG II-induced contractions independent of AT2R activation (13, 18). This is further supported by the differences in subtype expression and function in uterine and peripheral arteries.
AT2R predominance is an attractive explanation for the attenuated UVSM responses to ANG II; however, AT2R activation might contribute by increasing endothelial NO synthesis and VSM relaxation (5, 10). For example, AT1R blockade in rodents induces vasodilation after ANG II exposure, and AT2R blockade enhances pressor responses (56). In addition, AT2R−/− mice have elevated blood pressure and enhanced pressor responses to infused ANG II (5, 10), and in pregnancy, blood pressure does not fall in midgestation (39, 40). In the present study, neither AT1R nor AT2R blockade altered baseline UVSM tension. Although AT1R blockade inhibited ANG II-induced contractions in all arteries, ANG II did not cause vasorelaxation. Notably, AT2R inhibition did not enhance ANG II-induced contractions in uterine or peripheral VSM in the absence or presence of endothelium, confirming studies in intact pregnant ewes (16). It is unclear why our results differ from those by other investigators studying sheep (27, 33). The differences with small mammals may reflect species specificity (37). Importantly, similar findings occur in human UVSM (42).
ANG II minimally affects placental blood flow in intact pregnant ewes (46). We now report that the ovine placental VSM is unresponsive to ANG II and less responsive to KCl than proximal uterine arteries. This refractoriness insures the maintenance of placental perfusion in the presence of elevated circulating ANG II in pregnancy. Surprisingly, sensitivity to NE was similar throughout the UVB, and placental responses were 20-fold greater than ANG II. This responsiveness by the maternal placental vasculature to α-agonists also occurs in intact pregnant Rhesus monkeys (34, 54). We believe the difference in sensitivity is related to the “flight or fight” response. UPBF accounts for ∼25% of cardiac output at term and placental blood flow for 90% of total UPBF (48); thus, ∼2 liters of blood can be rapidly redistributed for emergent needs.
Although AT1R expression was unchanged, contractile responses to ANG II by third generation UVSM increased approximately twofold at term, paralleling similar increases to KCl and NE. This was associated with increases in UVSM actin/myosin and cellular protein content, suggesting vascular remodeling (1, 2). The increase in cellular protein suggests VSM hypertrophy and/or hyperplasia (24), which will be examined in future studies. This was not seen in placental VSM, although 90% of UPBF travels in these arteries (43). Thus, vascular remodeling is not solely due to flow-mediated mechanisms. Our data also suggest that proximal uterine arteries contribute to UPBF regulation in pregnancy (38).
Perspectives and Significance
Fetal growth and well-being and thus, survival of mammalian species depend on establishment of the fetal and maternal placental vasculature, the subsequent growth of the fetal-placental vascular bed and progressive vasodilation of the maternal placental arteries, and the maintenance of UPBF to insure placental oxygen delivery and fetal uptake (43). We have shown that the maternal UVB is inherently refractory to the constricting effects of ANG II throughout reproduction, including pregnancy when plasma ANG II increases approximately threefold, and this may reflect the more than fourfold greater expression of AT2R vs. AT1R within the UVB. Unlike in small mammals, this is not due to AT2R-induced vasorelaxation (5, 10), but rather a paucity of AT1R (13, 18). In contrast, the systemic VSM, which is predominantly AT1R, contributes to basal vascular tone and blood pressure during pregnancy. In contrast, the UVB is inherently sensitive to α-stimulation, which may contribute to maternal adaptation and survival during stress. Similar findings occur in human UVSM, raising important questions about the role of increased sympathetic outflow and altered UPBF in the pathogenesis of fetal growth restriction in women with preeclampsia and/or hypertension (23, 42, 50). These findings also suggest that α-agonists may not be appropriate pressor agents in treating hypotension in pregnancy. They not only constrict the entire UVB, but also cross the placenta and directly affect fetal placental blood flow (34, 54, 55). Thus, ANG II may be the preferred pressor since clearance occurs across the placenta (45, 51), fetal effects are uncommon at pressor doses (39), and pressor effects exceed responses in the UVB (20, 21, 36). Finally, these studies again demonstrate the striking similarities in the cardiovascular system of pregnant women and sheep (49, 15, 18).
GRANTS
These studies were funded by National Institutes of Health grant 5RO-1-HD-008783-34 and funds from the George L. MacGregor Professorship in Pediatrics awarded to C. R. Rosenfeld.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.R.R. conception and design of research; C.R.R., K.D., and X.-t.L. analyzed data; C.R.R. and X.-t.L. interpreted results of experiments; C.R.R., K.D., and X.-t.L. prepared figures; C.R.R. drafted manuscript; C.R.R., K.D., and X.-t.L. edited and revised manuscript; C.R.R., K.D., and X.-t.L. approved final version of manuscript; K.D. and X.-t.L. performed experiments.
REFERENCES
- 1. Annibale DJ, Rosenfeld CR, Kamm KE. Alterations in vascular smooth muscle contractility during ovine pregnancy. Am J Physiol Heart Circ Physiol 256: H1282– H1288, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Annibale DJ, Rosenfeld CR, Stull JT, Kamm KE. Protein content and myosin light chain phosphorylation in uterine arteries during pregnancy. Am J Physiol Cell Physiol 259: C484– C489, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Arens Y, Chapados R, Cox BE, Kamm KE, Rosenfeld CR. Differential development of umbilical and systemic arteries: II. Contractile proteins. Am J Physiol Regul Integr Comp Physiol 274: R1815– R1823, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Baker PN, Pipkin FB, Symonds EM. Platelet angiotensin II binding and plasma rennin concentration, plasma rennin substrate and plasma angiotensin II in human pregnancy. Clin Sci 79: 403– 408, 1990 [DOI] [PubMed] [Google Scholar]
- 5. Batenberg WW, Tom B, Schuijt MP, Danser J. Angiotensin II type 2 receptor-mediated vasodilation. Focus on bradykinin, NO, and endothelium-derived hyperpolarizing factor(s). Vasc Pharmacol 42: 109– 118, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Bird IM, Zheng J, Cale JM, Magness RR. Pregnancy induces an increase in angiotensin II type-1 receptor expression in uterine but not systemic artery endothelium. Endocrinology 138: 490– 498, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Bottari SP, de Gasparo M, Steckelings UM, Levens NR. Angiotensin II receptor subtypes: characterization, signaling mechanisms, and possible physiological implications. Front Neuroendocrinol 14: 123– 171, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Burrell JH, Lumbers ER. Angiotensin receptor subtypes in the uterine artery during ovine pregnancy. Eur J Pharmacol 330: 257– 267, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Carey LC, Rose JC. The midgestational maternal blood pressure decline is absent in mice lacking expression of the angiotensin II AT2 receptor. J Renin Angiotensin Aldosterone Syst 12: 29– 35, 2011 [DOI] [PubMed] [Google Scholar]
- 10. Carey RM. Update on the role of the AT2 receptor. Curr Opin Nephrol Hypertens 14: 67– 71, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Chang K, Zhang L. Steroid hormones and uterine vascular adaptation to pregnancy. Reprod Sci 15: 336– 348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen K, Merrill DC, Rose JC. The importance of angiotensin II subtype receptors for blood pressure control during mouse pregnancy. Reprod Sci 14: 694– 704, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Cox BE, Ipson MA, Shaul PW, Kamm KE, Rosenfeld CR. Myometrial angiotensin II receptor subtypes change during ovine pregnancy. J Clin Invest 92: 2240– 2248, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox BE, Liu X, Fluharty SJ, Rosenfeld CR. Vessel specific regulation of angiotensin II receptor subtypes during ovine development. Pediatr Res 57: 124– 132, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Cox BE, Rosenfeld CR, Kalinyak JE, Magness RR, Shaul PW. Tissue specific expression of vascular smooth muscle angiotensin II receptor subtypes during ovine pregnancy. Am J Physiol Heart Circ Physiol 271: H212– H221, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Cox BE, Roy TA, Rosenfeld CR. Angiotensin II mediates uterine vasoconstriction through α-stimulation. Am J Physiol Heart Circ Physiol 287: H126– H134, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Cox BE, Williams CE, Rosenfeld CR. Angiotensin II indirectly vasoconstricts the ovine uterine circulation. Am J Physiol Regul Integr Comp Physiol 278: R337– R344, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Cox BE, Word RA, Rosenfeld CR. Angiotensin II receptor characteristics and subtype expression in uterine arteries and myometrium during pregnancy. J Clin Endocrinol Metab 81: 49– 58, 1996 [DOI] [PubMed] [Google Scholar]
- 19. Damron DP, Bernstein IM, Shapiro RE, Schonberg A. Uterine blood flow responses to α-adrenergic blockade in nulligravid women of reproductive age. J Soc Gynecol Invest 11: 388– 392, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Erkkola RU, Pirhonen JP. Flow velocity waveforms in uterine and umbilical arteries during the angiotensin II sensitivity test. Am J Obstet Gynecol 162: 1193– 1197, 1990 [DOI] [PubMed] [Google Scholar]
- 21. Erkkola RU, Pirhonen JP. Uterine and umbilical flow velocity waveforms in normotensive and hypertensive subjects during the angiotensin II sensitivity test. Am J Obstet Gynecol 166: 910– 916, 1992 [DOI] [PubMed] [Google Scholar]
- 22. Grady EF, Sech LA, Griffen CA, Schambelan M, Kalynyak JE. Expression of AT2 receptors in the developing rat fetus. J Clin Invest 88: 921– 933, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenwood JP, Stoker JB, Walker JJ, Mary DA. Sympathetic nerve discharge in normal and pregnancy-induced hypertension. J Hypertens 16: 617– 624, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Hutanu C, Cox BE, Liu Xt DeSpain K, Rosenfeld CR. Vascular development beginning in early ovine gestation: carotid smooth muscle function, phenotype and biochemical markers. Am J Physiol Regul Integr Comp Physiol 293: R323– R333, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Inagami T, Gero DF, Kitami Y. Molecular biology of angiotensin II receptors: an overview. J Hypertens 12: S83– S94, 1994 [PubMed] [Google Scholar]
- 26. Khan LH, Rosenfeld CR, Liu X, Magness RR. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab 298: E222– E228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lambers DS, Greenberg SG, Clark KE. Functional role of angiotensin II type 1 and 2 receptors in regulation of uterine blood flow in nonpregnant sheep. Am J Physiol Heart Circ Physiol 278: H353– H359, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Mackenjee HR, Shaul PW, Magness RR, Rosenfeld CR. Angiotensin II vascular smooth-muscle receptors are not down-regulated in near-term pregnant sheep. Am J Obstet Gynecol 165: 1641– 1648, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Magness RR, Cox K, Rosenfeld CR, Gant NF. Angiotensin II metabolic clearance rate and pressor responses in nonpregnant and pregnant women. Am J Obstet Gynecol 171: 668– 679, 1994 [DOI] [PubMed] [Google Scholar]
- 30. Magness RR, Osei-Boaten K, Mitchell MD, Rosenfeld CR. In vitro prostacyclin production by ovine uterine and systemic arteries: effects of angiotensin II. J Clin Invest 76: 2206– 2212, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Magness RR, Rosenfeld CR, Hassan A, Shaul PW. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am J Physiol Heart Circ Physiol 270: H1914– H1923, 1996 [DOI] [PubMed] [Google Scholar]
- 32. Magness RR, Rosenfeld CR. Systemic and uterine responses to α-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol 155: 897– 904, 1986 [DOI] [PubMed] [Google Scholar]
- 33. McMullen JR, Gibson KJ, Lumbers ER, Burrell LH, Wu J. Interactions between AT1 and AT2 receptors in uterine arteries from pregnant ewes. Eur J Pharmacol 378: 195– 202, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Misenhimer HR, Margulies SL, Panigel M, Ramsey EM, Donner MW. Effects of vasoconstrictive drugs on the placental circulation of the Rhesus monkey. Invest Radiol 7: 469– 499, 1972 [DOI] [PubMed] [Google Scholar]
- 35. Naden RP, Coultrup S, Arant BS, Jr, Rosenfeld CR. Metabolic clearance of angiotensin II in pregnant and nonpregnant sheep. Am J Physiol Endocrinol Metab 249: E49– E55, 1985 [DOI] [PubMed] [Google Scholar]
- 36. Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest 68: 468– 474, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nielsen AH, Schauser KH, Poulsen K. The uteroplacental renin-angiotensin system. Placenta 21: 468– 477, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology 24: 58– 71, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perales AJ, Naden RP, Laptook AR, Rosenfeld CR. Fetal responses to maternal infusions of angiotensin II. Am J Obstet Gynecol 154: 195– 203, 1986 [DOI] [PubMed] [Google Scholar]
- 40. Rosenfeld CR, Barton MD, Meschia G. Effects of epinephrine on distribution of blood flow in the pregnant ewe. Am J Obstet Gynecol 124: 156– 163, 1976 [DOI] [PubMed] [Google Scholar]
- 41. Rosenfeld CR, Cox BE, Magness RR, Shaul PW. Ontogeny of angiotensin II vascular smooth muscle receptors in ovine fetal and placental and uterine arteries. Am J Obstet Gynecol 168: 1562– 1569, 1993 [DOI] [PubMed] [Google Scholar]
- 42. Rosenfeld CR, DeSpain K, Liu X-t. Differential sensitivity to angiotensin II and norepinephrine in human uterine arteries. J Clin Endocrinol Metab. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenfeld CR.(Editor). The Uterine Circulation. Ithaca, NY: Perinatology, 1989, p. 1–310 [Google Scholar]
- 44.Rosenfeld CR, Gant NF., Jr The chronically instrumented ewe, a model for studying vascular reactivity to angiotensin II in pregnancy. J Clin Invest 67: 486– 492, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rosenfeld CR, Gresores A, Magness RR, Roy TA. Comparison of angiotensin II in fetal and pregnant sheep. Metabolic clearance and vascular sensitivity. Am J Physiol Endocrinol Metab 268: E237– E247, 1995 [DOI] [PubMed] [Google Scholar]
- 46. Rosenfeld CR, Naden RP. Uterine and non-uterine vascular responses to angiotensin II in ovine pregnancy. Am J Physiol Heart Circ Physiol 257: H17– H24, 1989 [DOI] [PubMed] [Google Scholar]
- 47. Rosenfeld CR, West JA. Circulatory responses to systemic infusions of norepinephrine in the pregnant ewe. Am J Obstet Gynecol 127: 376– 383, 1977 [DOI] [PubMed] [Google Scholar]
- 48. Rosenfeld CR. Distribution of cardiac output in ovine pregnancy. Am J Physiol Heart Circ Physiol 232: H231– H235, 1977 [DOI] [PubMed] [Google Scholar]
- 49. Rosenfeld CR. Mechanisms regulating angiotensin II responsiveness by the uteroplacental circulation. Am J Physiol Regul Integr Comp Physiol 281: R1025– R1040, 2001 [DOI] [PubMed] [Google Scholar]
- 50. Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia–A state of sympathetic overactivity. N Engl J Med 335: 1480– 1485, 1996 [DOI] [PubMed] [Google Scholar]
- 51. Stanley JR, Giammattie CE, Sheikh AU, Green JL, Zehnder T, Rose JC. Effects of chronic infusion of angiotensin II on rennin and blood pressure in the late-gestation fetal sheep. Am J Obstet Gynecol 176: 931– 937, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Sullivan JA, Rupnow HL, Cale JM, Magness RR, Bird IM. Pregnancy and ovarian steroid regulation of angiotensin II type 1 and type 2 receptor expression in ovine uterine artery endothelium and vascular smooth muscle. Endothelium 12: 41– 56, 2005 [DOI] [PubMed] [Google Scholar]
- 53. Talledo OE, Chesley LC, Zuspan FP. Renin-angiotensin system in normal and toxemic pregnancies. III. Differential sensitivity to angiotensin II and norepinephrine in toxemia of pregnancy. Am J Obstet Gynecol 100: 218– 221, 1968 [Google Scholar]
- 54. Wallenburg HCS, Hutchinson DL. A radiographic study of the effects of catecholamines on uteroplacental blood flow in Rhesus monkey. J Med Primatol 8: 57– 65, 1979 [DOI] [PubMed] [Google Scholar]
- 55. Warwick D, Kee N, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology 111: 506– 512, 2009 [DOI] [PubMed] [Google Scholar]
- 56. Zwart AS, Davis EA, Widdop RE. Modulation of AT1 receptor-mediated contraction of rat uterine artery by AT2 receptors. Br J Pharmacol 125: 1429– 1436, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]